Abstract
The original hydrogen isotope (D/H) ratios of different planetary bodies may indicate where each body formed in the Solar System. However, geological and atmospheric processes can alter these ratios through time. Over the past few decades, D/H ratios in meteorites from Vesta and Mars, as well as from S- and C-type asteroids, have been measured. The aim of this article is to bring together all previously published data from these bodies, as well as the Earth, in order to determine the original D/H ratio for each of these inner Solar System planetary bodies. Once all secondary processes have been stripped away, the inner Solar System appears to be relatively homogeneous in terms of water D/H, with the original water D/H ratios of Vesta, Mars, the Earth, and S- and C-type asteroids all falling between δD values of −100‰ and −590‰. This homogeneity is in accord with the ‘Grand tack’ model of Solar System formation, where giant planet migration causes the S- and C-type asteroids to be mixed within 1 AU to eventually form the terrestrial planets.
This article is part of the themed issue ‘The origin, history and role of water in the evolution of the inner Solar System’.
Keywords: hydrogen isotopes, water, meteorites, terrestrial planets, asteroid belt
1. D/H ratio models of the early Solar System
Theoretical studies of interstellar chemistry show that in astrophysical environments, at temperatures less than 50 K, water becomes enriched in deuterium (2H or D) relative to molecular hydrogen, with D/H ratios reaching 0.001–0.01 [1–5]. These theoretical studies are supported by astronomical observations of D-rich water (D/H = 0.001–0.08) in the envelopes surrounding protostars [6,7]. Thus, prior to the formation of our Sun, water in the molecular cloud would have had a high D/H ratio. However, when this D-rich water is incorporated into the hot inner region of a protoplanetary disc, isotopic exchange reactions occur with other hydrogen-bearing species (e.g. H2O + HD ↔ HDO + H2), which dramatically lower the D/H ratio (e.g. [8–15]). Isotopic exchange occurs more rapidly at high temperatures, meaning water in the inner regions of the disc would equilibrate with H2 gas, producing low D/H ratios of approximately 2 × 10–5 [16]. By contrast, because isotopic exchange is sluggish at low temperatures, water in the outer regions of the disc would preserve its original high D/H ratio from the molecular cloud. Therefore, after the Sun's formation the D/H ratio of water in the evolving protoplanetary disc would have been dependent (at least initially) on the temperature of the surrounding environment, and thus distance from the Sun.
Measurements of D/H ratios in Oort cloud comet (OCC) water are highly variable (1.4–6.5 × 10−4), with the lowest measured ratio being similar to terrestrial ocean water [17–29]. The Jupiter family comets (JFCs) Hartley 2 and 45P/Honda–Mrkos–Pajdušáková have low water D/H ratios (1.61 and less than 2.0 × 10−4) [30,31], whereas the ROSINA mass spectrometer aboard the Rosetta spacecraft measured a high D/H ratio (three times that of the Earth's oceans) in water vapour sublimated from the JFC 67P/Churyumov–Gerasimenko [32]. The overlap in D/H ratios, along with other indistinguishable chemical and physical characteristics [33], argues for a common parent population for the OCCs and JFCs [34]. However, D/H ratio variation implies the parent population formed over a long period of time or distance from the Sun. As an added complication, all these measurements are of sublimated water from the surface of comets, thus probably do not represent the bulk comet D/H ratio.
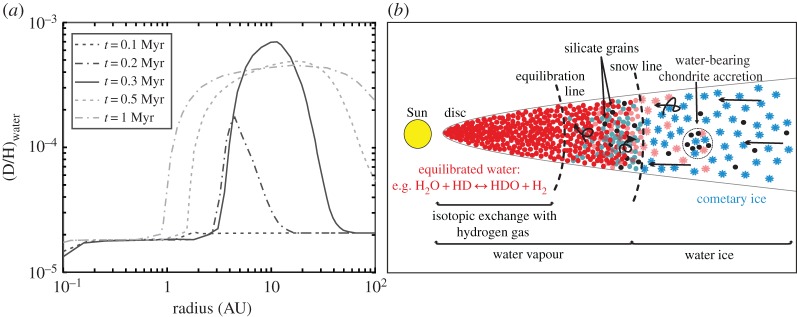
Yang et al. [16] coupled a dynamic model of material transport and mixing with a kinetic study of D–H isotopic exchange in an attempt to explain the variation in cometary D/H ratios, and to produce a picture of the first 1 Myr of Solar System history (figure 1a). These authors found that, during the very earliest stages of the protoplanetary disc (approx. 0.1 Myr after formation), viscous spreading may have redistributed low D/H ratio water from the inner to the very outer disc regions (figure 1a). Inside of 2 AU the D/H ratio of water is shown to be almost equal to that of molecular hydrogen. Viscous spreading would have redistributed this low D/H water throughout the protoplanetary disc by 0.1 Myr. At a later stage (approx. 0.2 Myr), molecular cloud infall begins to add high D/H water to the disc. In the inner disc, this molecular cloud water exchanges with molecular hydrogen, and the D/H ratio remains low. Beyond 2 AU low temperatures prevent this exchange and the D/H of water reflects the high ratio of the molecular cloud, with maximum values at approximately 3 AU and 10 AU in the 0.2 Myr and 0.3 Myr snapshots, respectively (figure 1a). However, this infall does not affect the outermost part of the disc, hence the D/H ratio of water remains low in this region. Therefore, observed cometary D/H variation can be explained if the parent population contained material from both the outer low D/H region and the approximately 2–10 AU high D/H region.
Figure 1.
D/H ratio evolution in the early Solar System. Isotopic equilibration with molecular hydrogen results in a low water D/H ratio in the hot inner disc (less than 2 AU) for the first approximately 0.3 Myr after the Sun's formation (a; adapted from [16]). By contrast, outer disc water (approx. 2–40 AU) remains unequilibrated, retaining the high D/H ratios inherited from the molecular cloud. Beyond approximately 0.3 Myr turbulent mixing produces a more uniform D/H distribution in the outer disc, with some molecular-hydrogen-equilibrated water being transported beyond the snowline to the region where the chondritic meteorites formed (b; adapted from [35]).
Beyond 0.3 Myr after the formation of the protoplanetary disc, once molecular cloud infall stops, turbulent mixing results in a more uniform D/H ratio in the outer disc. Gas turbulence would also result in some of the low D/H water from the inner disc being transported beyond the snowline, to the region where the chondritic meteorites are thought to have formed (figure 1b). Therefore, water in these meteorites should be composed of both an H2 equilibrated water component from the inner disc and a cometary water component that drifted inwards from the outer disc [35]. Transport and mixing would have been particularly efficient in the early stages of disc evolution, if the disc was first built compact and then expanded because of turbulence [36]. This expansion is consistent with the presence of crystalline silicates in comets [20].
2. D/H ratio measurements
D/H ratios are commonly quoted relative to Vienna Standard Mean Ocean Water (VSMOW; D/H = 1.5576 × 10−4) using the notation δD = {[(D/H)unknown/(D/H)VSMOW] − 1} × 1000, in units of parts per thousand (per mil (‰)). This means VSMOW has a δD value of 0‰. Deuterium-enriched reservoirs have high δD values (e.g. interstellar water δD ≈ +10 000‰ [37]), and D-poor reservoirs have low values (e.g. protosolar disc δD ≈ −870‰ [38]). This notation will be used in the following sections to compare the D/H ratios of various different extraterrestrial materials.
(a). Eucrites
Eucrites belong to the howardite–eucrite–diogenite group of meteorites that are derived from the asteroid belt, dominantly from the asteroid Vesta [39–41]. These basaltic meteorites are some of the oldest igneous rocks in the Solar System, mostly having crystallized only approximately 8–20 Myr after the first solids formed [42,43]. Sarafian et al. [44] measured the D/H ratio of structurally bound water in eucrite apatite [Ca5(PO4)3(OH,F,Cl)], reporting δD values of between −231‰ and −37‰ from five different meteorites. The authors state that these values probably reflect the original D/H ratio of the parent body (Vesta). As the apatite grains have young cosmic ray exposure ages, high water contents and a small range of D/H ratios across the different exposure ages and metamorphic grades, contamination by exogenic H from solar wind and/or by D produced during spallation processes is unlikely [44]. Degassing could have raised the D/H ratio from its original value, because of the preferential evaporation of the lighter hydrogen isotope. However, the small observed spread in δD with the relatively large spread of water contents in the analysed apatite indicates that degassing did not occur in these meteorites [45,46]. Stable isotope fractionation between apatite and melt is expected to be small (approx. 20‰) at high temperatures; thus, apatite-melt fractionation should be minor relative to the variation in δD observed [47]. Therefore, none of these three processes substantially affected the H isotopic compositions measured [44]. As each apatite grain is stoichiometric, hydrogen contamination via terrestrial weathering is ruled out.
