Abstract
Malignant melanoma (MM) is the most aggressive form of skin cancer. Adult anthropometry influences MM development; however, associations between childhood body size and future melanomagenesis are largely unknown. We investigated whether height, body mass index (BMI; weight (kg)/height (m)2), and body surface area (BSA) at ages 7–13 years and birth weight are associated with adult MM. Data from the Copenhagen School Health Records Register, containing annual height and weight measurements of 372,636 Danish children born in 1930–1989, were linked with the Danish Cancer Registry. Cox regression analyses were performed. During follow-up, 2,329 MM cases occurred. Height at ages 7–13 years was significantly associated with MM, even after BMI and BSA adjustments. No significant BMI-MM or BSA-MM associations were detected when adjusting for height. Children who were persistently tall at both age 7 years and age 13 years had a significantly increased MM risk compared with children who grew taller between those ages. Birth weight was positively associated with MM. We conclude that associations between body size and MM originate early in life and are driven largely by height and birth weight, without any comparable influence of BMI or BSA. Melanoma transformation is unlikely to be due to height per se; however, height-regulating processes in childhood present new areas for mechanistic explorations of this disease.
Keywords: birth weight, body height, body mass index, body surface area, child, melanoma
During the last several decades, the incidence of malignant melanoma (MM) has dramatically increased. In 2012, approximately 232,000 new cases were diagnosed worldwide (1). In contrast with many other human malignancies, the incidence rate for MM starts to increase during adolescence (2). A genetic predisposition, melanocytic nevi, fair skin pigmentation, and exposure to ultraviolet radiation are known risk factors for melanoma development (melanomagenesis) (3, 4). However, the underlying processes responsible for a predisposition to MM are multifaceted and not fully understood; they conceivably involve additional etiological factors.
Childhood is a critical period of growth in which the skin expands widely to accommodate the growing child. The proliferation rate of epidermal melanocytes changes with development and peaks during childhood and adolescence (5). Notably, murine skin grafts from newborns are more prone to develop melanoma when exposed to ultraviolet radiation compared with skin grafts from adults (6), indicating that preadult life is a vulnerable period of particular significance in predisposing individuals to future melanomagenesis. Accordingly, several studies have examined whether birth weight is linked to MM risk, and some, but not all, have found positive associations (7–11). At the other end of the life span, several studies have investigated whether adult height, body mass index (BMI), and body surface area (BSA) are associated with MM risk (12–21). Generally, adult height and BSA are positively associated with MM, whereas results for BMI are equivocal. Despite these studies, there is a gap in knowledge as to whether the association between body size and MM risk originates in childhood. Therefore, we investigated whether childhood height, BMI, BSA, or birth weight was associated with an increased risk of cutaneous and invasive forms of MM in a large cohort of Danish children.
METHODS
Cohort
The Copenhagen School Health Records Register (CSHRR) contains computerized health information on 372,636 Danish children who attended public and private schools in the Copenhagen municipality and were born during the period 1930–1989 (22). Each child had an individual school health card that included information on the child's name and date of birth. Furthermore, it included information on annual height and weight measurements from the ages of 7 years to 13 years which were taken by physicians and nurses using standardized procedures.
During the health examination, weight and height measurements were conducted without shoes and while the children were either naked or wearing underwear; only after 1973 was light clothing allowed (22). After 1983, body size measurements were only available at ages corresponding to school entry and exit due to procedural changes in the municipality. Children were measured more frequently if they had special health conditions or needs. From the year 1942 (year of birth 1936) onwards, information on birth weight is available. Birth weight was either parentally reported or copied from the child's health book.
BMI was calculated as weight (kilograms) divided by the square of height (meters), and BSA was calculated according to the formula (weight (kg)0.5378 × height (cm)0.3964 × 0.024265) as described by Haycock et al. (23). BMI and BSA values were transformed to z scores based on age- and sex-specific reference values, whereas height data were transformed to z scores based on age-, sex- and birth cohort-specific (5-year interval) reference values. For BMI, the reference population was selected from a period when the obesity rate was low and stable (children born from 1955 to 1960) (24). The BMI, BSA, and height z scores were computed using the lambda mu sigma method (25). Height and BMI z scores were directly used if the measurements were taken exactly on the child's birthday. Otherwise, if 2 measurements existed within a period of ±12 months, the z scores were interpolated to the child's age in whole years (i.e., age 7, 8, …, 13 years) or extrapolated if only 1 measurement was available.
