Abstract
We describe the current state of the search for direct, surviving samples of early, inner Solar System fluids—fluid inclusions in meteorites. Meteoritic aqueous fluid inclusions are not rare, but they are very tiny and their characterization is at the state of the art for most analytical techniques. Meteoritic fluid inclusions offer us a unique opportunity to study early Solar System brines in the laboratory. Inclusion-by-inclusion analyses of the trapped fluids in carefully selected samples will, in the immediate future, provide us detailed information on the evolution of fluids as they interacted with anhydrous solid materials. Thus, real data can replace calculated fluid compositions in thermochemical calculations of the evolution of water and aqueous reactions in comets, asteroids, moons and the terrestrial planets.
This article is part of the themed issue ‘The origin, history and role of water in the evolution of the inner Solar System’.
Keywords: water, asteroids, inner Solar System, Europa, Ceres, fluid inclusions
1. Introduction
Over the past 60 years, we have become increasingly aware of the fundamental importance of water, and aqueous alteration, on primitive Solar System bodies. Asteroid samples such as the carbonaceous and ordinary chondrites, long touted as primordial material relatively unchanged since formation, have finally been recognized to have been profoundly affected by interactions with liquid water early on—certainly within their first 10 million years [1–4]. Nevertheless, fundamental information concerning the location within the Solar System and the timing of the aqueous alteration, as well as the origin and chemical and isotopic composition of the aqueous fluids, are lacking [5–7]. Workers have attempted to model and understand this aqueous process through analysis of hydrated minerals present in the meteorites, as well as through computer simulations of the alteration process [8,9]. A major impediment to our understanding of aqueous alteration has been the near-absence of actual samples of aqueous fluids in meteorites.
Henry Clifton Sorby (1826–1908) has been called the father of microscopic petrography. He was certainly one of the first to profitably use a microscope in the elucidation of rocks and minerals, despite criticism—to quote Sorby: ‘In those early days people laughed at me. They quoted Saussure who had said that it was not a proper thing to examine mountains with microscopes, and ridiculed my action in every way. Most luckily I took no note of them’ [10]. In 1858, Sorby began the first detailed analysis of aqueous fluid inclusions (he is also called the father of fluid inclusion analysis), and in 1864 [11] published the first account of fluid inclusions in meteorites. Sorby wrote ‘…it is important to remark that the olivine of meteorites contains most excellent “glass-cavities”…The olivine also contains “gas-cavities”, like those so common in volcanic materials, thus indicating the presence of some gas or vapour (aussun, parnalee)’. Apparently, these observations were never followed up [11]. One hundred and fifty years later fluid inclusions were finally verifiably found in meteorites, but in some of the least likely ones.
2. Monahans and Zag meteorites
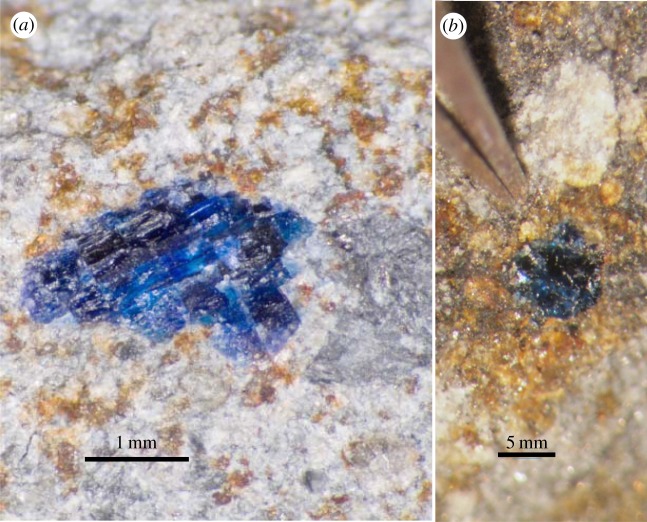
The Monahans (1998) (hereafter simply Monahans) chondrite fell on 22 March 1998, in the dry west Texas town of Monahans; the fall was witnessed by seven boys, and the first of two stones was recovered immediately. This particular stone was carried to the Johnson Space Center, and broken open in a class 10 000 clean room facility less than 48 h after the fall. Thus, there was no opportunity for interaction with liquid water or other contamination of the interior of the meteorite, which is very rarely the case. This particular meteorite was also opened not by sawing with water or cutting fluid, but rather by hammer and chisel—again an atypical situation. The Monahans meteorite is a regolith breccia consisting of light (lightly shocked) and dark (highly shocked) clasts set within a grey, fine-grained, clastic matrix. The light lithology has witnessed shock level S2 (light to moderate, 5–10 GPa), the grey matrix S3 (moderate, 10–15 GPa) and the dark lithology S4 (high, 15–30 GPa) [12,13]. All lithologies are H5, which indicates moderate thermal metamorphism to approximately 700°C [14]. We now know that this metamorphism predated final aggregation of the rock. Upon opening the first sample in a clean room, we noted that the grey matrix contains locally abundant aggregates of purple halite (NaCl) crystals, measuring up to 1 cm in size (though most are only a few millimetres at most; figure 1). We have not previously seen megascopic halite in any extraterrestrial sample, although microscopic halite has been reported in the Murchison CM2 chondrite [15], several ureilites [16] and some Martian meteorites [17–20]. In fact, some of these prior reports had been considered to be rather amazing, but there is no reason to doubt them. Backscattered electron imaging of Monahans revealed that crystals of sylvite (KCl) are present within the halite crystals, similar to their occurrence in terrestrial evaporites [21]. The purple colour of the halite is probably due to exposure to solar and galactic cosmic rays, and/or exposure to beta-decaying 40K in the sylvite [21,22], although that hypothesis has never been verified. The presence of halite and sylvite solely within Monahans grey matrix indicates that the halite formed or was deposited at the surface of the asteroid, in the regolith, before final aggregation of the meteorite. However, its deposition must postdate both thermal metamorphism and shock, because halite is such a brittle material that it could not reasonably have been expected to survive these geological processes.
Figure 1.
Halite (blue) crystals in Monahans (a) and Zag (b) H chondrites. (Online version in colour.)
The three lithologies of Monahans were analysed separately for noble gases [23,24], which verified that Monahans is a regolith breccia. The relative measured amounts of He, 20Ne and 36Ar in the grey and dark Monahans lithologies indicated a major contribution of solar-wind-implanted gases, although these are only approximately 5–10% as large as those in meteorites with the highest concentration of these gases (Pesyanoe and Fayetteville [25,26]). The grey matrix Monahans lithology contains the highest concentration of solar gas [23,24], indicating that it was pre-irradiated for a few million years in the regolith of its parent asteroid, probably during the time of the halite deposition, and prior to its ejection from the final asteroid as a meteorite.
One milligram of Monahans halite/sylvite was consumed by Larry Nyquist for Rb/Sr analysis by mass spectrometry [23,24]. The calculated Rb–Sr model age for the halite is 4.7 ± 0.2 Ga, within error limits of other H chondrites measured in the same manner [27]. Because the isotopic composition of Sr in the halite/sylvite is extremely radiogenic, the model age must be a very good approximation of the formation age of the halite. In addition, Bogard et al. [28] used the 39Ar/40Ar technique to determine a minimum formation age for the Monahans halite of 4.33 ± 0.01 Ga.
The same year that Monahans fell into west Texas also saw the fall of the Zag H3-5 chondrite into Morocco [13,23,24], fortunately another dry environment, and fortunately this meteorite was also recovered very rapidly. An alert meteorite collector and dealer, Edwin Thompson, had the wisdom to saw open his pieces using alcohol from a freshly opened bottle, which preserved the halite he hoped to find inside. This meteorite, also a regolith breccia, contains rather more abundant halite than Monahans. Two samples of the Zag halite were dated by Whitby et al. [29] to 4.03 ± 0.005 Ga and 4.66 ± 0.08 Ga by using the I–Xe method, consistent with the dates obtained by Bogard and Nyquist for Monahans halite. It is interesting that such an unassuming mineral as halite is amenable to such varied dating techniques. However, it is clear that this meteoritic mineral is only preserved under unusual circumstances. In the intervening decades, no further meteorites have been found to contain halite. We also note that Zag was a large fall, and that stones recovered later have not been found to contain halite, indicating that even stones with no apparent terrestrial weathering can have been significantly altered.
(a). Fluid inclusions in halite
The most convincing evidence that a rock or mineral sample has formed in the presence of fluids or has been exposed to fluids at some time after formation is when the rock or mineral contains fluid inclusions. Fluid inclusions are microsamples (down to nanomole quantities) of fluid that are trapped at the crystal/fluid interface during growth (primary inclusions) or at some later time along a healed fracture in the mineral (secondary inclusions) [30]. Both primary and secondary fluid inclusions are found in Monahans and Zag halite, with the latter predominating. The presence of secondary inclusions in the halite indicates that aqueous fluids were locally present following halite deposition, suggesting that aqueous activity could have been episodic. By definition, secondary fluid inclusions form at some time after the host mineral has been precipitated. Therefore, the presence of secondary inclusions in the halite indicates that fluids persisted in the environment of formation, or were introduced into that environment episodically, for some unknown length of time after the halite was precipitated. If such halite were to have formed as evaporites, then secondary inclusions could have formed during later emanations or eruptions.
