Abstract
Hydrogen production through water splitting has been considered as a green, pure and high-efficient technique. As an important half-reaction involved, hydrogen evolution reaction is a complex electrochemical process involving liquid-solid-gas three-phase interface behaviour. Therefore, new concepts and strategies of material design are needed to smooth each pivotal step. Here we report a multiscale structural and electronic control of molybdenum disulfide foam to synergistically promote the hydrogen evolution process. The optimized three-dimensional molybdenum disulfide foam with uniform mesopores, vertically aligned two-dimensional layers and cobalt atoms doping demonstrated a high hydrogen evolution activity and stability. In addition, density functional theory calculations indicate that molybdenum disulfide with moderate cobalt doping content possesses the optimal activity. This study demonstrates the validity of multiscale control in molybdenum disulfide via overall consideration of the mass transport, and the accessibility, quantity and capability of active sites towards electrocatalytic hydrogen evolution, which may also be extended to other energy-related processes.
The hydrogen evolution reaction is a complicated process involving liquid-solid-gas three-phase interface behaviour. Here, the authors report the multiscale structural and electronic control of molybdenum disulfide foam and demonstrate its high activity and stability for hydrogen evolution.
The properties of two-dimensional (2D) MoS2 are significantly different in comparison with its three-dimensional (3D) form. Thus, it has been considered for a number of applications, such as solar cells1,2,3, photocatalysis4,5,6, lithium ion batteries7,8,9 and electrocatalysis10,11,12,13. Owing to its natural abundance, low cost and good catalytic performance, recently MoS2 has become a representative non-precious material for electrocatalytic hydrogen evolution reaction (HER) of water splitting14,15,16,17,18,19,20,21. Such liquid-to-gas electrochemical conversion, with a complex reaction process at the interface of liquid (H+), solid (catalyst) and gas (H2), require a multiscale structural and electronic control of MoS2 to make each involved reaction step to proceed smoothly. This includes sufficient transport of reactants and products, accessibility of catalyst surface, abundant active sites and enough catalytic capability. Similar to the recent developments in the mesoporous framework of graphene22,23,24 or polymer25,26 foam, the design and preparation of a uniform mesoporous MoS2 foam could simultaneously facilitate the mass transport and accessibility of active sites. Yet, unlike the flexibility of carbon atoms skeleton in graphene and organic small molecules in polymers, MoS2 with single-crystal layer composed of three molecular layers (S–Mo–S) appears much more inflexible, which leads to such engineering still remaining a great challenge. Also, the S-edges of 2D MoS2 is usually considered as the active sites, while the in-plane structure is not active in catalysis15,27,28,29,30,31. Our recent work demonstrated that introducing different dopant atoms into the MoS2 matrix can enhance the intrinsic activity of its in-plane S atoms32. Therefore, further atomic-scale engineering via doping hetero atoms into the mesoporous MoS2 foam may achieve a multiscale modulation to synergistically boost the HER electrochemical process. However, such all-round structural and electronic control within MoS2 to enhance the HER performance has not been reported before.
Herein, we present a multiscale structural and electronic control of MoS2 foam for highly efficient HER process: (i) the macro-scale: a uniform mesoporous MoS2 foam (mPF-MoS2, average pore size ∼30 nm) facilitate the transport of H3O+ and H2, and increases the accessibility of MoS2 surface; (ii) the nano-scale: oriented vertical growth of MoS2 nanosheets around the mesopores increase the number of edges as the active sites; (iii) the atomic-scale: further chemical doping with transition metal Co atoms into the mPF-MoS2 framework enhance the intrinsic HER activity (mPF-Co-MoS2). Such mPF-Co-MoS2 electrocatalyst exhibits an excellent durability and a low overpotential of only 156 mV at the current density of 10 mA cm−2, comparable to the most active MoS2-based HER electrocatalysts in acidic medium (Supplementary Table 1). Furthermore, the density functional theory (DFT) calculations confirmed the experimental results that an appropriate Co doping content can greatly promote the HER activity of MoS2. The strategies, introduced in the present work, may open new opportunities for the rational design of MoS2 through a multiscale structural and electronic control to strengthen the electrocatalytic HER and other energy-related process, and possibly for the structural control of other 2D materials.
Results
Synthesis of mesoporous MoS2 foam
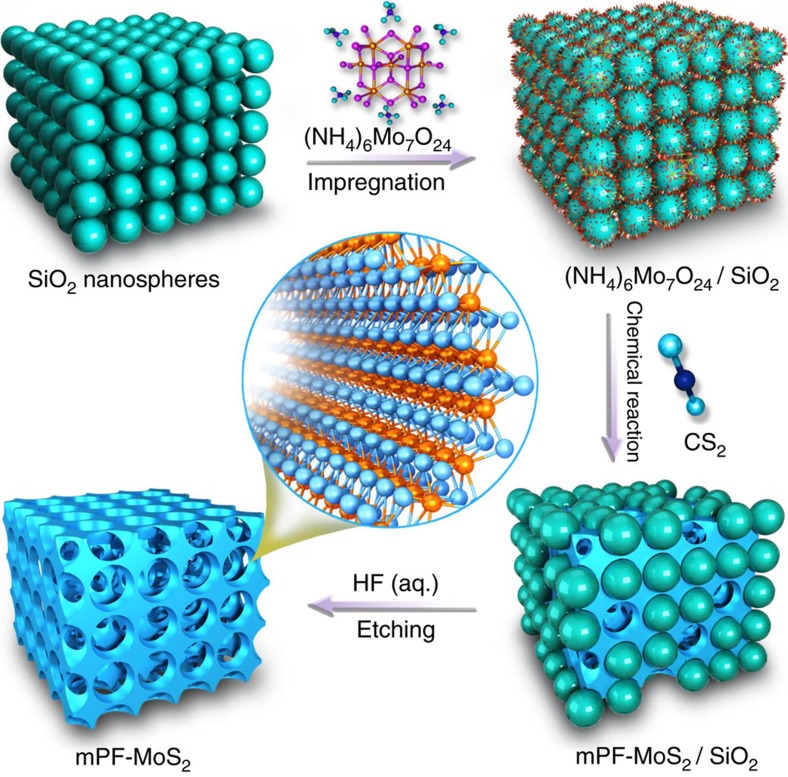
The uniform mesoporous MoS2 foam (mPF-MoS2) was prepared with the synthetic procedure illustrated in Fig. 1. First, (NH4)6Mo7O24 molecules were homogeneously adsorbed onto the colloidal SiO2 nanospheres via a wet impregnation method. Then, the direct chemical reaction with CS2 on SiO2 surface was conducted to convert Mo precursors into small MoS2 domains. Because of the induction of the monodisperse SiO2 nanospheres template, these small domains would further self-assembly into vertically aligned MoS2 layers around the SiO2 nanospheres. Finally, the mPF-MoS2 can be obtained via etching the SiO2 template with HF solution. Note that the etching process will not influence the structure of MoS2 because the MoS2 can not be dissolved by HF solution (Supplementary Fig. 1).
