Abstract
Of the potential volatile sources for the terrestrial planets, the CI and CM carbonaceous chondrites are closest to the planets' bulk H and N isotopic compositions. For the Earth, the addition of approximately 2–4 wt% of CI/CM material to a volatile-depleted proto-Earth can explain the abundances of many of the most volatile elements, although some solar-like material is also required. Two dynamical models of terrestrial planet formation predict that the carbonaceous chondrites formed either in the asteroid belt (‘classical’ model) or in the outer Solar System (5–15 AU in the Grand Tack model). To test these models, at present the H isotopes of water are the most promising indicators of formation location because they should have become increasingly D-rich with distance from the Sun. The estimated initial H isotopic compositions of water accreted by the CI, CM, CR and Tagish Lake carbonaceous chondrites were much more D-poor than measured outer Solar System objects. A similar pattern is seen for N isotopes. The D-poor compositions reflect incomplete re-equilibration with H2 in the inner Solar System, which is also consistent with the O isotopes of chondritic water. On balance, it seems that the carbonaceous chondrites and their water did not form very far out in the disc, almost certainly not beyond the orbit of Saturn when its moons formed (approx. 3–7 AU in the Grand Tack model) and possibly close to where they are found today.
This article is part of the themed issue ‘The origin, history and role of water in the evolution of the inner Solar System’.
Keywords: water, volatiles, terrestrial planets, asteroids, Grand Tack, chondrites
1. Introduction
The origin of Earth's water has, of course, tremendous astrobiological significance, but it also has considerable significance for understanding how the terrestrial planets formed and more generally for the early evolution of the Solar System. Much of the debate about the origin of water in the inner Solar System has tended to focus on the abundance and isotopic composition of H, and that is the case here. However, as will become apparent, it is important to also consider the abundances and isotopic compositions of other volatile elements, such as C, N and the noble gases, as they will have been accreted along with the H/H2O in the same potential source materials.
Table 1 gives the sizes and estimated bulk H and N isotopic compositions of the largest objects in the inner Solar System that are known to have accreted at least some water when they formed. Mercury does have small amounts of water at its surface, but it is almost certainly exogenous [16]. The largest asteroid, Ceres, contains water in its interior [17], as do some smaller asteroids (e.g. [18–22]), but their H isotopic compositions have not been measured.
Table 1.
The orbital semi-major axes (astronomical units), sizes and estimated bulk H and N isotopic compositions of some of the largest objects in the inner Solar System. The isotopic compositions are given as both absolute ratios and delta values (relative to SMOW (standard mean ocean water) and air for H and N, respectively) because of the differing preferred usages in the astronomical and geochemical communities.
| orbit (AU) | radius (km) | D/H × 10−4 | δD(‰) | 15N/14N × 10−3 | δ15N (‰) | |
|---|---|---|---|---|---|---|
| Venusa | 0.72 | 6052 | 160 ± 20 | ∼100 000 | 3.66−0.62+0.95 | −4−169+258 |
| Earth [1] | 1.0 | 6371 | 1.49 ± 0.03 | −43 ± 20 | 3.68 | 0 |
| Moon [2–8] | 1.0 | 1737 | 1.40 to 1.85 | −100 to 190 | 3.67 to 3.78 | −2 to 27 |
| Marsb | 1.52 | 3390 | ≤1.99 | ≤275 | 3.70 | 7 |
| Vesta [9–11] | 2.36 | 263 | 1.31 to 1.50 | −162 to −34 | <3.61 | <−18 |
Hydrogen isotopes are fractionated relatively easily by both physical and chemical processes. For instance, the H isotopic composition of Venus's atmosphere is hugely enriched in D (table 1) due to the almost complete loss of H to space through photodissociation of H2O [13]. On the other hand, the isotopic compositions of the other bodies are remarkably similar (table 1) given their range of sizes, formation locations and formation histories, as well as the potential sources of their water (table 2).
Table 2.
The average bulk H and N isotopic compositions of analysed CI, CM, CR and Tagish Lake samples, along with the bulk solar compositions, the average H isotopic compositions of H2O and N isotopic compositions of HCN/NH3 in Oort Cloud (OCC) and Jupiter family (JFC) comets, and the range of isotopic compositions in the envelopes of forming protostars (PS). For the averages, the uncertainties are the 1σ standard deviations of the samples.
| D/H × 10−4 | δD (‰) | 15N/14N × 10−3 | δ15N (‰) | |
|---|---|---|---|---|
| CI [23–25] | 1.68 ± 0.01 | 78 ± 7 | 3.83 ± 0.02 | 42 ± 5 |
| CM [23–25] | 1.59 ± 0.20 | −53 ± 130 | 3.84 ± 0.24 | 44 ± 66 |
| CR [23–25] | 2.57 ± 0.21 | 652 ± 134 | 4.32 ± 0.03 | 175 ± 8 |
| Tagish Lake [23–25] | 2.32 ± 0.13 | 495 ± 83 | 3.91 ± 0.03 | 61 ± 7 |
| Bulk solar [26,27] | 0.21 ± 0.05 | −865 ± 32 | 2.27 ± 0.03 | −382 ± 8 |
| OCCs [28–36] | 2.88 ± 0.71 | 851 ± 161 | 6.81 ± 0.41 | 853 ± 32 |
| JFCs [32,35,37,38] | 3.64 ± 2.61 | 1218 ± 1675 | 6.97 ± 0.25a | 896 ± 19a |
| PS [39–41] | 10–100 | 5500–63 000 | 3.44–6.25 | −64 to 700 |
aThe average JFC N isotopic composition does not include the outlier comet 73P whose 15N/14N = 4.65 ± 0.69 × 10−3.
At least two mechanisms have been proposed in which solar nebula H was accreted by the Earth and other planets—adsorption of H onto grain surfaces in the nebula [42] and dissolution of H2 by a magma ocean [43,44]. However, the H isotopic composition of the nebula is almost an order of magnitude more depleted in D than those of the major inner Solar System bodies (table 2). It has been suggested that isotopic fractionation associated with hydrodynamic escape of H2 can explain the more D-rich compositions of the Earth and Mars. However, given their different sizes and formation histories, it would be an extraordinary coincidence if they all experienced such similar degrees of H isotopic fractionation. Also, it is not clear that hydrodynamic escape can explain the H isotopic composition of Vesta, or the abundances and isotopic compositions of other volatile elements in any of the objects (see below). Thus, it is unlikely that direct accretion of solar nebula gas can have been the major source of water for the planets and planetesimals in the inner Solar System.
At the other isotopic extreme is the D-rich interstellar ice (table 2) that the forming Solar System would have inherited from its parental molecular cloud. Again, H isotopes rule out such ices as the major source of water in the inner Solar System. However, as recent models suggest that it is not possible to produce significant D enrichments in H2O in discs, a small amount of interstellar water may ultimately be required to explain the non-solar H isotopic compositions of the inner Solar System bodies [45,46].
This leaves comets and the parent bodies of the chondritic meteorites as the most likely major sources of water for the terrestrial planets. To distinguish between these two sources requires consideration of the isotopic compositions and abundances of other volatile elements in addition to H. The element most commonly used is N (figure 1). Again, it is clear that the isotopic compositions of the larger inner Solar System objects are distinct from the solar composition and from comets. This then leaves primitive chondritic meteorites as the most likely sources of volatiles for the terrestrial planets, in particular the CI and CM carbonaceous chondrites [23,47]. If this is correct, then, as will become apparent later, a significant fraction of the water and almost all the N in the terrestrial planets comes from organic matter and not from ice. The organic matter would also be the major source of C and noble gases.
Figure 1.
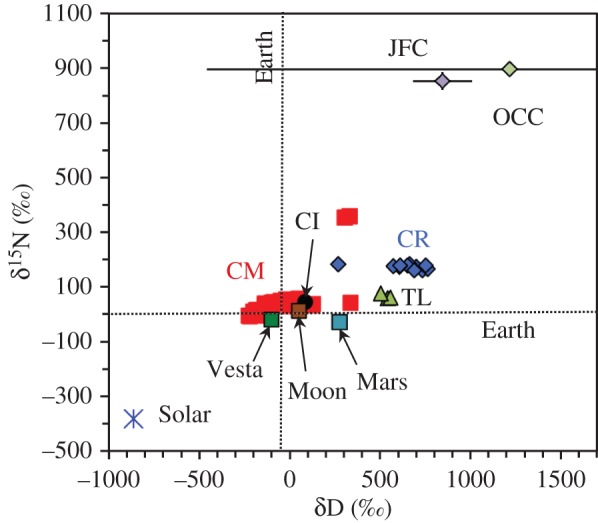
Comparison of the estimated bulk H and N isotopic compositions of the major inner Solar System bodies with those of the average Jupiter family (JFC) and Oort Cloud (OCC) comets and individual members of the most volatile-rich chondritic meteorites. The H isotopic compositions of Mars and the Moon are probably upper limits. The isotopic composition of N in Venus's atmosphere is roughly terrestrial, but its H isotopes have been hugely fractionated by the loss of H to space. The sources for the isotopic compositions are given in tables 1 and 2, and Alexander et al. [23].
One note of caution in the above discussion is that the comet compositions in figure 1 are probably not their bulk compositions—the H isotopes are those of H2O and the N isotopes are of relatively minor volatile species (HCN and NH3). We know very little of the isotopic compositions of the refractory organic material that could make up approximately 30% of comets (e.g. [48]) and will certainly be a major contributor to the H and N budgets of bulk comets. However, if the refractory organics are anything like the organics in the most primitive chondrites and in interplanetary dust particles (IDPs) that may come from comets, they will be enriched in D and 15N relative to the terrestrial planets (e.g. [49,50]). Thus, even comets like Hartley 2 with near-terrestrial H2O H isotopic compositions [37] are likely to have bulk compositions that are too D-rich to be the major source of the volatiles in the terrestrial planets [23].
