ABSTRACT
The possibility that Methanothrix (formerly Methanosaeta) and Geobacter species cooperate via direct interspecies electron transfer (DIET) in terrestrial methanogenic environments was investigated in rice paddy soils. Genes with high sequence similarity to the gene for the PilA pilin monomer of the electrically conductive pili (e-pili) of Geobacter sulfurreducens accounted for over half of the PilA gene sequences in metagenomic libraries and 42% of the mRNA transcripts in RNA sequencing (RNA-seq) libraries. This abundance of e-pilin genes and transcripts is significant because e-pili can serve as conduits for DIET. Most of the e-pilin genes and transcripts were affiliated with Geobacter species, but sequences most closely related to putative e-pilin genes from genera such as Desulfobacterium, Deferribacter, Geoalkalibacter, and Desulfobacula, were also detected. Approximately 17% of all metagenomic and metatranscriptomic bacterial sequences clustered with Geobacter species, and the finding that Geobacter spp. were actively transcribing growth-related genes indicated that they were metabolically active in the soils. Genes coding for e-pilin were among the most highly transcribed Geobacter genes. In addition, homologs of genes encoding OmcS, a c-type cytochrome associated with the e-pili of G. sulfurreducens and required for DIET, were also highly expressed in the soils. Methanothrix species in the soils highly expressed genes for enzymes involved in the reduction of carbon dioxide to methane. DIET is the only electron donor known to support CO2 reduction in Methanothrix. Thus, these results are consistent with a model in which Geobacter species were providing electrons to Methanothrix species for methane production through electrical connections of e-pili.
IMPORTANCE Methanothrix species are some of the most important microbial contributors to global methane production, but surprisingly little is known about their physiology and ecology. The possibility that DIET is a source of electrons for Methanothrix in methanogenic rice paddy soils is important because it demonstrates that the contribution that Methanothrix makes to methane production in terrestrial environments may extend beyond the conversion of acetate to methane. Furthermore, defined coculture studies have suggested that when Methanothrix species receive some of their energy from DIET, they grow faster than when acetate is their sole energy source. Thus, Methanothrix growth and metabolism in methanogenic soils may be faster and more robust than generally considered. The results also suggest that the reason that Geobacter species are repeatedly found to be among the most metabolically active microorganisms in methanogenic soils is that they grow syntrophically in cooperation with Methanothrix spp., and possibly other methanogens, via DIET.
KEYWORDS: direct interspecies electron transfer, DIET, Geobacter, Methanothrix (Methanosaeta), metatranscriptomics, methane, methanogenesis
INTRODUCTION
Methanothrix (formerly Methanosaeta) species are thought to be among the most prodigious methane-producing microorganisms on Earth. This is because of the abundance of Methanothrix in many terrestrial soils and sediments that are important sources of atmospheric methane, and the ability of Methanothrix to very effectively utilize acetate, the precursor for ca. two-thirds of the methane produced in these environments (1, 2).
Methanothrix spp. also have the potential to produce methane from carbon dioxide reduction. The genomes of Methanothrix species cultures (1, 3) and the metagenomes of Methanothrix spp. in anaerobic digesters (4) contain a complete set of genes coding for the required enzymes. It is unlikely that hydrogen or formate is the electron donor for carbon dioxide reduction, because none of the four Methanothrix spp. available in culture can use hydrogen or formate as electron donors (2, 5–8), and Methanothrix species lack genes coding for membrane-bound hydrogenase complexes known to be required for hydrogen uptake into the cell by other methanogen species (1, 3, 9). However, Methanothrix harundinacea can reduce carbon dioxide to methane with electrons received from direct interspecies electron transfer (DIET), and DIET-derived electrons can serve as the sole energy source to support growth (4, 10). High transcript abundance for genes involved in the reduction of carbon dioxide to methane is likely to indicate that Methanothrix spp. are participating in DIET (4).