Figure 1. Schematic illustration of the fabrication of mesoporous MoS2 foam.
The direct chemical synthesis was adopted with the (NH4)6Mo7O24 and CS2 as precursors, assisted by the colloidal SiO2 nanospheres.
Structural analysis of mesoporous MoS2 foam
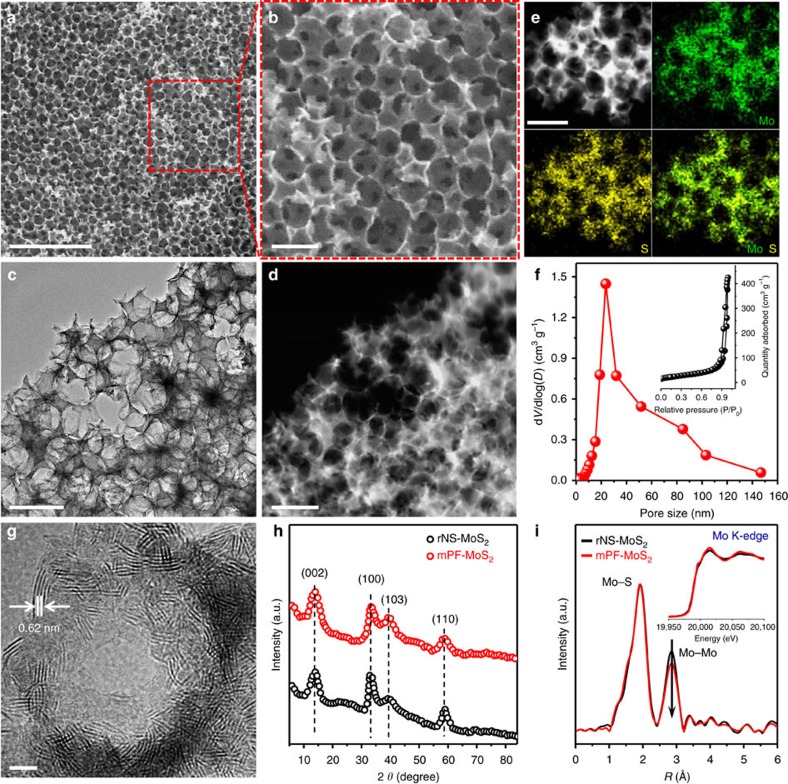
The scanning electron microscopy (SEM) and transmission electron microscopy (TEM) as well as high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) show that the mPF-MoS2 possessed abundant spherical voids derived from the residual spaces after the removal of SiO2 nanospheres, leading to a uniform porous framework (Fig. 2a–d, Supplementary Figs 2 and 3a). Note that these uniform nanopores are interconnected throughout the entire 3D MoS2 foam at different orientations by 3D tomography (Supplementary Figs 4 and 5, Supplementary Movies 1 and 2), which facilitates the mass transport and accessibility of active sites during the catalytic process. The energy-dispersive X-ray (EDX) maps exhibits that the Mo and S elements were distributed homogeneously in the porous framework (Fig. 2e). The N2 adsorption-desorption isotherms indicate the presence of mesopores with a narrow pore size distribution at ∼24 nm (Fig. 2f).
Figure 2. Morphology and structural characterizations of mesoporous MoS2 foam.
(a,b) SEM images of mPF-MoS2. (c,d) TEM image and corresponding HAADF-STEM image of mPF-MoS2 at the same position. (e) HAADF-STEM image and corresponding EDX maps of mPF-MoS2. (f) Pore size distribution and N2 adsorption–desorption type IV isotherms (inset) of mPF-MoS2. (g) HRTEM image of mPF-MoS2 with inset showing a typical MoS2 layer distance of 0.62 nm and a distinct mesopore. (h) XRD pattern of mPF-MoS2 in comparison to rNS-MoS2. (i) The k2-weighted EXAFS spectra of mPF-MoS2 in comparison with rNS-MoS2. The inset is the normalized Mo K-edge XANES spectra of mPF-MoS2 in comparison to rNS-MoS2. Scale bar: (a) 500 nm, (b–e) 100 nm, (g) 5 nm.