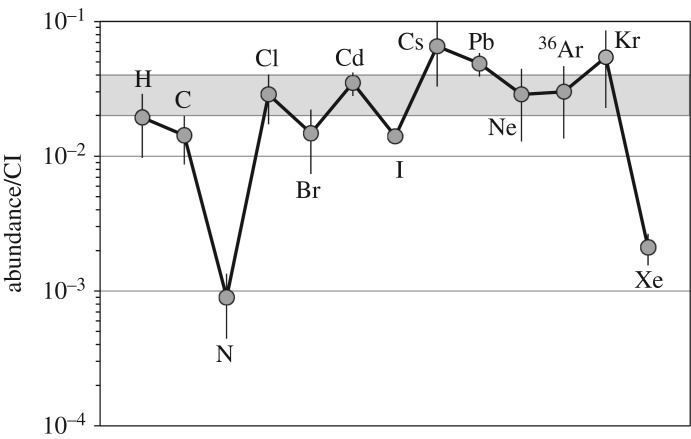
Additional constraints on the sources of the terrestrial planet volatiles come from volatile abundances, as well as the isotopic compositions of other elements that vary between the chondrite groups (e.g. O, Ti, Cr, Os, Ru and Mo). The abundances of H, N and other volatiles in most types of chondrite are so low that they cannot have supplied the volatiles to the Earth, Moon and Mars without violating other bulk chemical and isotopic constraints. Of the potential chondritic sources, only the CI and CM carbonaceous chondrites are able to roughly reproduce the H and N isotopes (figure 1), as well as the relative abundances of H, C, volatile lithophiles and the noble gases (figure 2). About 2–4 wt% of CI/CM-like material would be needed to account for Earth's volatile budget [47], a small enough amount that it would avoid violating other elemental/isotopic constraints. However, the CI/CM-like material would have to have been accreted prior to the end of core formation, normally assumed to coincide with Moon formation. This is to avoid disagreement with the isotopic compositions of the highly siderophile elements in the Earth's mantle and because the estimated mass of material accreted after the Moon-forming impact, approximately 0.5 wt% in the so-called Late Veneer, is too small (e.g. [54]). Relative to the chondrites, N and Xe are notably depleted (figure 2) in estimates of the bulk Earth [47,55]. Xenon may have been progressively lost from the atmosphere over geologic time [56]. It cannot be ruled out that the N budget of the mantle has been underestimated [47,57,58] or that N has been sequestered into the core [47], but it is also possible that the N depletion reflects multiple episodes of impact-induced atmospheric loss [59].
Figure 2.
The CI chondrite-normalized estimated abundances of H, C, N, noble gases and volatile lithophiles in the bulk Earth. Within the uncertainties, the volatile element abundances are consistent with a 2–4% CI contribution to a largely volatile-depleted proto-Earth. The depletions in N and Xe could be due to an underestimate of the N content of the Earth and loss of Xe from the atmosphere to space, respectively (see the text for details). The terrestrial abundances of H, C, N and noble gases are from Marty [47], and the volatile lithophile abundances are from Palme & O'Neill [51] except for I that is from Deruelle et al. [52]. The CI chondrite H, C and N abundances are from Alexander et al. [23], while all other elements are from Lodders [53].
2. Delivery of water to the terrestrial planets
The parent bodies of the chondrites are in the asteroid belt (approx. 2–4 AU), and the CI and CM chondrites are thought to come from C-complex asteroids that dominate the outer asteroid belt. So if the CI/CM chondrites are representative of the major sources of volatiles in the terrestrial planets, how were they delivered?
In the ‘classical’ explanation (e.g. [60]), there was a radial gradient in the volatile contents of the planetesimals of the inner Solar System that reflected the thermal gradient in the solar nebula at the time the planetesimals formed. Once the gas disc had dissipated, mutual gravitational interactions between the planetesimals and between the planetesimals and the giant planets led to scattering, collisions and growth. Growth would have been faster in the inner part of the planetesimal disc (less than 2 AU) where the density of planetesimals was higher and dynamical time scales were shorter. As the water-rich objects beyond 2 AU must have been scattered into this region, they would have tended to be accreted somewhat later.
Perennial problems with the ‘classical’ model are explaining the low mass of Mars and the structure of the asteroid belt, particularly the absence of embryo-sized objects in it. The so-called Grand Tack model was designed to overcome these problems [61]. In the Grand Tack model, interactions with the gas disc caused Jupiter to migrate in to approximately 1.5 AU, at which point a still growing Saturn caught up with it. Jupiter and Saturn then became locked in an orbital resonance that changed how they interacted with the gas disc and caused them to migrate outwards. This outward migration only stopped when the gas disc dissipated. As one might expect, the inward migration would have removed most of the existing planetesimals between approximately 4 AU and approximately 1.5 AU. Then as the giant planets migrated out again, they would have scattered some outer Solar System planetesimals (5–15 AU) into the inner Solar System. The clearing-out of most planetesimals beyond 1.5 AU ensured that a growing Mars remained relatively small and produced a low-mass asteroid belt that was crudely zoned, with inner Solar System objects dominating the inner belt and outer Solar System objects dominating the outer belt. Presumably, these outer Solar System planetesimals would have been volatile-rich and, therefore, potent sources of volatiles for the growing terrestrial planets.
If the Grand Tack model is correct, the source(s) of water in the inner Solar System could ultimately have been in the outer Solar System. The rest of this paper focuses on the meteorite record to try to distinguish between the ‘classical’ and Grand Tack models, searching in particular for indicators of meteorite formation distance from the Sun, because it is the formation distances of the volatile-rich objects that most clearly distinguish the two models.
3. Meteorites in brief
The classification of meteorites has been described in detail by Krot et al. [62], so it is only briefly outlined here. The major subdivision of meteorites is between the unmelted chondrites and the non-chondrites (achondrites, stony irons and irons) that come from asteroids that experienced variable degrees of melting and differentiation. The non-chondrites are very poor in H and other highly volatile elements, and so will not be discussed in detail here. Historically, the chondrites have been divided into three classes (ordinary, carbonaceous and enstatite) based on their compositions and mineralogies. These in turn have been subdivided into a number of groups: ordinary chondrites into H, L and LL; carbonaceous chondrites into CI, CM, CR, CV, CO, CB, CH and CK; and enstatite chondrites into EH and EL. The name ‘carbonaceous chondrite’ is a historical one and is a bit misleading because some ordinary and enstatite chondrites contain more C than some carbonaceous chondrites. The chondrite classification scheme is still evolving as more meteorites are found; two new classes (R and K chondrites) have been recognized, and a number of individual meteorites do not belong to any recognized group. While there are variations, the bulk compositions of the chondrites are remarkably similar to that of the rock-forming component of the solar photosphere (i.e. excluding H, C, N, O and the noble gases). Indeed, the CIs have bulk compositions that are within error identical to the rock-forming component of the Sun [53,63].
Chondrites comprise three main components: refractory inclusions, chondrules and fine-grained matrix. Refractory inclusions and chondrules formed at high temperatures (1400–1800°C) in the solar nebula. The most abundant refractory inclusions, the calcium–aluminium-rich inclusions (CAIs), are the oldest Solar System objects to have been dated and are generally assumed to mark the formation of the Solar System. Given their high formation temperatures, water and organics would not have survived refractory inclusion or chondrule formation. Consequently, at the time when the chondrite parent bodies formed, it was only in the matrix that water (as ice) and organic matter would have been present. It is also in the matrix that one finds other primitive materials, such as presolar circumstellar grains.
After formation, the chondrites experienced secondary modification (thermal metamorphism and aqueous alteration) due to the internal heating of their parent bodies by the decay of short-lived (now extinct) radionuclides and shock heating associated with impacts. A petrographic classification scheme for secondary processes divides the chondrites into six types—types 3 to 6 reflect increasing extent of thermal metamorphism, and types 3 to 1 reflect increasing degrees of aqueous alteration. By convention, the chemical classification is followed by the petrologic one (e.g. CI1, CM2, CV3).
Spectroscopically, the enstatite chondrites have been linked to the Hungaria asteroids that inhabit the very inner edge of the asteroid belt, the ordinary chondrites (and the related R chondrites) have been linked to the S-complex asteroids that dominate the inner asteroid belt, and the carbonaceous chondrites have been linked with the C-complex asteroids that dominate the outer asteroid belt [64,65]. It is important to note that this spectroscopic/compositional zoning in the asteroid belt is only apparent among the larger asteroids, i.e. those asteroids that have not experienced catastrophic collisional disruption and whose orbits will not have changed much since the end of planet formation. Smaller asteroids are predominantly collisional fragments and are subject to orbital migration [66]. The relationship between S-complex asteroids and ordinary chondrites has been confirmed by two space missions to near-Earth asteroids [67–69], but the link between C-complex asteroids and carbonaceous chondrites has yet to be definitively demonstrated. Interestingly, differences in the sizes of small nucleosynthetic anomalies in bulk Ti and Cr indicate that the carbonaceous chondrites are distinct from all other inner Solar System materials (the ordinary and enstatite chondrites, non-chondritic meteorites, Earth, Moon and Mars) [70]. This is certainly consistent with the Grand Tack model, but how the nucleosynthetic anomalies were produced is not understood, and they could reflect spatial and/or temporal variations in the solar nebula.
That there could have been a temporal element to the production of the Ti and Cr anomalies is suggested by estimates of the accretion times of the chondrites. The Solar System formed with abundant short-lived radionuclides, particularly 26Al. Planetesimals that formed before approximately 2 Ma after Solar System formation would have melted and differentiated, unless they were small, in which case they are unlikely to have survived to the present day. To survive impacts in space and atmospheric entry, the unmelted chondrites must have undergone a degree of lithification (rock formation from unconsolidated material). This restricts their formation to a window between roughly 2 Ma and 4 Ma after Solar System (CAI) formation, the upper limit being when there was just enough short-lived radioactivity to melt ice and enable some aqueous alteration. Unlithified bodies that formed after approximately 4 Ma could be one of the sources, along with comets, of the IDPs that are being continuously accreted by the Earth [71]. More detailed estimates of accretion times can be made using thermal models and estimates of the maximum temperatures experienced by any member of a chondrite group (figure 3). However, these estimates are at best approximate, as they must make a number of assumptions (e.g. planetesimal sizes, initial ice contents, and that the centres of all the chondrite parent bodies have been sampled). It is also possible that some chondrites (e.g. the CVs) are the unmelted crusts of differentiated objects [73,74], in which case their parent bodies must have formed much earlier than indicated in figure 3.
Figure 3.