Although there is substantial evidence that Methanothrix spp. can reduce carbon dioxide to methane with electrons derived from DIET in anaerobic brewery waste digesters (4, 11, 12), it is unknown whether DIET by Methanothrix spp. also contributes to carbon dioxide reduction in anaerobic soils and sediments. There are substantial differences between anaerobic digesters and methanogenic soils and sediments that might influence Methanothrix participation in DIET. For example, brewery wastes have a relatively simple organic composition, with ethanol as the major substrate (12), and organics are provided at high rates, supporting rapid metabolic fluxes. In contrast, the primary organic substrates in freshwater methanogenic environments are composed of a much more complex assemblage of polymeric fermentable material that is only slowly degraded, and ethanol is unlikely to be an important metabolic intermediate in these environments (13). Evidence consistent with the concept of Methanothrix spp. receiving electrons from DIET in anaerobic soils was high expression of the mer gene, which encodes a key enzyme in the carbon dioxide reduction pathway, by Methanothrix spp. in incubations of arctic peat soils (14).
The only bacteria known to donate electrons to methanogens via DIET are Geobacter species (4, 10, 15). Geobacter species require electrically conductive pili (e-pili) and outer membrane c-type cytochromes to participate in DIET (4, 15–18) unless DIET is artificially stimulated with the addition of conductive materials, such as granular activated carbon, carbon cloth, or biochar (19–21). Magnetite also appears to promote DIET between Geobacter and Methanosarcina species (22), but it is likely that e-pili are still required (23). Data consistent with Geobacter species playing a role in DIET in terrestrial methanogenic environments were the finding that Geobacter spp. were among the most metabolically active bacteria in methanogenic rice paddy soils (24–26). However, not all Geobacter species produce the e-pili required for DIET (27, 28) and thus, more direct analysis of the potential for e-pilin expression is required.
Therefore, in order to further evaluate whether Methanothrix and Geobacter species might be participating in DIET in terrestrial methanogenic environments, we analyzed the metagenome and metatranscriptome from bacteria and archaea present in methanogenic rice paddy soils. Rice paddies were selected as the model terrestrial environment because methane generated by rice paddies accounts for ∼10% of global atmospheric methane production (29, 30). The results suggest that Methanothrix spp. were receiving electrons for the reduction of carbon dioxide via DIET from Geobacter, and possibly other microorganisms, that were actively expressing e-pilin genes.
RESULTS AND DISCUSSION
Metabolically active microorganisms expressing e-pilin genes.
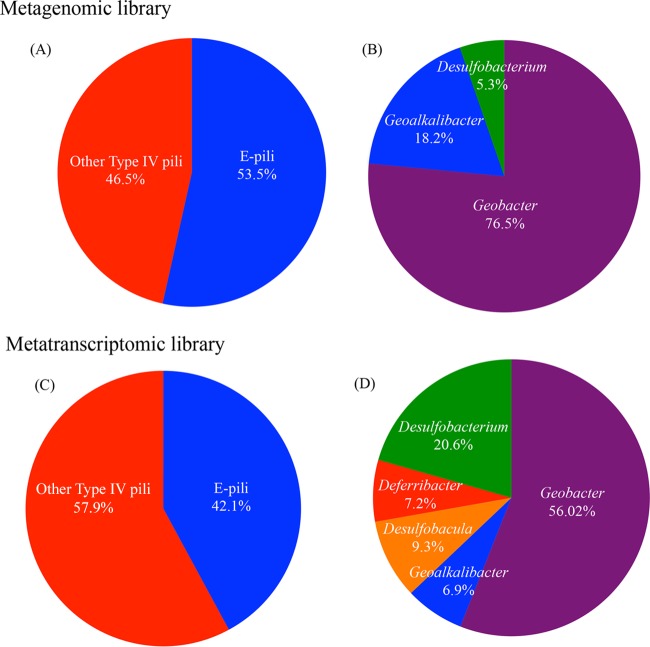
Analysis of quality control (QC)-filtered and merged rice paddy metagenomic and metatranscriptomic libraries showed that about half of the total pilin reads were phylogenetically related to electrically conductive Geobacter e-pilin gene sequences. BLASTx and Bowtie2 analyses showed that ca. 53.5% of the metagenomic reads clustered with putative e-pili (Fig. 1A and Table 1). Analysis of metatranscriptomic libraries with BLASTx and Bowtie2 algorithms also demonstrated that putative e-pili accounted for ∼42% of the type IV pilA genes that were being actively transcribed in the rice paddy sediments (Fig. 1 and Table 1).