The high resolution (HR) TEM image shows a typical interlayer distance of 0.62 nm corresponding to the (002) plane of MoS2 (Fig. 2g), and the hexagonal 2H-MoS2 crystal characteristics could also be gain from the X-ray diffraction (XRD) pattern (Fig. 2h). Remarkably, the MoS2 layers were almost vertically aligned around the mesopores with a large fraction of exposed edge sites (Fig. 2g and Supplementary Fig. 3b). Compared with random-oriented MoS2 nanosheet (rNS-MoS2) sample prepared without SiO2 template, the mPF-MoS2 showed no obvious difference in the XRD patterns (Fig. 2h), Raman spectra (Supplementary Fig. 6), X-ray photoelectron spectroscopy (XPS) (Supplementary Fig. 7) and X-ray absorption near-edge structure (XANES) spectra (inset of Fig. 2i). But according to the HRTEM images comparison (Supplementary Fig. 3), the mPF-MoS2 with vertical aligned layer and smaller lateral size possessed much more exposed edge sites. In addition, the extended X-ray absorption fine structure (EXAFS) spectra (Fig. 2i) exhibited that mPF-MoS2 had less Mo-Mo coordination than rNS-MoS2, also confirming the mPF-MoS2 possessed more edge sites. This should increase the catalytic activity of mPF-MoS2 significantly.
Electrocatalytic performance of mesoporous MoS2 foam
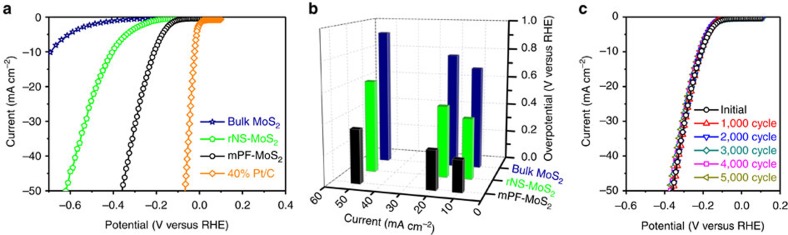
A typical three-electrode setup in 0.5 M H2SO4 electrolyte was adopted to conduct the electrocatalytic measurements. Bulk MoS2 shows a poor HER activity with only a minor improvement observed for rNS-MoS2 (Fig. 3a). From Fig. 3b, one could see that compared with rNS-MoS2, the required overpotential to drive a HER current density of 10, 20 and 50 mA cm−2 within mPF-MoS2 reduced 195, 219 and 262 mV, respectively. Particularly, the overpotential at a current density of 10 mA cm−2 for mPF-MoS2 is 210 mV, which is superior to the reported dense vertically aligned MoS2 film30. Furthermore, the mPF-MoS2 showed a long-term stable performance within the accelerated degradation measurements by 5,000 cyclic voltammetric (CV) sweeps (Fig. 3c), which also indicated mPF-MoS2 is a good non-precious alternative for HER electrocatalyst.
Figure 3. Electrocatalytic HER performance of mesoporous MoS2 foam.
(a) HER polarization curves for mPF-MoS2 in comparison with bulk MoS2, rNS-MoS2 and 40% Pt/C. (b) Overpotential at current density of 10, 20 and 50 mA cm−2 for mPF-MoS2 compared with rNS-MoS2 and bulk MoS2. (c) Durability measurement of mPF-MoS2. The polarization curves were recorded initially and after every 1,000 sweeps between −0.1 and +0.5 V (versus RHE) at 100 mV s−1. All the HER measurements were conducted in an Ar-saturated 0.5 M H2SO4 electrolyte at 25 °C.
It is usually considered that only the edge sites of pure MoS2 own the HER activity, while the basal plane is catalytically inert14,15. Therefore, the mPF-MoS2, possessing rich vertical edge sites, is expected to be more catalytically active. Moreover, numerous mesopores in mPF-MoS2 facilitate the mass transport. Meanwhile, according to the contact angle measurements (Supplementary Fig. 8), the mPF-MoS2 (23°) become more hydrophilic relative to the rNS-MoS2 (32°) and bulk MoS2 (105°), leading to the more easy accessibility of reactants on active sites for the mPF-MoS2 catalyst. In addition, the massive mesopores with curved surface in MoS2 2D plane may induce the strain, which can further increase the electrocatalytic activity referring to the literatures19,33.
Chemical doping of mesoporous MoS2 foam
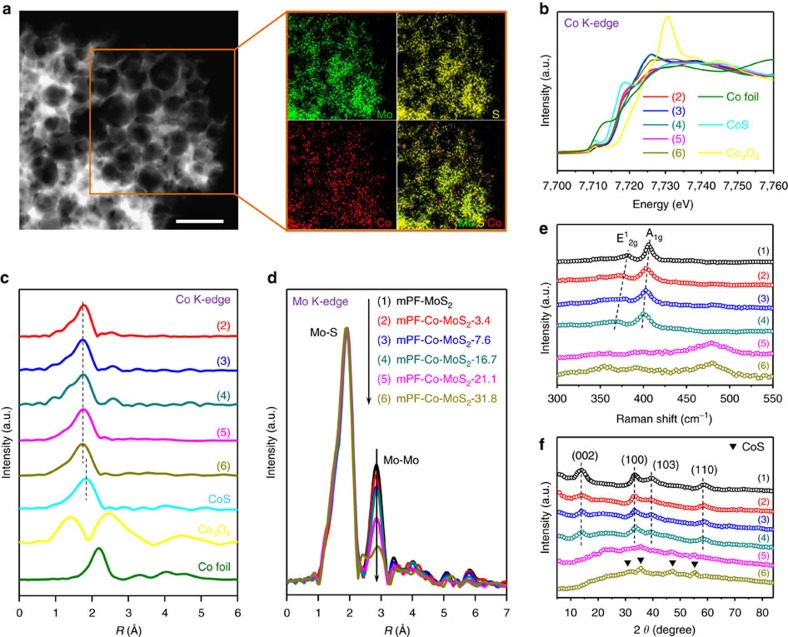
Doping of different transition metal atoms into the MoS2 matrix can enhance the intrinsic activity of its in-plane S atoms32. Here, we introduced Co atoms into the mPF-MoS2 framework by in situ adding Co precursors within the impregnation procedure (see the experimental section for details), yielding a Co-doped mesoporous MoS2 foam (mPF-Co-MoS2). As shown in the SEM (Supplementary Fig. 9), TEM (Supplementary Fig. 10a) and HAADF-STEM (Fig. 4a) images, the mesoporous MoS2 foam has been well retained after Co doping, with MoS2 flakes still assembling as vertically aligned layers around the mesopores (Supplementary Fig. 10b). The Co dopants bring indiscernible chemical state variation of the MoS2 framework according to the XPS spectra (Supplementary Fig. 11) and Mo K-edge XANES spectra (Supplementary Fig. 12). No Co-containing nanoparticles were observed from TEM images, consistent with the EDX maps showing the homogeneous distribution of Co, Mo and S elements over the entire mesoporous framework (Fig. 4a).