(b). Carbonaceous chondrites
Reported bulk rock D/H ratios for the carbonaceous chondrites vary between δD values of +2150‰ and −229‰ [48,49]. However, these values are not representative of chondritic water alone, as the bulk rock includes hydrogen within insoluble organic material (IOM). Hydrous phyllosilicate is present in the CI, CM and Tagish Lake carbonaceous chondrites, as well as in a number of CR and CV meteorites. However, it is intergrown with IOM at the micrometre scale, meaning physical separation is not possible. Alexander et al. [49] calculated the D/H ratio of water in the CM and CR chondrite groups by comparing the bulk rock D/H and C/H ratios of group members showing different degrees of aqueous alteration. Assuming the bulk hydrogen isotopic compositions are produced by simple mixing of hydrated silicates and IOM, the bulk compositions should form a line on a plot of δD versus C/H, with the hydrogen isotope intercept giving the isotopic composition of water. The CM and CR chondrites do form a line on this type of plot, producing calculated water D/H ratios of −444 ± 23‰ and +96 (+110/−65)‰, respectively [49].
The CI chondrites, along with the most primitive CO and CV chondrites, are not common enough to use the above approach. However, the CI's Orgueil and Ivuna, along with the CO ALH 77307, fall on the CM trend, suggesting that they all probably had similar initial water compositions (table 1). If all chondrites accreted a common IOM component [60], chondrite water contents can also be calculated via subtraction of this component. This method is especially useful for those C-chondrite groups with only one or a few members (CIs, COs, CVs and Tagish Lake). By applying this methodology, Alexander et al. [49] calculated that C-chondrite water has current D/H ratios between δD −587‰ and +207‰ (table 1).
Table 1.
The D/H ratio of inner Solar System water reservoirs.
| D/H (×10−4) | δD (‰) | reference | |
|---|---|---|---|
| protosolar disc | 0.21 ± 0.04 | −863 to −868 | [38,50] |
| the Earth | |||
| bulk Earth | 1.49 ± 0.03 | −24 to −62 | [51] |
| VSMOW | 1.56 | 0 | [52] |
| GISP | 1.26 | −190 | [53] |
| MORB | 1.46–1.47 | −55 to −65 | [54] |
| deep mantle | <1.22 | <−218 | [55] |
| Mars | |||
| interior | <1.99 | <275 | [56–58] |
| atmosphere | 7.58–10.90 | 4950 ± 1080 | [59] |
| Vesta | 1.2–1.5 | −231 to −37 | [44] |
| C-chondrite water | |||
| CI | 0.64–0.98 | −373 to −587 | [49] |
| CM | 0.83–0.90 | −421 to −468 | [49] |
| CO | 0.85–1.32 | −152 to −455 | [49] |
| CR | 1.61–1.88 | 34–208 | [49] |
| CV | ≤0.82 | ≤−473 | [49] |
| Tagish Lake | ≤1.14 | ≤−268 | [49] |
| O-chondrite water | |||
| Semarkona | 2.80–3.44 | 798–1209 | [49] |
| R-chondrite water | 7.26 ± 0.13 | 3579–3746 | [49] |
(c). Non-carbonaceous chondrites
High D/H ratios are present in ordinary chondrite (OC) IOM, and these ratios increase with increasing metamorphism, to values as high as δD 12 000‰ [60,61]. In addition, the D/H ratio of water in the OC Semarkona is much higher than the ratios of the carbonaceous chondrites (δD = 798–1209‰ [49]). These high D/H ratios appear to be a product of oxidation in the meteorite, rather than a reflection of the original parent body D/H ratio—water becomes isotopically heavy due to the oxidation of Fe and the loss of isotopically very light H2. This heavy water then exchanges with the IOM.
The Rumuruti (R) chondrites are highly oxidized, containing rare or no Fe–Ni metal, as well as abundant ferromagnesian minerals rich in Fe3+ (e.g. [62,63]). Direct measurements of water D/H ratios are possible in the R-chondrite LaPaz Icefield (LAP) 04840, because it (uniquely) contains abundant OH-bearing silicate minerals (approx. 13% ferri-magnesiohornblende and approx. 0.4% phlogopite by volume), as well as rare apatite [63]. The D/H ratio of water in LAP 04840 hornblende and phlogopite ranges from δD 3595‰ to 3743‰ and from δD 2739‰ to 3043‰, respectively (table 1). As with the OCs, oxidation of Fe by water could also produce such D enrichments in the remaining water. Oxidation also destroys IOM, releasing D-rich hydrogen into the chondrite matrix. This hydrogen is subsequently incorporated into hornblende and phlogopite during their metamorphic formation [63]. R-chondrites are more oxidized (near the quartz–fayalite–magnetite buffer) than the OCs, which explains their higher water D/H ratios [63].
(d). Mars
In contrast to the asteroidal parent bodies of the eucrites and chondrites, Mars is large enough to have retained a substantial atmosphere. Therefore, it has at least two separate hydrogen isotope reservoirs (possibly three; see [64]). The atmospheric reservoir and the interior mantle reservoir are completely separated, because Mars has no plate tectonics. Therefore, there has been no recycling of atmospherically equilibrated crustal material back into the mantle.
The current Martian atmosphere is deuterium enriched, with ratios ranging from δD 0–2000‰ in high-altitude regions to δD 7000‰ in the polar regions [65]. The D/H ratio of Mars' atmosphere at Gale Crater, measured in situ by the Mars Curiosity rover, was reported as δD 4950 ± 1080‰ [59]. These high D/H ratios are caused by the preferential loss of the lighter hydrogen isotope to space via Jeans escape [66]. Hence, over geological time the D/H ratio of the Martian atmosphere is expected to have increased.
High atmospheric D/H ratios are represented in Martian meteorites via secondary alteration minerals. The nakhlite group of meteorites contain Martian aqueous alteration assemblages, consisting of smectite phyllosilicate, siderite carbonate, sulfates, Fe-oxides and hydroxides, and halite (e.g. [67–79]). These assemblages have been reported to contain high D/H ratios (up to δD 1165‰ [56]), although wide variability highlights the ease of isotopic exchange between certain alteration minerals and the terrestrial atmosphere. However, the maximum value in this case provides a minimum for the Martian atmosphere at the time of alteration formation (633 Ma [80]). Allan Hills (ALH) 84001 is the most ancient Martian meteorite, having crystallized at approximately 4.1 Ga (e.g. [81,82]). This orthopyroxenite contains small carbonate rosettes (less than 200 µm diameter), which are zoned from Ca and Fe rich to Mg rich [83–87], and are reported to have formed at approximately 3.9 Ga [88]. D/H ratios vary in these carbonates between δD 182‰ and 2092‰ [89,90]. This variation may be caused by different levels of terrestrial contamination, which would drag the D/H ratio down towards 0‰. Alternatively, or perhaps in addition, the high shock pressures experienced by this meteorite [86,91–93] could have caused shock implantation of atmospheric hydrogen into carbonate, driving the D/H ratio higher in the most shocked grains (see below).
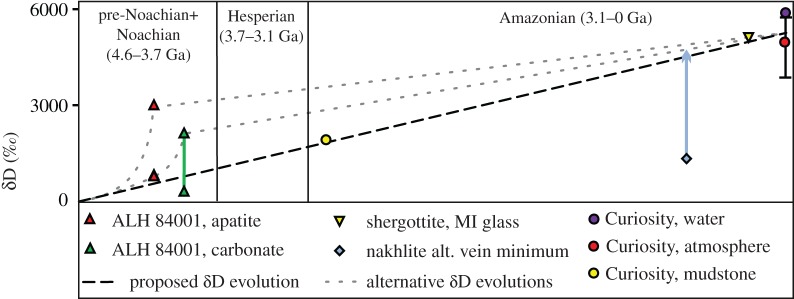
If meteorite alteration mineral D/H ratios are plotted in conjunction with current atmospheric measurements, as well as in situ lithological measurements from the Curiosity rover [94], a picture of atmospheric loss over time is produced (figure 2). These data suggest a linear increase in the atmospheric D/H ratio with time, and an origin of approximately 0‰. This low initial D/H ratio supports a volcanic origin for Mars' atmosphere, because measurements of melt inclusion (MI) glass and apatite in the most primitive and least shocked Martian meteorites indicate that the D/H ratio of Mars' mantle is low (δD < 275‰ [57,58]). However, not all hydrous primary (igneous) minerals contain D/H ratios representative of the Martian mantle reservoir (figure 3). In fact, the majority of these minerals have D/H ratios somewhere between low mantle and high crustal/atmospheric ratios (assuming the crust and atmosphere are equilibrated, but see [64]). There are a number of ways that these intermediate D/H ratios can be produced, both pre- and post-crystallization.
Figure 2.
The evolution of D/H ratio in the Martian atmosphere over time. Previous estimates of atmospheric D/H increase used ALH 84001 apatite D/H to suggest a rapid initial hydrogen (and general atmospheric) loss prior to 4.1 Ga (e.g. [65]). However, as ALH 84001 is a highly shocked meteorite that is reported to contain a crustal assimilant, the D/H ratio of its apatite may not be representative of the Martian atmosphere at the time of crystallization.