On April 2, 1968, the Danish Civil Registration System introduced unique personal identification numbers to all living citizens of Denmark and those born thereafter (26). These identification numbers were recorded on the health cards and retrieved for children in school prior to this time on the basis of their name, date of birth, and sex. A conservative approach was used to ensure the validity of the retrieved numbers, and as a result they were identified for 88% of all children (22). The identification numbers enabled linkage at the individual level to the Danish Cancer Registry for follow-up. The Danish Cancer Registry was founded in 1942 and contains records of the incidence of malignant and selected benign tumors in the Danish population (27). Notably, 89% of the cancers are morphologically confirmed, ensuring a high validity of the registry (27). The diagnosis of cutaneous MM was defined according to the International Classification of Diseases, Seventh Revision (code 190) and the International Classification of Diseases, Tenth Revision (code C43). Information on vital status was obtained through linkage to the Vital Statistics Register (26).
Study population
To be eligible for this study, individuals needed an identification number (to enable follow-up) and needed to be at least 15 years of age. Accordingly, the follow-up period was initiated in 1968 when individuals received this number or at age 15 years, depending on which occurred last. Additionally, follow-up ended on the date of an MM diagnosis, emigration, death, or loss to follow-up or on December 31, 2012 (date of the last available update from the Danish Cancer Registry), whichever came first. Of the total number of CSHRR individuals (n = 372,636), we excluded 42,668 individuals without identification numbers (e.g., children for whom numbers could not be retrieved), 2,709 individuals who emigrated, died, or were lost to follow-up prior to age 15 years or April 2, 1968, 4 individuals with a missing date of MM diagnosis, 19 cases who were diagnosed with MM before the age of 15 years or April 2, 1968, 6,143 individuals with missing height or weight measurements, and 9 individuals with outlying height or BMI values (z score <−4.5 or >4.5) at all ages. This resulted in a cohort of 321,084 eligible participants (162,638 men and 158,446 women) (see Web Figure 1, available at http://aje.oxfordjournals.org/). For the growth analyses of height, only individuals who had measurements at both age 7 years and age 13 years were included (total = 258,594; 129,876 men and 128,718 women). Because children rarely change by ±1 z score in height, we present the results for an “average” child with a height z score of 0, a “tall” child with a z score of 0.5, and a “persistently tall” child with a z score of 0.5 at both age 7 years and age 13 years.
In the subanalysis of birth weight, the further eligibility criteria of being born in 1936 or onwards and having a birth weight of 2.0–5.5 kg were applied. This resulted in a study population of 244,847 individuals (124,999 men and 119,848 women) (Web Figure 2).
Statistical methods
Cox proportional hazards regression analyses were conducted to analyze the associations between height or BMI or BSA z scores at each age from 7 to 13 years and the risk of adult MM. The association between birth weight (kg) and the future risk of MM was investigated using the same approach. Age was the underlying time metric, and all analyses were stratified by birth cohort (5-year strata: 1930–1934 … 1980–1984 and 1985–1989). Because height and BMI were weakly correlated (rage7 = 0.19, rage13 = 0.29), the analyses for the explanatory variables were repeated using mutual adjustment (i.e., with the height models adjusting for BMI or BSA, the BMI models adjusting for height, and the BSA models adjusting for height). Furthermore, we found that height and BSA were strongly correlated (rage7 = 0.83, rage13 = 0.79), so we repeated the same adjustments. Additionally, using Cox proportional hazards regression, we investigated the associations between different growth patterns in height z scores from ages 7 to 13 years and MM. Departures from linearity were assessed by testing against a restricted cubic spline with 5 knots placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles. The proportional hazards assumptions were checked by including a time-varying covariate in the Cox regression model.
Using a likelihood ratio test, potential interactions between birth cohort and associations between height, BMI, BSA, or birth weight and the risk of developing MM were investigated in 5-year intervals up to 1975. Due to a low number of cases, the birth years from 1975 to 1989 were combined into 1 category (1930–1934 … 1970–1974 and 1975–1989). Furthermore, potential interactions between sex and height, BMI, BSA, or birth weight and the risk of MM were also investigated (using a likelihood ratio test). Overall, no departures from linearity (all P’s > 0.14), violations of the proportional hazards assumption, or differences in the associations by birth cohort (all P’s > 0.12) were detected. None of the associations differed significantly by sex; thus, all analyses were stratified by sex. Since sex-specific estimates may be of interest, they are presented in Web Tables 1–4. The statistical analyses were conducted using Stata, version 12.1 (StataCorp LP, College Station, Texas). The study was approved by the Danish Data Protection Agency. In accordance with Danish law, informed consent is not required for purely register-based studies of preexisting information.
RESULTS
During approximately 11 million person-years of follow-up, 2,329 MM cases (1,057 men and 1,272 women) were identified. The age span for MM diagnosis was broad, ranging from 16 years to 82 years, with a median age of 58 years for men (5th–95th percentile range, 33–75) and 52 years for women (5th–95th percentile range, 28–73). Between ages 7 and 13 years, median height, BMI, and BSA values increased from approximately 122 cm to 156 cm, from 15 to 18, and from 0.87 m2 to 1.38 m2, respectively (Table 1).