The aqueous fluid inclusions range up to 15 µm in longest dimension (figure 2). At room temperature, a few of the inclusions (approx. 25%) contain bubbles that are in constant motion (as viewed using an optical microscope), proving that the inclusions contain a low-viscosity liquid and ‘vapour’. During cooling experiments under the microscope, the inclusions in the Monahans and Zag halite solidified (froze) at −45 to −50°C [23,24]. Thus, the liquid in the inclusions cannot be carbon dioxide, because solid and vapour carbon dioxide cannot coexist at any temperature above the triple point of carbon dioxide, which is −56.6°C. Moreover, the vapour bubbles appear to shrink and in some cases disappear during freezing, consistent with the interpretation that the inclusions contain an aqueous solution (if the inclusions were pure carbon dioxide, the bubble would become larger upon freezing of liquid carbon dioxide to form solid carbon dioxide). When the frozen inclusions were heated, first melting was observed at about −35 to −40°C, indicating that the inclusions probably contain divalent cations such as Fe2+, Ca2+ or Mg2+, in addition to Na+ and K+. Given the environment of formation in an asteroidal setting [1], dissolved Fe and Ca are the most likely cause of the lowered first-melting temperatures, unlike terrestrial evaporite environments in which the pair Ca and Mg are more likely. The final ice-melting temperature was very difficult to determine with any degree of accuracy. It was estimated that final ice melting occurred at or slightly below −20°C, because at this temperature the vapour bubble moved freely within the inclusions [23,24]. Below this temperature, the bubbles were generally pinned into one place, or moved only in a restricted region of the inclusion, presumably because of the presence of ice (or hydrate phases) which could not be discerned optically. The practice of using the lowest temperature at which the vapour bubble can be seen to move freely in the inclusion as an approximation of the ice-melting temperature is commonly used in studies of small inclusions in terrestrial samples [30].
Figure 2.
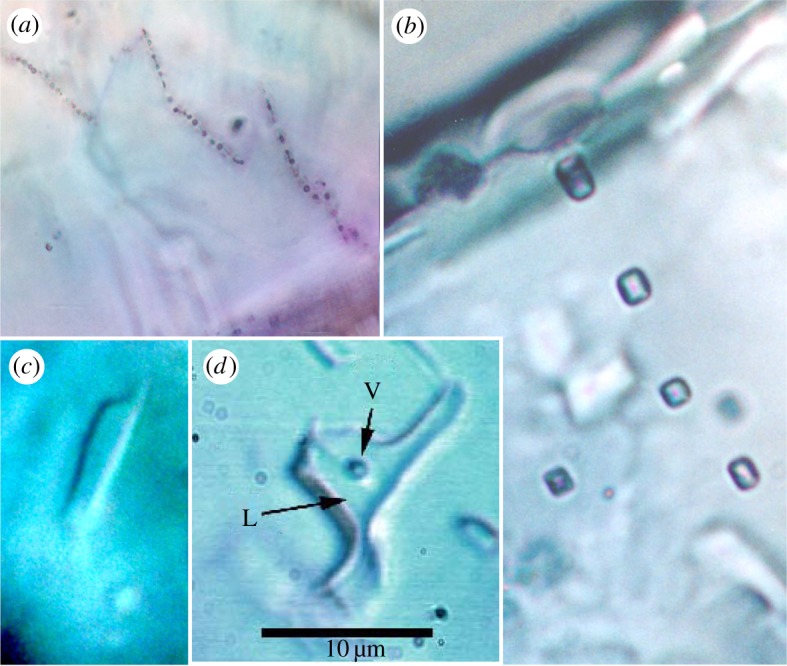
Aqueous fluid inclusions in Monahans halite crystals, viewed in transmitted light. (a) Secondary fluid inclusions, probably along healed fractures. (b–d) Probable primary inclusions. V, vapour; L, liquid. The 10 µm scale bar applies to all images. (Online version in colour.)
The presence of water in the inclusions was confirmed by Raman microprobe analysis [23,24]. As shown in figure 3, Raman spectra of inclusions in Monahans halite showed a significant peak at approximately 3400 cm−1, which is diagnostic of aqueous salt solutions [31]. Pure water gives a very broad peak that is skewed to lower wavenumbers. With the addition of salt, the peak becomes more symmetrical and narrower [32], as shown by the spectrum for a droplet of NaCl-saturated water collected at the same conditions used for the investigation of the two inclusions in halite. The Raman spectrum of an area of the halite away from inclusions did not show a peak in the 3000–4000 cm−1 region (figure 3), indicating that the species responsible for the peak occurred in the inclusions but not in the halite itself.
Figure 3.
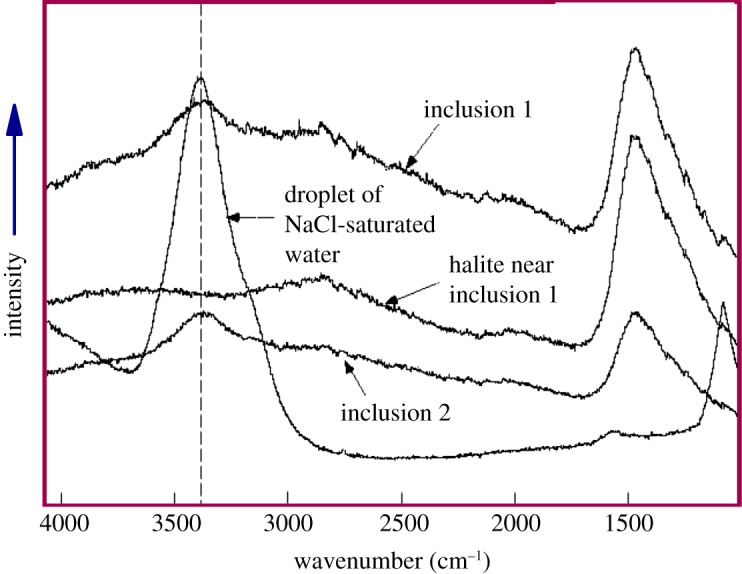
Raman spectra of two regions of Monahans halite containing aqueous fluid inclusions 1 and 2, one barren area of Monahans halite near inclusion 1 (but not including the inclusion), and a standard sample of NaCl-saturated water. Peaks near 3400 cm−1 wavenumbers are diagnostic of NaCl-saturated water. After [23,24]. (Online version in colour.)
The minimum temperature of formation of a given fluid inclusion may be estimated based on the relative size of the vapour bubble in the inclusion at room temperature. Thus, a pure water inclusion with a homogenization temperature of 374°C would contain 69 vol% vapour at room temperature, whereas a pure water inclusion with a homogenization temperature of 200°C would contain about 15 vol% vapour at room temperature. Fluid inclusions with homogenization temperatures less than about 100°C (such as those in the meteoritic halites) often fail to nucleate a vapour bubble when cooled to room temperature and remain as metastable, single-phase liquid inclusions. The rarity of vapour bubbles in fluid inclusions in Monahans and Zag halite suggests a low formation temperature—less than 100°C, and probably less than 50°C, assuming that the formation pressure was low—a few tens of bars at most. The bubbles that are present are small, and may have resulted from freeze stretching of the inclusions during space exposure [30]. The vapour bubbles represent the water vapour in equilibrium with liquid water at room temperature and, as such, are essentially a poor vacuum with a calculated pressure of about 0.03 bar. No gases, such as CO2, N2 or CH4, were detected during Raman analysis of the inclusions, and because the detection limits for these gases are generally in the range of a mole per cent in fluid inclusions, if these gases are present, their concentrations are low.
Why were fluid inclusions not found in meteorites before 1999, especially in meteorites where the activity of liquid water was evident? In fact, over the years, there have been scattered reports of fluid inclusions in meteorites [33,34]. One of the more widely known studies of fluid inclusions in meteorites, and the one that led to reports of fluid inclusions in meteorites being viewed with scepticism for many years, was that of Warner et al. [34]. In that study, the authors reported the presence of two-phase, liquid–vapour inclusions in silicate minerals in diogenite ALHA 77256, and some lunar rocks. These inclusions exhibited moving vapour bubbles (similar to those observed in Monahans and Zag halite), and Raman analysis of the inclusions showed that they contained an aqueous phase (again similar to the Monahans halite). The Raman spectra also showed evidence for higher hydrocarbons in the inclusions (also present in the Monahans halite, see below). The great interest in fluid inclusions generated in the planetary science community by this report was short-lived, however, as later investigation of the same samples showed that the inclusions were artefacts from specimen preparation fluids [35]. Rudnick et al. [35] state in their conclusions that ‘We emphasize that our observations should not be construed as proof that fluid inclusions do not exist in extraterrestrial samples; those reported by Fieni et al. and those observed by us in unsectioned samples of the Peetz and Jilin chondrites may indeed be indigenous fluids. The search for fluid inclusions in extraterrestrial materials should not be abandoned, but actively pursued on carefully prepared samples'. Perron et al. [36] observed vesicles filled with heavy nitrogen gas and water vapour in the Bencubbin CB chondrite, proposing that these formed during the impact of an asteroid fragment onto the Bencubbin parent body. In their scenario, the heavy nitrogen in the bubbles came from one or several of its carrier phases in Bencubbin, and the water came from hydrous silicates. As hypothesized by Meibom et al. [37], these hydrous phases, similar to the hydrated clasts now found in CH and CBb chondrites, were probably common in the Bencubbin parent body at that time, but were later almost totally destroyed by one or more large-scale shock events. Perron et al. [36] found that the oxygen isotopic composition of the impact melt is much heavier than that of the silicate clasts, probably reflecting the composition of the water at the origin of the phyllosilicates.