Figure 4. Structural and electronic properties of various Co-doped mesoporous MoS2 foam.
(a) HAADF-STEM image and corresponding EDX maps with orange rectangle in HAADF-STEM image of mPF-Co-MoS2-16.7. Scale bar, 100 nm. (b) Co K-edge XANES spectra of a series of mPF-Co-MoS2 samples in comparison to Co foil, CoS, and Co3O4, respectively. (c) Co K-edge k2-weighted EXAFS spectra of a series of mPF-Co-MoS2 samples in comparison with CoS, Co3O4 and Co foil, respectively. (d) Mo K-edge k2-weighted EXAFS spectra of various mPF-Co-MoS2 samples compared with mPF-MoS2. (e) Raman spectra of different mPF-Co-MoS2 samples in comparison to mPF-MoS2. (f) XRD patterns of a series of mPF-Co-MoS2 samples in comparison with mPF-MoS2. The numbers (1), (2), (3), (4), (5) and (6) represent mPF-MoS2 and mPF-Co-MoS2 with the Co doping contents of 3.4, 7.6, 16.7, 21.1 and 31.8%, respectively.
Co doping contents within the mesoporous MoS2 foam can be easily modulated by varying the amount of Co precursors, resulting in a series of mPF-Co-MoS2-x samples (x represents the Co doping contents in wt.%). As shown in the Co K-edge XANES spectra (Fig. 4b) and EXAFS spectra (Fig. 4c), all Co atoms in different mesoporous MoS2 foam possess the valence and the Co–S bonds are distinguished from those in commercial CoS crystal. This indicates that Co atoms are covalently doped into the MoS2 2D plane rather than being adsorbed on the surface. This finding is also confirmed by the Mo K-edge EXAFS spectra (Fig. 4d) showing a decrease of Mo-Mo coordination caused by the substituted-doping of Co atoms within the MoS2 2D plane. In addition, the decrease of Mo-Mo coordination accompanied with the increase of Co doping contents (Fig. 4d) was also consistent with the stepwise red shift of E12g and A1g modes in Raman spectra (Fig. 4e) resulted from the progressively increased Co dopants in MoS2 2D plane to soften the Mo-S related modes and decrease their vibration frequency34. Nevertheless, the E12g and A1g modes of MoS2 will change significantly when the Co doping contents exceeded 16.7% (Fig. 4e), suggesting a structural variation in the mesoporous MoS2 foam. This correlates with the XRD patterns showing that the crystal structure of MoS2 was well maintained with no other phases appearing after Co doping, until the Co doping contents were 21.1% or more (Fig. 4f). Meanwhile, distinct change in pore structure of mPF-Co-MoS2 samples appeared when the Co doping contents exceeds 16.7% (Supplementary Fig. 13). Note that mesoporous MoS2 foam with different Co doping contents showed no obvious difference in the contact angle measurements (Supplementary Fig. 14). The above analyses indicated that there is an optimum doping content (16.7% from our experience) which will provide significant Co contents but still preserving the mPF-Co-MoS2 integrated mesoporous vertically aligned framework.
Effect of Co dopant on electrocatalytic performance
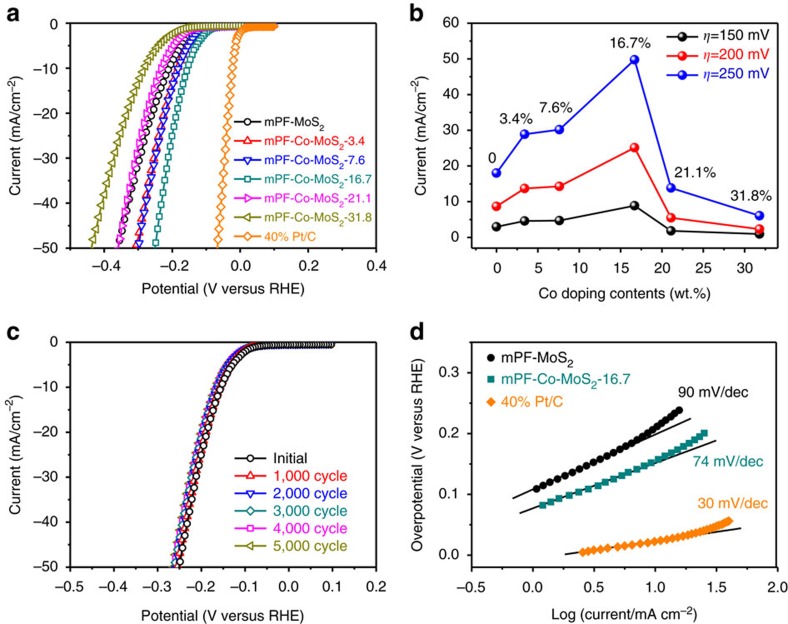
In view of the additional atomic-scale modulation in mesoporous MoS2 foam, a further enhanced HER process was expected. Thus, mPF-Co-MoS2-3.4 exhibited a distinctly enhanced activity, reducing the overpotential of 26 and 53 mV at the current density of 10 and 50 mA cm−2 relative to mPF-MoS2 (Fig. 5a). The sample with Co doping content of 16.7% demonstrates the optimum activity (Fig. 5b). The volcano-shaped relationship between HER activity and Co doping contents confirmed our finding that there is an optimum Co doping level which, from one hand enhances the intrinsic catalytic activity of mPF-MoS2 and at the same time maintains the inherent framework within mesoporous MoS2 foam. Remarkably, the mPF-Co-MoS2-16.7 showed a high HER activity with the overpotential at a current density of 10 mA cm−2 of only 156 mV (Fig. 5a), comparable to the most active MoS2-based non-precious HER electrocatalysts in acidic medium (Supplementary Table 1). Moreover, mPF-Co-MoS2-16.7 also showed a long-term stable performance even after 5,000 CV sweeps within the accelerated degradation measurements (Fig. 5c). Tafel plots showed that mPF-Co-MoS2-16.7 with a Tafel slope value of 74 mV dec−1 and mPF-MoS2 (90 mV dec−1) followed a similar reaction process via the Volmer–Heyrovsky mechanism35,36,37, deviating from the Pt/C electrocatalyst (30 mV dec−1) via the Volmer-Tafel mechanism (Fig. 5d). These results demonstrated that the Co doping content will significantly affect the activity modulation of MoS2, and a moderate value can maximally promote the multiscale structural and electronic control in mesoporous MoS2 foam for the HER activity optimization.
