Abstract
Darwinian theory has yet to explain adequately the fact of sex. If males provide little or no aid to offspring, a high (up to 2-fold) extra average fitness has to emerge as a property of a sexual parentage if sex is to be stable. The advantage must presumably come from recombination but has been hard to identify. It may well lie in the necessity to recombine defenses to defeat numerous parasites. A model demonstrating this works best for contesting hosts whose defense polymorphisms are constrained to low mutation rates. A review of the literature shows that the predictions of parasite coevolution fit well with the known ecology of sex. Moreover, parasite coevolution is superior to previous models of the evolution of sex by supporting the stability of sex under the following challenging conditions: very low fecundity, realistic patterns of genotype fitness and changing environment, and frequent mutation to parthenogenesis, even while sex pays the full 2-fold cost.
Full text
PDF

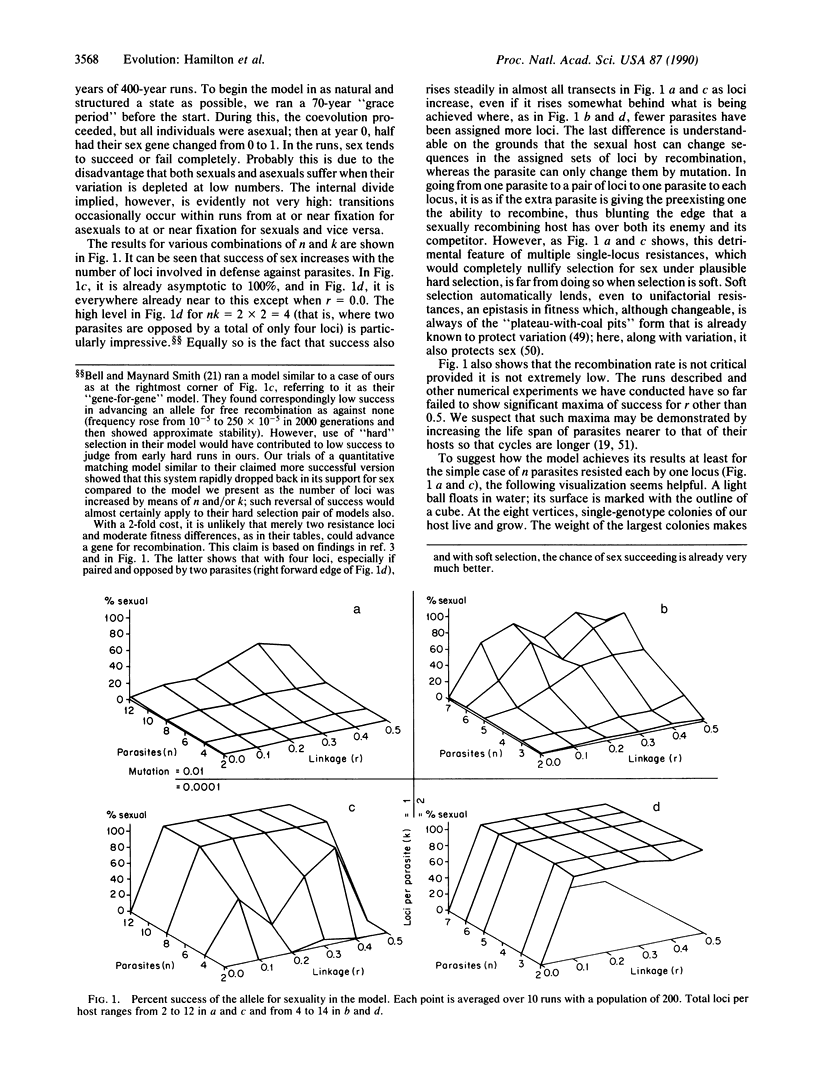

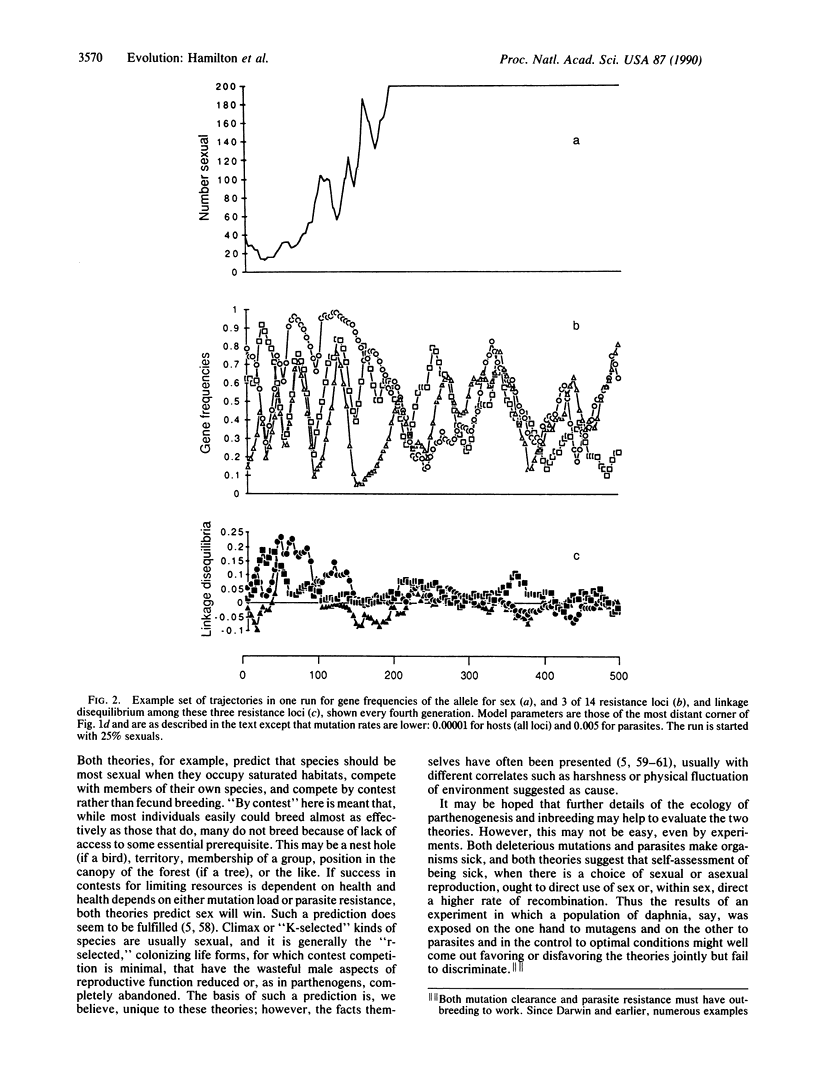



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. F., Albright J. W. Natural resistance to animal parasites. Contemp Top Immunobiol. 1984;12:1–52. doi: 10.1007/978-1-4684-4571-8_1. [DOI] [PubMed] [Google Scholar]
- Anderson R. M., May R. M. Coevolution of hosts and parasites. Parasitology. 1982 Oct;85(Pt 2):411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- Barnes G. L., Kay R. Blood-groups in giardiasis. Lancet. 1977 Apr 9;1(8015):808–808. doi: 10.1016/s0140-6736(77)92999-3. [DOI] [PubMed] [Google Scholar]
- Barnicot N. A. Some biochemical and serological aspects of primate evolution. Sci Prog. 1969 Winter;57(228):459–493. [PubMed] [Google Scholar]
- Barran L. R., Miller R. W. Temperature-induced alterations in phospholipids of Fusarium oxysporum f. sp. lycopersici. Can J Microbiol. 1976 Apr;22(4):557–562. doi: 10.1139/m76-083. [DOI] [PubMed] [Google Scholar]
- Blackwell C. C., Jonsdottir K., Hanson M. F., Weir D. M. Non-secretion of ABO blood group antigens predisposing to infection by Haemophilus influenzae. Lancet. 1986 Sep 20;2(8508):687–687. doi: 10.1016/s0140-6736(86)90193-5. [DOI] [PubMed] [Google Scholar]
- Blackwell C. C., Jónsdóttir K., Hanson M., Todd W. T., Chaudhuri A. K., Mathew B., Brettle R. P., Weir D. M. Non-secretion of ABO antigens predisposing to infection by Neisseria meningitidis and Streptococcus pneumoniae. Lancet. 1986 Aug 2;2(8501):284–285. doi: 10.1016/s0140-6736(86)92103-3. [DOI] [PubMed] [Google Scholar]