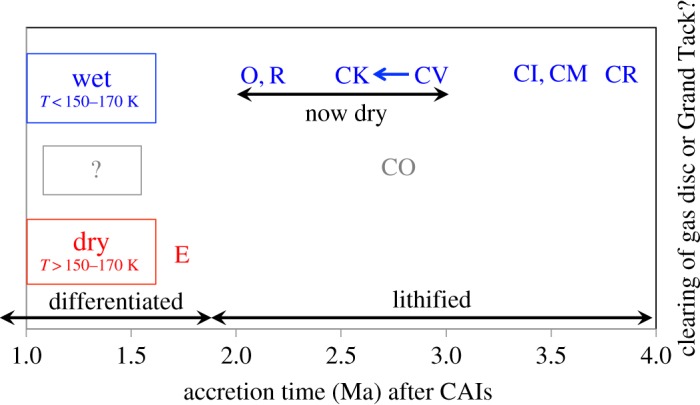
Estimates of the accretion times of the chondrite parent bodies (modified after [72]). All the chondrites have avoided melting, but they experienced sufficient lithification as a result of metamorphism and/or aqueous alteration to be robust enough to survive impacts and atmospheric entry. Hence, the chondrites must have formed in a window of time between when there was not quite enough short-lived radioactivity to melt and differentiate planetesimals, and when there was not enough radioactivity to even melt water ice. With the exception of the highly reduced enstatite chondrites (E) and possibly the COs, all the chondrites accreted at least some water, presumably as ice, implying accretion temperatures that were below the sublimation temperature of water ice in the nebula (150–170 K). (Online version in colour.)
There is direct or indirect evidence that, with the exception of the enstatite chondrites, all chondrites accreted some water (e.g. [75]). Thus, by the time the ordinary chondrites formed, the ambient temperatures at approximately 2 AU had fallen below the sublimation temperature of water ice in the disc (approx. 150–170 K). Intuitively, one might expect that, as the disc continued to cool, chondrites that formed later would have accreted more ice, particularly if they also formed further from the Sun. Unfortunately, estimating how much water the chondrite parent bodies accreted is difficult. This is largely because in most chondrite parent bodies there was enough short-lived radioactivity to metamorphose and dehydrate them. Only the CI, CM and CR chondrites, along with the ungrouped carbonaceous chondrite Tagish Lake, seem to have formed late enough to have avoided extensive dehydration. Even in these meteorites, evidence for the original water is now preserved primarily as OH in phyllosilicates, hydrated amorphous silicates and, possibly, hydroxides, although some water was also consumed in the formation of anhydrous secondary minerals such as magnetite and sulfates. Estimating the initial water budgets is further complicated by the range of H2O/OH contents seen in CM and CR chondrites [24], the possibility that water was redistributed within or lost from the chondrite parent bodies, and that any water ice that remained after alteration would have been lost when the meteorites were small objects in space prior to atmospheric entry. Nevertheless, with the notable exception of the CR chondrites, there is a general increase in matrix contents with accretion age, and because water ice was accreted with the matrix, this suggests that the water contents may also have increased with accretion age.
The conventional explanation for the apparent stratigraphy in the asteroid belt and the range of peak temperatures experienced by the chondrite groups is that there was a wave of planetesimal formation that slowly progressed outwards through the asteroid belt [76,77]. Of course, the Grand Tack model greatly increases the potential range of radial distances the chondrites may have formed over. However, at present it cannot be demonstrated that accretion age is correlated with formation distance. Nor is it clear that a monotonic increase in accretion age with formation distance is consistent with recent models that suggest that planetesimal formation should have been rapid and efficient throughout much of the disc [78–80].
4. Hydrogen isotopes: indicators of formation distance?
At present, the H isotopes of water are the most promising indicators of formation distance. This is because there is likely to have been a radial gradient in the H isotopic composition of water in the disc. This gradient would have been the result of radial mixing between water in the outer Solar System that retains a large D-rich interstellar component and D-poor (solar D/H) water in the warm inner Solar System produced by re-equilibration between H2 and H2O. While some models predict a simple monotonic increase in D/H in water with radial distance (e.g. [81]), this is almost certainly a gross oversimplification (e.g. [82]). Nevertheless, the basic prediction that planetesimals that formed further from the Sun should tend to be more D-rich is at least consistent with the H isotopic compositions of water ice in most comets (e.g. [38]) and Saturn's moon Enceladus [83].
Determining the initial H isotopic composition of the water accreted by the chondrites is not as straightforward as one might think. This is because the OH-bearing clay minerals in the chondrites are intimately intermixed with D-rich organic material. Not only is it not possible to physically or chemically separate the clay minerals from the organics, but there may also have been isotopic exchange between the water and the organics during aqueous alteration in the chondrite parent bodies. However, the situation is not hopeless. The CM and CR chondrites experienced a wide range in the extent of alteration, but the variation in organic contents is more restricted. Provided that (i) this range in alteration either reflects heterogeneous accretion of the ice or, more likely, a very early redistribution of water in the parent bodies before any exchange with the organics took place, and (ii) once alteration began, the meteorites remained as closed systems, then plotting bulk H isotopes versus bulk C/H should produce a linear mixing line between the original water and organic H isotopic compositions. As can be seen from figure 4, this does seem to be the case for both the CM and CR chondrites, with the y-axis intercepts giving the initial water isotopic compositions. The same exercise cannot be done for the other chondrite groups because they do not show the same ranges of alteration. However, model compositions can be estimated by subtracting a primitive organic component from their bulk compositions.
Figure 4.
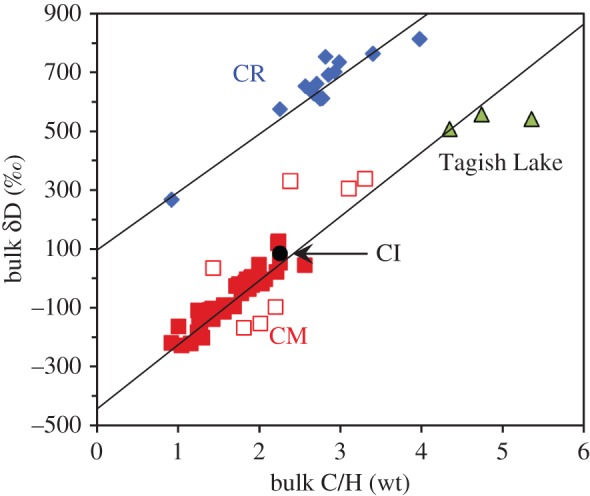
The bulk H isotopic compositions versus the bulk C/H ratios of CI, CM, CR and Tagish Lake carbonaceous chondrites (after [23]). The linear arrays displayed by the CM and CR chondrites reflect mixing between relatively D-poor water/OH and D-rich organic matter (δD ≈ 3500‰). The y-axis intercepts of the best-fit lines to the CM and CR trends give their water/OH compositions. The open red squares are CM chondrites that were not used in the fit for various reasons (see [23]). (Online version in colour.)
The initial water H isotopic compositions estimated in this way are shown in figure 5, along with a direct water measurement for the R chondrites [84], and compared to those of comets and Enceladus. The estimates for water in CI and CM chondrites, as well as Tagish Lake, are all intermediate between the terrestrial and solar values, and quite distinct from those of any comets. The estimate for the CR chondrite water is slightly above terrestrial and overlaps with the compositions of two of the least D-enriched comets. However, perhaps most surprising are the ordinary and R chondrite compositions—the ordinary chondrite composition is similar to that of most comets, as well as Enceladus, while the R chondrite composition is more D-enriched than any measured comet. Taken at face value, this would seem to imply that the ordinary and R chondrites formed further from the Sun than the carbonaceous chondrites and even most comets, which would be contrary to the expectations of both the ‘classical’ and Grand Tack models. At the very least and counter-intuitively, it is possible that the ordinary and R chondrites accreted more interstellar water than the carbonaceous chondrites [85,86].
Figure 5.
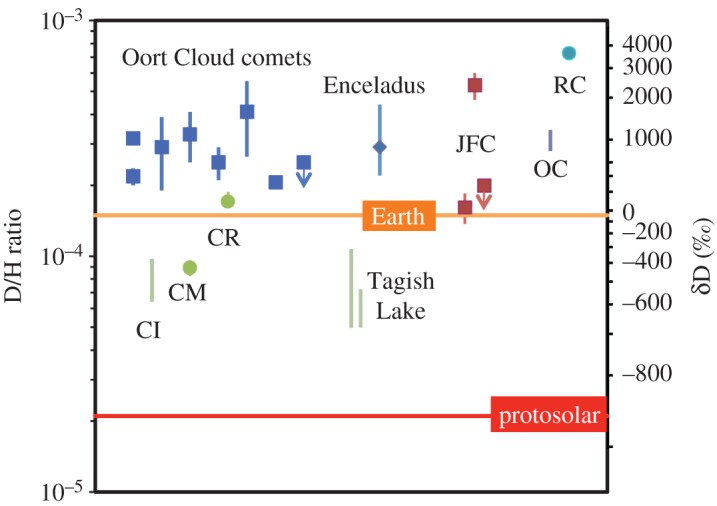
Comparison of the estimated H isotopic compositions of water in various chondrites with those for water in comets and Saturn's moon Enceladus (after [23]). The CI, CM and Tagish Lake isotopic compositions are clearly distinct from those measured for outer Solar System objects. The CR composition overlaps with those of the most D-poor comets. Surprisingly, the water in ordinary and R chondrites has H isotopic compositions that resemble those of most comets and Enceladus. However, this does not necessarily mean that the ordinary and R chondrites come from the outer Solar System. It is more likely that the H isotopic compositions of their water has been fractionated by parent body processes (see the text for details). This parent body fractionation may also have affected the carbonaceous chondrites, particularly the CRs.
However, there may be a more mundane explanation for the ordinary and R chondrite results [23,50,87]. Magnetite and other Fe3+-bearing minerals are ubiquitous in the altered chondrites. Petrologic evidence indicates that the Fe in these minerals was largely derived from the oxidation of Fe metal by water. These oxidation reactions (e.g. 3Fe + 4H2O = Fe3O4 + 4H2) would have produced copious H2. At low temperatures (less than 200°C), there is a very large equilibrium isotopic fraction between water and H2, which probably explains why the δD values of H2 produced during serpentinization on Earth can be as low as −600‰ to −800‰ [88–90]. Loss of such isotopically light H2 would have enriched the residual water in D, and the higher the initial metal/water ratio the greater the final water δD will have been. Given their low matrix contents, the ordinary and R chondrites probably did have the highest initial metal/water ratios of the chondrites measured. After correcting for the estimated water loss, the initial H isotopic composition of the ordinary chondrite water may have been similar to that of the carbonaceous chondrites [91]. However, this process will have affected all the chondrites to varying degrees and it may partly explain why the CR chondrites have higher δD values than the other carbonaceous chondrites.