Table 1.
Median Values for Height, Body Mass Index, and Body Surface Area for 321,084 Boys and Girls at Each Age From Ages 7 to 13 Years, Copenhagen, Denmark, 1968–2012a
| Characteristic and Age, years | Boys | Girls | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of Childrenb | Median | 5th Percentile | 95th Percentile | No. of Childrenb | Median | 5th Percentile | 95th Percentile | |
| Height, cm | ||||||||
| 7 | 151,893 | 122.6 | 114.0 | 131.2 | 147,998 | 121.7 | 113.0 | 130.4 |
| 8 | 153,742 | 127.9 | 118.9 | 137.0 | 149,777 | 126.9 | 117.9 | 136.0 |
| 9 | 147,874 | 133.1 | 123.8 | 142.9 | 144,778 | 132.1 | 122.7 | 142.0 |
| 10 | 143,832 | 138.1 | 128.4 | 148.4 | 141,261 | 137.2 | 127.3 | 148.0 |
| 11 | 142,678 | 142.9 | 132.8 | 153.8 | 140,371 | 142.9 | 132.0 | 154.8 |
| 12 | 141,210 | 147.7 | 137.0 | 159.8 | 139,313 | 149.3 | 137.1 | 161.7 |
| 13 | 138,607 | 153.5 | 141.5 | 167.6 | 137,413 | 155.5 | 143.0 | 167.0 |
| Body mass indexc | ||||||||
| 7 | 151,893 | 15.4 | 13.8 | 17.7 | 147,998 | 15.3 | 13.5 | 18.0 |
| 8 | 153,742 | 15.7 | 14.0 | 18.3 | 149,777 | 15.6 | 13.7 | 18.7 |
| 9 | 147,874 | 16.0 | 14.2 | 19.0 | 144,778 | 16.0 | 13.9 | 19.6 |
| 10 | 143,832 | 16.4 | 14.4 | 19.9 | 141,261 | 16.4 | 14.2 | 20.4 |
| 11 | 142,678 | 16.8 | 14.7 | 20.7 | 140,371 | 16.8 | 14.4 | 21.3 |
| 12 | 141,210 | 17.2 | 15.0 | 21.6 | 139,313 | 17.5 | 14.8 | 22.2 |
| 13 | 138,607 | 17.8 | 15.3 | 22.3 | 137,413 | 18.3 | 15.3 | 23.2 |
| Body surface aread, m2 | ||||||||
| 7 | 151,893 | 0.88 | 0.78 | 1.02 | 147,998 | 0.87 | 0.76 | 1.01 |
| 8 | 153,742 | 0.95 | 0.83 | 1.10 | 149,777 | 0.94 | 0.82 | 1.10 |
| 9 | 147,874 | 1.02 | 0.89 | 1.20 | 144,778 | 1.01 | 0.87 | 1.20 |
| 10 | 143,832 | 1.09 | 0.95 | 1.29 | 141,261 | 1.08 | 0.93 | 1.30 |
| 11 | 142,678 | 1.16 | 1.01 | 1.39 | 140,371 | 1.16 | 0.99 | 1.41 |
| 12 | 141,210 | 1.24 | 1.07 | 1.50 | 139,313 | 1.27 | 1.06 | 1.54 |
| 13 | 138,607 | 1.33 | 1.13 | 1.63 | 137,413 | 1.38 | 1.15 | 1.65 |
a Based on data from the Copenhagen School Health Records Register.
b Numbers of individuals at each age may vary from the total, because not every child had anthropometric values at each age.
c Body mass index was defined as weight (kg)/height (m)2.
d Body surface area was defined as weight (kg)0.5378 × height (cm)0.3964 × 0.024265.