- Blackwell C. C., Weir D. M., James V. S., Cartwright K. A., Stuart J. M., Jones D. M. The Stonehouse study: secretor status and carriage of Neisseria species. Epidemiol Infect. 1989 Feb;102(1):1–10. doi: 10.1017/s0950268800029629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. F., Weismiller D. G., Holmes K. V. Genetic resistance to mouse hepatitis virus correlates with absence of virus-binding activity on target tissues. J Virol. 1987 Jan;61(1):185–189. doi: 10.1128/jvi.61.1.185-189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremermann H. J. The adaptive significance of sexuality. Experientia. 1985 Oct 15;41(10):1245–1254. doi: 10.1007/BF01952067. [DOI] [PubMed] [Google Scholar]
- Bull J. J., Harvey P. H. Evolutionary biology. A new reason for having sex. Nature. 1989 May 25;339(6222):260–261. doi: 10.1038/339260a0. [DOI] [PubMed] [Google Scholar]
- Bumstead N., Huggins M. B., Cook J. K. Genetic differences in susceptibility to a mixture of avian infectious bronchitis virus and Escherichia coli. Br Poult Sci. 1989 Mar;30(1):39–48. doi: 10.1080/00071668908417123. [DOI] [PubMed] [Google Scholar]
- Clausen H., Hakomori S. ABH and related histo-blood group antigens; immunochemical differences in carrier isotypes and their distribution. Vox Sang. 1989;56(1):1–20. doi: 10.1111/j.1423-0410.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Clemens J. D., Sack D. A., Harris J. R., Chakraborty J., Khan M. R., Huda S., Ahmed F., Gomes J., Rao M. R., Svennerholm A. M. ABO blood groups and cholera: new observations on specificity of risk and modification of vaccine efficacy. J Infect Dis. 1989 Apr;159(4):770–773. doi: 10.1093/infdis/159.4.770. [DOI] [PubMed] [Google Scholar]
- Crow J. F., Kimura M. Efficiency of truncation selection. Proc Natl Acad Sci U S A. 1979 Jan;76(1):396–399. doi: 10.1073/pnas.76.1.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker-Jackson J. E., Honigberg B. M. Glycoproteins released by Leishmania donovani: immunologic relationships with host and bacterial antigens and preliminary biochemical analysis. J Protozool. 1978 Nov;25(4):514–525. doi: 10.1111/j.1550-7408.1978.tb04178.x. [DOI] [PubMed] [Google Scholar]
- Eshel I., Akin E. Coevolutionary instability of mixed Nash solutions. J Math Biol. 1983;18(2):123–133. doi: 10.1007/BF00280661. [DOI] [PubMed] [Google Scholar]
- Esterre P., Dedet J. P. The relationship of blood-group type to American cutaneous leishmaniasis. Ann Trop Med Parasitol. 1989 Aug;83(4):345–348. doi: 10.1080/00034983.1989.11812355. [DOI] [PubMed] [Google Scholar]
- Figueroa F., Günther E., Klein J. MHC polymorphism pre-dating speciation. Nature. 1988 Sep 15;335(6187):265–267. doi: 10.1038/335265a0. [DOI] [PubMed] [Google Scholar]
- Greenblatt C. L., Kark J. D., Schnur L. F., Slutzky G. M. Do leishmania serotypes mimic human blood group antigens? Lancet. 1981 Feb 28;1(8218):505–506. doi: 10.1016/s0140-6736(81)91897-3. [DOI] [PubMed] [Google Scholar]