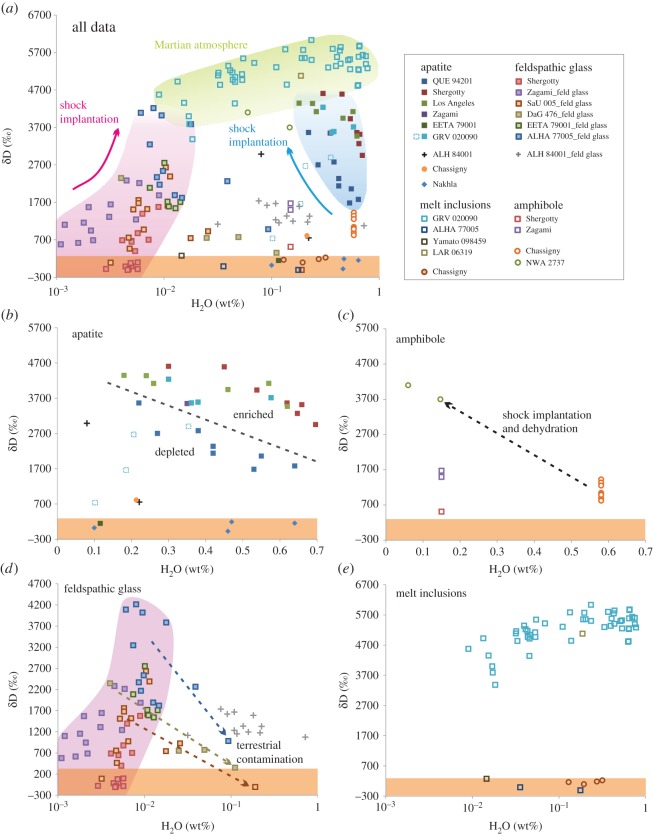
Figure 3.
(a–e) D/H ratio (δD) versus water content in Martian igneous minerals. High-impact shock pressures, mantle source enrichment and crustal assimilation can add D-rich atmospheric hydrogen to igneous minerals, meaning the majority do not have D/H ratios representative of the Martian mantle (δD < 275‰, orange envelope). See the electronic supplementary material for data and references.
In contrast to eucrite apatite D/H ratios, apatite in the shergottites and ALH 84001 shows a distinct trend of increasing D/H with decreasing water content (figure 3b). This trend is also weakly apparent in ALH 84001 feldspathic glass (figure 3d), and is characteristic of H2 degassing from a melt [45,46]. By contrast, Nakhla apatite D/H ratios stay constantly low over a range of water contents, indicating a lack of degassing in this melt. These low D/H ratios probably represent the Martian mantle reservoir.
A similar D/H trend is visible in chassignite amphiboles (figure 3c). However, instead of each meteorite exhibiting a range of D/H and water contents, two separate chassignites display very different amphibole compositions [95]. The high shock pressures experienced by meteorites upon ejection from the Martian surface may affect their isotopic ratios, especially for volatile elements. Experimental studies have shown that shock devolatilization of amphibole decreases its water content while simultaneously increasing the D/H ratio, because the lighter isotope is preferentially lost [96,97]. Hydrogen implantation from the surrounding atmosphere was also reported during these experiments, which would exaggerate the D/H increase under Martian atmospheric conditions. This trend of dehydration with a strong D/H increase is visible in chassignite amphibole, with amphibole in the highly shocked chassignite NWA 2737 showing much higher D/H ratios and lower water contents than that in Chassigny (figure 3c [95]). Shergottite feldspathic glass shows an increase in D/H ratio with increasing shock—ALHA 77005 is the most shocked shergottite [98] and contains glass with the highest D/H ratios, whereas Zagami and Shergotty are two of the least shocked and contain glass with lower D/H ratios (figure 3d). However, in contrast to hydrous amphibole, feldspathic glass contains much lower amounts of water, meaning hydrogen implantation overprints any hydrogen loss through devolatilization, and the overall water abundance increases with increasing shock.
The D/H ratio of MI glass follows a different trend from that of feldspathic glass, largely because the former contains more water (figure 3e). The D/H ratios are low (δD ≤ 275‰) in the MI glass of Chassigny [90], as well as the depleted and intermediate shergottites Yamato 098459 and ALHA 77005, respectively [58,90]. These low D/H ratios are probably representative of the Martian mantle. By contrast, MI glass in the enriched shergottites GRV 020090 and LAR 06319 contains highly elevated D/H ratios (δD 3386–6034‰ [58,99]). Based on the light rare-earth-element abundance of LAR 06319 melt inclusions, Basu Sarbadhikari et al. [100] reported that this meteorite probably derived its enrichment properties from partial melting of an enriched and oxidized mantle reservoir. Thus, the enriched component in these shergottites must have been rich in water equilibrated with the Martian atmosphere. Such enrichment explains why apatite in the depleted shergottite QUE 94201 contains lower D/H ratios than apatite in the enriched shergottites (figure 3b).
The D/H ratio of erupting melts on Mars could also be increased by assimilation of crustal material rich in atmospheric hydrogen, such as soil, sediments or ice. Based on sulfur isotope ratios, the shergottite Los Angeles is reported to contain assimilated crustal material, as are Nakhla and ALH 84001 [101]. However, Los Angeles is an enriched shergottite, so its parental melt would have had high D/H ratios prior to any assimilation. Indeed, other enriched shergottites that have not assimilated crustal material (e.g. Shergotty and Zagami [101]), contain apatite with similar D/H ratios to that in Los Angeles (figure 3b). Therefore, crustal assimilation does not appear to have had a significant effect on apatite D/H ratio in this meteorite, probably because the melt and assimilant had similar D/H ratios. Nakhla apatite has low D/H ratios reported to represent the Martian mantle [57]. Incorporation of atmosphere-equilibrated assimilant water should have increased the D/H ratio of these apatites, indicating that the Nakhla assimilant did not contain abundant water. ALH 84001 apatite D/H ratios appear to be elevated from the range of the mantle because of degassing. However, only two apatite grains have been measured in this meteorite (δD 751‰ and 2998‰; [90] and [102], respectively), hence much more data are needed before any conclusions can be drawn about the effects of assimilation on the water content of ALH 84001.
(e). The Earth
A range of D/H ratio values are found on Earth (table 1). The hydrological cycle fractionates hydrogen, creating glacial ice (standard Greenland Ice Sheet Precipitation (GISP) δD = –190‰ [53]; Standard Light Antarctic Precipitation 2 δD = −427.5‰ [103]), ocean water (VSMOW δD = 0‰ [52]) and fresh water (δD = 0 to −300‰ [51]) reservoirs. In contrast to Mars, the Earth's atmospheric and mantle water reservoirs are not kept separate, because subduction provides a means to mix surface water back into the mantle. This mixing produces a variation in mantle δD from –126‰ to +46‰ via slab dehydration and sediment recycling [104,105]. Mid-ocean ridge basalt (MORB) source D/H ratios are more uniformly mixed, forming a narrow range of δD −60 ± 5‰ [54]. However, none of these reservoirs are likely to represent the Earth's original D/H ratio.
The Earth's atmosphere is not a closed system. Experimentally based chemical models suggest Jeans escape could have caused an increase in the Earth's atmospheric D/H ratio of between a factor of 2 and 9 since the formation of the planets [106]. Plate tectonic mixing ensures this change has been incorporated into the mantle. In addition, collisions with hydrogen-bearing planetesimals or cometary material after the Earth's accretion could have altered the D/H ratio of the planet's surface and upper mantle [107]. Therefore, to determine the Earth's original D/H ratio a reservoir that has been completely unaffected by these surface and upper mantle changes is required.
Although alternative theories exist (e.g. [108]), most studies suggest that high 3He/4He ratios in some ocean island basalts indicate the existence of relatively undegassed regions in the deep mantle compared with the upper mantle, which retain a greater proportion of their primordial He [109,110]. Early Tertiary (60 Myr) picrites from Baffin Island and west Greenland, which represent volcanic rocks from the proto/early Iceland mantle plume, contain the highest recorded terrestrial 3He/4He ratios [109,110]. These picrites also have Pb and Nd isotopic ratios consistent with primordial mantle ages (4.45–4.55 Ga) [111], indicating the persistence of an ancient, isolated reservoir in the mantle. The undegassed and primitive nature [112] of this reservoir means that it could preserve the Earth's initial D/H ratio. Indeed, the D/H ratios of MI glass in these picrites extend lower than any previously measured mantle values (δD −97‰ to −218‰ [55]).
3. Do the measurements fit the models?
Measurements of meteorites from Vesta, C-type asteroids (carbonaceous chondrites) and Mars, along with terrestrial primitive deep mantle material, indicate the initial D/H ratios of water in these planetary bodies lie between approximately δD +200‰ and −590‰ [44,49,55,57,58]. This range could be narrowed if the most negative values from the Martian mantle (δD approx. −100‰ [57,90]) are assumed to be the most representative (with the least input from the D-enriched Martian atmosphere). In addition, the CR chondrites have more positive water D/H ratios than the other C-chondrite groups, which may be a product of oxidation rather than a reflection of the original parent body D/H ratio [113,114]. If this is the case, the range of inner Solar System water D/H can be reduced to approximately δD −100‰ to −590‰. Therefore, compared with the outer Solar System (e.g. [32]), the water D/H ratios of inner Solar System materials appear relatively homogeneous.