Nevertheless, the observations from Bencubbin, Peetz and Jilin have never been confirmed. The fluid inclusions reported here in Monahans and Zag halite thus represent the first verified proof that aqueous fluid inclusions do indeed exist in some extraterrestrial samples, and have led to a renewed search for these in other extraterrestrial samples.
The fluid inclusion-containing halite in Monahans analysed by microthermometry and Raman spectroscopy underwent no sample preparation that could have introduced fluid inclusion artefacts. As noted above, the Monahans meteorite fell into a dry west Texas town, and was collected and immediately returned to JSC for study. The meteorite was first broken, and the halite exposed, in a clean laboratory, and the crystals containing the fluid inclusions were removed from the broken sample by hand picking; no cutting or polishing was conducted before the fluid inclusions were studied. The possibility that the fluids were introduced into the halite after reaching Earth is nil. Similarly, Zag fell in the same year into Morocco, and the halite-bearing pieces were carefully collected, sawed and preserved by Edwin Thompson, who gave them to M.E.Z. We note that samples of Zag other than those preserved by Mr Thompson have also been shown to contain halite [29], indicating that halite was at the time of fall very common in Zag. It remains possible that some unexamined Zag samples could still preserve halite, but recent searches for these have proved fruitless. Only the halite maintained in dry nitrogen or other special conditions have been preserved in these meteorites. In the past year, we carefully opened well-preserved samples of Zag in a clean laboratory, discovering sufficient newly exposed halite to permit analyses for indigenous amino acids [38].
(b). Chemical and isotopic analyses of water in meteoritic fluid inclusions
In a first attempt to make direct measurements of fluid compositions of preselected, individual fluid inclusions, we initiated analyses at Virginia Tech by laser ablation inductively coupled plasma mass spectrometry (LA ICPMS) using an Agilent 7500ce quadrupole ICPMS and a Lambda Physik GeoLas 193 nm excimer laser ablation system. This system has been designed and built expressly for the purpose of fluid inclusion analysis. Preliminary results seem to reveal that the inclusion aqueous fluids contain highly charged cations of Ca, Mg and Fe, as predicted (R. Bodnar and M. Zolensky, 2015, unpublished data). However, the measurements were barely above background, and so these analyses are in fact still just barely beyond the current state of the art. No doubt such analyses will be practicable in a few more years.
Large variations of hydrogen isotopic composition have been observed in hydrous minerals from chondrites [39], and cometary and interstellar water is highly D-enriched [40,41], representing cloud or outer solar disc chemistry. The hydrogen isotopic composition of hydrous minerals in chondrites is believed to reflect inner Solar System water [39,42]. Oxygen isotopes in the Solar System are also highly variable [43,44], reflecting contributions of at least one H2O component, probably O16-poor [45–47]. However, it is impossible to measure isotopes of hydrogen and oxygen simultaneously in hydrous minerals, because they invariably contain structural oxygen not bound in water. Fortunately, halite contains no structural oxygen or hydrogen, permitting measurement of both the isotopic composition of oxygen and hydrogen in aqueous fluid inclusions. Once again, halite is shown to be a special mineral.
The isotopic measurement of individual aqueous fluid inclusions in halite required a secondary ion mass spectrometer (SIMS) equipped with a cryosample stage, which is not a typical instrument configuration, and which was certainly not available when these meteorites fell in 1998. It took a fair degree of patience, but ultimately a Cameca ims-1270 SIMS instrument equipped with a cryosample stage was prepared for the desired isotopic measurements by Hisayoshi Yurimoto and co-workers at Hokkaido University [48]. The cryosample stage (Techno. I. S. Corporation) was placed in the sample chamber in place of the original sample stage and cooled down to about −190°C using liquid nitrogen. At this temperature, the brine in the fluid inclusions was frozen.
Accordingly, Yurimoto et al. [48] laboriously measured both oxygen and hydrogen isotopic compositions of preselected, individual, fluid inclusion fluids in halite crystals from Monahans and Zag halite crystals. Isotopic compositions of both hydrogen and oxygen were determined for six and two fluid inclusions from Monahans and Zag halite, respectively, among the total of 17 inclusions measured, and are plotted in δD–Δ17O space in figure 4. The obtained oxygen and hydrogen isotopic compositions span the entire range of aqueously altered minerals of chondrites and are comparable to those of comets and icy satellites of outer planets, but is extremely deuterium (D)-rich compared with outer planets. The measured δD values of fluid inclusions of Monahans and Zag halite range widely from −400 to +1300‰ (figure 4). This variation is larger than the reproducibility of standard analysis and the estimated error of each measurement. Multiple oxygen measurements for single fluid inclusions were successfully collected for two inclusions in Monahans halite. The observed variations of mass-independent fractionation component of oxygen isotopic composition (Δ17O) of fluid inclusions of the Monahans and Zag halite range widely from −20 to +30‰ (figure 4). This variation clearly shows disequilibrium of oxygen isotopes between inclusions, shifted in the 16O-poor direction compared with the bulk oxygen isotopic composition of ordinary chondrites [49].
Figure 4.
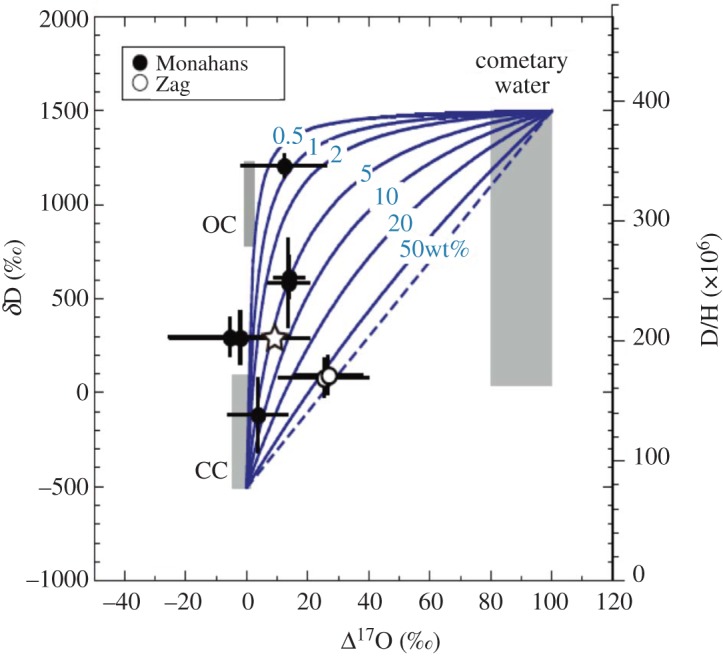
Isotopic compositions of hydrogen and oxygen determined for six and two fluid inclusions from Monahans and Zag halite, respectively, among the total of 17 measured inclusions measured, plotted on a diagram of δD versus Δ17O. Fields defined by water vapour in comet comae (cometary water), OH in hydrous minerals in ordinary chondrites (OC) and in carbonaceous chondrites (CC) are shown as grey rectangles. Water–rock interaction relationships between cometary water and hydrous carbonaceous chondrites are shown as blue curves. After [48]. (Online version in colour.)
The degree of 16O depletion of the water in the inclusions is larger than the most 16O-depleted astromaterial magnetite formed by aqueous alteration on asteroids identified so far [50]. This variation of oxygen isotopes of fluid inclusions therefore appears to be unique among Solar System objects analysed to date. The highly heterogeneous isotopic compositions between inclusions, being heavily fractionated for hydrogen and mass-independently fractionated for oxygen, indicate that the halite mother brines were in isotopic disequilibrium. None of the measurements are equivalent to isotopic compositions of ordinary chondrite water [42], demonstrating that these fluid inclusion fluids are not related to indigenous ordinary chondrite fluids. This conclusion is consistent with the petrography of the meteorites [13,23,24]. The isotopic compositions of the water in the halites are highly scattered in space, but the D-rich fluids tend to be 16O-poor. These highly disequilibrium characteristics are consistent with equally highly disequilibrium mineralogy of solid inclusions also trapped in these halites (see below), suggesting a relation to water–rock reactions in some carbonaceous chondrites [51].