Figure 6 plots as a function of radial distance from the Sun the estimated water isotopic compositions of the carbonaceous chondrites, comets and Saturn's moon Enceladus. The H isotopic composition of CH4 in Titan's atmosphere [93] is also plotted, although it is unclear how closely it reflects the isotopic composition of the water that Titan initially accreted. The formation locations of the two classes of comet are unknown, but are likely to have been in the region of the current orbit of Neptune (e.g. [92]). Hence, they have both been given nominal formation locations of approximately 30 AU. If Enceladus and Titan formed from Saturn's subdisc, this would presumably have been towards the end of Saturn's growth, which in the Grand Tack model would have been between roughly 3 AU and 7 AU (arrowed) rather than at Saturn's current orbital radius of approximately 10 AU. Again, it is clear that, with the possible exception of the CRs, the carbonaceous chondrites had significantly lighter initial water H isotopic compositions than comets and Enceladus. Consequently, it is tempting to infer that water beyond somewhere between 3 and 7 AU was enriched in D relative to the bulk Earth and somewhere between 3 and 7 AU there was a steep decrease in the isotopic composition of the water in the disc. However, it is possible that Enceladus formed from the debris of a tidally disrupted planetesimal that was captured by Saturn [94]. If correct, this would weaken or remove the constraints on the H isotopic composition of water in the inner part of the outer Solar System.
Figure 6.
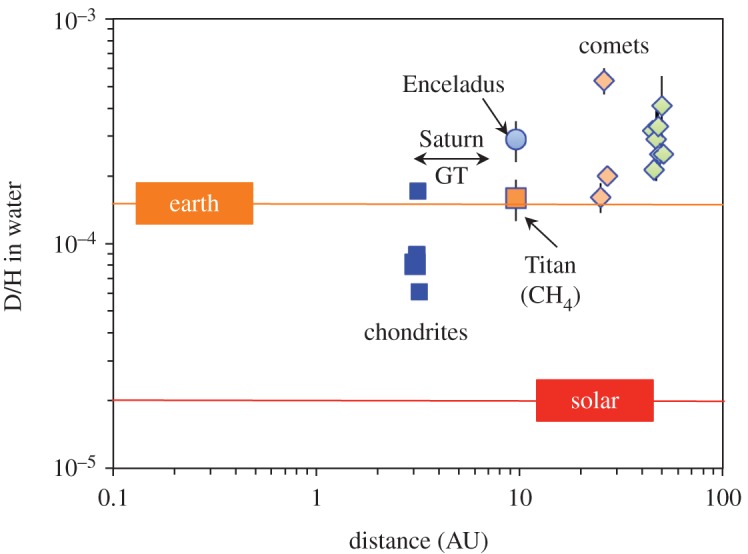
The H isotopic compositions of water in various objects as a function of radial distance from the Sun. The H isotopic composition of methane in Titan's atmosphere is also shown, although it is not known how closely it reflects that of the water that Titan accreted. The asteroidal parent bodies of the carbonaceous chondrites are not known and so they have been given nominal radial distances of 3 AU. Saturn's moons Titan and Enceladus have been given their current orbital distances, although if there was a Grand Tack they may have formed between approximately 3 AU and approximately 7 AU (arrowed). The formation locations of the comets are unknown, but are thought to have been between approximately 20 AU and approximately 30 AU (e.g. [92]). With the possible exception of the CRs, the carbonaceous chondrite with the most D-rich water, the carbonaceous chondrites have compositions that are distinct from any measured outer Solar System body. Sources are given in tables 1 and 2, and Alexander et al. [23].
Nitrogen isotopes might provide some further constraints on the formation distances of planetesimals. The N2–NH3 system in the nebula might have behaved in an analogous way to the H2–H2O system, although N2 is more stable than H2 and there has been no detailed isotopic modelling of the N system in the nebula. Nevertheless, as is evident from figures 1 and 7, the N isotopic compositions of the bulk chondrites are less 15N-enriched than HCN/NH3 in comets, as well as Titan's atmosphere [96], which may have originally been accreted as NH3 [95]. Hence, when the N isotopes are plotted against radial distance (figure 7), there is a similar pattern to that for H isotopes (figure 6), with outer Solar System objects being more enriched than the chondrites. However, to truly compare like with like we should be using NH3 compositions in chondrites not their bulk compositions. There is little NH3 or ammonium salts in chondrites, and what there is has not been extensively studied. However, they do contain amino acids that have garnered a lot of attention and they are thought to have formed in their parent bodies via Strecker-cyano synthesis that would have involved HCN and NH3. The N isotopic compositions of these amino acids, as well as a few measurements of NH3 and other N-bearing soluble organic compounds, have a similar range to the bulk chondrites [97–101].
Figure 7.
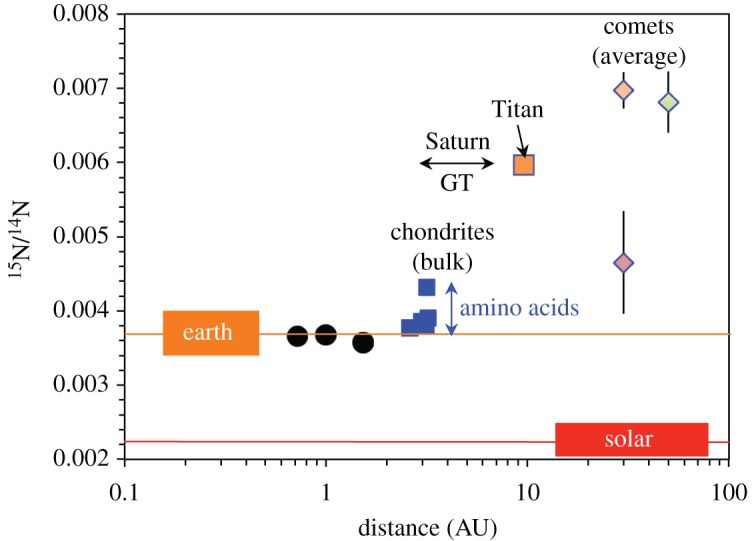
The same as for figure 6 but for N isotopes. The N isotopic compositions of individual comets are generally very consistent but the uncertainties can be large, so they have been averaged (table 2), except for the JFC outlier comet 73P. Titan's atmospheric N isotopic composition is thought to reflect that of the NH3 it accreted [95]. The chondrite compositions (carbonaceous in blue, ordinary in red) are for bulk meteorites. However, amino acids and other N-bearing soluble organic compounds (blue double arrow) probably formed from HCN/NH3 and exhibit a similar range of isotopic compositions to the bulk meteorites (see the text for details).
Bulk chondrites exhibit a range of mass-dependent and mass-independent O isotope variations, and their individual components can show even larger variations. In an O three-isotope diagram, bulk chondrites lie fairly close to the terrestrial mass fractionation line (TFL)—the TFL is defined by the equation δ17O = 0.52 × δ18O, while mass-independent deviations from the TFL are usually indicated by Δ17O = δ17O − 0.52 × δ18O. On the other hand, the Sun and most refractory inclusions have 16O-rich compositions (Δ17O = −28 ± 2‰ for the Sun) that fall far from the TFL [102]. At present, the favoured explanation for producing these large mass-independent variations is that UV self-shielding by CO, either in the protosolar molecular cloud [103] or in the outer solar nebula [104], produced 16O-rich CO and 16O-poor H2O. The H2O may have had a mass-independent anomaly as high as Δ17O ≈ 80‰ [105]. By some still poorly understood process, fractionation of silicates and H2O ice from CO in the gas, followed by isotopic exchange between the silicates and H2O, would explain why all inner Solar System materials, as well as IDPs and comet Wild 2 dust, have O isotopic compositions that fall fairly close to the TFL [106–109]. Much of this exchange has to have occurred prior to accretion of the chondrite and comet parent bodies because most of their components, other than refractory inclusions, have O isotopic compositions that lie closer to the TFL than to the solar composition. The isotopic compositions of secondary minerals (e.g. magnetite and carbonates) in chondrites have relatively modest mass-independent fractionations (e.g. summary by [23] in their electronic supplementary material), suggesting that the bulk of the water that they accreted had already been re-equilibrated with silicates in the disc [110,111]. Qualitatively, this is a very similar conclusion to that arrived at from the H isotopes, i.e. most of the water had been re-equilibrated in the inner Solar System, but that a small fraction of interstellar water was present when the chondrites formed.
On balance, the combination of H, N and O isotopes in carbonaceous chondrites suggests that they did not form beyond the formation locations of Saturn's moons, which in the Grand Tack model would have been between approximately 3 AU and approximately 7 AU. Thus, if there was a Grand Tack, it did not scatter objects into the asteroid belt from as far out as envisaged in the original model, but the meteoritic data are equally consistent with the chondrites having formed more or less where their parent bodies are presently located in the asteroid belt.
5. Minor contributors to volatile budgets
If CI/CM-like planetesimals were the major sources of the terrestrial planets' water and other volatiles, were there other more minor contributors? As already mentioned, the CK, CO and CV chondrites can be ruled out because their volatile contents are too low. Figure 1 shows that, based on their bulk H and N isotopic compositions, the CR chondrites and Tagish Lake can also be ruled out. This really only leaves the CI and CM chondrites as potential representatives of the major sources of Earth's volatiles. The CIs have roughly twice the volatile contents of the CMs, and so half the amount of CI-like material would be required to reproduce the Earth's volatile budget. On the other hand, the bulk CI and CM H and N isotopic compositions are not identical to terrestrial (figure 1 and table 2), and the CI composition is further from terrestrial than the average CM composition. As there are no obvious fractionation mechanisms for producing the Earth's isotopically lighter H and N compositions from bulk CI/CM material, an additional volatile source is required. The most obvious source would be a solar component acquired either directly from the nebula or as solar wind implanted into the surfaces of accreted dust and small planetesimals. Indeed, noble gases clearly show that there is a solar component in the Earth's mantle (e.g. [112]).