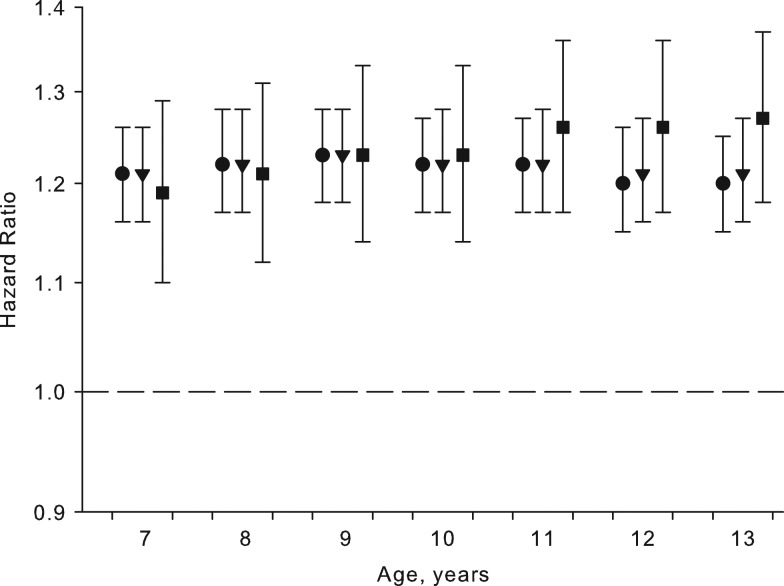
We investigated associations between childhood height at the ages of 7–13 years and the risk of MM and found significant and positive associations between height at all ages and MM. At ages 7 and 13 years, the hazard ratios per height z score were 1.21 (95% confidence interval (CI): 1.16, 1.26) and 1.20 (95% CI: 1.15, 1.25), respectively (Figure 1, Web Table 5). One z-score increment in height corresponded to approximately 5.1 cm at age 7 years and 8 cm at age 13 years in boys and approximately 5.2 cm at age 7 years and 6.9 cm at age 13 years in girls. The values were larger at age 13 years due to greater variation in height at this age. Similar results were found at ages 8–12 years (Figure 1, Web Table 5). Furthermore, when the associations were adjusted for childhood BMI or BSA, the associations between childhood height and MM remained virtually unchanged (Figure 1, Web Table 5), indicating that the height-MM association was independent of childhood BMI and BSA.
Figure 1.
Hazard ratios for the risk of malignant melanoma per increment of height z score in childhood, unadjusted (•) and adjusted for body mass index (▾) or body surface area (▪) and stratified by sex and 5-year birth cohort, Copenhagen, Denmark, 1968–2012. Body mass index was defined as weight (kg)/height (m)2; body surface area was defined as weight (kg)0.5378 × height (cm)0.3964 × 0.024265. Based on data from the Copenhagen School Health Records Register. Bars, 95% confidence intervals.
The investigation of associations between childhood BMI or BSA at ages 7–13 years and the diagnosis of MM in adulthood revealed that for BMI the hazard ratios were small and in the positive direction but generally not statistically significant, whereas the associations between BSA and MM were positively significant (Table 2). The results were similar at all ages. Notably, when these results were adjusted for childhood height, the hazard ratios were attenuated for both BMI and BSA, and all became nonsignificant (Table 2).
Table 2.
Hazard Ratios for the Risk of Adult Malignant Melanoma per Increment of Body Mass Index or Body Surface Area z Score in Childhood, by Age, Copenhagen, Denmark, 1968–2012a
| Age, years | No. of Childrenb | No. of Casesb | Body Mass Indexc | Body Surface Aread | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Height-Adjustede | Unadjusted | Height-Adjustede | |||||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||
| 7 | 299,891 | 2,194 | 1.04 | 1.00, 1.09 | 1.01 | 0.96, 1.05 | 1.19 | 1.14, 1.24 | 1.02 | 0.93, 1.11 |
| 8 | 303,519 | 2,227 | 1.05 | 1.00, 1.10 | 1.00 | 0.96, 1.05 | 1.20 | 1.15, 1.25 | 1.01 | 0.93, 1.10 |
| 9 | 292,652 | 2,207 | 1.05 | 1.00, 1.10 | 0.99 | 0.95, 1.04 | 1.19 | 1.14, 1.25 | 0.99 | 0.91, 1.08 |
| 10 | 285,093 | 2,176 | 1.04 | 1.00, 1.09 | 0.99 | 0.94, 1.04 | 1.18 | 1.13, 1.24 | 0.98 | 0.91, 1.07 |
| 11 | 283,049 | 2,178 | 1.03 | 0.98, 1.08 | 0.97 | 0.93, 1.02 | 1.17 | 1.12, 1.22 | 0.96 | 0.88, 1.04 |
| 12 | 280,523 | 2,164 | 1.03 | 0.98, 1.08 | 0.97 | 0.92, 1.02 | 1.16 | 1.11, 1.21 | 0.95 | 0.87, 1.03 |
| 13 | 276,020 | 2,144 | 1.01 | 0.97, 1.06 | 0.96 | 0.91, 1.00 | 1.14 | 1.09, 1.19 | 0.93 | 0.86, 1.00 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Based on data from the Copenhagen School Health Records Register. All analyses were stratified by sex and 5-year birth cohort.
b Numbers of individuals at each age may vary from the total, as not every child had anthropometric values at each age.
c Body mass index was defined as weight (kg)/height (m)2.
d Body surface area was defined as weight (kg)0.5378 × height (cm)0.3964 × 0.024265.
e Adjusted for childhood height.