Isotopic compositions of water in carbonaceous chondrites are in the range of −500 to +100‰ for δD [42] and −5 to +2‰ for Δ17O [44]. The halite fluid inclusion brines plot outside the region of estimated carbonaceous chondrite water. Therefore, a more D-rich and 16O-poor source is required for these fluids. Extremely D-rich water has been reported from comets [41], although the observed D/H composition among comets is highly variable [52]. Another observation of extremely D-rich water is from Enceladus [53]. It is known that extremely 16O-poor oxygen has a close relationship with cometary water [43]. Therefore, highly D-rich and 16O-poor water is expected in comets and icy satellites of the outer planets (hereafter collectively called ‘cometary water’). The inferred range of isotopic composition of cometary water is shown in figure 4. In figure 4, the measured isotopic compositions of the fluid inclusion fluids are distributed in a space between the bottom side of carbonaceous chondrite water (CC in figure 4) and the top side of the cometary water.
Water–rock interaction relationships between cometary water and hydrous carbonaceous chondrite are shown as curves in figure 4. Water contents of carbonaceous chondrites are parameters, and the values are shown on each curve. Isotope fractionation of hydrogen during the interaction is not taken into account because isotopic composition differences between carbonaceous chondrite water and cometary water are much larger than the isotopic fractionation factors. If the water contents of carbonaceous chondrites are small (always less than approx. 10 wt%, and typically less than 4 wt% [42]) and the proportion of cometary water component is small in the interaction system, the hydrogen isotopic composition of water becomes easily enriched in D from the initial composition by water–rock interaction, whereas oxygen isotopic composition is barely changed because of buffering by rocky oxygen components. If cometary water and carbonaceous chondrite water interact, i.e. the proportion of rock is small in the system, the change of hydrogen isotopic composition of water becomes proportional to the change of the oxygen isotopic composition.
Calculations made by Yurimoto et al. [48] indicate that the observed compositions of the Monahans and Zag water can be explained by simple water–rock interaction of carbonaceous chondrite water and cometary water, plausibly (but not uniquely) on a C-type asteroid. This model suggests that the halite parent body consisted of at least 1–10 wt% water, which is consistent with the normal range of carbonaceous chondrites [42]. Because chondritic water is believed to have originated in the inner Solar System, and cometary water in the outer Solar System, outer solar nebula or presolar molecular cloud [39,42,54], the observed isotopic compositions of the halite fluids suggest that dynamic delivery and accretion of water originating throughout the Solar System and in the parent molecular cloud onto planetesimals was a fundamental mechanism in the evolution of the water in the inner Solar System. The isotopic composition of lunar rock water [55] suggests that the global water mixing or delivery processes in the Solar System continued at least until the time of the Moon's formation and early evolution.
(c). Locating fluid inclusions in other astromaterials
The presence of fluid inclusions in the rather fragile halite crystals in Monahans and Zag suggested that fluids must have also been preserved in other meteoritic minerals, such as carbonates and silicates. This is supported by the discovery of decrepitated fluid inclusions in the nakhlites, appearing as dark trails through augite grains [20]. Therefore, we have been carefully examining newly prepared thin sections of CM and CI chondrites for fluid inclusions. New sections are generally necessary, because most existing meteorite thin sections have been examined using electron or ion beam instruments, and local heating during analysis usually decrepitates fluid inclusions in secondary minerals. In order to search for fluid inclusions, one must prepare new thin sections using no water or oil, and very small amounts of pure methanol, as we learned from the erroneous report of fluid inclusions in lunar rocks, where preparation fluids became trapped inside of cracks in the thin sections, resulting in confusion [35]. It is also critical to not significantly heat the sample during thin section preparation, which can also lead to inclusion decrepitation. These precautions mean that essentially no existing thin sections of meteorites are appropriate for this investigation.
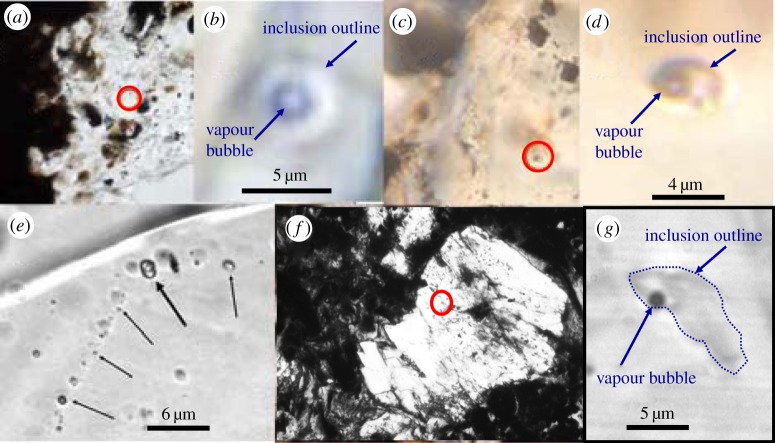
We have located potential aqueous fluid inclusions in Ivuna (CI), Murray (CM2), Mighei (CM2), Sayama (CM2), ALH 84029 (CM2), Tagish Lake (C2), LON 94101 (CM2) and Sutter's Mill (C2) in carbonate, sulfide, olivine and enstatite crystals (figures 5 and 6). In particular, optical and scanning electron microscope (SEM) imaging indicated that calcite and sulfides in Sutter's Mill have abundant, though very small, fluid inclusions (figure 6). The apparent success of this survey demonstrates that we can expect to find aqueous fluid inclusions in numerous, varied meteorites. These new inclusions had not been discovered before because they are very small (none larger than a few micrometres), and because of the high potential for creating spurious fluid inclusions during standard sample preparation procedures. However, the majority of these newly found, apparent fluid inclusions await confirmation.
Figure 5.
Two-phase fluid inclusions in carbonaceous chondrite calcite crystals, viewed in transmitted light. (a) Probable primary fluid inclusion in the Murray CM2 chondrite at lower magnification (red circle). (b) Same inclusion in Murray at higher magnification. (c) Probable primary fluid inclusion in the Tagish Lake C2 chondrite at lower magnification (red circle). (d) Same inclusion in Tagish Lake at higher magnification. (e) Probable secondary fluid inclusions along a healed fracture in the Tagish Lake C2 chondrite. (f) Probable primary fluid inclusion in Ivuna at lower magnification (red circle). (g) Same inclusion in Ivuna at higher magnification. (Online version in colour.)
Figure 6.
Probable primary, now vacant, fluid inclusions (arrowed) in a calcite crystal in the Sutter's Mill CM chondrite. Scale bar measures 10 µm.
(d). X-ray computed microtomography
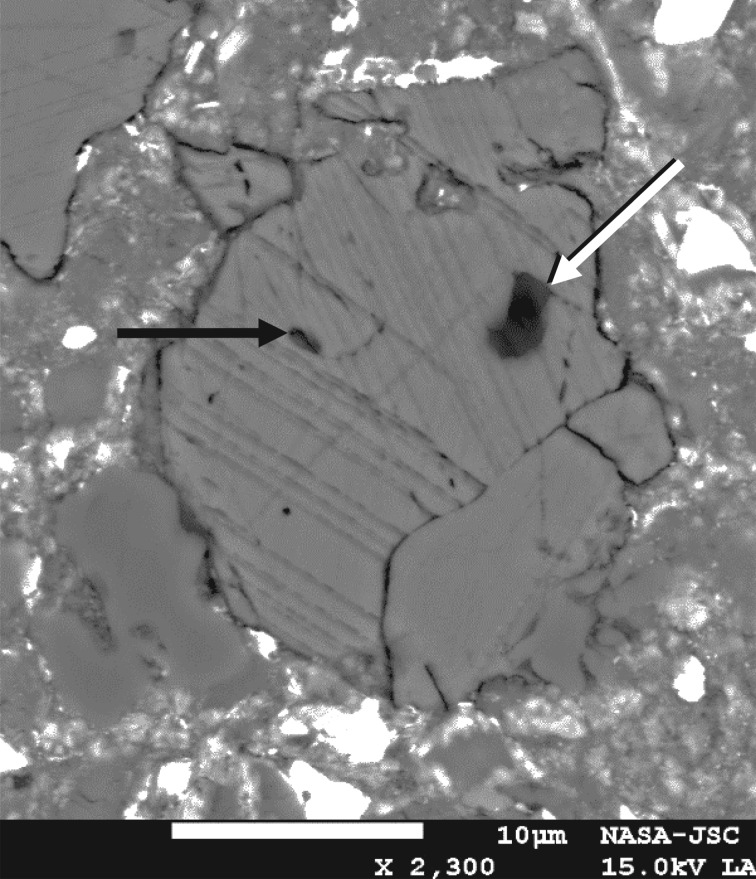
X-ray computed tomography (XRCT) can be a powerful tool in the search for potential fluid inclusion-bearing phases in meteorites. We subjected a kilogram-sized, carefully curated specimen of the Zag meteorite to XRCT at the High-Resolution X-ray Computed Tomography Facility at the University of Texas at Austin (figure 7), locating what appear to be additional grains of halite inside this mass. We did not open this piece until we were ready to perform the proposed organic analyses, and then only under strict cleanliness conditions. However, this one example will serve to demonstrate the potential value of this technique for the non-destructive interrogation of new samples.