- Guillet J. G., Lai M. Z., Briner T. J., Buus S., Sette A., Grey H. M., Smith J. A., Gefter M. L. Immunological self, nonself discrimination. Science. 1987 Feb 20;235(4791):865–870. doi: 10.1126/science.2433769. [DOI] [PubMed] [Google Scholar]
- Hamilton W. D. The moulding of senescence by natural selection. J Theor Biol. 1966 Sep;12(1):12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Onodera T., Prabhakar B. S., Horita M., Suzuki H., Notkins A. L. Virus-induced autoimmunity: monoclonal antibodies that react with endocrine tissues. Science. 1983 Apr 15;220(4594):304–306. doi: 10.1126/science.6301002. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Hastie N. D. Accelerated evolution in the reactive centre regions of serine protease inhibitors. Nature. 1987 Mar 5;326(6108):96–99. doi: 10.1038/326096a0. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M., Jenkins C. D. Genetic segregation and the maintenance of sexual reproduction. Nature. 1989 May 25;339(6222):300–301. doi: 10.1038/339300a0. [DOI] [PubMed] [Google Scholar]
- Kondrashov A. S. Deleterious mutations and the evolution of sexual reproduction. Nature. 1988 Dec 1;336(6198):435–440. doi: 10.1038/336435a0. [DOI] [PubMed] [Google Scholar]
- Kondrashov A. S. Selection against harmful mutations in large sexual and asexual populations. Genet Res. 1982 Dec;40(3):325–332. doi: 10.1017/s0016672300019194. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Hoeffler W. K. SV40 large T shares an antigenic determinant with a cellular protein of molecular weight 68,000. Nature. 1980 Nov 13;288(5787):167–170. doi: 10.1038/288167a0. [DOI] [PubMed] [Google Scholar]
- Lane D., Koprowski H. Molecular recognition and the future of monoclonal antibodies. Nature. 1982 Mar 18;296(5854):200–202. doi: 10.1038/296200a0. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I., Ardelt W., Cook J., Denton A., Empie M. W., Kohr W. J., Park S. J., Parks K., Schatzley B. L. Ovomucoid third domains from 100 avian species: isolation, sequences, and hypervariability of enzyme-inhibitor contact residues. Biochemistry. 1987 Jan 13;26(1):202–221. doi: 10.1021/bi00375a028. [DOI] [PubMed] [Google Scholar]
- Lawlor D. A., Ward F. E., Ennis P. D., Jackson A. P., Parham P. HLA-A and B polymorphisms predate the divergence of humans and chimpanzees. Nature. 1988 Sep 15;335(6187):268–271. doi: 10.1038/335268a0. [DOI] [PubMed] [Google Scholar]