Furthermore, we also investigated whether growth in height between the ages of 7 and 13 years was associated with MM. These analyses included 2,022 MM cases (910 men and 1,112 women) who had height measurements available at both ages. Most children remained in their growth trajectory, and 32% of the children had height changes of at least ±0.5 z score between these ages. We found that compared with a child who had average height at both age 7 years and age 13 years (reference, z score = 0), a child who had an average height at age 7 years but was taller at age 13 years (z score = 0.5, corresponding to approximately 4 cm greater growth in boys and girls) did not have a significantly higher risk of MM (P = 0.29) (Table 3). However, compared with the reference child, significantly increased risks of MM were observed for a child who was taller at age 7 years but of average height at age 13 years (P = 0.001) and for a child who was persistently taller at ages 7 and 13 years (P < 0.001) (Table 3). Further, compared with a child who was of average height at age 7 years but taller at age 13 years, a child who was persistently taller at ages 7 and 13 years had a significantly higher risk of MM (hazard ratio = 1.08, 95% CI: 1.03, 1.13; P = 0.001).
Table 3.
Hazard Ratios for the Risk of Adult Malignant Melanoma According to a 0.5-z-Score Change in Height Between the Ages of 7 and 13 Years (n = 258,594 Individuals and 2,022 Cases), Copenhagen, Denmark, 1968–2012a
| Height z Score at Age 7 Yearsb,c | Height z Score at Age 13 Years | |||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | |||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| 0 | 1 | Referent | 1.02 | 0.98, 1.07 | 0.29 | |
| 0.5 | 1.08 | 1.03, 1.13 | 0.001 | 1.10 | 1.08, 1.13 | <0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Based on data from the Copenhagen School Health Records Register. All analyses were stratified by sex and 5-year birth cohort.
b Deviations from linearity in the associations were not detected (all P’s > 0.16).
c An “average” child had a height z score of 0, a “taller” child had a z score of 0.5, and a “persistently taller” child had a z score of 0.5 at both age 7 years and age 13 years.
In the subanalysis on birth weight, 1,682 individuals (755 men and 927 women) were diagnosed with MM. Birth weight was positively and significantly associated with adult MM, with a hazard ratio of 1.13 (95% CI: 1.03, 1.23) per kg of birth weight. Sex-specific estimates are presented in Web Table 4. No interactions between birth weight and height, BMI, or BSA were detected (all P’s > 0.20).
DISCUSSION
In this study, we found that greater childhood height at the ages of 7–13 years was significantly associated with an increased risk of MM in adulthood. Moreover, the height-MM association remained virtually unchanged after adjustment for BMI or BSA, indicating that the association works independently of BMI and BSA in children. For childhood BMI, we detected only small positive associations that were mostly not statistically significant, and after adjustment for height they disappeared altogether. Furthermore, the associations between childhood BSA and MM were statistically significant; however, they were attenuated after adjustment for height. Collectively, these results suggest that height plays a more dominant role than BMI and BSA in MM development. Growth during childhood was also associated with MM risk—children who were persistently tall had a higher risk of MM in adulthood than children who grew tall between ages 7 and 13 years. Finally, we observed that birth weight was positively and significantly associated with MM development.
The associations between body size and MM development have been investigated in various adult populations, but the results have been equivocal. Several studies have found associations between greater adult height and MM (12, 13, 16–20), and generally height displays the strongest association with MM as compared with cancers at other anatomical sites (17–20). Similarly, although there are discrepancies in the literature (12–16, 28, 29), BSA is generally positively associated with MM development. However, in agreement with our results, the lack of analyses adjusting BSA for height could result in incorrect identification of BSA as an MM risk factor, even though it is in fact the height parameter of BSA that is the main driver of the positive association with MM risk. In regard to the BMI-MM association, results of previous studies contradict each other (12–16, 21, 28, 30), making it difficult to disentangle whether BMI is a risk factor for melanomagenesis. Nevertheless, sex-specific differences have been reported, with BMI being significantly associated with MM development in men but not in women, conceivably resulting from different sunbathing habits in obese women (12). However, proposed biological mechanisms are lacking.
Our height-MM results derive from measurements taken from children, making direct comparisons with adult studies challenging, and only a few studies have been conducted in adolescents. In agreement with our identified height-MM association, a study of Israeli adolescents (ages 16–19 years) found that greater height was a risk factor for MM (31). Additionally, BSA was also found to be associated with MM risk (31). In contrast, in a study of American agricultural workers which used self-reported heights and weights upon enrollment plus recalled weight at age 20 years, Dennis et al. (32) did not find associations between height and MM. However, they found that BMI and BSA at age 20 years were positively associated with MM (32). Different findings for the height-MM associations may be due to ethnicity, population size, and the inaccuracy of self-reported and recalled measurements.