Figure 7.

(a) The 1 kg Zag sample, measuring 13 cm across. (b) X-ray computed tomographic ‘slice’ through this sample; a probable halite grain (dark because of low relative density) is indicated by an arrow. (Online version in colour.)
In order to search for additional fluid inclusions in carbonaceous chondrites, and also (and very importantly) to verify the identity of potential new inclusions located by bulk XRCT and optical microscopy, a non-destructive technique using X-ray microabsorption tomography combined with focused ion beam (FIB) subsampling was developed by Tsuchiyama and co-workers [56–59]. As noted above, fluid inclusion candidates were located in calcite, olivine and sulfide (FeS and ZnS) grains in several carbonaceous chondrites which had experienced considerable aqueous alteration. However, because these candidates were very small (all less than 10 µm across, most much less), we could not determine by Raman spectroscopy whether these were really trapped aqueous fluids or merely voids, glass or other solid inclusions. Subsamples of these grains containing the potential fluid inclusions were removed by FIBing. The subsamples were then examined using a synchrotron-based imaging microtomography system at beamline BL47XU, SPring-8, Japan. Phase and absorption contrast images can be simultaneously obtained in three dimensions using scanning-imaging X-ray microscopy (SIXM) [60]. In this technique, phase and absorption contrasts are obtained simultaneously, permitting discrimination of minerals, fluids, organics and empty space. As performed by Tsuchiyama et al. [58], samples are imaged at two X-ray energies, 7 and 8 keV, to identify mineral phases (dual-energy microtomography [61]). The size of individual voxels (pixels in three dimensions) was 50–80 nm, which gave an effective spatial resolution of approximately 200 nm. An advantage of this technique is that fluid inclusions can also be located in opaque phases, such as sulfides and oxides. This capability is critical to any study of evolving fluid compositions, as was usually the case for early Solar System materials.
The majority of the grains examined to date by SIXM have inclusions more than 1 µm in size (the maximum being approx. 5 µm). Submicrometre-sized inclusions are present in all the grains examined. These results show that mineral grains in chondrites have more inclusions than expected solely from two-dimensional observations. The X-ray absorption of the meteoritic candidate inclusions showed that they were not solid inclusions. However, because no vapour bubbles were observed inside the majority of the samples, the investigators could not routinely determine whether they are really aqueous fluids or merely voids. One Sutter's Mill calcite grain had an inclusion approximately 2 µm in size, which seemed to have a bubble and a tiny solid daughter crystal inside (three-phase inclusion). As the exact three-dimensional position of located inclusions is well determined in the course of the SIXM work, it is possible to make subsequent analyses of the inclusions by SIMS after freezing the sample, as has been done for a halite sample [48]. The new XRCT and SIXM techniques have thus become proven techniques for locating small inclusions not only in meteorites, but also for terrestrial materials.
3. Solids and organics associated with the fluid inclusion-bearing minerals
Abundant solid inclusions are present in the extraterrestrial halites, associated with the aqueous fluid inclusions. These solids were probably entrained within the mother brines during the proposed eruption that deposited salt on the surface of the parent asteroid, or ejected it into space. Thus, these solids should include material from the rocky mantle and surface of the erupting body. The solid inclusions mainly consist of abundant and widely variable organics [62–64] that could not have been significantly heated following encapsulation in the halite, because this would have resulted in the loss of fluids from the halite. Analyses of solids from a single Monahans halite grain by Raman microprobe, SEM/energy dispersive X-ray, synchrotron X-ray diffraction and transmission electron microscopy reveal that these grains include macromolecular carbon similar in structure to CV3 chondrite matrix carbon, aliphatic carbon compounds, olivine of widely varying composition (Fo59–99), high- and low-Ca pyroxene, feldspars, magnetite, Fe–Ni sulfides, Fe–Ni metal, lepidocrocite, carbonates, diamond, apatite, phyllosilicates and zeolites [65]. It is clear that this phase assemblage is not at chemical equilibrium, consistent with rapid mixing and eruption of the assemblage.
The elucidation of the organics from the halite has the potential to revolutionize our understanding of the early history of carbon in the Solar System. Of particular importance is the observation that some organic inclusions in the halite have haloes of chloromethane, as revealed by Raman spectroscopy [51,66]. Chloromethane is water-soluble and requires cold formation temperatures at high hydrogen fugacity. When heated, methanol aromaticizes into polyaromatic hydrocarbons, which are not observed, another proof that these halites have not been heated. Fries [66] proposed that the haloes formed from methane leaking out of the organic inclusions, and interacting with the host halite. Raman spectroscopy of the organics trapped in the halite reveal that these have experienced a dramatic range of formation temperatures, from the chloromethane at the cold end, to materials that probably experienced temperatures in excess of 500°C, characteristic of organics found in thermally metamorphosed CV chondrites [62,66]. Because these high temperatures must predate trapping of the phases in the precipitating halite, they must reflect the temperature range present inside of the halite parent asteroid, and thus a wide temperature range also for the brines. The precipitation of the halite occurred at a temperature below 50°C, probably at the surface of the parent body. Ito and Kebukawa [63] have discovered that organics associated with the Zag halite have enormous excesses of deuterium and 15N, a consequence of formation at very cold temperatures. Such temperatures would have been achievable at the edge of the early solar nebula or beyond in interstellar clouds. Preservation of these isotopic signatures during residence inside of a hydrologically active asteroid would have required unusual circumstances and these organic grains are probably the resistant survivors of a once large population of such material.
4. Carbonaceous chondrite clasts associated with the meteoritic halite
Zag also contains a centimetre-sized carbonaceous chondrite clast, found by Norton and co-workers [67]. This clast in Zag is predominantly a fine-grained mixture of serpentine, saponite, magnetite, Ca phosphates, organic-dominated grains, pyrrhotite and Ca–Mn–Mg–Na carbonates. The very abundant carbonates have Mn-rich cores, mantles of Ca carbonate, and very thin Na–Mg-rich rims. The bulk oxygen isotopic composition of this clast plots in a unique place above the terrestrial fractionation line, and has a very high Δ17O value of +1.41 [67,68]. The Na-rich rims of carbonates suggested a link between this clast and the halite in Zag, and indeed careful examination of this clast using a field-emission gun scanning electron microscope (FEG-SEM) revealed 10 µm-sized (Na,K)Cl crystals, conclusively forging a link between this clast and the larger halites in Zag and, probably, Monahans. Owing to the extreme care with which this particular sample was handled, the halite is clearly indigenous to the clast and thus the Zag clast and halite are linked.
Because both Zag and Monahans are H chondrite regolith breccias, it is logical to examine other H chondrites (at least). In fact, a CI-like clast has been reported in the Tsukuba H chondrite [69], and the recently fallen Carancas H chondrite contains another [68]. The clasts in Tsukuba and Carancas appear to be mineralogically identical to the Zag clast. We suspect that this material was relatively widespread in the Solar System, but that its friability has generally prevented survival in meteorites. The O isotope composition of the Zag clasts shows that it is not merely CI chondrite material, although it is mineralogically very similar, suggesting similar formational history. These clasts probably represent rocky, mantle samples of the halite parent body.
5. Ceres as the source of the meteoritic halite
We have direct observations of hydrovolcanism of several small Solar System bodies (e.g. the moons Triton, Titan and Enceladus) [70], and indirect evidence for this process on the moons Europa, Ganymede and Miranda, and the Kuiper Belt object Charon [71], revealing continuing and widespread aqueous processes across the Solar System. The carbonaceous chondritic bodies, presumed to mainly be C-, P- and D-class asteroids and possibly short-period comets [72], would probably have been hydrovolcanically active early in their histories, and the meteoritic halites described above have been interpreted by some [51,62,65] to have originated from this ancient hydrovolcanism based on the following points. (i) Although some primitive carbonaceous chondrites clearly experienced late-stage Na–Cl-bearing fluid metasomatism [73], the occurrence of actual halite is exceptionally rare in astromaterials [23,24]. (ii) Salt crystals (probably halite) are associated with the current cryovolcanism on Enceladus [74,75]. (iii) On-site spacecraft analysis of icy grains associated with Enceladus halite found minor organic or silicate components hypothesized to derive from Enceladus' putative rocky mantle [75–77], as found in the meteoritic halite. These materials included methane, which we reported in the Monahans halite [62]. (iv) The Enceladus cryovolcanic fluids are in chemical disequilibrium, reflecting incomplete reactions between interior volatiles and rocky materials [53]. The coexistence of N2 and HCN in cryovolcanic fluids on Enceladus requires that the plume consists of a mixture of materials whose sources experienced different degrees of aqueous processing, including primordial material trapped in ice that has not been in contact with liquid water. The mineral assemblage and isotopic variations of fluids within the Monahans and Zag halites are also far from equilibrium [48,65]. (v) Hydrogen isotopic compositions of the Monahans and Zag fluids are comparable to hydrovolcanic fluids on Enceladus today [48,53].