Figure 5. Effect of Co doping on the HER performance of mesoporous MoS2 foam.
(a) HER polarization curves for mPF-Co-MoS2 with different Co doping contents in comparison with mPF-MoS2 and 40% Pt/C. (b) Current densities at overpotential of 150, 200 and 250 mV for mPF-Co-MoS2 with different Co doping contents compared with mPF-MoS2. (c) Durability measurement of mPF-Co-MoS2-16.7. The polarization curves were recorded initially and after every 1,000 sweeps between −0.1 and +0.5 V (versus RHE) at 100 mV s−1. (d) Tafel plots for mPF-MoS2, mPF-Co-MoS2-16.7 and 40% Pt/C, respectively. All the HER measurements were conducted in an Ar-saturated 0.5 M H2SO4 electrolyte at 25 °C.
Theoretical studies of Co doping effect
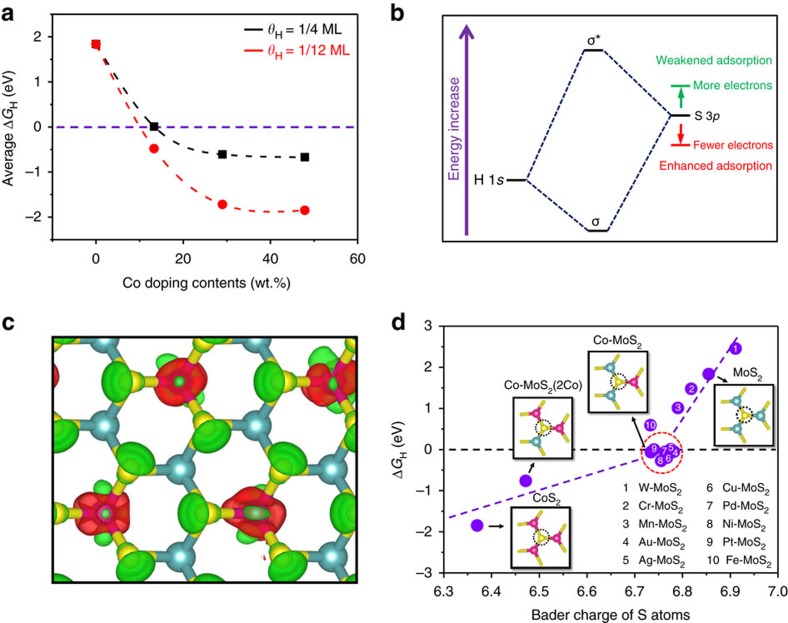
DFT calculations were carried out to gain further insights into the influence of different Co doping contents within the basal plane of MoS2 on the HER activity. The hydrogen adsorption free energy (ΔGH) is a widely accepted descriptor of HER activity for various catalytic materials, where the optimal value of ΔGH is around zero (∼0) eV to compromise the reaction barriers and achieve the best HER activity38,39. For the basal plane of pristine MoS2, the ΔGH is ∼2 eV, far away from the optimal value. With Co atoms introduced into the MoS2 in-plane, taking the coverage (θH) of 1/4 monolayer (ML) as an example, the ΔGH decreased continuously and reached ∼0 eV at the Co doping content of 13.3 wt.% (atomic ratio of Co:Mo is 1:2), beyond which the ΔGH will depart away from the optimal values again (Fig. 6a). These simulations indicate that there indeed exists a moderate Co doping content to promote MoS2 to gain the optimal HER activity, confirming the experimental results.
Figure 6. Theoretical calculations for the effect of Co doping contents on HER of MoS2.
(a) Average ΔGH on S atoms versus the Co doping contents, considering different coverage of 1/4 ML and 1/12 ML. The corresponding optimized catalyst structures can be seen in Supplementary Fig. 15. (b) Schematic diagram of the bonding of H 1s orbital and S 3p orbital (from MoS2), where depletion of electrons on S atoms will lower the orbital position and enhance the H–S bond. (c) Differential charge density of Co-doped MoS2 (Co doping content of 13.3 wt.%, Co:Mo atomic ratio of 1:2). Red and green contours represent electron accumulation and depletion, respectively. The isosurface level is set to be 0.11 e/Bohr3. (d) ΔGH on S atoms versus the Bader charge of S atoms for different structures, with the detailed data for each point shown in Supplementary Table 2. The insets are the atomic configurations of one S atom bonding with three Co, two Co and one Mo, one Co and two Mo, as well as three Mo atoms, respectively. Green balls: Mo; yellow balls: S; pink balls: Co.