In order to determine whether the terrestrial planets sourced their volatiles from C-chondrites, the bulk D/H of these chondrites should be considered, including contributions from organics and possibly nebular hydrogen. The range of C-chondrite bulk D/H is considerably wider than that for just H2O, and stretches to much more positive values (δD = −229‰ to +2150‰ [48,49]). For the CI and CM chondrites at least, the water/metal ratios appear to have been high, based on bulk H and C contents [115] and the degree of aqueous alteration (e.g. [116–119]). Hence, hydrogen isotopic fractionation due to oxidation should not have been significant on these parent bodies. C-chondrite parent bodies (C-type asteroids) could originally have had lower bulk δD values if they lost significant water/ice during and after accretion. The heat of accretion could have caused expulsion of water from the warm interiors to the surface/near surface, where it would re-freeze. These ice-rich surfaces may have been stripped off over time by impacts and sublimation (C. Alexander 2017, personal communication). Heating, freezing and sublimation all preferentially leave behind the heavier hydrogen isotope, meaning residual parent body water would become D-enriched (increased δD value). Organics would become similarly enriched via isotopic exchange. There is remote-sensing evidence for ice at the surface of at least some C-type asteroids today [120,121]. However, in the absence of sample return or in situ exploration, this theory remains speculative.
Based on observations of extrasolar planetary discs, it is thought that Jupiter and Saturn formed a few million years after the formation of the protoplanetary disc [122]. This places gas giant formation and migration after the period of hydrogen isotope equilibration in the disc [16] and the formation of the chondritic parent bodies [35] (figure 1). Gas-driven inward and outward migration of the giant planets is estimated to have occurred on a time scale of hundreds of thousands of years [123]. The terrestrial planets' characteristics, including Mars' relatively small mass, are best reproduced if these planets formed within a disc that had an outer edge at 1 AU [124,125]. These conditions can be reproduced by Jupiter's inward migration to 1.5 AU and subsequent outward migration—the ‘Grand tack’ model of Solar System formation [126]. Inward migration scatters and mixes S- and C-type asteroids (the parent bodies of O- and C-chondrites, respectively) within this 1 AU terrestrial planet-forming region. S-type asteroids from 1 to 3 AU are reported to make up most of this mixed material [126]. If oxidation occurred before terrestrial planet accretion the water content of the S-type asteroids would be much lower, and the D/H ratio of the remaining water much higher (δD = 798–1209‰ [49]), than that measured for the terrestrial planets. Complete oxidation of the metal in OCs is estimated to take hundreds to 10 000 years [61], with several million years required before this for parent body accretion and heating to the point that oxidation can take place. However, the Grand tack model indicates that the terrestrial planets accreted much later than chondrites, 30–50 Myr after the formation of Ca,Al-rich inclusions [126,127]. In support of this time scale, W isotope data from lunar samples suggest that the Earth–Moon system formed approximately 30–100 Ma after the formation of the Solar System [128]. Therefore, OC oxidation does appear to have occurred prior to terrestrial planet formation, hence OC's D/H ratio would have been high at the time of planetary accretion. This problem can be overcome if a significant proportion of C-chondrite material accreted during terrestrial planet formation. As the OCs are extremely water-poor compared with the C-chondrites, the latter's D/H could overprint that of the OCs—Semarkona is by far the most water-rich OC with 0.09–0.1 wt% H, versus 0.17–1.36 wt% H for the C-chondrites [49].
Based on the Earth's bulk elemental and isotopic composition, numerous studies have attempted to recreate the Earth from different types and proportions of chondritic meteorites (e.g. [129]). However, none of these studies have been able to successfully recreate the abundances of all elements. Nitrogen isotope ratios (15N/14N) appear to be relatively homogeneous between Vesta, the Moon, Mars and the Earth, but the C-chondrites, particularly the CRs and CMs, have heavier N isotope ratios ([44,130] and references therein). In addition, terrestrial noble gas (Ne and Xe) signatures from primitive mantle sources suggest the presence of a solar component [131,132]. Therefore, accretion of some nebula gas or solar wind is the only way to explain the bulk Earth, Mars (and probably the other terrestrial planet) compositions.
Protosolar nebula water adsorption has been suggested as an alternative theory for the source of inner Solar System planetary body water. The temperature was high at 1 AU during the early Solar System, but 1000–500 K would still allow adsorption of 25–300% of the Earth's ocean water onto fractal grains during the Earth's accretion [133]. Asaduzzaman et al. [134] used density functional theory calculations to determine the amount of water that olivine surfaces could retain in the solar nebula. These authors found that water can be retained on adsorption surfaces at temperatures up to 900 K. In addition, if enough water is present in the nebula it may penetrate beyond the olivine grain surface to produce serpentine and brucite. This mechanism would be valid for all inner Solar System rocky materials. However, current models have been unable to accurately reproduce the D/H ratios of these inner Solar System bodies, as the protosolar nebula has a much lower D/H ratio than the range measured in inner Solar System meteorites (δD ≈ −870‰ [38]). In addition, it is unlikely that protosolar adsorption is a valid mechanism for the delivery of other volatile elements (e.g. C, N and noble gases).
4. Summary
Once the effects of secondary processes are removed, the original hydrogen isotope ratios of water in the Earth, Mars and Vesta, along with the C- and S-type asteroids, cover a relatively narrow range (approx. δD −100‰ to −590‰). This relative water homogeneity indicates that these materials shared a water source. The differentiated bodies (the Earth, Mars and Vesta) could have sourced their water from the C- and S-type asteroids, as the latter formed prior to terrestrial planet accretion. Oxidation of S-type asteroids almost certainly occurred prior to terrestrial planet accretion in the early Solar System, causing an increase in the bulk rock D/H ratio. However, the low water content of the S-type asteroids means their high D/H ratio could have been overprinted, at least in part, by the low D/H ratio of the C-type asteroids, which have much higher water contents. This simplistic view is complicated by the fact that no combination of chondrite meteorite compositions (as a proxy for parental asteroid compositions) has been able to recreate the bulk Earth. In particular, nitrogen and noble gases suggest that solar wind or nebula gas played a role in the Earth's formation. Inner Solar System nebula hydrogen gas (H2) or solar wind would have had a low D/H ratio at the time of terrestrial planet formation, thus this addition could have further overprinted the high D/H ratios of the S-type asteroid component in the terrestrial planets.
Supplementary Material
Acknowledgements
I would like to thank Conel Alexander for his helpful reviewer comments and suggestions, as well as associate editor Bailey Fallon. I acknowledge funding from the UK STFC through grant no. ST/N000846/1.
Competing interests
I declare I have no competing interests.
Funding
This work was funded by Science and Technology Facilities Council grant no. ST/M002268/1.
References
- 1.Brown PD, Millar TJ. 1989. Models of the gas–grain interaction—deuterium chemistry. Mon. Not. R. Astron. Soc. 237, 661–671. ( 10.1093/mnras/237.3.661) [DOI] [Google Scholar]
- 2.Millar TJ, Bennett A, Herbst E. 1989. Deuterium fractionation in dense interstellar clouds. Astrophys. J. 340, 906–920. ( 10.1086/167444) [DOI] [Google Scholar]
- 3.Roberts H, Millar TJ. 2000. Modelling of deuterium chemistry and its application to molecular clouds. Astron. Astrophys. 361, 388–398. [Google Scholar]
- 4.Roberts H, Herbst E, Millar TJ. 2004. The chemistry of multiply deuterated species in cold, dense interstellar cores. Astron. Astrophys. 424, 905–917. ( 10.1051/0004-6361:20040441) [DOI] [Google Scholar]
- 5.Ceccarelli C, Caselli P, Bockelée-Morvan D, Mousis O, Pizzarello S, Robert F, Semenov D.2014. Deuterium fractionation: the Ariadne's thread from the pre-collapse phase to meteorites and comets today. See http://arxiv.org/pdf/1403.7143v1.pdf .