The Monahans and Zag halites resulted from the expulsion of subterranean brines from one or more icy early Solar System bodies approximately 4.5 billion years ago [28,29]. The expelled fluids entrained organics and mineral grains from the rocky mantle of the body, which were captured in the halite as it crystallized in space. After considerable travel, the halites were deposited into the regolith of an S-type asteroid. Low neutron fluence experienced by the halite and the absence of solar wind 132Xe suggest that the Zag halites (at least) were not part of the regolith when the regolith matrix material now enclosing the halite acquired its solar wind and spallation components [29]. This probably indicates fairly late arrival of the halite at the S-type asteroid, but before final lithification of the regolith. Later, an event stripped a block of the surface away from this second body, eventually resulting in a meteorite shower that delivered the material to a third body (Earth). This final event need not have been due to an impact; rather it could have resulted from rotational spin-up by the Yarkovsky–O'Keefe–Radzievskii–Paddack (YORP) effect [78,79], which would have not further heated the trapped halite grains. It is not clear whether Zag and Monahans originated from the same S-type asteroid, or whether this scenario of deposition followed by expulsion occurred repeatedly. This body-hopping scenario is thought to have been a common occurrence in the early Solar System, as xenoliths are frequently found in meteorites [80], and the process of explosive eruptions from asteroids is supported by numerical models [81]. These well-travelled halite crystals thus preserve samples of early Solar System aqueous and solid materials from a cryovolcanically active body and highlight the complex dynamic physical and chemical environment in the early Solar System.
The mineralogy we have found in the halite and carbonaceous clasts appears to be entirely consistent with the reported mineralogy of the Ceres regolith [82]. For example, the Dawn Mission Team has reported finding considerable quantities of nitrogen-bearing compounds (reportedly ammoniated saponite) in the regolith of Ceres [82], and reported that the mineralogy thus determined appears to be similar to that of the CI chondrites. We propose that the clasts in Zag, Carancas and Tsukuba provide critical samples of the mantle and crust of the water-bearing world that produced the halite in Zag and Monahans—a water- and organic-rich world in the early Solar System that has survived at the largest remaining asteroid.
6. Conclusion
It is well recognized that aqueous fluids, especially brines, were important in the early Solar System [50]. Such fluids are probably present today below the surfaces of the icy moons Europa and Callisto [52]. Brown & Hand [83] proposed that NaCl and KCl dominate the non-ice component of the leading hemisphere of Europa, and that the most abundant salts in Europa ocean brines are not sulfates, but chlorides. Samples of ancient, inner Solar System water have survived in the form of aqueous fluid inclusions in chondrites and, probably, other classes of meteorites. Meteoritic fluid inclusions thus offer a unique opportunity to study early Solar System brines in the laboratory. Inclusion by inclusion analyses of the trapped fluids in carefully selected samples will, in the immediate future, provide detailed information on the evolution of fluids as they interacted with anhydrous solid materials. Thus, real data can replace calculated fluid compositions in thermochemical calculations of the evolution of water and aqueous reactions in comets, asteroids, moons and the terrestrial planets [84]. Analysis of the organics, in particular, that accompany these brine samples will shed important new light on the origin of life.
Acknowledgements
We acknowledge the constant support of NASA for this research, principally through the NASA Cosmochemistry Program. The Japanese authors thank the Japanese Ministry of Education. We thank SPring-8 Laboratory for SXRD support.
Competing interests
We declare we have no competing interests.
Funding
We received funding from the NASA Cosmochemistry Program for this study.
References
- 1.DuFresne E, Anders E. 1962. On the chemical evolution of the carbonaceous chondrites. Geochim. Cosmochim. Acta 26, 1085–1092. ( 10.1016/0016-7037(62)90047-9) [DOI] [Google Scholar]
- 2.McSween HY., Jr 1987. Aqueous alteration in carbonaceous chondrites: mass balance constraints in matrix mineralogy. Geochim. Cosmochim. Acta 51, 2469–2477. ( 10.1016/0016-7037(87)90298-5) [DOI] [Google Scholar]
- 3.Zolensky M, McSween HY. 1988. Aqueous alteration. In Meteorites and the early Solar System (eds Kerridge J, Matthews M), pp. 114–143. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 4.Krot AN, Scott ERD, Zolensky ME. 1995. Mineralogic and chemical variations among CV3 chondrites and their components: nebular and asteroidal processing. Meteoritics 30, 748–775. ( 10.1111/j.1945-5100.1995.tb01173.x) [DOI] [Google Scholar]
- 5.Brearley A. 2006. The action of water. In Meteorites and the early Solar System II (eds Lauretta D, McSween H), pp. 587–624. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 6.Krot A, Hutcheon I, Brearley A, Pradivtseva O, Petaev M, Hohenberg CM. 2006. Timescales and settings for alteration of chondritic meteorites. In Meteorites and the early Solar System II (eds Lauretta D, McSween H), pp. 525–554. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 7.Zolensky ME, Krot A, Benedix G. 2008. Low-temperature alteration on asteroids. In Oxygen in the Solar System (eds MacPherson GJ, Mittlefehldt D, Jones J, Simon S), Reviews in Mineralogy and Geochemistry, vol. 68, pp. 429–462. Washington, DC: Mineralogical Society of America. [Google Scholar]
- 8.Zolensky ME, Bourcier WL, Gooding J. 1989. Aqueous alteration on the hydrated asteroids: results of EQ3/6 computer simulations. Icarus, 78, 411–425. ( 10.1016/0019-1035(89)90188-7) [DOI] [Google Scholar]
- 9.Browning L, Bourcier WL. 1998. Constraints on the anhydrous precursor mineralogy of fine-grained materials in carbonaceous chondrites. Meteorit. Planet. Sci. 33, 1213–1220. ( 10.1111/j.1945-5100.1998.tb01306.x) [DOI] [Google Scholar]
- 10.Hentschel K. 2014. Visual cultures in science and technology: a comparative history, p. 106 Oxford, UK: Oxford University Press. [Google Scholar]
- 11.Sorby HC. 1864. On the microscopical structure of meteorites. Proc. R. Soc. Lond. 13, 333–334. ( 10.1098/rspl.1863.0075) [DOI] [Google Scholar]
- 12.Stöffler D, Keil K, Scott ERD. 1991. Shock metamorphism of ordinary chondrites. Geochim. Cosmochim. Acta 55, 3845–3867. ( 10.1016/0016-7037(91)90078-J) [DOI] [Google Scholar]
- 13.Rubin AE, Zolensky ME, Bodnar RJ. 2002. The halite-bearing Monahans 1998 and Zag meteorite breccias: shock metamorphism, thermal metamorphism and aqueous alteration on the H-chondrite parent body. Meteorit. Planet. Sci. 37, 125–142. ( 10.1111/j.1945-5100.2002.tb00799.x) [DOI] [Google Scholar]
- 14.McSween HY, Sears D, Dodd R. 1988. Thermal metamorphism. In Meteorites and the early Solar System (eds Kerridge J, Matthews M), pp. 102–113. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 15.Barber DJ. 1981. Matrix phyllosilicates and associated minerals in C2M carbonaceous chondrites. Geochim. Cosmochim. Acta 45, 945–970. ( 10.1016/0016-7037(81)90120-4) [DOI] [Google Scholar]
- 16.Berkeley J, et al. 1979. Fluorescent accessory phases in the carbonaceous matrix of ureilites. Geophys. Res. Lett. 5, 1075–1078. ( 10.1029/GL005i012p01075) [DOI] [Google Scholar]
- 17.Gooding JL, Wentworth SJ, Zolensky ME. 1991. Aqueous alteration of the Nakhla meteorite. Meteoritics 26, 135–143. ( 10.1111/j.1945-5100.1991.tb01029.x) [DOI] [Google Scholar]
- 18.Bridges JC, Grady MM. 1998. Melted sediment from Mars in Nakhla. In Proc. 29th Lunar and Planetary Science Conf., paper 1399. See http://www.lpi.usra.edu/meetings/lpsc98/pdf/1399.pdf.
- 19.Bridges JC, Grady MM. 2000. Evaporite mineral assemblages in the nakhlite (Martian) meteorites. Earth Planet. Sci. Lett. 176, 267–279. ( 10.1016/S0012-821X(00)00019-4) [DOI] [Google Scholar]
- 20.Bridges JC, Smith MP, Grady MM. 2000. Progressive evaporation and relict fluid inclusions in the nakhlites. In Proc. 31st Lunar and Planetary Science Conf., paper 1590. See http://www.lpi.usra.edu/meetings/lpsc2000/pdf/1590.pdf.