FIG 1.
(A and C) Proportion of total type IV pilin reads that clustered with long type IV pili or e-pili in metagenomic (A) and metatranscriptomic (C) libraries. (B and D) Taxonomic assignment of e-pilin reads in metagenomic (B) and metatranscriptomic (D) libraries. Results represent an average of values obtained from nhmmer, BLASTx, and Bowtie2 alignments of QC-filtered merged reads.
TABLE 1.
Number and proportion of QC-filtered merged reads from metagenomic and metatranscriptomic libraries that mapped to various nucleic acid databases determined with Bowtie2 software
| Read information | Mean | SEM |
|---|---|---|
| No. of reads | ||
| Metatranscriptomic reads mapped to Geobacter species | 86,988.17 | 6,732.52 |
| Metatranscriptomic reads mapped to Methanothrix species | 84,646.18 | 4,169.30 |
| Metagenomic reads mapped to Geobacter | 27,848.18 | 4,232.47 |
| Metagenomic reads mapped to Methanothrix | 21,421.68 | 3,568.21 |
| Metatranscriptomic reads mapped to metagenomic contigs | 23,073.77 | 1,521.34 |
| Metatranscriptomic reads mapped to unassembled metagenomic reads | 27,968.10 | 1,910.16 |
| Metatranscriptomic reads mapped to e-pilin genes | 1,147.87 | 155.55 |
| Metatranscriptomic reads mapped to long type IV pilin genes | 1,278.93 | 325.76 |
| Metagenomic reads mapped to e-pilin genes | 334.11 | 10.42 |
| Metagenomic reads mapped to long type IV pilin genes | 275.27 | 26.25 |
| % of e-pilin reads mapped to Geobacter (metatranscriptome) | 66.54 | 2.50 |
| % of e-pilin reads mapped to Geobacter (metagenome) | 82.39 | 5.42 |
| % of long type IV pilin reads mapped to Geobacter (metatranscriptome) | 3.77 | 1.12 |
| % of long type IV pilin reads mapped to Geobacter (metagenome) | 4.66 | 0.16 |
Most of the e-pilin genes and gene transcripts clustered within the genus Geobacter (Fig. 1), but sequences most closely related to e-pili from genera such as Desulfobacterium, Deferribacter, Geoalkalibacter, and Desulfobacula, were also detected (Fig. 1). The ability of microorganisms from genera outside Geobacter to participate in DIET has yet to be evaluated, but extracellular electron transfer has been observed in all of these genera, with the exception of Desulfobacula. For example, Desulfobacterium autotrophicum (31), several species of Deferribacter (32–34), and Geoalkalibacter (35–37) are all capable of Fe(III) reduction. In addition, Deferribacter spp. were enriched from sediments on current-harvesting anodes (38, 39), and Geoalkalibacter spp. produce high current densities in microbial fuel cells (40, 41). The capacity to produce high current densities and to participate in DIET appear to be linked in Geobacter species (28). Therefore, it seems feasible that some of these organisms may also be participating in DIET in the rice paddy soil.