To understand the origin of the increased HER activity with increased Co doping content, an analysis of the electronic properties has been made. First, the projected density of state of S atoms show a significant increase in the electronic states of in-plane S sites around Fermi level after Co atoms doping (Supplementary Fig. 16), resulting in the enhanced catalytic activity, in accordance with our previous study32. Furthermore, according to the molecular orbital theory, when H atom is absorbed on surface S atom, the combination of H 1s orbital and S 3p orbital will form a bonding orbital (σ) and anti-bonding orbital (σ*), where the degree of energy level matching between H atom and S atom determines the H–S bonding strength (Fig. 6b). Because of the very high energy level of S 3p orbital relative to H 1s orbital, the H adsorption on basal plane of pristine MoS2 is too weak (ΔGH=∼2 eV), leading to a poor HER performance of MoS2 for HER. When doping another metal atom such as Co into the MoS2 in-plane, the electron number on S atom will decrease (Fig. 6c) to offset the energy level mismatching for enhancing the H adsorption and HER activity. Different metal atoms own the different capability to modify the electron density on S atoms (see Fig. 6d and Supplementary Table 2 for detailed data), and the metal dopants that tune the Bader charge of S atoms into the range of ∼6.73 to ∼6.78 will lead to a moderate ΔGH (Fig. 6d) and high HER activity (Supplementary Fig. 17). Among them, the Co atom is indeed a good regulator to bring the ΔGH get ∼0 eV. Nevertheless, further increasing Co doping contents will cause the excessive decrease of electron on S atoms to make the interaction between H atoms and S atoms too strong (Fig. 6d). In addition, high Co doping contents can also lead to less stable of MoS2 surface according to the surface energy (γ) calculations (Supplementary Fig. 18), which is also harmful to the HER activity.
Discussion
In summary, we introduce a multiscale structural and electronic control of MoS2 strategy to achieve the high-efficient HER electrocatalysis. First, a uniform mesoporous MoS2 foam (mPF-MoS2) was fabricated with a significantly enhanced HER performance compared with that of random-oriented MoS2 nanosheet (rNS-MoS2). It originates from the macro-scale modulation fabricating massive mesopores to gain the sufficient transport of H3O+ and H2, the favourable accessibility of MoS2 surface and the strain-induced promotion, as well as the nano-scale modulation with vertically aligned layers to provide abundant active edge sites. Second, chemical doping was introduced to add further atomic-scale engineering in the mesoporous MoS2 foam for the intrinsic activity increase. The optimum Co-doped mesoporous MoS2 foam (mPF-Co-MoS2) with a Co content of 16.7% showed a further distinct enhancement of HER activity, which possessed a long-term durability with more than 5,000 recycles and an overpotential of only 156 mV at the current density of 10 mA cm−2, comparable to the most active MoS2-based electrocatalysts in acidic medium. DFT calculations confirmed the experimental results that a moderate Co doping content can modulate the H adsorption on MoS2 to a suitable degree and simultaneously maintain the structure stability to promote the HER activity reach optimum value. The findings in the present work pave a rational pathway to strengthen the electrocatalytic HER performance of MoS2 via the multiscale structural and electronic control, and the involved concept and strategy can be extended to other energy-related process or other 2D materials.
Methods
Materials synthesis
The mPF-MoS2 was synthesized through a direct chemical synthesis method. First, 400 mg (NH4)6Mo7O24·4H2O and 5,333.4 mg SiO2 colloidal disperse (30 wt.% SiO2 in ethylene glycol, Alfa Aesar) were dispersed in 20 ml deionized water, followed by stirring under room temperature to remove the solvent and drying under 80 °C. Then, the gained solid and 10 ml CS2 were transferred into a 40 ml stainless steel autoclave under Ar and maintained at 400 °C for 4 h. The final product was treated with HF (aq.) under room temperature for 5 h, followed by washing with water and absolute ethanol for several times and drying at 80 °C. For comparison, the rNS-MoS2 was synthesized by using 900 mg (NH4)6Mo7O24·4H2O dissolved in 20 ml deionized water and 10 ml CS2 conducted within the same chemical reaction as the mPF-MoS2 without using SiO2 template. The series of mPF-Co-MoS2 samples were synthesized by using 400 mg (NH4)6Mo7O24·4H2O, specified amount of Co(NO3)2·6H2O and 5,333.4 mg SiO2 colloidal dispers to gain the impregnated solid, and then with 10 ml CS2 to proceed within the same process as the mPF-MoS2. The Co doping contents in final mPF-Co-MoS2 samples were measured by inductively coupled plasma atomic emission spectroscopy.
Materials characterization
SEM was conducted on Hitachi S4800 operated at 20 kV. TEM, HAADF-STEM and EDX mapping were carried out on a FEI Tecnai 30 microscope and a 20 microscope operated at an accelerating voltage of 300 and 200 kV, respectively. The 3D tomography in the STEM mode was carried out on the FEI Talos F200 × microscope operated at 200 kV. N2 adsorption–desorption was measured with a Micromeritics Tristar 3020 Surface Area and Porosimetry analyzer. XRD measurements were conducted on a Rigaku Ultima IV diffractometer with Cu Kα radiation at 35 kV and 15 mA. XANES and EXAFS were measured at the BL14W1 beamline of the Shanghai Synchrotron Radiation Facility (SSRF). Raman spectroscopy was performed on a Renishaw inVia Raman microscope with a 532 nm excitation laser at a power of 0.29 mW. XPS measurements were carried out on an Omicron XPS System used Al Kα X-rays as the excitation source with a voltage of 15 kV and power of 300 W. Contact angle of water solution droplet on the surface of catalyst layer were conducted on SDC-100 contact angle measurement instrument (Shengding Precision Instrument Co., Ltd., China) at room temperature. Inductively coupled plasma atomic emission spectroscopy was carried out in Varian AA240z graphite furnace atomic absorption spectrometer.