- 21.Chang L, Howie R, Zussman J. 1996. Rock-forming minerals, vol. 5B Non-silicates, pp. 369–378. London, UK: Longman Group. [Google Scholar]
- 22.Nassau K. 1983. The physics and chemistry of color, pp. 189–199. New York, NY: Wiley Interscience. [Google Scholar]
- 23.Zolensky ME, Bodnar RJ, Gibson EK, Gounelle M, Nyquist LE, Reese Y, Shih C-Y, Wiesmann H. 1999. Asteroidal water within fluid inclusion-bearing halite in an H5 chondrite. Science 285, 1377–1379. ( 10.1126/science.285.5432.1377) [DOI] [PubMed] [Google Scholar]
- 24.Zolensky ME, Bodnar RJ, Rubin AE. 1999. Asteroidal water within fluid-inclusion-bearing halite in ordinary chondrites. Meteorit. Planet. Sci. Suppl. 34, A124 see http://adsabs.harvard.edu/abs/1999M&PSA..34..124Z. [DOI] [PubMed] [Google Scholar]
- 25.Marti K. 1969. Solar-type xenon: a new isotopic composition of xenon in the Pesyanoe meteorite. Science 166, 1263–1265. ( 10.1126/science.166.3910.1263) [DOI] [PubMed] [Google Scholar]
- 26.Wieler R, Baur H, Pedroni A, Signer P, Pellas P. 1989. Exposure history of the regolithic chondrite Fayetteville: I. Solar-gas-rich matrix. Geochim. Cosmochim. Acta 53, 1441–1448. ( 10.1016/0016-7037(89)90076-8) [DOI] [Google Scholar]
- 27.Nyquist L, Bansal B, Weismann H, Shih C-Y. 1994. Neodymium, strontium and chromium isotopic studies of the LEW86010 and Angra dos Reis meteorites and the chronology of the angrite parent body. Meteoritics 29, 872–885. ( 10.1111/j.1945-5100.1994.tb01102.x) [DOI] [Google Scholar]
- 28.Bogard D, Garrison D, Masarik J. 2001. The Monahans chondrite and halite: 39Ar–40Ar age, solar gases, cosmic-ray exposure ages, and parent body regolith neutron flux and thickness. Meteorit. Planet. Sci. 36, 107–122. ( 10.1111/j.1945-5100.2001.tb01813.x) [DOI] [Google Scholar]
- 29.Whitby J, Burgess R, Turner G, Gilmour J, Bridges J. 2000. Extinct 129I in halite from a primitive meteorite: evidence for evaporite formation in the early solar system. Science 288, 1819–1821. ( 10.1126/science.288.5472.1819) [DOI] [PubMed] [Google Scholar]
- 30.Roedder E. 1984. Fluid inclusions. Washington, DC: Mineralogical Society of America. [Google Scholar]
- 31.Mernagh T, Wilde A. 1989. The use of the laser Raman microprobe for the determination of salinity in fluid inclusions. Geochim. Cosmochim. Acta 53, 765–771. ( 10.1016/0016-7037(89)90022-7) [DOI] [Google Scholar]
- 32.Lawler J, Crawford M. 1983. Stretching of fluid inclusions resulting from a low-temperature microthermometric technique. Econ. Geol. 78, 527–529. ( 10.2113/gsecongeo.78.3.527) [DOI] [Google Scholar]
- 33.Fiéni C, Bourote-Denise M, Pellas P, Touret J. 1978. Aqueous fluid inclusions in feldspars and phosphates from Peetz chondrite. Meteoritics 13, 460 See http://adsabs.harvard.edu/abs/1978Metic..13..460F. [Google Scholar]
- 34.Warner J, Ashwal L, Bergman S, Gibson E, Henry D, Lee-Berman R. 1983. Fluid inclusions in stony meteorites. J. Geophys. Res. Suppl. 88, A731–A735. ( 10.1029/JB088iS02p0A731) [DOI] [PubMed] [Google Scholar]
- 35.Rudnick R, Ashwal L, Henry D, Gibson E Jr, Roedder E, Belkin H, Colucci M. 1985. Fluid inclusions in stony meteorites: a cautionary note. J. Geophys. Res. Suppl. 90, C669–C675. ( 10.1029/JB090iS02p0C669) [DOI] [PubMed] [Google Scholar]
- 36.Perron C, Fiéni C, Guilhaumou N. 2008. Nitrogen and water bubbles, oxygen isotopes, shock effects: deciphering the history of the Bencubbin meteorite breccia. Geochim. Cosmochim. Acta 72, 959–977. ( 10.1016/j.gca.2007.11.030) [DOI] [Google Scholar]
- 37.Meibom A, et al. 2005. Shock melts in QUE 94411, Hammadah al Hamra 237, and Bencubbin: remains of the missing matrix? Meteorit. Planet. Sci. 40, 1377–1391. ( 10.1111/j.1945-5100.2005.tb00408.x) [DOI] [Google Scholar]
- 38.Chan Q, Zolensky M, Burton A, Locke D. 2016. Amino acids in the asteroidal water-bearing salt crystals hosted in the Zag meteorite. In Proc. 47th Lunar and Planetary Science Conf., paper 1402. See http://www.lpi.usra.edu/meetings/lpsc2016/pdf/1402.pdf.
- 39.Robert F. 2006. Solar system deuterium/hydrogen ratio. In Meteorites and the early Solar System II (eds Lauretta D, McSween H), pp. 341–352. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 40.Butner H, Charnley S, Ceccarelli C, Rodgers S, Pardo J, Parise B, Cernicharo J, Davis G. 2007. Discovery of interstellar heavy water. Astrophys. J. 659, L137–L140. ( 10.1086/517883) [DOI] [Google Scholar]
- 41.Villanueva G, Mumma M, Bonev B, DiSanti M, Gibb E, Böhnhardt H, Lippi M. 2009. A sensitive search for deuterated water in comet 8P/Tuttle. Astrophys. J. 690, L5 ( 10.1088/0004-637X/690/1/L5) [DOI] [Google Scholar]
- 42.Alexander CMO'D, Bowden R, Fogel M, Howard K, Herd C, Nittler L. 2012. The provenances of asteroids, and their contributions to the volatile inventories of the terrestrial planets. Science 337, 721–723. ( 10.1126/science.1223474) [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto N, Seto Y, Itoh S, Kuramoto K, Fujino K, Nagashima K, Krot A, Yurimoto H. 2007. Remnants of the early solar system water enriched in heavy oxygen isotopes. Science 317, 231–233. ( 10.1126/science.1142021) [DOI] [PubMed] [Google Scholar]
- 44.Yurimoto H, Krot A, Choi B-G, Aléon J, Kunihiro T, Brearley A. 2008. Oxygen isotopes of chondritic components. In Oxygen in the Solar System (eds MacPherson GJ, Mittlefehldt DW, Jones JH, Simon SB), Reviews in Mineralogy and Geochemistry, vol. 68, pp. 141–186. Washington, DC: Mineralogical Society of America. [Google Scholar]
- 45.Kuramoto K, Yurimoto H. 2005. Oxygen isotopic heterogeneity in the solar system: the molecular cloud origin hypothesis and its implications for meteorites and the planets. In Chondrites and the protoplanetary disk (eds Krot AN, Scott E, Reipurth B), Astron. Soc. Pacific Conf. Ser., vol. 341, pp. 181–192. San Francisco, CA: Astronomical Society of the Pacific. [Google Scholar]
- 46.Lyons J, Young E. 2005. CO self-shielding as the origin of oxygen isotope anomalies in the early solar nebula. Nature 435, 317–320. ( 10.1038/nature03557) [DOI] [PubMed] [Google Scholar]
- 47.Yurimoto H, Kuramoto K. 2004. Molecular cloud origin for the oxygen isotope heterogeneity in the solar system. Science 305, 1763–1766. ( 10.1126/science.1100989) [DOI] [PubMed] [Google Scholar]
- 48.Yurimoto H, Itoh S, Zolensky M, Kusakabe M, Karen A, Bodnar R. 2014. Isotopic compositions of asteroidal liquid water trapped in fluid inclusions of chondrites. Geochem. J. 48, 1–12. ( 10.2343/geochemj.2.0335) [DOI] [Google Scholar]
- 49.Clayton R, Mayeda T, Goswami J, Olsen E. 1991. Oxygen isotope studies of ordinary chondrites. Geochim. Cosmochim. Acta 55, 2317–2337. ( 10.1016/0016-7037(91)90107-G) [DOI] [Google Scholar]
- 50.Choi B-G, McKeegan K, Krot A, Wasson J. 1997. Extreme oxygen-isotope compositions in magnetite from unequilibrated ordinary chondrites. Nature 392, 577–579. ( 10.1038/33356) [DOI] [Google Scholar]
- 51.Zolensky M, et al. 2013. Early solar system cryovolcanics in the laboratory. In Proc. 76th Annual Meteoritical Society Meeting, Edmonton, Canada, Paper 5200. See http://www.hou.usra.edu/meetings/metsoc2013/pdf/5200.pdf.