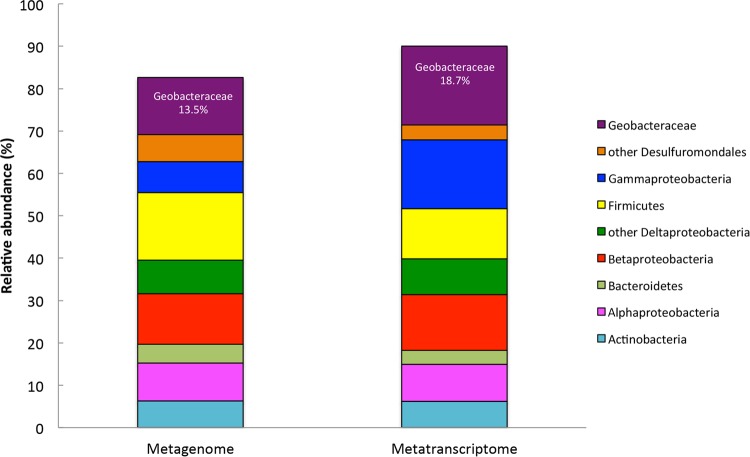
Analysis of the metagenomic and metatranscriptomic libraries (Fig. 2) indicated that of the bacteria harboring e-pilin sequences, Geobacter spp. were by far the most abundant. Geobacteraceae species accounted for 19% and 14% of the bacterial sequences in the metatranscriptomic and metagenomic libraries, respectively. In order to determine whether Geobacter spp. were metabolically active in the rice paddy soil and to further evaluate the potential significance of e-pili in Geobacter respiration, merged metatranscriptomic reads were mapped against the G. sulfurreducens genome, and log2 reads per kilobase per million (RPKM) values were determined (see Table S6 in the supplemental material).
FIG 2.
Phylogenetic distribution of QC-filtered merged bacterial reads (∼150 bp) in metagenomic and metatranscriptomic libraries constructed with DNA or mRNA extracted from methanogenic rice paddy soils. These values represent the mean of results obtained from four different software programs (Tables S3A and B): GenomePeek, MG-RAST, BLASTx, and Kraken.
Citrate synthase has been shown to be an indicator of Geobacter metabolism in the subsurface (42–44). Citrate synthase (gltA) was one of the most highly expressed Geobacter genes (log2 RPKM, 8.4) in the rice paddy soils, well above the median log2 RPKM value of 5.7 (Table S6). Other genes that are indicative of Geobacter growth in the subsurface, such as genes coding for ribosomal proteins, like rpsC, which encodes 30S ribosomal protein S3 (45), were also being actively transcribed by Geobacter spp. in the subsurface (log2 RPKM, 6.7) (Table S6). In addition to rpsC, many other growth-related genes were being significantly transcribed by Geobacter species: 103 genes were involved in protein synthesis, 14 genes were involved in transcription, 15 genes were involved in DNA replication, and 23 genes were involved in cell division.
The e-pilin gene (log2 RPKM, 9.1) and genes for all of the e-pilin accessory proteins (pilA-C, pilB, pilC, pilD, pilE, pilM, pilN, pilO, pilQ, pilR, pilS, pilT, pilV, pilW, and pilY) were highly expressed by Geobacter species. Transcript abundance was also high (log2 RPKM, 7.05) for genes that mapped to the G. sulfurreducens gene for the multiheme c-type cytochrome OmcS. OmcS is localized along the e-pili of G. sulfurreducens (46). A mutation associated with increased rates of DIET in defined cocultures greatly increases OmcS expression, and OmcS-deficient mutants are incapable of DIET (16). Thus, the high expression of genes for OmcS and e-pili are consistent with the hypothesis that Geobacter spp. in the rice paddy soils are participating in DIET.
Methanogens involved in DIET.
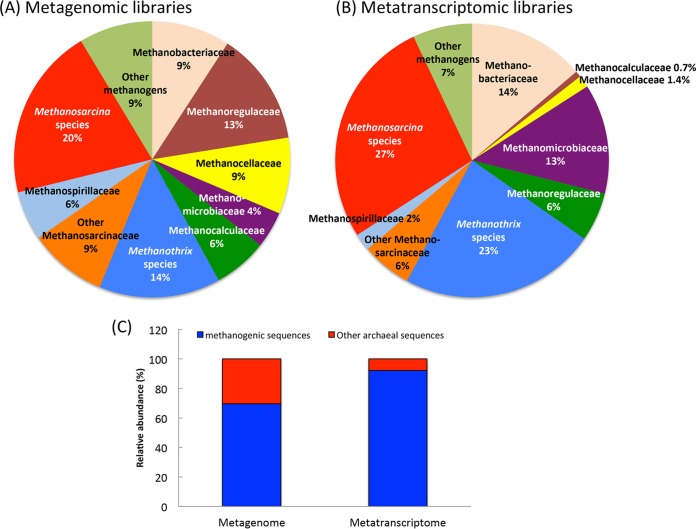
Consistent with the finding that the rice paddy soil incubations were actively producing methane (∼0.3 mmol methane per liter of soil per day), methanogens were the predominant archaea and accounted for 70% and 92% of the archaeal sequences detected in metagenomic and metatranscriptomic libraries, respectively (Fig. 3). Similar to other studies of methanogenic communities associated with flooded rice paddy soils (47, 48), Methanothrix and Methanosarcina spp. were the most abundant methanogens and together accounted for almost half of the sequences. These results are significant because these are the only genera shown thus far to participate in DIET (4, 10, 15).