- 6.Liu F-C, Parise B, Kristensen L, Visser R, van Dishoeck EF, Güsten R. 2011. Water deuterium fractionation in the low-mass protostars NGC1333-IRAS2A*. Astron. Astrophys. 527, A19 ( 10.1051/0004-6361/201015519) [DOI] [Google Scholar]
- 7.Coutens A, Vastel C, Caux E, Ceccarelli C, Bottinelli S, Wiesenfeld L, Faure A, Scribano Y, Kahane C. 2012. A study of deuterated water in the low-mass protostar IRAS 16293-2422*. Astron. Astrophys. 539, A132 ( 10.1051/0004-6361/201117627) [DOI] [Google Scholar]
- 8.Geiss J, Reeves H. 1981. Deuterium in the Solar System. Astron. Astrophys. 93, 189–199. [Google Scholar]
- 9.Lécluse C, Robert F. 1994. Hydrogen isotope exchange reaction rates: origin of water in the inner Solar System. Geochim. Cosmochim. Acta 58, 2927–2939. ( 10.1016/0016-7037(94)90126-0) [DOI] [Google Scholar]
- 10.Drouart A, Dubrulle B, Gautier D, Robert F. 1999. Structure and transport in the solar nebula from constraints on deuterium enrichment and giant planets formation. Icarus 140, 129–155. ( 10.1006/icar.1999.6137) [DOI] [Google Scholar]
- 11.Mousis O, Gautier D, Bockelée-Morvan D, Robert F, Dubrulle B, Drouart A. 2000. Constraints on the formation of comets from D/H ratios measured in H2O and HCN. Icarus 148, 513–525. ( 10.1006/icar.2000.6499) [DOI] [Google Scholar]
- 12.Hersant F, Gautier D, Huré J. 2001. A two-dimensional model for the primordial nebula constrained by D/H measurements in the Solar System: implications for the formation of giant planets. Astrophys. J. 554, 391–407. ( 10.1086/321355) [DOI] [Google Scholar]
- 13.Horner J, Mousis O, Hersant F. 2007. Constraints on the formation regions of comets from their D:H ratios. Earth Moon Planets 100, 43–56. ( 10.1007/s11038-006-9096-4) [DOI] [Google Scholar]
- 14.Kavelaars JJ, Mousis O, Petit J, Weaver HA. 2011. On the formation location of Uranus and Neptune as constrained by dynamical and chemical models of comets. Astrophys. J. Lett. 734, L30–L34. ( 10.1088/2041-8205/734/2/L30) [DOI] [Google Scholar]
- 15.Petit J-M, Kavelaars JJ, Mousis O, Weaver HA. 2011. Formation location of Uranus and Neptune from D/H in satellites and comets. In Proc. EPSC-DPS Joint Meeting 2011, Nantes, France, 2–7 October 2011 Göttingen, Germany: Copernicus. [Google Scholar]
- 16.Yang L, Ciesla FJ, Alexander CMO'D. 2013. The D/H ratio of water in the solar nebula during its formation and evolution. Icarus 226, 256–267. ( 10.1016/j.icarus.2013.05.027) [DOI] [Google Scholar]
- 17.Balsiger H, Altwegg K, Geiss J. 1995. D/H and O-18/O-16 ratio in the hydronium ion and in neutral water from in situ ion measurements in comet Halley. J. Geophys. Res. 100, 5827–5834. ( 10.1029/94JA02936) [DOI] [Google Scholar]
- 18.Eberhardt P, Reber M, Krankowsky D, Hedges RR. 1995. The D/H and 18O/16O ratios in water from comet Halley. Astron. Astrophys. 302, 301. [Google Scholar]
- 19.Bockelée-Morvan D, et al. 1998. Deuterated water in comet C/1996 B2 (Hyakutake) and its implications for the origin of comets. Icarus 133, 147 ( 10.1006/icar.1998.5916) [DOI] [Google Scholar]
- 20.Bockelée-Morvan D, et al. 2012. Herschel measurements of the D/H and 16O/18O ratios in water in the Oort-cloud comet C/2009 P1 (Garradd). Astron. Astrophys. 544, L15 ( 10.1051/0004-6361/201219744) [DOI] [Google Scholar]
- 21.Meier R, et al. 1998. A determination of the HDO/H2O ratio in comet C/1995 O1 (Hale-Bopp). Science 279, 842–844. ( 10.1126/science.279.5352.842) [DOI] [PubMed] [Google Scholar]
- 22.De Laeter JR, et al. 2003. Atomic weights of the elements: review 2000 (IUPAC technical report). Pure Appl. Chem. 75, 683–800. ( 10.1351/pac200375060683) [DOI] [Google Scholar]
- 23.Biver N, et al. 2006. Radio wavelength molecular observations of comets C/1999 T1 (McNaught-Hartley), C/2001 A2 (LINEAR), C/2000 WM1 (LINEAR) and 153P/Ikeya-Zhang. Astron. Astrophys. 449, 1255 ( 10.1051/0004-6361:20053849) [DOI] [Google Scholar]
- 24.Robert F. 2006. Solar System deuterium/hydrogen ratio. In Meteorites and the early solar system II (eds DS Lauretta, HY McSween), pp. 341–351. Tucson, AZ: University of Arizona Press. [Google Scholar]
- 25.Hutsemékers D, Manfroid J, Jehin E, Zucconi J-M, Arpigny C. 2008. The 16OH/18OH and OD/OH isotope ratios in comet C/2002 T7 (LINEAR). Astron. Astrophys. 490, L31–L34. ( 10.1051/0004-6361:200810833) [DOI] [Google Scholar]
- 26.Weaver HA, A'Hearn MF, Arpigny C, Combi MR, Feldman PD, Tozzi G-P, Dello Russo N, Festou MC. 2008. Atomic deuterium emission and the D/H ratio in comets. In Proc. Asteroids, Comets, Meteors 2008, Baltimore, MD, 14–18 July 2008 LPI contribution no. 1405, paper 8216 Houston, TX: Lunar and Planetary Institute. [Google Scholar]
- 27.Villanueva GL, et al. 2009. A sensitive search for deuterated water in comet 8P/Tuttle. Astrophys. J. 690, L5 ( 10.1088/0004-637X/690/1/L5) [DOI] [Google Scholar]
- 28.Brown RH, Lauretta DS, Schmidt B, Moores J. 2012. Experimental and theoretical simulations of ice sublimation with implications for the chemical, isotopic, and physical evolution of icy objects. Planet. Space Sci. 60, 166–180. ( 10.1016/j.pss.2011.07.023) [DOI] [Google Scholar]
- 29.Biver N, et al. 2016. Isotopic ratios of H, C, N, O, and S in comets C/2012 F6 (Lemmon) and C/2014 Q2 (Lovejoy). Astron. Astrophys. 589, A78 ( 10.1051/0004-6361/201528041) [DOI] [Google Scholar]
- 30.Hartogh P, et al. 2011. Ocean-like water in the Jupiter-family comet 103P/Hartley 2. Nature 478, 218–220. ( 10.1038/nature10519) [DOI] [PubMed] [Google Scholar]
- 31.Lis DC, et al. 2013. A Herschel study of D/H in water in the Jupiter-family comet 45P/Honda-Mrkos-Pajdušáková and prospects for D/H measurements with CCAT. Astrophys. J. Lett. 774, L3 ( 10.1088/2041-8205/774/1/L3) [DOI] [Google Scholar]
- 32.Altwegg K, et al. 2015. 67P/Churyumov-Gerasimenko, a Jupiter family comet with a high D/H ratio. Science 347, p1261952-1 ( 10.1126/science.1261952) [DOI] [PubMed] [Google Scholar]
- 33.A'Hearn MF, et al. 2012. Cometary volatiles and the origin of comets. Astrophys. J. 758, 29–37. ( 10.1088/0004-637X/758/1/29) [DOI] [Google Scholar]
- 34.Brasser R, Morbidelli A. 2013. Oort cloud and scattered disc formation during a late dynamical instability in the Solar System. Icarus 225, 40–49. ( 10.1016/j.icarus.2013.03.012) [DOI] [Google Scholar]
- 35.Jacquet E, Robert F. 2013. Water transport in protoplanetary disks and the hydrogen isotopic composition of chondrites. Icarus 223, 722–732. ( 10.1016/j.icarus.2013.01.022) [DOI] [Google Scholar]
- 36.Yang L, Ciesla FJ, Alexander CMO. 2012. The D/H ratio of water in a forming and evolving protoplanetary disk. In Proc. Lunar and Planetary Science Conf. 2012, The Woodlands, TX, 19–23 March 2012, abstract 2023 Houston, TX: Lunar and Planetary Institute. [Google Scholar]
- 37.Cleeves LI, Bergin EA, Alexander CMO'D, Du F, Graninger D, Öberg KI, Harries TG. 2014. The ancient heritage of water ice in the solar system. Science 345, 1590–1593. ( 10.1126/science.1258055) [DOI] [PubMed] [Google Scholar]
- 38.Geiss J, Gloeckler G. 1998. Abundances of deuterium and helium-3 in the protosolar cloud. Space Sci. Rev. 84, 239–250. ( 10.1023/A:1005039822524) [DOI] [Google Scholar]
- 39.McCord TB, Adams JB, Johnson TV. 1970. Asteroid Vesta: spectral reflectivity and compositional implications. Science 168, 1445–1447. ( 10.1126/science.168.3938.1445) [DOI] [PubMed] [Google Scholar]