- Leist T., Althage A., Haenseler E., Hengartner H., Zinkernagel R. M. Major histocompatibility complex-linked susceptibility or resistance to disease caused by a noncytopathic virus varies with the disease parameter evaluated. J Exp Med. 1989 Jul 1;170(1):269–277. doi: 10.1084/jem.170.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. W. On the coevolution of pathogen and host: I. General theory of discrete time coevolution. J Theor Biol. 1981 Dec 21;93(4):927–951. doi: 10.1016/0022-5193(81)90348-9. [DOI] [PubMed] [Google Scholar]
- Lokki J., Saura A., Lankinen P., Suomalainen E. Genetic polymorphism and evolution in parthenogenetic animals. VI. Diploid and triploid Polydrosus mollis (Coleoptera: Curculionidae). Hereditas. 1976 Jun 14;82(2):209–216. doi: 10.1111/j.1601-5223.1976.tb01559.x. [DOI] [PubMed] [Google Scholar]
- Manning J. T. Males and the advantage of sex. J Theor Biol. 1984 May 21;108(2):215–220. doi: 10.1016/s0022-5193(84)80067-3. [DOI] [PubMed] [Google Scholar]
- May R. M., Anderson R. M. Epidemiology and genetics in the coevolution of parasites and hosts. Proc R Soc Lond B Biol Sci. 1983 Oct 22;219(1216):281–313. doi: 10.1098/rspb.1983.0075. [DOI] [PubMed] [Google Scholar]
- Nevo E. Genetic variation in natural populations: patterns and theory. Theor Popul Biol. 1978 Feb;13(1):121–177. doi: 10.1016/0040-5809(78)90039-4. [DOI] [PubMed] [Google Scholar]
- Nöthel H. Adaptation of Drosophila melanogaster populations to high mutation pressure: evolutionary adjustment of mutation rates. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1045–1049. doi: 10.1073/pnas.84.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakeshott J. G., Collet C., Phillis R. W., Nielsen K. M., Russell R. J., Chambers G. K., Ross V., Richmond R. C. Molecular cloning and characterization of esterase-6, a serine hydrolase of Drosophila. Proc Natl Acad Sci U S A. 1987 May;84(10):3359–3363. doi: 10.1073/pnas.84.10.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overfield T., Klauber M. R. Prevalence tuberculosis in Eskimos having blood group B gene. Hum Biol. 1980 Feb;52(1):87–92. [PubMed] [Google Scholar]
- Pereira F. E., Bortolini E. R., Carneiro J. L., da Silva C. R., Neves R. C. A, B, O blood groups and hepatosplenic form of schistosomiasis mansoni (Symmer's fibrosis). Trans R Soc Trop Med Hyg. 1979;73(2):238–238. doi: 10.1016/0035-9203(79)90224-4. [DOI] [PubMed] [Google Scholar]
- Rothwell T. L., Pope S. E., Collins G. H. Trixacarus caviae infection of guinea pigs with genetically determined differences in susceptibility to Trichostrongylus colubriformis infection. Int J Parasitol. 1989 May;19(3):347–348. doi: 10.1016/0020-7519(89)90147-1. [DOI] [PubMed] [Google Scholar]
- Rusting R. Skin deep. A recombinant growth factor hastens wound healing. Sci Am. 1989 Sep;261(3):38–38. [PubMed] [Google Scholar]