Electrochemical measurements
HER polarization curve tests were conducted on a Princeton Parstat MC potentiostat/galvanostat with a three-electrode electrochemical cell equipped with a gas flow controlling system. Graphite rod was used as the counter electrode and Ag/AgCl (saturated KCl-filled) as the reference electrode. A glassy carbon rotating disk electrode with a diameter of 5 mm covered by a thin catalyst film was used as the working electrode. Typically, 4 mg catalyst was suspended in 1 ml ethanol with 20 μl Nafion solution (5 wt.%, Du Pont) to form a homogeneous ink assisted by ultrasound. Then 25 μl of the ink was spread onto the surface of glassy carbon by a micropipette and dried under room temperature. The final loading for the catalysts and 40% Pt/C electrocatalysts on work electrode is 0.5 mg cm−2. HER tests were conducted in an Ar-saturated 0.5 M H2SO4 electrolyte at 25 °C. The potential range was from 0 to −1.0 V (versus Ag/AgCl) and the scan rate was 2 mV s−1. Before measurements, the samples were repeatedly swept from −0.4 to 0.3 V (versus Ag/AgCl) in the electrolyte until a steady voltammogram curve was obtained. All the final potentials have been calibrated with respect to a reversible hydrogen electrode (RHE).
DFT calculations
All theoretical calculations were performed using Vienna ab initio simulation packages (VASP)40 with projector-augmented wave scheme41. The generalized gradient approximation with the Perdew–Burke–Ernzerhof (PBE)42 functional was used for the exchange–correlation interaction. The plane wave cutoff was set to 400 eV. The convergence of total energy and forces were set to 1 × 10−5 eV and 0.05 eV Å−1, respectively. A periodically repeated single-layer MoS2 (a trilayer unit of S-Mo-S as a single layer43) crystal model with a 20 Å vacuum space has been built for DFT calculations. The Brillouin zone was sampled by a 3 × 3 × 1 k-point grid with the Monkhorst–Pack scheme44 for structural optimization and a 6 × 6 × 1 k-point grid for electronic structure calculations. More details see the Supplementary Methods.
Data availability
The data that support the findings of this study are available from the corresponding authors on request.
Additional information
How to cite this article: Deng, J. et al. Multiscale structural and electronic control of molybdenum disulfide foam for highly efficient hydrogen production. Nat. Commun. 8, 14430 doi: 10.1038/ncomms14430 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary Figures, Supplementary Tables and Supplementary Methods
Reconstructed structure of mPF-MoS2 by rotating 360° around the sample tilting axis from the 3D STEM tomography. The pore structure can be clearly seen through the movie.
Reconstructed structure of mPF-MoS2 by the 3D STEM tomography, showing the cross-section images at different depth of the sample. The distribution and size of the pore in the mesoporous MoS2 foam can be clearly seen through the movie.
Acknowledgments
We gratefully acknowledge the financial support from the Ministry of Science and Technology of China (No. 2016YFA0204100 and 2016YFA0200200), the National Natural Science Foundation of China (No. 21573220 and 21621063), the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences (No. QYZDB-SSW-JSC020), the strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA09030100). We thank staff at the BL14W1 beamline of the Shanghai Synchrotron Radiation Facilities (SSRF) for assistance with the EXAFS and XANES measurements. We also acknowledge the computational resources from National Supercomputing Center in Shenzhen.
Footnotes
The authors declare no competing financial interests.
Author contributions X.B. and D.D. conceived the project and designed the experiments. J.D. performed the materials synthesis, materials characterization and electrochemical measurements. H.L. conducted the DFT calculations. S.W. assisted with the materials characterization. D.D. and M.C. performed the XPS measurements. C.L. and Z.T. conducted the Raman measurements. C.M. performed the 3D tomography. Z.T. and K.S.N. gave the valuable discussions and suggestions. J.D., D.D. and X.B. co-wrote the manuscript.
References
- Liu Z. K., Lau S. P. & Yan F. Functionalized graphene and other two-dimensional materials for photovoltaic devices: device design and processing. Chem. Soc. Rev. 44, 5638–5679 (2015). [DOI] [PubMed] [Google Scholar]
- Wang Q. H., Kalantar-Zadeh K., Kis A., Coleman J. N. & Strano M. S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 7, 699–712 (2012). [DOI] [PubMed] [Google Scholar]
- Gu X. et al. A solution-processed hole extraction layer made from ultrathin MoS2 nanosheets for efficient organic solar cells. Adv. Energy Mater. 3, 1262–1268 (2013). [Google Scholar]
- Xiang Q. J., Yu J. G. & Jaroniec M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 134, 6575–6578 (2012). [DOI] [PubMed] [Google Scholar]
- Ding Q. et al. Efficient photoelectrochemical hydrogen generation using heterostructures of Si and chemically exfoliated metallic MoS2. J. Am. Chem. Soc. 136, 8504–8507 (2014). [DOI] [PubMed] [Google Scholar]
- Chang K. et al. MoS2/graphene cocatalyst for efficient photocatalytic H2 evolution under visible light irradiation. ACS Nano 8, 7078–7087 (2014). [DOI] [PubMed] [Google Scholar]
- Stephenson T., Li Z., Olsen B. & Mitlin D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 7, 209–231 (2014). [Google Scholar]
- Chhowalla M. et al. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 5, 263–275 (2013). [DOI] [PubMed] [Google Scholar]
- Zhu C. B., Mu X. K., van Aken P. A., Yu Y. & Maier J. Single-layered ultrasmall nanoplates of MoS2 embedded in carbon nanofibers with excellent electrochemical performance for lithium and sodium storage. Angew. Chem. Int. Ed. 53, 2152–2156 (2014). [DOI] [PubMed] [Google Scholar]