- 40.Clayton RN, Onuma N, Mayeda TK. 1976. A classification of meteorites based on oxygen isotopes. Earth Planet. Sci. Lett. 30, 10–18. ( 10.1016/0012-821X(76)90003-0) [DOI] [Google Scholar]
- 41.McSween HY Jr, Mittlefehldt DW, Beck AW, Mayne RG, McCoy TJ. 2012. HED meteorites and their relationship to the geology of Vesta and the dawn mission. In The dawn mission to minor planets 4 Vesta and 1 Ceres (eds C Russell, C Raymond), pp. 141–174. New York, NY: Springer. [Google Scholar]
- 42.Misawa K, Yamaguchi A, Hiroshi K. 2005. U-Pb and 207Pb-206Pb ages of zircons from basaltic eucrites: implications for early basaltic volcanism on the eucrite parent body. Geochim. Cosmochim. Acta 69, 5847–5861. ( 10.1016/j.gca.2005.08.009) [DOI] [Google Scholar]
- 43.Zhou Q, et al. 2013. SIMS Pb–Pb and U–Pb age determination of eucrite zircons at <5 µm scale and the first 50 Ma of the thermal history of Vesta. Geochim. Cosmochim. Acta 110, 152–175. ( 10.1016/j.gca.2013.02.016) [DOI] [Google Scholar]
- 44.Sarafian AR, Nielsen SG, Marschall HR, McCubbin FM, Monteleone BD. 2014. Early accretion of water in the inner solar system from a carbonaceous chondrite-like source. Science 346, 623–626. ( 10.1126/science.1256717) [DOI] [PubMed] [Google Scholar]
- 45.Sharp ZD, McCubbin FM, Shearer CK. 2013. A hydrogen-based oxidation mechanism relevant to planetary formation. Earth Planet. Sci. Lett. 380, 88–97. ( 10.1016/j.epsl.2013.08.015) [DOI] [Google Scholar]
- 46.Tartèse R, Anand M, McCubbin FM, Elardo SM, Shearer CK, Franchi IA. 2014. Apatites in lunar KREEP basalts: the missing link to understanding the H isotope systematics of the Moon. Geology 42, 363–366. ( 10.1130/G35288.1) [DOI] [Google Scholar]
- 47.Chacko T, Cole DR, Horita J. 2001. Equilibrium oxygen, hydrogen and carbon isotope fractionation factors applicable to geologic systems. Rev. Mineral. Geochem. 43, 1–81. ( 10.2138/gsrmg.43.1.1) [DOI] [Google Scholar]
- 48.Kerridge JF. 1985. Carbon, hydrogen and nitrogen in carbonaceous chondrites: abundances and isotopic compositions in bulk samples. Geochim. Cosmochim. Acta 49, 1707–1714. ( 10.1016/0016-7037(85)90141-3) [DOI] [PubMed] [Google Scholar]
- 49.Alexander CMO'D, Bowden R, Fogel ML, Howard KT, Herd CDK, Nittler LR. 2012. The provenances of asteroids, and their contributions to the volatile inventories of the terrestrial planets. Science 337, 721–723. ( 10.1126/science.1223474) [DOI] [PubMed] [Google Scholar]
- 50.Lellouch E, Bezard B, Fouchet T, Feuchtgruber H, Encrenaz T, de Graauw T. 2001. The deuterium abundance in Jupiter and Saturn from ISO-SWS observations. Astron. Astrophys. 370, 610–622. ( 10.1051/0004-6361:20010259) [DOI] [Google Scholar]
- 51.Lécuyer C, Gillet P, Robert F. 1998. The hydrogen isotope composition of seawater and the global water cycle. Chem. Geol. 145, 249–261. ( 10.1016/S0009-2541(97)00146-0) [DOI] [Google Scholar]
- 52.Michael PJ. 1988. The concentration, behavior and storage of H2O in the suboceanic upper mantle: implications for mantle metasomatism. Geochim. Cosmochim. Acta 52, 555–566. ( 10.1016/0016-7037(88)90110-X) [DOI] [Google Scholar]
- 53.Hoefs J. 2009. Stable isotope geochemistry, 6th edn Berlin, Germany: Springer. [Google Scholar]
- 54.Clog M, Aubaud C, Cartigny P, Dosso L. 2013. The hydrogen isotopic composition and water content of southern Pacific MORB: a reassessment of the D/H ratio of the depleted mantle reservoir. Earth Planet. Sci. Lett. 381, 156–165. ( 10.1016/j.epsl.2013.08.043) [DOI] [Google Scholar]
- 55.Hallis LJ, Huss GR, Nagashima K, Taylor GJ, Halldórsson SA, Hilton DR, Mottl MJ, Meech KJ. 2015. Evidence for primordial water in Earth's deep mantle. Science 350, 795–797. ( 10.1126/science.aac4834) [DOI] [PubMed] [Google Scholar]
- 56.Hallis LJ, Taylor GJ, Nagashima K, Huss GR, Needham AW, Franchi IA, Grady MM. 2012. Hydrogen isotope analyses of alteration phases in the nakhlite Martian meteorites. Geochim. Cosmochim. Acta 97, 105–119. ( 10.1016/j.gca.2012.08.017) [DOI] [Google Scholar]
- 57.Hallis LJ, Taylor GJ, Nagashimaa K, Huss GR. 2012. Magmatic water in the Martian meteorite Nakhla. Earth Planet. Sci. Lett. 359–360, 84–92. ( 10.1016/j.epsl.2012.09.049) [DOI] [Google Scholar]
- 58.Usui T, Alexander CMO'D, Wang J, Simon JI, Jones JH. 2012. Origin of water and mantle–crust interactions on Mars inferred from hydrogen isotopes and volatile element abundances of olivine-hosted melt inclusions of primitive shergottites. Earth Planet. Sci. Lett. 357–358, 119–129. ( 10.1016/j.epsl.2012.09.008) [DOI] [Google Scholar]
- 59.Webster CR, et al. 2013. Isotope ratios of H, C, and O in CO2 and H2O of the Martian atmosphere. Science 341, 260–263. ( 10.1126/science.1237961) [DOI] [PubMed] [Google Scholar]
- 60.Alexander CMO'D, Fogel M, Yabuta H, Cody GD. 2007. The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim. Cosmochim. Acta 71, 4380–4403. ( 10.1016/j.gca.2007.06.052) [DOI] [Google Scholar]
- 61.Alexander CMO'D, Newsome SD, Fogel ML, Nittler LR, Busemann H, Cody GD. 2010. Deuterium enrichments in chondritic macromolecular material—implications for the origin and evolution of organics, water and asteroids. Geochim. Cosmochim. Acta 74, 4417–4437. ( 10.1016/j.gca.2010.05.005) [DOI] [Google Scholar]
- 62.Brearley AJ, Jones RH. 1998. Chondritic meteorites. In Planetary materials (ed. Papike JJ.). Reviews in Mineralogy, 36 Washington, DC: Mineralogical Society of America. [Google Scholar]
- 63.McCanta MC, Treiman AH, Dyar MD, Alexander CMO'D, Rumble D III, Essene EJ. 2008. The LaPaz Icefield 04840 meteorite: mineralogy, metamorphism, and origin of an amphibole- and biotite-bearing R chondrite. Geochim. Cosmochim. Acta 72, 5757–5780. ( 10.1016/j.gca.2008.07.034) [DOI] [Google Scholar]
- 64.Usui T, Alexander CMO'D, Wang J, Simon JI, Jones JH. 2015. Meteoritic evidence for a previously unrecognized hydrogen reservoir on Mars. Earth Planet. Sci. Lett. 410, 140–151. ( 10.1016/j.epsl.2014.11.022) [DOI] [Google Scholar]
- 65.Villanueva GL, Mumma MJ, Novak RE, Käufl HU, Hartogh P, Encrenaz T, Tokunaga A, Khayat A, Smith MD. 2015. Strong water isotopic anomalies in the Martian atmosphere: probing current and ancient reservoirs. Science 348, 218–221. ( 10.1126/science.aaa3630) [DOI] [PubMed] [Google Scholar]
- 66.Owen T, Maillard JP, de Bergh C, Lutz BL. 1988. Deuterium on Mars: the abundance of HDO and the value of D/H. Science 240, 1767–1771. ( 10.1126/science.240.4860.1767) [DOI] [PubMed] [Google Scholar]
- 67.Gooding JL, Wentworth SJ, Zolensky ME. 1991. Aqueous alteration of the Nakhla meteorite. Meteoritics 26, 135–143. ( 10.1111/j.1945-5100.1991.tb01029.x) [DOI] [Google Scholar]
- 68.Bridges JC, Grady MM. 1999. A halite-siderite-anhydrite-chloroapatite assemblage in Nakhla: mineralogical evidence for evaporites on Mars. Meteoritics Planet. Sci. 34, 407–415. ( 10.1111/j.1945-5100.1999.tb01349.x) [DOI] [Google Scholar]
- 69.Bridges JC, Grady MM. 2000. Evaporite mineral assemblages in the nakhlite (Martian) meteorites. Earth Planet. Sci. Lett. 176, 267–279. ( 10.1016/S0012-821X(00)00019-4) [DOI] [Google Scholar]
- 70.Bridges JC, Catling DC, Saxton JM, Swindle TD, Lyon IC, Grady MM. 2001. Alteration assemblages in Martian meteorites: implications for near-surface processes. Space Sci. Rev. 96, 365–392. ( 10.1023/A:1011965826553) [DOI] [Google Scholar]
- 71.Treiman. 2005. The nakhlite meteorites: augite-rich igneous rocks from Mars. Chemie der Erde 65, 203–270. ( 10.1016/j.chemer.2005.01.004) [DOI] [Google Scholar]