- STEBBINS G. L. Longevity, habitat. and release of genetic variability in the higher plants. Cold Spring Harb Symp Quant Biol. 1958;23:365–378. doi: 10.1101/sqb.1958.023.01.035. [DOI] [PubMed] [Google Scholar]
- Sagai T., Sakaizumi M., Miyashita N., Bonhomme F., Petras M. L., Nielsen J. T., Shiroishi T., Moriwaki K. New evidence for trans-species evolution of the H-2 class I polymorphism. Immunogenetics. 1989;30(2):89–98. doi: 10.1007/BF02421536. [DOI] [PubMed] [Google Scholar]
- Sage R. D., Whitney J. B., 3rd, Wilson A. C. Genetic analysis of a hybrid zone between domesticus and musculus mice (Mus musculus complex): hemoglobin polymorphisms. Curr Top Microbiol Immunol. 1986;127:75–85. doi: 10.1007/978-3-642-71304-0_9. [DOI] [PubMed] [Google Scholar]
- Sasaki A., Iwasa Y. Optimal recombination rate in fluctuating environments. Genetics. 1987 Feb;115(2):377–388. doi: 10.1093/genetics/115.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V. K., Yamaki K., Abe T., Shinohara T. Molecular mimicry between uveitopathogenic site of retinal S-antigen and Escherichia coli protein: induction of experimental autoimmune uveitis and lymphocyte cross-reaction. Cell Immunol. 1989 Aug;122(1):262–273. doi: 10.1016/0008-8749(89)90166-4. [DOI] [PubMed] [Google Scholar]
- Skamene E. Genetic control of susceptibility to mycobacterial infections. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S394–S399. doi: 10.1093/clinids/11.supplement_2.s394. [DOI] [PubMed] [Google Scholar]
- Tashian R. E., Shreffler D. C., Shows T. B. Genetic and phylogenetic variation in the different molecular forms of mammalian erythrocyte carbonic anhydrases. Ann N Y Acad Sci. 1968 Jun 14;151(1):64–77. doi: 10.1111/j.1749-6632.1968.tb11878.x. [DOI] [PubMed] [Google Scholar]
- Tooby J. Pathogens, polymorphism, and the evolution of sex. J Theor Biol. 1982 Aug 21;97(4):557–576. doi: 10.1016/0022-5193(82)90358-7. [DOI] [PubMed] [Google Scholar]
- Treisman M. The evolution of sexual reproduction: a model which assumes individual selection. J Theor Biol. 1976 Aug 7;60(2):421–431. doi: 10.1016/0022-5193(76)90068-0. [DOI] [PubMed] [Google Scholar]
- Vernick K. D., Collins F. H. Association of a Plasmodium-refractory phenotype with an esterase locus in Anopheles gambiae. Am J Trop Med Hyg. 1989 Jun;40(6):593–597. doi: 10.4269/ajtmh.1989.40.593. [DOI] [PubMed] [Google Scholar]
- Vidović D., Matzinger P. Unresponsiveness to a foreign antigen can be caused by self-tolerance. Nature. 1988 Nov 17;336(6196):222–225. doi: 10.1038/336222a0. [DOI] [PubMed] [Google Scholar]
- Walker I. The evolution of sexual reproduction as a repair mechanism. Part I. A model for self-repair and its biological implications. Acta Biotheor. 1978;27(3-4):133–158. doi: 10.1007/BF00115831. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]