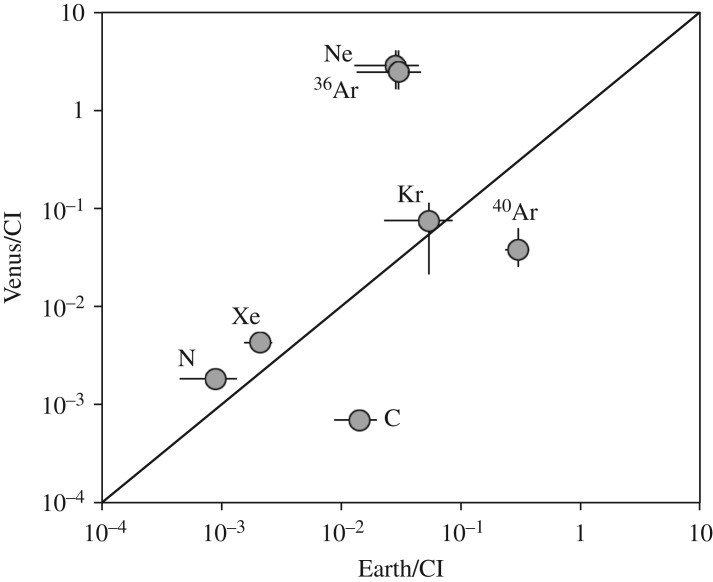
The estimates of the interior N isotopic compositions of the Moon and Mars, and the upper limits we have for their interior H isotopic compositions (table 1), are both consistent with CI- and/or CM-like materials being the major volatile sources. The N isotopic composition of Venus is also consistent with CI- and/or CM-like sources for its volatiles. However, other aspects of its volatile budget seem to require additional sources. Figure 8 compares the CI-normalized volatile budgets of Earth and Venus. The Venus volatile budget assumes that the planet has fully degassed and that all of its volatiles are in its atmosphere. For elements like N, Kr and even Xe, there is a reasonable agreement between the Earth and Venus. Carbon and 40Ar (produced by decay of 40K) are clearly depleted in Venus's atmosphere, probably because they have not fully degassed. It is the large enrichments in 22Ne and 36Ar in Venus that are problematic, but might be explained by additional accretion of material rich in implanted solar wind [114] and/or cometary planetesimals [115].
Figure 8.
Comparison of the CI chondrite-normalized estimated volatile abundances in Earth and Venus. The Venus abundances assume that its interior has entirely degassed. The relative depletions in Venus's C and 40Ar (from decay of 40K) suggest that this was not always the case. The large relative enrichments in Ne and 36Ar indicate that Venus did not have the same mix of sources as Earth (see the text for discussion). The data for the Earth are from Marty [47], and those for Venus from Fegley [113] except for Xe which is from Bogard [114] but its value is very uncertain.
The origin of the water in Vesta is an interesting problem. All the samples of Vesta that we have are igneous rocks, and the evidence for water is largely confined to trace phosphate minerals. Because they are igneous rocks from a small body, degassing of the magmas (as well as shock) could have contributed to the variability of the phosphate H isotopic compositions that have been reported (table 1, [10,11])—degassing can result in either D enrichments or depletions in the residual melt depending on the fO2. In terms of its Cr, Ti and O isotopes, Vesta is clearly related to other inner Solar System objects and not the carbonaceous chondrites [70]. Nevertheless, Vesta's H and N isotopic compositions are roughly consistent with a CM/CI origin. Vesta is the second largest object in the asteroid belt and appears to have undergone wholesale melting and differentiation. Estimates for the timing of differentiation and the onset of magma ocean crystallization range from 2.2 ± 1.1 Ma [116,117] to less than or equal to 0.6−0.4+0.5 Ma [118] after CAIs, and are broadly consistent with estimates of accretion times for Vesta of less than 1 Myr after CAIs [119]. As discussed earlier, this is long before the CI and CM parent bodies seem to have formed. Sarafian et al. [10] assumed that Vesta lost what volatiles it may originally have formed with during differentiation, and that the volatiles that are now found in it were collisionally accreted approximately 10–20 Ma later while there was still some melt present in Vesta's interior. However, it has not been definitively shown that differentiating planetesimals will fully degas them. Also, ordinary chondrites accreted matrix with primitive materials (organics, presolar grains, etc.) and water whose initial H isotopes may have resembled that in CI/CM chondrites. Thus, it should not be ruled out at this stage that the water in Vesta is primary and that CI/CM chondrites represent a primitive dust component in the disc that was widely distributed in the solar nebula even at early times. This possibility also has relevance for Mars because it may have reached roughly 50% of its current mass by 1.8−1.0+0.9 Ma after CAIs [120,121], although it is possible that most of its volatiles were accreted later towards the end of its growth.
6. Summary and conclusion
Any discussion of the origin of water in the inner Solar System should not consider water in isolation, but must also consider the origin(s) of other volatiles. Of the potential sources of the volatiles for the terrestrial planets, the CI and CM carbonaceous chondrites provide the closest fit to the planets' bulk H and N isotopic compositions. For the Earth, at least, the abundances of H, C, the noble gases (except Xe) and many volatile lithophile elements are consistent with the addition of approximately 2–4 wt% of CI/CM material to a volatile-depleted proto-Earth. Other constraints require that the CI/CM material must have been accreted prior to the Moon-forming impact, and not in the post-impact Late Veneer. However, the Earth and even more so Venus show clear evidence that they also accreted some solar-like material, probably either as solar wind implanted into accreted dust/planetesimals or in cometary ices.
Hence, the origin of water in the inner Solar System largely becomes a question of the origin of the chondrites, in particular the CI and CM chondrites. Two dynamical models for delivery of volatiles to the growing terrestrial planets predict very different formation locations for the carbonaceous chondrites. In the ‘classical’ model, the volatiles were accreted in volatile-rich planetesimals that were scattered into the terrestrial planet region from the asteroid belt, i.e. the carbonaceous chondrite parent bodies formed roughly where they are located today. On the other hand, in the Grand Tack model, the carbonaceous chondrite parent bodies are remnants of a swarm of planetesimals that were scattered into the inner Solar System from as far out as 13–15 AU by the orbital migrations of Jupiter and Saturn.
The meteorite record can potentially distinguish between these two models if an indicator of formation distance can be found. There is evidence that the carbonaceous chondrites are chemically distinct from all other inner Solar System materials. However, as the carbonaceous chondrite parent bodies appear to have formed after all other measured inner Solar System planetesimals, these chemical differences could be the result of temporal rather than radial variations in the disc. At present, the H isotopes of water appear to be the most promising indicators of formation distance, as they are expected to become increasingly D-rich with distance from the Sun. This radial gradient would have been due to radial mixing between outer Solar System water with a large D-rich interstellar component, and inner Solar System water that had been isotopically re-equilibrated with D-poor H2. The estimated initial H isotopic compositions of water accreted by the CI, CM, CR and Tagish Lake carbonaceous chondrites were, with the possible exception of the CRs, much more D-poor than known outer Solar System objects (comets, Enceladus and Titan), although they do require a small interstellar component. The ordinary and R chondrites contain small amounts of water that have outer Solar System-like H isotopic compositions. However, this is likely to be largely the result of parent body processes and at least the ordinary chondrite water may have been isotopically quite similar to that in the carbonaceous chondrites.
Based on the H isotopes, if Enceladus and Titan formed around Saturn towards the end of its growth, then in the Grand Tack model the carbonaceous chondrites did not form beyond 3–7 AU. However, there are alternative formation scenarios, at least for Enceladus, that, if correct, would greatly weaken this conclusion. Nevertheless, the O isotopes of the water in chondrites also suggests that it had undergone significant re-equilibration prior to accretion, and the inferred N isotopic compositions of HCN/NH3 in carbonaceous chondrites are also quite distinct from those of comets and Titan. Thus, on balance, it seems that the carbonaceous chondrites and their water did not form very far out in the disc, almost certainly not beyond the orbit of Saturn when its moons formed (approx. 3–7 AU in the Grand Tack model) and possibly close to where they are found today.
Acknowledgements
The author would like to thank Monica Grady, Sara Russell and Chris Ballentine for the invitation to participate in the ‘The origin, history and role of water in the evolution of the inner Solar System’ workshop, and the Royal Society for partial funding for travel to the workshop.
Competing interests
The author declares that there are no competing interests.
Funding
This work was also partially supported by NASA Cosmochemistry grant no. NNX14AJ54G.