In the growth analyses of height and MM development, our results indicated that the risk of MM was 8% higher among children who were persistently taller, as compared with children who grew taller between 7 and 13 years of age. These results indicate that a child's growth pattern matters as well, since 2 children at age 13 years will have different melanoma risk profiles depending on whether they have been persistently tall or grew tall between 7 and 13 years of age. Collectively, these results suggest that growth before age 7 years is of particular importance for MM risk. None of the previous studies were performed on changes in childhood height in relation to future MM risk, and this is most likely due to the lack of appropriate data resources.
There are putative pathophysiological mechanisms through which height may affect the subsequent risk of MM. Studies have identified genes that are associated with both height (33) and the underlying processes linked to MM progression (34–36). Additionally, insulin-like growth factor 1 is positively associated with growth in childhood (37). Interestingly, insulin-like growth factor 1 has been shown to be a potent growth factor for melanoma cells (38), constituting a plausible link between greater childhood height and future melanomagenesis. Moreover, a recent study found a direct association between the number of melanocytic nevi and adult height (39), providing another potential explanation for the height-MM association. Finally, greater height often indicates a higher socioeconomic status (40), providing more traveling opportunities that could potentially lead to increased sun exposure and MM risk. Nevertheless, the lack of differences in the associations by birth cohort in this study, even in the years when charter vacation packages became popular (41), suggests that the height-MM association occurs as a result of other factors. Accordingly, it is likely that it is not height per se but rather a function of underlying height- regulating mechanisms that eventually drives the malignant transformation.
Further, we found that birth weight was positively associated with the risk of MM. This finding suggests that accelerated growth during fetal life somehow sensitizes children to MM development in adulthood. Our result is consistent with an earlier study on this cohort (but with fewer cases) (8). There are plausible biological links between birth weight and MM. In humans, the melanocortin 1 receptor gene (MCR1) is highly polymorphic (42). In separate studies, 2 of its alleles were associated with higher birth weight (43) and an increased risk of MM (42); thus, it is a potential pathway linking birth weight and MM.
Our study had several strengths. Because of the unique CSHRR resource, which has a large population size, and minimal loss to follow-up, our findings were not influenced by selection bias. Additionally, the inclusion of carefully recorded height and weight measurements (in contrast to recalled and self-reported values) eliminated the influence of recall bias on the results. Birth weight was obtained through parental recall (often supported by written documentation), and prior studies have found that this is a valid method (44). The current study may have been limited by the lack of information on factors that are potentially related to both childhood body size and MM risk, such as the number of melanocytic nevi, socioeconomic status, sun exposure history, and genetic predisposition.
Our findings are relevant to contemporary populations. Although there were secular increases in height across the years included in our study, we did not find differences in the height-MM association by birth cohort, which strengthens the plausibility that biological mechanisms underlie the associations. The Danish population is among the tallest in the world (45), but since we did not detect nonlinearity in the height-MM associations, the risk applies across the entire spectrum of height, not just to the tallest children. For example, our results show that compared with an average-height 7-year-old boy (123.3 cm; 48.5 inches), a boy who is 5.1 cm (2 inches) taller has a 21% greater risk of MM. The strength of these associations suggests that height-regulating processes in childhood could be a target for mechanistic explorations of MM.
In conclusion, we found that childhood height, independently of BMI and BSA, is positively and significantly associated with the risk of adult MM. In contrast, both childhood BMI and BSA, adjusted for height, did not play a noteworthy role in the etiology of adult MM. Furthermore, our findings showed that height growth pattern in childhood is associated with an individual's predisposition to melanomagenesis in adulthood. Finally, we found that a high birth weight is associated with increased risk of adult MM. Collectively, these results show that height and birth weight play a role in the origins of MM, thus contributing to the emerging clarification of the association between early-life risk factors and MM development in adulthood.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Clinical Epidemiology [formerly Institute of Preventive Medicine], Bispebjerg and Frederiksberg Hospital, The Capital Region, Copenhagen, Denmark (Kathrine D. Meyle, Michael Gamborg, Thorkild I. A. Sørensen, Jennifer L. Baker); Department of Public Health, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (Thorkild I. A. Sørensen); and Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (Thorkild I. A. Sørensen, Jennifer L. Baker).
This work was supported by the European Research Council under the European Union's Seventh Framework Programme and grant agreement 281419 (awarded to J.L.B.).
The Copenhagen School Health Records Register was built in collaboration between the Copenhagen City Archives and the Department of Clinical Epidemiology at Bispebjerg and Frederiksberg Hospital.
The funder of the study had no influence on the study design, data collection, analyses, or interpretation of results, manuscript preparation, or the decision to submit the manuscript for publication.