- 72.Changela HG, Bridges JC. 2011. Alteration assemblages in the nakhlites: variation with depth on Mars. Meteorit. Planet. Sci. 45, 1847–1867. ( 10.1111/j.1945-5100.2010.01123.x) [DOI] [Google Scholar]
- 73.Hallis LJ, Taylor GJ. 2011. Comparisons of the four Miller range nakhlites, MIL 03346, 090030, 090032 and 090136: textural and compositional observations of primary and secondary mineral assemblages. Meteorit. Planet. Sci. 46, 1787–1893. ( 10.1111/j.1945-5100.2011.01293) [DOI] [Google Scholar]
- 74.Bridges JC, Schwenzer SP. 2012. The nakhlite hydrothermal brine on Mars. Earth Planet. Sci. Lett. 359–360, 117–123. ( 10.1016/j.epsl.2012.09.044) [DOI] [Google Scholar]
- 75.Lee MR, Tomkinson T, Mark DF, Stuart FM, Smith CL. 2013. Evidence for silicate dissolution on Mars from the Nakhla meteorite. Meteorit. Planet. Sci. 48, 224–240. ( 10.1111/maps.12053) [DOI] [Google Scholar]
- 76.Lee MR, Tomkinson T, Hallis LJ, Mark DF. 2015. Formation of iddingsite veins in the Martian crust by centripetal replacement of olivine: evidence from the nakhlite meteorite Lafayette. Geochim. Cosmochim. Acta 154, 49–65. ( 10.1016/j.gca.2015.01.022) [DOI] [Google Scholar]
- 77.Needham AW, Abel RL, Tomkinson T, Grady MM. 2013. Martian subsurface fluid pathways and 3D mineralogy of the Nakhla meteorite. Geochim. Cosmochim. Acta 116, 96–110. ( 10.1016/j.gca.2012.07.004) [DOI] [Google Scholar]
- 78.Tomkinson T, Lee MR, Mark DF, Smith CL. 2013. Sequestration of Martian CO2 by mineral carbonation. Nat. Commun. 4, 2662 ( 10.1038/ncomms3662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hallis LJ, Ishii HA, Bradley JP, Taylor GJ. 2014. Transmission electron microscope analyses of alteration phases in Martian meteorite MIL 090032. Geochim. Cosmochim. Acta 134, 275–288. ( 10.1016/j.gca.2014.02.007) [DOI] [Google Scholar]
- 80.Borg L, Drake MJ. 2005. A review of meteorite evidence for the timing of magmatism and of surface or near-surface liquid water on Mars. J. Geophys. Res. 110, E12S03 ( 10.1029/2005JE002402) [DOI] [Google Scholar]
- 81.Ash RD, Knott SF, Turner G. 1996. A 4-Gyr shock age for a Martian meteorite and implications for the cratering history of Mars. Nature 380, 57–59. ( 10.1038/380057a0) [DOI] [Google Scholar]
- 82.Bogard DD, Garrison DH. 1999. Argon-39-argon-40 ‘ages’ and trapped argon in Martian shergottites, Chassigny and Allan Hills 84001. Meteorit. Planet. Sci. 34, 451–473. ( 10.1111/j.1945-5100.1999.tb01353.x) [DOI] [Google Scholar]
- 83.Harvey RP, McSween HY. 1996. A possible high-temperature origin for the carbonates in Martian meteorite ALH84001. Nature 382, 49–51. ( 10.1038/382049a0) [DOI] [PubMed] [Google Scholar]
- 84.McKay GA, Lofgren GE. 1997. Carbonates in ALH84001: evidence for kinetically controlled growth (abs). In Proc. Lunar Planet. Sci. XXVII, 27th Lunar and Planetary Science Conf, Houston, TX, 18–22 March 1996, pp. 921–922. Houston, TX: Lunar Planetary Institute. [Google Scholar]
- 85.Scott ERD, Yamaguchi A, Krot AN. 1997. Petrological evidence for shock melting of carbonates in the Martian meteorite ALH84001. Nature 387, 377–379. ( 10.1038/387377a0) [DOI] [PubMed] [Google Scholar]
- 86.Scott ERD, Krot AN, Yamaguchi A. 1998. Carbonates in fractures of Martian meteorite Allan Hills 84001: petrologic evidence for impact origin. Meteorit. Planet. Sci. 33, 709–719. ( 10.1111/j.1945-5100.1998.tb01677.x) [DOI] [PubMed] [Google Scholar]
- 87.Gleason JD, Kring DA, Hill DH, Boynton WV. 1997. Petrography and bulk chemistry of Martian orthopyroxenite ALH 84001: implications for the origin of secondary carbonates. Geochem. Cosmochim. Acta 61, 3503–3512. ( 10.1016/S0016-7037(97)00173-7) [DOI] [PubMed] [Google Scholar]
- 88.Borg LE, Connelly JN, Nyquist LE, Shih C-Y, Wiesmann H, Reese Y. 1999. The age of the carbonates in Martian meteorite ALH84001. Science 268, 90–94. ( 10.1126/science.286.5437.90) [DOI] [PubMed] [Google Scholar]
- 89.Sugiura N, Hoshino H. 2000. Hydrogen-isotopic compositions in ALH 84001 and the evolution of the Martian atmosphere. Meteorit. Planet. Sci. 35, 373–380. ( 10.1111/j.1945-5100.2000.tb01783.x) [DOI] [PubMed] [Google Scholar]
- 90.Boctor NZ, Alexander CMO'D, Wang J, Hauri E. 2003. The sources of water in Martian meteorites: clues from hydrogen isotopes. Geochim. Cosmochim. Acta 67, 3971–3989. ( 10.1016/S0016-7037(03)00234-5) [DOI] [Google Scholar]
- 91.Treiman AH. 1995. A petrographic history of Martian meteorite ALH84001: two shocks and an ancient age. Meteoritics 30, 294–302. ( 10.1111/j.1945-5100.1995.tb01127.x) [DOI] [Google Scholar]
- 92.Treiman AH. 1998. The history of Allan Hills 84001 revised: multiple shock events. Meteorit. Planet. Sci. 33, 753–764. ( 10.1111/j.1945-5100.1998.tb01681.x) [DOI] [PubMed] [Google Scholar]
- 93.Greenwood JP, McSween HY. 2001. Petrogenesis of Allan Hills 84001: constraints from impact melted feldspathic and silica glasses. Meteorit. Planet. Sci. 36, 43–61. ( 10.1111/j.1945-5100.2001.tb01809.x) [DOI] [Google Scholar]
- 94.Ming DW, et al. 2015. Volatile and organic compositions of sedimentary rocks in Yellowknife Bay, Gale Crater, Mars. Science 343, 1245267 ( 10.1126/science.1245267) [DOI] [PubMed] [Google Scholar]
- 95.Giesting PA, Schwenzer SP, Filiberto J, Starkey NA, Franchi IA, Treiman AH, Tindle AJ, Grady MM. 2015. Igneous and shock processes affecting chassignite amphibole evaluated using chlorine/water partitioning and hydrogen isotopes. Meteorit. Planet. Sci. 50, 433–460. ( 10.1111/maps.12430) [DOI] [Google Scholar]
- 96.Minitti ME, Rutherford MJ, Taylor BE, Dyar MD, Schultz PH. 2007. Assessment of shock effects on amphibole water contents and hydrogen isotope compositions: 1. Amphibolite experiments. Earth Planet. Sci. Lett. 266, 46–60. ( 10.1016/j.epsl.2007.10.047) [DOI] [Google Scholar]
- 97.Minitti ME, Leshin LA, Dyar MD, Ahrens TJ, Guan Y, Luo S-N. 2008. Assessment of shock effects on amphibole water contents and hydrogen isotope compositions: 2. Kaersutitic amphibole experiments. Earth Planet. Sci. Lett. 266, 288–302. ( 10.1016/j.epsl.2007.11.012) [DOI] [Google Scholar]
- 98.Fritz J, Artemieva N, Greshake A. 2005. Ejection of Martian meteorites. Meteorit. Planet. Sci. 40, 1393–1411. ( 10.1111/j.1945-5100.2005.tb00409.x) [DOI] [Google Scholar]
- 99.Hu S, Lin Y, Zhang J, Hao J, Feng L, Xu L, Yang W, Yang J. 2014. NanoSIMS analyses of apatite and melt inclusions in the GRV 020090 Martian meteorite: hydrogen isotope evidence for recent past underground hydrothermal activity on Mars. Geochim. Cosmochim. Acta 140, 321–333. ( 10.1016/j.gca.2014.05.008) [DOI] [Google Scholar]
- 100.Basu Sarbadhikari A, Day JMD, Liu Y, Rumble D, Taylor LA. 2009. Petrogenesis of olivine phyric Shergottite Larkman Nunatak 06319: implications for enriched components in Martian basalts. Geochim. Cosmochim. Acta 73, 2190–2214. ( 10.1016/j.gca.2009.01.012) [DOI] [Google Scholar]
- 101.Franz HB, et al. 2014. Isotopic links between atmospheric chemistry and the deep sulphur cycle on Mars. Nature 508, 364–368. ( 10.1038/nature13175) [DOI] [PubMed] [Google Scholar]
- 102.Greenwood JP, Itoh S, Sakamoto N, Vicenzi EP, Yurimoto H. 2008. Hydrogen isotope evidence for loss of water from Mars through time. Geophys. Res. Lett. 35, L05203 ( 10.1029/2007GL032721) [DOI] [Google Scholar]