References
- 1.Lécuyer C, Gillet P, Robert F. 1998. The hydrogen isotope composition of seawater and the global water cycle. Chem. Geol. 145, 249–261. ( 10.1016/S0009-2541(97)00146-0) [DOI] [Google Scholar]
- 2.Kerridge JF, Eugster JS, Kim JS, Marti K. 1990. Nitrogen isotopes in the 74001/74002 double-drive tube from Shorty Crater, Apollo 17. In Proc. 21st Lunar and Planetary Science Conf, pp. 291–299. Houston, TX: Lunar and Planetary Institute; See http://adsabs.harvard.edu/abs/1991LPSC...21..291K. [Google Scholar]
- 3.Mathew KJ, Marti K. 2001a. Lunar nitrogen: indigenous signature and cosmic-ray production rate. Earth Planet. Sci. Lett. 184, 659–669. ( 10.1016/S0012-821X(00)00327-7) [DOI] [Google Scholar]
- 4.Saal AE, Hauri EH, Van Orman JA, Rutherford MJ. 2013. Hydrogen isotopes in lunar volcanic glasses and melt inclusions reveal a carbonaceous chondrite heritage. Science 340, 1317–1320. ( 10.1126/science.1235142) [DOI] [PubMed] [Google Scholar]
- 5.Tartèse R, Anand M, Barnes JJ, Starkey NA, Franchi IA, Sano Y. 2013. The abundance, distribution, and isotopic composition of hydrogen in the Moon as revealed by basaltic lunar samples: implications for the volatile inventory of the Moon. Geochim. Cosmochim. Acta 122, 58–74. ( 10.1016/j.gca.2013.08.014) [DOI] [Google Scholar]
- 6.Füri E, Deloule E, Gurenko A, Marty B. 2014. New evidence for chondritic lunar water from combined D/H and noble gas analyses of single Apollo 17 volcanic glasses. Icarus 229, 109–120. ( 10.1016/j.icarus.2013.10.029) [DOI] [Google Scholar]
- 7.Füri E, Barry PH, Taylor LA, Marty B. 2015. Indigenous nitrogen in the Moon: constraints from coupled nitrogen–noble gas analyses of mare basalts. Earth Planet. Sci. Lett. 431, 195–205. ( 10.1016/j.epsl.2015.09.022) [DOI] [Google Scholar]
- 8.Mortimer J, Verchovsky AB, Anand M, Gilmour I, Pillinger CT. 2015. Simultaneous analysis of abundance and isotopic composition of nitrogen, carbon, and noble gases in lunar basalts: insights into interior and surface processes on the Moon. Icarus 255, 3–17. ( 10.1016/j.icarus.2014.10.006) [DOI] [Google Scholar]
- 9.Miura Y, Sugiura N. 1993. Nitrogen isotopic compositions in three Antarctic and two non-Antarctic eucrites. In Proc. NIPR Symp. Antarctic Meteorites, No. 6, pp. 338–356. Tokyo, Japan: National Institute of Polar Research. [Google Scholar]
- 10.Sarafian AR, Nielsen SG, Marschall HR, McCubbin FM, Monteleone BD. 2014. Early accretion of water in the inner solar system from a carbonaceous chondrite-like source. Science 346, 623–626. ( 10.1126/science.1256717) [DOI] [PubMed] [Google Scholar]
- 11.Barrett TJ, Barnes JJ, Tartèse R, Anand M, Franchi IA, Greenwood RC, Charlier BLA, Grady MM. 2016. The abundance and isotopic composition of water in eucrites. Meteor. Planet. Sci. 51, 1110–1124. ( 10.1111/maps.12649) [DOI] [Google Scholar]
- 12.Hoffman JH, Hodges RR, McElroy MB, Donahue TM, Koplin M. 1979. Composition and structure of the Venus atmosphere: results from Pioneer Venus. Science 205, 49–52. ( 10.1126/science.205.4401.49) [DOI] [PubMed] [Google Scholar]
- 13.Donahue TM, Hoffman JH, Hodges RR, Watson AJ. 1982. Venus was wet—a measurement of the ratio of deuterium to hydrogen. Science 216, 630–633. ( 10.1126/science.216.4546.630) [DOI] [PubMed] [Google Scholar]
- 14.Mathew KJ, Marti K. 2001b. Early evolution of Martian volatiles: nitrogen and noble gas components in ALH84001 and Chassigny. J. Geophys. Res. 106, 1401–1422. ( 10.1029/2000JE001255) [DOI] [Google Scholar]
- 15.Usui T, Alexander CMO'D, Wang J, Simon JI, Jones JH. 2012. Origin of water and mantle–crust interactions on Mars inferred from hydrogen isotopes and volatile element abundances of olivine-hosted melt inclusions of primitive shergottites. Earth Planet. Sci. Lett. 357–358, 119–129. ( 10.1016/j.epsl.2012.09.008) [DOI] [Google Scholar]
- 16.Lawrence DJ, et al. 2013. Evidence for water ice near Mercury's north pole from MESSENGER Neutron Spectrometer measurements. Science 339, 292 ( 10.1126/science.1229953) [DOI] [PubMed] [Google Scholar]
- 17.Küppers M, et al. 2014. Localized sources of water vapour on the dwarf planet (1) Ceres. Nature 505, 525–527. ( 10.1038/nature12918) [DOI] [PubMed] [Google Scholar]
- 18.Campins H, Hargrove K, Pinilla-Alonso N, Howell ES, Kelley MS, Licandro J, Mothé-Diniz T, Fernández Y, Ziffer J. 2010. Water ice and organics on the surface of the asteroid 24 Themis. Nature 464, 1320–1321. ( 10.1038/nature09029) [DOI] [PubMed] [Google Scholar]
- 19.Rivkin AS, Emery JP. 2010. Detection of ice and organics on an asteroidal surface. Nature 464, 1322–1323. ( 10.1038/nature09028) [DOI] [PubMed] [Google Scholar]
- 20.Licandro J, Campins H, Kelley M, Hargrove K, Pinilla-Alonso N, Cruikshank D, Rivkin AS, Emery J. 2011. (65) Cybele: detection of small silicate grains, water-ice, and organics. Astron. Astrophys. 525, 34 ( 10.1051/0004-6361/201015339) [DOI] [Google Scholar]
- 21.Hargrove KD, Emery JP, Campins H, Kelley MSP. 2015. Asteroid (90) Antiope: another icy member of the Themis family? Icarus 254, 150–156. ( 10.1016/j.icarus.2015.03.008) [DOI] [Google Scholar]
- 22.Sheppard SS, Trujillo C. 2015. Discovery and characteristics of the rapidly rotating active asteroid (62412) 2000 SY178 in the main belt. Astron. J. 149, 44 ( 10.1088/0004-6256/149/2/44) [DOI] [Google Scholar]
- 23.Alexander CMO'D, Bowden R, Fogel ML, Howard KT, Herd CDK, Nittler LR. 2012. The provenances of asteroids, and their contributions to the volatile inventories of the terrestrial planets. Science 337, 721–723. ( 10.1126/science.1223474) [DOI] [PubMed] [Google Scholar]
- 24.Alexander CMO'D, Howard K, Bowden R, Fogel ML. 2013. The classification of CM and CR chondrites using bulk H, C and N abundances and isotopic compositions. Geochim. Cosmochim. Acta 123, 244–260. ( 10.1016/j.gca.2013.05.019) [DOI] [Google Scholar]
- 25.Alexander CMO'D, Cody GD, Kebukawa Y, Bowden R, Fogel ML, Kilcoyne ALD, Nittler LR, Herd CDK. 2014. Elemental, isotopic and structural changes in Tagish Lake insoluble organic matter produced by parent body processes. Meteor. Planet. Sci. 49, 503–525. ( 10.1111/maps.12282) [DOI] [Google Scholar]
- 26.Geiss J, Gloeckler G. 1998. Abundances of deuterium and helium-3 in the protosolar cloud. Space Sci. Rev. 84, 239–250. ( 10.1023/A:1005039822524) [DOI] [Google Scholar]
- 27.Marty B, Chaussidon M, Wiens RC, Jurewicz AJG, Burnett DS. 2011. A 15N-poor isotopic composition for the Solar System as shown by Genesis solar wind samples. Science 332, 1533–1536. ( 10.1126/science.1204656) [DOI] [PubMed] [Google Scholar]
- 28.Eberhardt P, Reber M, Krankowsky D, Hedges RR. 1995. The D/H and 18O/16O ratios in water from comet Halley. Astron. Astrophys. 302, 301–316. See http://adsabs.harvard.edu/abs/1995A&A...302..301E. [Google Scholar]
- 29.Bockelée-Morvan D, et al. 1998. Deuterated water in comet C/1996 B2 (Hyakutake) and its implications for the origin of comets. Icarus 133, 147–162. ( 10.1006/icar.1998.5916) [DOI] [Google Scholar]
- 30.Meier R, Owen TC, Matthews HE, Jewitt DC, Bockelée-Morvan D, Biver N, Crovisier J, Gautier D. 1998. A determination of the HDO/H2O ratio in comet C/1995 O1 (Hale-Bopp). Science 279, 842–844. ( 10.1126/science.279.5352.842) [DOI] [PubMed] [Google Scholar]
- 31.Hutsemékers D, Manfroid J, Jehin E, Zucconi J-M, Arpigny C. 2008. The 16OH/18OH and OD/OH isotope ratios in comet C/2002 T7 (LINEAR). Astron. Astrophys. 490, L31–L34. ( 10.1051/0004-6361:200810833) [DOI] [Google Scholar]
- 32.Manfroid J, Jehin E, Hutsemékers D, Cochran A, Zucconi J-M, Arpigny C, Schulz R, Stüwe JA, Ilyin I. 2009. The CN isotopic ratios in comets. Astron. Astrophys. 503, 613–624. ( 10.1051/0004-6361/200911859) [DOI] [Google Scholar]
- 33.Villanueva GL, Mumma MJ, Bonev BP, Di Santi MA, Gibb EL, Böhnhardt H, Lippi M. 2009. A sensitive search for deuterated water in comet 8P/Tuttle. Astrophys. J. Lett. 690, L5–L9. ( 10.1088/0004-637X/690/1/L5) [DOI] [Google Scholar]
- 34.Brown RH, Lauretta DS, Schmidt B, Moores J. 2012. Experimental and theoretical simulations of ice sublimation with implications for the chemical, isotopic, and physical evolution of icy objects. Planet. Space Sci. 60, 166–180. ( 10.1016/j.pss.2011.07.023) [DOI] [Google Scholar]
- 35.Rousselot P, et al. 2014. Toward a unique nitrogen isotopic ratio in cometary ices. Astrophys. J. Lett. 780, L17 ( 10.1088/2041-8205/780/2/L17) [DOI] [Google Scholar]
- 36.Shinnaka Y, Kawakita H, Kobayashi H, Nagashima M, Boice DC. 2014. 14NH2/15NH2 ratio in comet C/2012 S1 (ISON) observed during its outburst in 2013 November. Astrophys. J. Lett. 782, L16 ( 10.1088/2041-8205/782/2/L16) [DOI] [Google Scholar]
- 37.Hartogh P, et al. 2011. Ocean-like water in the Jupiter-family comet 103P/Hartley 2. Nature 478, 218–220. ( 10.1038/nature10519) [DOI] [PubMed] [Google Scholar]
- 38.Altwegg K, et al. 2015. 67P/Churyumov-Gerasimenko, a Jupiter family comet with a high D/H ratio. Science 347, 1261952 ( 10.1126/science.1261952) [DOI] [PubMed] [Google Scholar]
- 39.Liu F-C, Parise B, Kristensen L, Visser R, van Dishoeck EF, Güsten R. 2011. Water deuterium fractionation in the low-mass protostar NGC1333-IRAS2A. Astron. Astrophys. 527, A19 ( 10.1051/0004-6361/201015519) [DOI] [Google Scholar]