Conflict of interest: none declared.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Ervik M, et al. . GLOBOCAN 2012: Estimated Cancer Incidence, Mortality, and Prevalence Worldwide in 2012.(IARC CancerBase no. 11, version 1.0). Lyon, France: International Agency for; Research on Cancer; 2013. http://globocan.iarc.fr. Accessed June 30, 2015. [Google Scholar]
- 2. Berg P, Lindelof B. Differences in malignant melanoma between children and adolescents. A 35-year epidemiological study. Arch Dermatol. 1997;133(3):295–297. [PubMed] [Google Scholar]
- 3. Holly EA, Kelly JW, Shpall SN, et al. . Number of melanocytic nevi as a major risk factor for malignant melanoma. J Am Acad Dermatol. 1987;17(3):459–468. [DOI] [PubMed] [Google Scholar]
- 4. Meyle KD, Guldberg P. Genetic risk factors for melanoma. Hum Genet. 2009;126(4):499–510. [DOI] [PubMed] [Google Scholar]
- 5. Autier P, Boyle P. Artificial ultraviolet sources and skin cancers: rationale for restricting access to sunbed use before 18 years of age. Nat Clin Pract Oncol. 2008;5(4):178–179. [DOI] [PubMed] [Google Scholar]
- 6. Noonan FP, Recio JA, Takayama H, et al. . Neonatal sunburn and melanoma in mice. Nature. 2001;413(6853):271–272. [DOI] [PubMed] [Google Scholar]
- 7. McCormack VA, dos Santos Silva I, Koupil I, et al. . Birth characteristics and adult cancer incidence: Swedish cohort of over 11,000 men and women. Int J Cancer. 2005;115(4):611–617. [DOI] [PubMed] [Google Scholar]
- 8. Ahlgren M, Wohlfahrt J, Olsen LW, et al. . Birth weight and risk of cancer. Cancer. 2007;110(2):412–419. [DOI] [PubMed] [Google Scholar]
- 9. Olesen AV, Parner ET, Mortensen PB, et al. . Prenatal risk factors for cutaneous malignant melanoma: follow-up of 2,594,783 Danes born from 1950 to 2002. Cancer Epidemiol Biomarkers Prev. 2009;18(1):155–161. [DOI] [PubMed] [Google Scholar]
- 10. O'Rorke MA, Black C, Murray LJ, et al. . Do perinatal and early life exposures influence the risk of malignant melanoma? A Northern Ireland birth cohort analysis. Eur J Cancer. 2013;49(5):1109–1116. [DOI] [PubMed] [Google Scholar]
- 11. Franco-Lie I, Iversen T, Robsahm TE, et al. . Birth weight and melanoma risk: a population-based case-control study. Br J Cancer. 2008;98(1):179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thune I, Olsen A, Albrektsen G, et al. . Cutaneous malignant melanoma: association with height, weight and body-surface area. a prospective study in Norway. Int J Cancer. 1993;55(4):555–561. [DOI] [PubMed] [Google Scholar]
- 13. Veierod MB, Thelle DS, Laake P. Diet and risk of cutaneous malignant melanoma: a prospective study of 50,757 Norwegian men and women. Int J Cancer. 1997;71(4):600–604. [DOI] [PubMed] [Google Scholar]
- 14. Naldi L, Altieri A, Imberti GL, et al. . Cutaneous malignant melanoma in women. Phenotypic characteristics, sun exposure, and hormonal factors: a case-control study from Italy. Ann Epidemiol. 2005;15(7):545–550. [DOI] [PubMed] [Google Scholar]
- 15. Gallus S, Naldi L, Martin L, et al. . Anthropometric measures and risk of cutaneous malignant melanoma: a case-control study from Italy. Melanoma Res. 2006;16(1):83–87. [DOI] [PubMed] [Google Scholar]
- 16. Olsen CM, Green AC, Zens MS, et al. . Anthropometric factors and risk of melanoma in women: a pooled analysis. Int J Cancer. 2008;122(5):1100–1108. [DOI] [PubMed] [Google Scholar]
- 17. Green J, Cairns BJ, Casabonne D, et al. . Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011;12(8):785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kabat GC, Heo M, Kamensky V, et al. . Adult height in relation to risk of cancer in a cohort of Canadian women. Int J Cancer. 2013;132(5):1125–1132. [DOI] [PubMed] [Google Scholar]
- 19. Kabat GC, Kim MY, Hollenbeck AR, et al. . Attained height, sex, and risk of cancer at different anatomic sites in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2014;25(12):1697–1706. [DOI] [PubMed] [Google Scholar]
- 20. Wirén S, Häggström C, Ulmer H, et al. . Pooled cohort study on height and risk of cancer and cancer death. Cancer Causes Control. 2014;25(2):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Praestegaard C, Kjaer SK, Christensen J, et al. . Obesity and risks for malignant melanoma and non-melanoma skin cancer: results from a large Danish prospective cohort study. J Invest Dermatol. 2015;135(3):901–904. [DOI] [PubMed] [Google Scholar]