- 103.Gröning M, Van Duren M, Andreescu L. 2007. Metrological characteristics of the conventional measurement scales for hydrogen and oxygen stable isotope amount ratios: the δ-scales. In Proc. of an International Workshop on ‘Combining and reporting analytical results: the role of traceability and uncertainty for comparing analytical results’, Rome, Italy, 6–8 March 2006, pp. 62–72. Cambridge, UK: Royal Society of Chemistry Publishing. [Google Scholar]
- 104.Xia Q-K, Deloule E, Wu Y-B, Chen D-G, Cheng H. 2008. Anomalously high δD values in the mantle. Geophys. Res. Lett. 29, 4-1–4-4. ( 10.1029/2001GL013887) [DOI] [Google Scholar]
- 105.Shaw AM, Hauri EH, Behn MD, Hilton DR, Macpherson CG, Sinton JM. 2012. Long-term preservation of slab signatures in the mantle inferred from hydrogen isotopes. Nat. Geosci. 5, 224–228. ( 10.1038/ngeo1406) [DOI] [Google Scholar]
- 106.Genda H, Ikoma M. 2008. Origin of the ocean on the Earth: early evolution of water D/H in a hydrogen-rich atmosphere. Icarus 194, 42–52. ( 10.1016/j.icarus.2007.09.007) [DOI] [Google Scholar]
- 107.Abramov O, Mojzsis SJ. 2009. Microbial habitability of the Hadean Earth during the late heavy bombardment. Nature 459, 419–422. ( 10.1038/nature08015) [DOI] [PubMed] [Google Scholar]
- 108.Gonnermann HM, Mukhopadhyay S. 2009. Preserving noble gases in a convecting mantle. Nature 459, 560–563. ( 10.1038/nature08018) [DOI] [PubMed] [Google Scholar]
- 109.Stuart FM, Lass-Evans S, Fitton JG, Ellam RM. 2003. High 3He/4He ratios in picritic basalts from Baffin Island and the role of a mixed reservoir in mantle plumes. Nature 424, 57–59. ( 10.1038/nature01711) [DOI] [PubMed] [Google Scholar]
- 110.Starkey NA, Stuart FM, Ellam RM, Fitton JG, Basu S, Larsen LM. 2009. Helium isotopes in early Iceland plume picrites: constraints on the composition of high 3He/4He mantle. Earth Planet. Sci. Lett. 277, 91–100. ( 10.1016/j.epsl.2008.10.007) [DOI] [Google Scholar]
- 111.Jackson MG, Carlson RW, Kurz MD, Kempton PD, Francis D, Blusztajn J. 2010. Evidence for the survival of the oldest terrestrial mantle reservoir. Nature 466, 853–856. ( 10.1038/nature09287) [DOI] [PubMed] [Google Scholar]
- 112.Robillard I, Francis D, Ludden JN. 1992. The relationship between E- and N-type magmas in the Baffin Bay lavas. Contrib. Mineral. Petrol. 112, 230–241. ( 10.1007/BF00310457) [DOI] [Google Scholar]
- 113.Bonal L, Alexander CMO'D, Huss GR, Nagashima K, Quirico E, Beck P. 2013. Hydrogen isotopic composition of the water on CR chondrites. Geochim. Cosmochim. Acta 106, 111–133. ( 10.1016/j.gca.2012.12.009) [DOI] [Google Scholar]
- 114.Le Guillou C, Changela HG, Brearley AJ. 2015. Widespread oxidised and hydrated amorphous silicates in CR chondrite matrices: implications for alteration conditions and H2 degassing of asteroids. Earth Planet. Sci. Lett. 420, 162–173. ( 10.1016/j.epsl.2015.02.031) [DOI] [Google Scholar]
- 115.Alexander CMO'D, Howard KT, Bowden R, Fogel ML. 2013. The classification of CM and CR chondrites using bulk H, C and N abundances and isotopic compositions. Geochim. Cosmochim. Acta 123, 244–260. ( 10.1016/j.gca.2013.05.019) [DOI] [Google Scholar]
- 116.Howard KT, Benedix GK, Bland PA, Cressey G. 2009. Modal mineralogy of CM2 chondrites by X-ray diffraction (PSD-XRD). Part 1: Total phyllosilicate abundance and the degree of aqueous alteration. Geochim. Cosmochim. Acta 73, 4576–4589. ( 10.1016/j.gca.2009.04.038) [DOI] [Google Scholar]
- 117.Howard KT, Benedix GK, Bland PA, Cressey G. 2011. Modal mineralogy of CM chondrites by X-ray diffraction (PSD-XRD). Part 2: degree, nature and settings of aqueous alteration. Geochim. Cosmochim. Acta 75, 2735–2751. ( 10.1016/j.gca.2011.02.021) [DOI] [Google Scholar]
- 118.Howard KT, Alexander CMO'D, Schrader DL, Dyl KA. 2015. Classification of hydrous meteorites (CR, CM and C2 ungrouped) by phyllosilicate fraction: PSD-XRD modal mineralogy and planetesimal environments. Geochim. Cosmochim. Acta 149, 206–222. ( 10.1016/j.gca.2014.10.025) [DOI] [Google Scholar]
- 119.King AJ, Schofield PF, Howard KT, Russell SS. 2015. Modal mineralogy of CI and CI-like chondrites by X-ray diffraction. Geochim. Cosmochim. Acta 165, 148–160. ( 10.1016/j.gca.2015.05.038) [DOI] [Google Scholar]
- 120.Rivkin AS, Howell ES, Emery JP, Volquardsen EL, DeMeo FE. 2012. Toward a taxonomy of asteroid spectra in the 3-μm region. In Proc. European Planetary Science Congress, Madrid, Spain, 23–28 September 2012, p. 359. EuroPlanet Research Infrastructure.
- 121.Rivkin AS, Howell E, Emery J. 2014. The LXD-mode main-belt/NEO observing program (LMNOP): results. In Proc. of Asteroids, Comets, Meteors 2014, Helsinki, Finland, 30 June–4 July 2014. See http://www.helsinki.fi/acm2014/pdf-material/ACM2014.pdf.
- 122.Haisch KE Jr, Lada EA, Lada CJ. 2001. Disk frequencies and lifetimes in young clusters. Astrophys. J. 553, L153–L156. ( 10.1086/320685) [DOI] [Google Scholar]
- 123.Armitage PJ. 2007. Massive planet migration: theoretical predictions and comparison with observations. Astrophys. J. 665, 1381–1390. [Google Scholar]
- 124.Wetherill GW. 1978. Accumulation of the terrestrial planets. In Protostars and planets (ed. Gehrels T.), pp. 565–598. IAU Colloquium 52. Paris, France: International Astronomical Union. [Google Scholar]
- 125.Hansen BMS. 2009. Formation of the terrestrial planets from a narrow annulus. Astrophys. J. 703, 1131–1140. ( 10.1088/0004-637X/703/1/1131) [DOI] [Google Scholar]
- 126.Walsh KJ, Morbidelli A, Raymond SN, O'Brien DP, Mandell AM. 2011. A low mass for Mars from Jupiter's early gas-driven migration. Nature 475, 206–209. ( 10.1038/nature10201) [DOI] [PubMed] [Google Scholar]
- 127.Kleine T, Touboul M, Bourdon B, Nimmo F, Mezger K, Palme H, Jacobsen SB, Yin Q-Z, Halliday AN. 2009. Hf-W chronology of the accretion and early evolution of asteroids and terrestrial planets. Geochim. Cosmochim. Acta 73, 5150–5188. ( 10.1016/j.gca.2008.11.047) [DOI] [Google Scholar]
- 128.Touboul M, Kleine T, Bourdon B, Palme H, Wieler R. 2007. Late formation and prolonged differentiation of the Moon inferred from W isotopes in lunar metals. Nature 450, 1206–1209. ( 10.1038/nature06428) [DOI] [PubMed] [Google Scholar]
- 129.Fitoussi C, Bourdon B. 2012. Silicon isotope evidence against an enstatite chondrite Earth. Science 335, 1477–1480. [DOI] [PubMed] [Google Scholar]
- 130.Saal AE, Hauri EH, Van Orman JA, Rutherford MJ. 2013. Hydrogen isotopes in lunar volcanic glasses and melt inclusions reveal a carbonaceous chondrite heritage. Science 340, 1317–1320. ( 10.1126/science.1235142) [DOI] [PubMed] [Google Scholar]
- 131.Trieloff M, Kunz J, Clague DA, Harrison D, Allègre CJ. 2000. The nature of pristine noble gases in mantle plumes. Science 288, 1036–1038. ( 10.1126/science.288.5468.1036) [DOI] [PubMed] [Google Scholar]
- 132.Holland G, Ballentine CJ. 2006. Seawater subduction controls the heavy noble gas composition of the mantle. Nature 411, 186–191. ( 10.1038/nature04761) [DOI] [PubMed] [Google Scholar]
- 133.Drake MJ. 2005. Origin of water in the terrestrial planets. Meteorit. Planet. Sci. 40, 519–527. ( 10.1111/j.1945-5100.2005.tb00960.x) [DOI] [Google Scholar]
- 134.Asaduzzaman A, Muralidharan K, Ganguly J. 2015. Incorporation of water into olivine during nebular condensation: insights from density functional theory and thermodynamics, and implications for phyllosilicate formation and terrestrial water inventory. Meteorit. Planet. Sci. 50, 578–589. ( 10.1111/maps.12409) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.