- 40.Coutens A, Vastel C, Caux E, Ceccarelli C, Bottinelli S, Wiesenfeld L, Faure A, Scribano Y, Kahane C. 2012. A study of deuterated water in the low-mass protostar IRAS 16293–2422. Astron. Astrophys. 539, A132 ( 10.1051/0004-6361/201117627) [DOI] [Google Scholar]
- 41.Wampfler SF, Jørgensen JK, Bizzarro M, Bisschop SE. 2014. Observations of nitrogen isotope fractionation in deeply embedded protostars. Astron. Astrophys. 572, A24. ( 10.1051/0004-6361/201423773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asaduzzaman A, Muralidharan K, Ganguly J. 2015. Incorporation of water into olivine during nebular condensation: insights from density functional theory and thermodynamics, and implications for phyllosilicate formation and terrestrial water inventory. Meteor. Planet. Sci. 50, 578–589. ( 10.1111/maps.12409) [DOI] [Google Scholar]
- 43.Sasaki S. 1990. The primary solar-type atmosphere surrounding the accreting Earth: H2O-induced high surface temperature. In Origin of the Earth (eds Newsom HE, Jones JH), pp. 195–209. Oxford, UK: Oxford University Press. [Google Scholar]
- 44.Ikoma M, Genda H. 2006. Constraints on the mass of a habitable planet with water of nebular origin. Astrophys. J. 648, 696–706. ( 10.1086/505780) [DOI] [Google Scholar]
- 45.Cleeves LI, Bergin EA, Alexander CMO'D, Du F, Graninger D, Öberg KI, Harries TJ. 2014. The ancient heritage of water ice in the Solar System. Science 345, 1590–1593. ( 10.1126/science.1258055) [DOI] [PubMed] [Google Scholar]
- 46.Cleeves LI, Bergin EA, Alexander CMO'D, Du F, Graninger D, Öberg KI, Harries TJ. 2015. Exploring the origins of deuterium enrichments in the solar nebular organics. Astrophys. J. 819, 13 ( 10.3847/0004-637X/819/1/13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marty B. 2012. The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth Planet. Sci. Lett. 313–314, 56–66. ( 10.1016/j.epsl.2011.10.040) [DOI] [Google Scholar]
- 48.Delsemme AH. 1991. Nature and history of the organic compounds in comets—an astrophysical view. In Comets in the post-Halley era (eds Newburn RL Jr, Neugebauer M, Rahe J), pp. 377–428. Dordrecht, The Netherlands: Kluwer Academic. [Google Scholar]
- 49.Alexander CMO'D, Fogel M, Yabuta H, Cody GD. 2007. The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim. Cosmochim. Acta 71, 4380–4403. ( 10.1016/j.gca.2007.06.052) [DOI] [Google Scholar]
- 50.Alexander CMO'D, Newsome SN, Fogel ML, Nittler LR, Busemann H, Cody GD. 2010. Deuterium enrichments in chondritic macromolecular material—implications for the origin and evolution of organics, water and asteroids. Geochim. Cosmochim. Acta 74, 4417–4437. ( 10.1016/j.gca.2010.05.005) [DOI] [Google Scholar]
- 51.Palme H, O'Neill HSC. 2014. Cosmochemical estimates of mantle composition. In Treatise on geochemistry, 2nd edn (ed Turekian HDHK.), pp. 1–39. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 52.Deruelle B, Dreibus G, Jambon A. 1992. Iodine abundances in oceanic basalts—implications for Earth dynamics. Earth Planet. Sci. Lett. 108, 217–227. ( 10.1016/0012-821X(92)90024-P) [DOI] [Google Scholar]
- 53.Lodders K. 2003. Solar System abundances and condensation temperatures of the elements. Astrophys. J. 591, 1220–1247. ( 10.1086/375492) [DOI] [Google Scholar]
- 54.Walker RJ, Bermingham K, Liu J, Puchtel IS, Touboul M, Worsham EA. 2015. In search of late-stage planetary building blocks. Chem. Geol. 411, 125–142. ( 10.1016/j.chemgeo.2015.06.028) [DOI] [Google Scholar]
- 55.Halliday AN. 2013. The origins of volatiles in the terrestrial planets. Geochim. Cosmochim. Acta 105, 146–171. ( 10.1016/j.gca.2012.11.015) [DOI] [Google Scholar]
- 56.Pujol M, Marty B, Burgess R. 2011. Chondritic-like xenon trapped in Archean rocks: a possible signature of the ancient atmosphere. Earth Planet. Sci. Lett. 308, 298–306. ( 10.1016/j.epsl.2011.05.053) [DOI] [Google Scholar]
- 57.Li Y, Wiedenbeck M, Shcheka S, Keppler H. 2013. Nitrogen solubility in upper mantle minerals. Earth Planet. Sci. Lett. 377, 311–323. ( 10.1016/j.epsl.2013.07.013) [DOI] [Google Scholar]
- 58.Mikhail S, Howell D. 2016. A petrological assessment of diamond as a recorder of the mantle nitrogen cycle. Am. Mineralogist 101, 780–787. ( 10.2138/am-2016-5464) [DOI] [Google Scholar]
- 59.Tucker JM, Mukhopadhyay S. 2014. Evidence for multiple magma ocean outgassing and atmospheric loss episodes from mantle noble gases. Earth Planet. Sci. Lett. 393, 254–265. ( 10.1016/j.epsl.2014.02.050) [DOI] [Google Scholar]
- 60.Raymond SN, O'Brien DP, Morbidelli A, Kaib NA. 2009. Building the terrestrial planets: constrained accretion in the inner Solar System. Icarus 203, 644–662. ( 10.1016/j.icarus.2009.05.016) [DOI] [Google Scholar]
- 61.Walsh KJ, Morbidelli A, Raymond SN, O'Brien DP, Mandell AM. 2011. A low mass for Mars from Jupiter's early gas-driven migration. Nature 475, 206–209. ( 10.1038/nature10201) [DOI] [PubMed] [Google Scholar]
- 62.Krot AN, Keil K, Scott ERD, Goodrich CA, Weisberg MK. 2014. Classification of meteorites and their genetic relationships. In Meteorites and cosmochemical processes (ed. Davis AM.), pp. 1–63. Amsterdam, The Netherlands: Elsevier-Pergamon. [Google Scholar]
- 63.Asplund M, Grevesse N, Sauval AJ, Scott P. 2009. The chemical composition of the Sun. Annu. Rev. Astron. Astrophys. 47, 481–522. ( 10.1146/annurev.astro.46.060407.145222) [DOI] [Google Scholar]
- 64.Bus SJ, Binzel RP. 2002. Phase II of the small main-belt asteroid spectroscopic survey: a feature-based taxonomy. Icarus 158, 146–177. ( 10.1006/icar.2002.6856) [DOI] [Google Scholar]
- 65.DeMeo FE, Alexander CMO'D, Walsh KJ, Binzel RP, Chapman CR. 2015. The compositional structure of the asteroid belt. In Asteroids IV (eds Michel P, DeMeo FE, Bottke WF), pp. 13–41. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 66.DeMeo FE, Carry B. 2014. Solar System evolution from compositional mapping of the asteroids. Nature 505, 629–634. ( 10.1038/nature12908) [DOI] [PubMed] [Google Scholar]
- 67.Nittler LR, et al. 2001. X-ray fluorescence measurements of the surface elemental composition of asteroid 433 Eros. Meteor. Planet. Sci. 36, 1673–1695. ( 10.1111/j.1945-5100.2001.tb01856.x) [DOI] [Google Scholar]
- 68.Foley CN, Nittler LR, McCoy TJ, Lim LF, Brown MRM, Starr RD, Trombka JI. 2006. Minor element evidence that asteroid 433 Eros is a space-weathered ordinary chondrite parent body. Icarus 184, 338–343. ( 10.1016/j.icarus.2006.05.011) [DOI] [Google Scholar]
- 69.Yurimoto H, et al. 2011. Oxygen isotopic compositions of asteroidal materials returned from Itokawa by the Hayabusa mission. Science 333, 1116–1119. ( 10.1126/science.1207776) [DOI] [PubMed] [Google Scholar]
- 70.Warren PH. 2011. Stable-isotopic anomalies and the accretionary assemblage of the Earth and Mars: a subordinate role for carbonaceous chondrites. Earth Planet. Sci. Lett. 311, 93–100. ( 10.1016/j.epsl.2011.08.047) [DOI] [Google Scholar]
- 71.Bradley JP. 2014. Early solar nebula grains—interplanetary dust particles. In Treatise on geochemistry, 2nd edn (ed Davis AM.), pp. 287–308. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 72.Sugiura N, Fujiya W. 2014. Correlated accretion ages and ε54Cr of meteorite parent bodies and the evolution of the solar nebula. Meteor. Planet. Sci. 49, 772–787. ( 10.1111/maps.12292) [DOI] [Google Scholar]
- 73.Elkins-Tanton LT, Weiss BP, Zuber MT. 2011. Chondrites as samples of differentiated planetesimals. Earth Planet. Sci. Lett. 305, 1–10. ( 10.1016/j.epsl.2011.03.010) [DOI] [Google Scholar]
- 74.Weiss BP, Elkins-Tanton LT. 2013. Differentiated planetesimals and the parent bodies of chondrites. Annu. Rev. Earth Planet. Sci. 41, 529–560. ( 10.1146/annurev-earth-040610-133520) [DOI] [Google Scholar]
- 75.Brearley AJ. 2006. The action of water. In Meteorites and the early Solar System II (eds Lauretta DS, McSween HY Jr), pp. 584–624. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 76.Grimm RE, McSween HY Jr. 1993. Heliocentric zoning of asteroid belt by 26Al heating. Science 259, 653–655. [Google Scholar]
- 77.Ghosh A, Weidenschilling SJ, McSween HY Jr, Rubin A. 2006. Asteroidal heating and thermal stratification of the asteroidal belt. In Meteorites and the early Solar System II (eds Lauretta DS, McSween HY Jr), pp. 555–566. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 78.Chambers JE. 2010. Planetesimal formation by turbulent concentration. Icarus 208, 505–517. ( 10.1016/j.icarus.2010.03.004) [DOI] [Google Scholar]
- 79.Cuzzi J, Hogan RC, Bottke WF. 2010. Towards initial mass functions for asteroids and Kuiper belt objects. Icarus 208, 518–538. ( 10.1016/j.icarus.2010.03.005) [DOI] [Google Scholar]
- 80.Johansen A, Low M-MM, Lacerda P, Bizzarro M. 2015. Growth of asteroids, planetary embryos, and Kuiper belt objects by chondrule accretion. Sci. Adv. 1, e1500109 ( 10.1126/sciadv.1500109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horner J, Mousis O, Hersant F. 2007. Constraints on the formation regions of comets from their D:H ratios. Earth Moon Planets 100, 43–56. ( 10.1007/s11038-006-9096-4) [DOI] [Google Scholar]