- Deng D. H. et al. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 11, 218–230 (2016). [DOI] [PubMed] [Google Scholar]
- Asadi M. et al. Cathode based on molybdenum disulfide nanoflakes for lithium-oxygen batteries. ACS Nano 10, 2167–2175 (2016). [DOI] [PubMed] [Google Scholar]
- Yuwen L. H. et al. General synthesis of noble metal (Au, Ag, Pd, Pt) nanocrystal modified MoS2 nanosheets and the enhanced catalytic activity of Pd-MoS2 for methanol oxidation. Nanoscale 6, 5762–5769 (2014). [DOI] [PubMed] [Google Scholar]
- Huang H., Feng X., Du C. C., Wu S. Y. & Song W. B. Incorporated oxygen in MoS2 ultrathin nanosheets for efficient ORR catalysis. J. Mater. Chem. A 3, 16050–16056 (2015). [Google Scholar]
- Hinnemann B. et al. Biornimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 127, 5308–5309 (2005). [DOI] [PubMed] [Google Scholar]
- Jaramillo T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007). [DOI] [PubMed] [Google Scholar]
- Li Y. G. et al. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011). [DOI] [PubMed] [Google Scholar]
- Huang X. et al. Solution-phase epitaxial growth of noble metal nanostructures on dispersible single-layer molybdenum disulfide nanosheets. Nat. Commun. 4, 1444 (2013). [DOI] [PubMed] [Google Scholar]
- Deng J. et al. High-performance hydrogen evolution electrocatalysis by layer-controlled MoS2 nanosheets. RSC Adv. 4, 34733–34738 (2014). [Google Scholar]
- Li H. et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 15, 48–53 (2016). [DOI] [PubMed] [Google Scholar]
- Lukowski M. A. et al. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 135, 10274–10277 (2013). [DOI] [PubMed] [Google Scholar]
- Liao L. et al. MoS2 formed on mesoporous graphene as a highly active catalyst for hydrogen evolution. Adv. Funct. Mater. 23, 5326–5333 (2013). [Google Scholar]
- Lin T. Q. et al. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 350, 1508–1513 (2015). [DOI] [PubMed] [Google Scholar]
- Jiao Y. C. et al. Highly ordered mesoporous few-layer graphene frameworks enabled by Fe3O4 nanocrystal superlattices. Angew. Chem. Int. Ed. 54, 5727–5731 (2015). [DOI] [PubMed] [Google Scholar]
- Wang G. et al. Controlled synthesis of N-doped carbon nanospheres with tailored mesopores through self-assembly of colloidal silica. Angew. Chem. Int. Ed. 54, 15191–15196 (2015). [DOI] [PubMed] [Google Scholar]
- Liu S. H. et al. Patterning two-dimensional free-standing surfaces with mesoporous conducting polymers. Nat. Commun. 6, 8817 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y. et al. Growth of single-layered two-dimensional mesoporous polymer/carbon films by self-assembly of monomicelles at the interfaces of various substrates. Angew. Chem. Int. Ed. 54, 8425–8429 (2015). [DOI] [PubMed] [Google Scholar]
- Kibsgaard J., Chen Z. B., Reinecke B. N. & Jaramillo T. F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 11, 963–969 (2012). [DOI] [PubMed] [Google Scholar]
- Gao M. R., Chan M. K. Y. & Sun Y. G. Edge-terminated molybdenum disulfide with a 9.4-angstrom interlayer spacing for electrochemical hydrogen production. Nat. Commun. 6, 7493 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J. F. et al. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 25, 5807–5813 (2013). [DOI] [PubMed] [Google Scholar]
- Kong D. S. et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 13, 1341–1347 (2013). [DOI] [PubMed] [Google Scholar]
- Ye G. L. et al. Defects engineered monolayer MoS2 for improved hydrogen evolution reaction. Nano Lett. 16, 1097–1103 (2016). [DOI] [PubMed] [Google Scholar]
- Deng J. et al. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 8, 1594–1601 (2015). [Google Scholar]
- Lee J. H., Jang W. S., Han S. W. & Baik H. K. Efficient hydrogen evolution by mechanically strained MoS2 nanosheets. Langmuir. 30, 9866–9873 (2014). [DOI] [PubMed] [Google Scholar]
- Li H. L. et al. Growth of alloy MoS2xSe2(1−x) nanosheets with fully tunable chemical compositions and optical properties. J. Am. Chem. Soc. 136, 3756–3759 (2014). [DOI] [PubMed] [Google Scholar]
- Conway B. E. & Tilak B. V. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 47, 3571–3594 (2002). [Google Scholar]
- Merki D., Vrubel H., Rovelli L., Fierro S. & Hu X. L. Fe, Co, and Ni ions promote the catalytic activity of amorphous molybdenum sulfide films for hydrogen evolution. Chem. Sci. 3, 2515–2525 (2012). [Google Scholar]
- Bockris J. O. M. & Potter E. C. The mechanism of the cathodic hydrogen evolution reaction. J. Electrochem. Soc. 99, 169–186 (1952). [Google Scholar]
- Norskov J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23–J26 (2005). [Google Scholar]
- Greeley J., Jaramillo T. F., Bonde J., Chorkendorff I. B. & Norskov J. K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 5, 909–913 (2006). [DOI] [PubMed] [Google Scholar]
- Kresse G. & Furthmuller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- Blochl P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994). [DOI] [PubMed] [Google Scholar]
- Perdew J. P., Burke K. & Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- Tsai C., Abild-Pedersen F. & Norskov J. K. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett. 14, 1381–1387 (2014). [DOI] [PubMed] [Google Scholar]
- Monkhorst H. J. & Pack J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures, Supplementary Tables and Supplementary Methods
Reconstructed structure of mPF-MoS2 by rotating 360° around the sample tilting axis from the 3D STEM tomography. The pore structure can be clearly seen through the movie.
Reconstructed structure of mPF-MoS2 by the 3D STEM tomography, showing the cross-section images at different depth of the sample. The distribution and size of the pore in the mesoporous MoS2 foam can be clearly seen through the movie.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors on request.