- 52.Hartogh P, et al. 2011. Ocean-like water in the Jupiter-family comet 103P/Hartley 2. Nature 478, 218–220. ( 10.1038/nature10519) [DOI] [PubMed] [Google Scholar]
- 53.Waite J, et al. 2009. Liquid water on Enceladus from observations of ammonia and 40Ar in the plume. Nature 460, 487 ( 10.1038/nature08153) [DOI] [Google Scholar]
- 54.Greenberg J. 1998. Making a comet nucleus. Astron. Astrophys. 330, 375–380. See http://adsabs.harvard.edu/abs/1998A&A..330..375G. [Google Scholar]
- 55.Greenwood J, Itoh S, Sakamoto N, Warren P, Taylor L, Yurimoto H. 2011. Hydrogen isotope ratios in lunar rocks indicate delivery of cometary water to the Moon. Nat. Geosci. 4, 79–82. ( 10.1038/ngeo1050) [DOI] [Google Scholar]
- 56.Tsuchiyama A, Miyake A, Zolensky M, Uesugi K, Nakano T, Takeuchi A, Suzuki Y, Yoshida K. 2014. Search for fluid inclusions in a carbonaceous chondrite using a new X-ray micro-tomography technique combined with FIB sampling. In Proc. 77th Annual Meteoritical Soceity Meeting, Casablanca, Morocco. See https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20140010587.pdf.
- 57.Zolensky M, et al. 2014. Mineralogy and petrography of C asteroid regolith: the Sutter's Mill meteorite. Meteorit. Planet Sci. 49, 1997–2016. ( 10.1111/maps.12386) [DOI] [Google Scholar]
- 58.Tsuchiyama A, Nakano T, Miyake A, Akihisa T, Uesugi K, Suzuki Y, Kitayama A, Matsuno J, Zolensky M. 2016. Application of scanning-imaging x-ray microscopy to fluid inclusion candidates incarbonates of carbonaceous chondrites. In JpGU (Japanese Geoscience Union) Meeting 2016, China, Japan, abstract.
- 59.Kitayama A, et al. 2016. Negative crystals of calcite and empty crystals in the shape of hexagonal plate in carbonaceous chondrites. In JpGU (Japanese Geoscience Union) Meeting 2016, China, Japan, abstract.
- 60.Takeuchi A, Uesugi K, Suzuki Y. 2013. Three-dimensional phase-contrast X-ray microtomography with scanning–imaging X-ray microscope optics. J. Synch. Radiat. 20, 793–800. ( 10.1107/S0909049513018876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsuchiyama A, et al. 2013. Analytical dual-energy microtomography: a new method for obtaining three-dimensional mineral phase images and its application to Hayabusa samples. Geochim. Cosmochim. Acta 116, 5–16. ( 10.1016/j.gca.2012.11.036) [DOI] [Google Scholar]
- 62.Fries M, Steele A, Zolensky M. 2012. Halogen-substituted methane in Monahans halite. In Proc. 75th Annual Meteoritical Society Meeting, Cairns, Australia, paper 5381. See http://www.lpi.usra.edu/meetings/metsoc2012/pdf/5381.pdf.
- 63.Kebukawa Y, et al. 2016. Organic aggregates with δD and δ15N anomalies in the Zag clast revealed by STXM and nanoSIMS. In Proc. 79th Annual Meteoritical Society Meeting, Berlin, Germany. See https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20160009082.pdf.
- 64.Kebukawa Y, et al. 2016. STXM-XANES analysis of organic matter in dark clasts and halite crystals in Zag and Monahans meteorites. In Proc. 47th Lunar and Planetary Science Conf., paper 1802. See http://www.hou.usra.edu/meetings/lpsc2016/pdf/1802.pdf.
- 65.Zolensky M, Fries M, Chan QH-S, Kebukawa Y, Steele A, Bodnar R. 2015. The mineralogy of Ceres* (*or something an awful lot like it). In Proc. 78th Annual Meteoritical Soceity Meeting, Berkeley, CA, paper 5270. See http://www.hou.usra.edu/meetings/metsoc2015/pdf/5270.pdf.
- 66.Fries M, Messenger S, Steele A, Zolensky M. 2013. Do we already have samples of Ceres? H chondrite halites and the Ceres–Hebe link. In Proc. 76th Annual Meteoritical Socity Meeting, Edmonton, Canada, paper 5266. See http://www.hou.usra.edu/meetings/metsoc2013/pdf/5266.pdf.
- 67.Zolensky M, Clayton RN, Mayeda T, Chokai J, Norton R. 2003. Carbonaceous chondrite clasts in the halite-bearing H5 chondrite Zag. Meteorit. Planet. Sci. Suppl.38, 5216. See http://adsabs.harvard.edu/abs/2003M&PSA..38.5216Z.
- 68.Zolensky M, et al. 2016. C chondrite clasts in H chondrite regolith breccias: something different. In Proc. 79th Annual Meteoritical Society Meeting, Berlin, Germany. See https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/20160007503.pdf.
- 69.Nakashima D, Nakamura T, Noguchi T. 2003. Formation history of CI-like phyllosilicate-rich clasts in the Tsukuba meteorite inferred from mineralogy and noble gas signatures. Earth Planet. Sci. Lett. 212, 321–336. ( 10.1016/S0012-821X(03)00282-6) [DOI] [Google Scholar]
- 70.Lellouch E, Paubert G, Moses J, Schneider N, Strobel D. 2002. Volcanically emitted sodium chloride as a source for Io's neutral clouds and plasma torus. Nature 421, 45–47. ( 10.1038/nature01292) [DOI] [PubMed] [Google Scholar]
- 71.Cook J, Desch S, Roush T, Trujillo C, Geballe T. 2007. Near-infrared spectroscopy of Charon: possible evidence for cryovolcanism on Kuiper Belt objects. Astrophys. J. 663, 1406–1419. ( 10.1086/518222) [DOI] [Google Scholar]
- 72.Gounelle M, Spurný P, Bland PA. 2006. The atmospheric trajectory and orbit of the Orgueil meteorite. Meteorit. Planet. Sci. 41, 135–150. ( 10.1111/j.1945-5100.2006.tb00198.x) [DOI] [Google Scholar]
- 73.Wasserburg GJ, Hutcheon ID, Aleon J, Ramon EC, Krot AN, Nagashima K,Brearley AJ. 2011. Extremely Na- and Cl-rich chondrule from the CV3 carbonaceous chondrite Allende. Geochim. Cosmochim. Acta 75, 4752–4770. ( 10.1016/j.gca.2011.06.004) [DOI] [Google Scholar]
- 74.Postberg R, Kempf S, Schmidt J, Brilliantov N, Beinsen A, Abel B, Buck U, Srama R. 2009. Sodium salts in E-ring ice grains from an ocean below the surface of Enceladus. Nature 459, 1099–1101. ( 10.1038/nature08046) [DOI] [PubMed] [Google Scholar]
- 75.Postberg F, Schmidt J, Hillier J, Kempf S, Srama R. 2011. A salt-water reservoir as the source of a compositionally stratified plume on Enceladus. Nature 474, 620–622. ( 10.1038/nature10175) [DOI] [PubMed] [Google Scholar]
- 76.Matson D, Castillo J, Lunine J, Johnson T. 2007. Enceladus’ plume: compositional evidence for a hot interior. Icarus 187, 569–573. ( 10.1016/j.icarus.2006.10.016) [DOI] [Google Scholar]
- 77.Postberg F, Kempf S, Hillier J, Srama R, Green S, McBride N, Grün E. 2008. The E ring in the vicinity of Enceladus. II. Probing the moon's interior—the composition of E-ring particles. Icarus 193, 438–454. ( 10.1016/j.icarus.2007.09.001) [DOI] [Google Scholar]
- 78.Paddack S, Rhee J. 1975. Rotational bursting of interplanetary dust particles. Geophys. Res. Lett 2, 365–367. ( 10.1029/GL002i009p00365) [DOI] [Google Scholar]
- 79.Lowry S, et al. 2007. Direct detection of the asteroidal YORP effect. Science 316, 272–274. ( 10.1126/science.1139040) [DOI] [PubMed] [Google Scholar]
- 80.Zolensky ME, Weisberg MK, Buchanan PC, Mittlefehldt DW. 1996. Mineralogy of carbonaceous chondrite clasts in howardites, eucrites and the Moon. Meteorit. Planet. Sci. 31, 518–537. ( 10.1111/j.1945-5100.1996.tb02093.x) [DOI] [Google Scholar]
- 81.Wilson L, Keil K. 1996. Clast sizes of ejecta from explosive eruptions on asteroids: implications for the fate of the basaltic products of differentiation. Earth Planet. Sci. Lett. 140, 191–200. ( 10.1016/0012-821X(96)00051-9) [DOI] [Google Scholar]
- 82.McSween HY, Castillo-Rogez J, Emergy JP, De Sanctis MC, the Dawn Science Team. 2016. Rationalizing the composition and alteration of Ceres. In Proc. 47th Lunar and Planetary Science Conf., paper 1258. See http://www.hou.usra.edu/meetings/lpsc2016/pdf/1258.pdf.
- 83.Brown M, Hand K. 2013. Salts and radiation products on the surface of Europa. Astrophys. J. 145, 110–117. ( 10.1088/0004-6256/145/4/110) [DOI] [Google Scholar]
- 84.Zolotov M. 2008. Oceanic composition on Europa: constraints from mineral solubilities. In Proc. 39th Lunar and Planetary Science Conf., paper 2349. See http://www.lpi.usra.edu/meetings/lpsc2008/pdf/2349.pdf.