- 82.Yang L, Ciesla FJ, Alexander CMO'D. 2013. The D/H ratio of water in the solar nebula during its formation and evolution. Icarus 226, 256–267. ( 10.1016/j.icarus.2013.05.027) [DOI] [Google Scholar]
- 83.Waite JH, Jr, et al. 2009. Liquid water on Enceladus from observations of ammonia and 40Ar in the plume. Nature 460, 487–490. ( 10.1038/nature08153) [DOI] [Google Scholar]
- 84.McCanta MC, Treiman AH, Dyar MD, Alexander CMO'D, Rumble D III, Essene EJ. 2008. The LaPaz Icefield 04840 meteorite: mineralogy, metamorphism, and origin of an amphibole- and biotite-bearing R chondrite. Geochim. Cosmochim. Acta 72, 5757–5780. ( 10.1016/j.gca.2008.07.034) [DOI] [Google Scholar]
- 85.Deloule E, Robert F, Doukhan JC. 1998. Interstellar hydroxyl in meteoritic chondrules: implications for the origin of water in the inner solar system. Geochim. Cosmochim. Acta 62, 3367–3378. ( 10.1016/S0016-7037(98)00232-4) [DOI] [Google Scholar]
- 86.Piani L, Robert F, Remusat L. 2015. Micron-scale D/H heterogeneity in chondrite matrices: a signature of the pristine solar system water? Earth Planet. Sci. Lett. 415, 154–164. ( 10.1016/j.epsl.2015.01.039) [DOI] [Google Scholar]
- 87.Bonal L, Alexander CMO'D, Huss GR, Nagashima K, Quirico E, Beck P. 2013. Hydrogen isotopic composition of the water in CR chondrites. Geochim. Cosmochim. Acta 106, 111–133. ( 10.1016/j.gca.2012.12.009) [DOI] [Google Scholar]
- 88.Coveney RM Jr, Goebel ED, Zeller EJ, Dreschhoff GAM, Angino EE. 1987. Serpentinization and the origin of hydrogen gas in Kansas. AAPG Bull. 71, 39–48. [Google Scholar]
- 89.Fritz P, Clark ID, Fontes J-C, Whiticar MJ, Faber E. 1992. Deuterium and 13C evidence for low temperature production of hydrogen and methane in a highly alkaline groundwater environment in Oman. Proc. 7th Int. Symp. Water–Rock Interaction, pp. 793–796. [Google Scholar]
- 90.Proskurowski G, Lilley MD, Kelley DS, Olson EJ. 2006. Low temperature volatile production at the Lost City Hydrothermal Field, evidence from a hydrogen stable isotope geothermometer. Chem. Geol. 229, 331–343. ( 10.1016/j.chemgeo.2005.11.005) [DOI] [Google Scholar]
- 91.Sutton S, Cloutis EA, Alexander CMO'D. 2013. The valence state of Fe and the origin of water in chondrites. In Proc. 44th Lunar and Planetary Science Conf., paper 2357 Houston, TX: Lunar and Planetary Institute; See http://www.lpi.usra.edu/meetings/lpsc2013/pdf/2357.pdf. [Google Scholar]
- 92.Brasser R, Morbidelli A. 2013. Oort cloud and scattered disc formation during a late dynamical instability in the Solar System. Icarus 225, 40–49. ( 10.1016/j.icarus.2013.03.012) [DOI] [Google Scholar]
- 93.Nixon CA, et al. 2012. Isotopic ratios in Titan's methane: measurements and modeling. Astrophys. J. 749, 159 ( 10.1088/0004-637X/749/2/159) [DOI] [Google Scholar]
- 94.Crida A, Charnoz S. 2012. Formation of regular satellites from ancient massive rings in the Solar System. Science 338, 1196–1199. ( 10.1126/science.1226477) [DOI] [PubMed] [Google Scholar]
- 95.Mandt KE, Mousis O, Lunine J, Gautier D. 2014. Protosolar ammonia as the unique source of Titan's nitrogen. Astrophys. J. Lett. 788, L24 ( 10.1088/2041-8205/788/2/L24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niemann HB, et al. 2010. Composition of Titan's lower atmosphere and simple surface volatiles as measured by the Cassini-Huygens probe gas chromatograph mass spectrometer experiment. J. Geophys. Res. (Planets) 115, 12006 ( 10.1029/2010JE003659) [DOI] [Google Scholar]
- 97.Epstein S, Krishnamurthy RV, Cronin JR, Pizzarello S, Yuen GU. 1987. Unusual stable isotope ratios in amino acid and carboxylic acid extracts from the Murchison meteorite. Nature 326, 477–479. ( 10.1038/326477a0) [DOI] [PubMed] [Google Scholar]
- 98.Pizzarello S, Feng X, Epstein S, Cronin JR. 1994. Isotopic analyses of nitrogenous compounds from the Murchison meteorite: ammonia, amines, amino acids, and polar hydrocarbons. Geochim. Cosmochim. Acta 58, 5579–5588. ( 10.1016/0016-7037(94)90251-8) [DOI] [PubMed] [Google Scholar]
- 99.Engel MH, Macko SA. 1997. Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite. Nature 389, 265–268. ( 10.1038/38460) [DOI] [PubMed] [Google Scholar]
- 100.Pizzarello S, Holmes W. 2009. Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochim. Cosmochim. Acta 73, 2150–2162. ( 10.1016/j.gca.2009.01.022) [DOI] [Google Scholar]
- 101.Elsila JE, Charnley SB, Burton AS, Glavin DP, Dworkin JP. 2012. Compound-specific carbon, nitrogen, and hydrogen isotopic ratios for amino acids in CM and CR chondrites and their use in evaluating potential formation pathways. Meteor. Planet. Sci. 47, 1517–1536. ( 10.1111/j.1945-5100.2012.01415.x) [DOI] [Google Scholar]
- 102.McKeegan KD, et al. 2011. The oxygen isotopic composition of the Sun inferred from captured solar wind. Science 332, 1528 ( 10.1126/science.1204636) [DOI] [PubMed] [Google Scholar]
- 103.Yurimoto H, Kuramoto K. 2004. Molecular cloud origin for the oxygen isotope heterogeneity in the Solar System. Science 305, 1763–1766. ( 10.1126/science.1100989) [DOI] [PubMed] [Google Scholar]
- 104.Lyons JR, Young ED. 2005. CO self-shielding as the origin of oxygen isotope anomalies in the early solar nebula. Nature 435, 317–320. ( 10.1038/nature03557) [DOI] [PubMed] [Google Scholar]
- 105.Sakamoto N, Seto Y, Itoh S, Kuramoto K, Fujino K, Nagashima K, Krot AN, Yurimoto H. 2007. Remnants of the early Solar System water enriched in heavy oxygen isotopes. Science 317, 231–233. ( 10.1126/science.1142021) [DOI] [PubMed] [Google Scholar]
- 106.Clayton RN, Mayeda TK. 1999. Oxygen isotope studies of carbonaceous chondrites. Geochim. Cosmochim. Acta 63, 2089–2104. ( 10.1016/S0016-7037(99)00090-3) [DOI] [Google Scholar]
- 107.Nakashima D, Ushikubo T, Joswiak DJ, Brownlee DE, Matrajt G, Weisberg MK, Zolensky ME, Kita NT. 2012. Oxygen isotopes in crystalline silicates of comet Wild 2: a comparison of oxygen isotope systematics between Wild 2 particles and chondritic materials. Earth Planet. Sci. Lett. 357–358, 355–365. ( 10.1016/j.epsl.2012.09.041) [DOI] [Google Scholar]
- 108.Starkey NA, Franchi IA. 2013. Insight into the silicate and organic reservoirs of the comet forming region. Geochim. Cosmochim. Acta 48, 1800–1822. ( 10.1016/j.gca.2012.11.040) [DOI] [Google Scholar]
- 109.Ogliore RC, Nagashima K, Huss GR, Westphal AJ, Gainsforth Z, Butterworth AL. 2015. Oxygen isotopic composition of coarse- and fine-grained material from comet 81P/Wild 2. Geochim. Cosmochim. Acta 166, 74–91. ( 10.1016/j.gca.2015.04.028) [DOI] [Google Scholar]
- 110.Alexander CMO'D. 2015. The vital roles of water and organics in the early Solar System. In Proc. 46th Lunar and Planetary Science Conf., paper 2744 Houston, TX: Lunar and Planetary Institute; See http://www.lpi.usra.edu/meetings/lpsc2015/pdf/2744.pdf. [Google Scholar]
- 111.Krot AN, Nagashima K, Alexander CMO'D, Ciesla FJ, Fujiya W, Bonal L. 2015. Sources of water and aqueous activity on the chondrite parent asteroids. In Asteroids IV (eds Michel P, DeMeo FE, Bottke WF), pp. 635–660. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 112.Russell SS, Ballentine CJ, Grady MM. 2017. The origin, history and role of water in the evolution of the inner Solar System. Phil. Trans. R. Soc. A 375, 20170108 ( 10.1098/rsta.2017.0108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fegley B. 2003. Venus. In Meteorites, comets and planets (ed. Davis AM.), pp. 487–507. New York, NY: Pergamon. [Google Scholar]
- 114.Bogard DD. 1988. On the origin of Venus’ atmosphere—possible contributions from simple component mixtures and fractionated solar wind. Icarus 74, 3–20. ( 10.1016/0019-1035(88)90027-9) [DOI] [Google Scholar]
- 115.Owen TC, Bar-Nun A. 2000. Volatile contributions from icy planetesimals. In Origin of the Earth and Moon (eds Canup RM, Righter K), pp. 459–471. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 116.Lugmair GW, Shukolyukov A. 2001. Early solar system events and timescales. Meteor. Planet. Sci. 36, 1017–1026. ( 10.1111/j.1945-5100.2001.tb01941.x) [DOI] [Google Scholar]
- 117.Trinquier A, Birck JL, Allègre CJ, Göpel C, Ulfbeck D. 2008. 53Mn–53Cr systematics of the early Solar System revisited. Geochim. Cosmochim. Acta 72, 5146–5163. ( 10.1016/j.gca.2008.03.023) [DOI] [Google Scholar]
- 118.Schiller M, Baker J, Creech J, Paton C, Millet M-A, Irving A, Bizzarro M. 2011. Rapid timescales for magma ocean crystallization on the Howardite-Eucrite-Diogenite parent body. Astrophys. J. Lett. 740, L22 ( 10.1088/2041-8205/740/1/L22) [DOI] [Google Scholar]
- 119.Neumann W, Breuer D, Spohn T. 2014. Differentiation of Vesta: implications for a shallow magma ocean. Earth Planet. Sci. Lett. 395, 267–280. ( 10.1016/j.epsl.2014.03.033) [DOI] [Google Scholar]
- 120.Dauphas N, Pourmand A. 2011. Hf–W–Th evidence for rapid growth of Mars and its status as a planetary embryo. Nature 473, 489–492. ( 10.1038/nature10077) [DOI] [PubMed] [Google Scholar]
- 121.Tang H, Dauphas N. 2014. 60Fe–60Ni chronology of core formation in Mars. Earth Planet. Sci. Lett. 390, 264–274. ( 10.1016/j.epsl.2014.01.005) [DOI] [Google Scholar]