- 22. Baker JL, Olsen LW, Andersen I, et al. . Cohort profile: the Copenhagen School Health Records Register. Int J Epidemiol. 2009;38(3):656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93(1):62–66. [DOI] [PubMed] [Google Scholar]
- 24. Baker JL, Olsen LW, Sørensen TIA. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357(23):2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11(10):1305–1319. [DOI] [PubMed] [Google Scholar]
- 26. Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 suppl):22–25. [DOI] [PubMed] [Google Scholar]
- 27. Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 suppl):42–45. [DOI] [PubMed] [Google Scholar]
- 28. Shors AR, Solomon C, McTiernan A, et al. . Melanoma risk in relation to height, weight, and exercise (United States). Cancer Causes Control. 2001;12(7):599–606. [DOI] [PubMed] [Google Scholar]
- 29. Sergentanis TN, Antoniadis AG, Gogas HJ. Obesity and risk of malignant melanoma: a meta-analysis of cohort and case-control studies. Eur J Cancer. 2013;49(3):642–657. [DOI] [PubMed] [Google Scholar]
- 30. Kirkpatrick CS, White E, Lee JA. Case-control study of malignant melanoma in Washington State. II. Diet, alcohol, and obesity. Am J Epidemiol. 1994;139(9):869–880. [DOI] [PubMed] [Google Scholar]
- 31. Levine H, Afek A, Shamiss A, et al. . Country of origin, age at migration and risk of cutaneous melanoma: a migrant cohort study of 1,100,000 Israeli men. Int J Cancer. 2013;133(2):486–494. [DOI] [PubMed] [Google Scholar]
- 32. Dennis LK, Lowe JB, Lynch CF, et al. . Cutaneous melanoma and obesity in the Agricultural Health Study. Ann Epidemiol. 2008;18(3):214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lettre G, Jackson AU, Gieger C, et al. . Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet. 2008;40(5):584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raskin L, Fullen DR, Giordano TJ, et al. . Transcriptome profiling identifies HMGA2 as a biomarker of melanoma progression and prognosis. J Invest Dermatol. 2013;133(11):2585–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viswanathan SR, Powers JT, Einhorn W, et al. . Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41(7):843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schultz J, Lorenz P, Gross G, et al. . MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res. 2008;18(5):549–557. [DOI] [PubMed] [Google Scholar]
- 37. Rogers I, Metcalfe C, Gunnell D, et al. . Insulin-like growth factor-I and growth in height, leg length, and trunk length between ages 5 and 10 years. J Clin Endocrinol Metab. 2006;91(7):2514–2519. [DOI] [PubMed] [Google Scholar]
- 38. Rodeck U, Herlyn M, Menssen HD, et al. . Metastatic but not primary melanoma cell lines grow in vitro independently of exogenous growth factors. Int J Cancer. 1987;40(5):687–690. [DOI] [PubMed] [Google Scholar]
- 39. Ribero S, Glass D, Aviv A, et al. . Height and bone mineral density are associated with naevus count supporting the importance of growth in melanoma susceptibility. PLoS One. 2015;10(1):e0116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Samaras TT, Elrick H, Storms LH. Height, health and growth hormone. Acta Paediatr. 1999;88(6):602–609. [DOI] [PubMed] [Google Scholar]
- 41. de Vries E, Coebergh JW. Cutaneous malignant melanoma in Europe. Eur J Cancer. 2004;40(16):2355–2366. [DOI] [PubMed] [Google Scholar]
- 42. Kennedy C, ter Huurne J, Berkhout M, et al. . Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol. 2001;117(2):294–300. [DOI] [PubMed] [Google Scholar]
- 43. Kinsler VA, Abu-Amero S, Budd P, et al. . Germline melanocortin-1-receptor genotype is associated with severity of cutaneous phenotype in congenital melanocytic nevi: a role for MC1R in human fetal development. J Invest Dermatol. 2012;132(8):2026–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walton KA, Murray LJ, Gallagher AM, et al. . Parental recall of birthweight: a good proxy for recorded birthweight. Eur J Epidemiol. 2000;16(9):793–796. [DOI] [PubMed] [Google Scholar]
- 45. Cavelaars AE, Kunst AE, Geurts JJ, et al. . Persistent variations in average height between countries and between socio-economic groups: an overview of 10 European countries. Ann Hum Biol. 2000;27(4):407–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.