FIG 3.
(A and B) Phylogenetic distribution of QC-filtered merged methanogenic reads (∼150 bp) in metagenomic (A) and metatranscriptomic (B) libraries constructed with DNA or mRNA extracted from methanogenic rice paddy soils. (C) Relative abundance of methanogenic and nonmethanogenic archaeal sequences in the metagenomic and metatranscriptomic libraries. These values represent the mean of results obtained from four different software programs (Tables S3A and B): GenomePeek, MG-RAST, BLASTx, and Kraken.
To date, no molecular strategy for definitively determining whether Methanosarcina species are engaged in DIET has been developed, because some Methanosarcina species can also use hydrogen as an electron donor for carbon dioxide reduction (49). However, as detailed in the introduction, high expression of genes that encode enzymes that are exclusively part of the pathway for the reduction of carbon dioxide to methane is considered to be diagnostic for DIET in Methanothrix species (4).
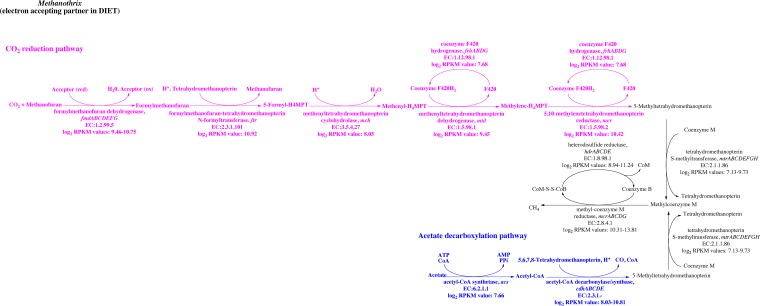
In order to further evaluate whether Methanothrix species were metabolically active and were transcribing carbon dioxide reduction genes, metatranscriptomic reads were mapped against the Methanothrix concilii genome, and log2 RPKM values were determined (Table S7). Similar to Geobacter species, Methanothrix species were expressing a number of genes involved in protein translation, transcription, cell division, and DNA replication, demonstrating that they were actively growing in the rice paddy soils. As expected, Methanothrix species in the rice paddy soils highly expressed genes coding for enzymes involved in the conversion of acetate to methane, as well as genes coding for enzymes common to both the pathway for acetate conversion to methane and carbon dioxide reduction to methane (Fig. 4 and Table S7). However, genes coding for enzymes specifically associated with the reduction of carbon dioxide to methane were also highly expressed. In fact, log2 RPKM values from many of the genes coding for subunits from formylmethanofuran dehydrogenase (fmd), the first enzyme from the CO2 reduction pathway, were higher than values from the first acetate metabolism gene, acetyl-coenzyme A (acetyl-CoA) synthetase (acs) (Fig. 4 and Table S7). Methanothrix spp. also significantly expressed all of the other genes coding for proteins from the carbon dioxide reduction pathway (ftr, mch, mtd, mer, and frh) in the paddy soils. These results suggest that the Methanothrix spp. in the rice paddy soils were actively involved in the reduction of carbon dioxide to methane, which is the methanogenic pathway that would need to be utilized by Methanothrix spp. accepting electrons from Geobacter during DIET.
FIG 4.
Enzymes involved in the carbon reduction or acetate decarboxylation pathways for methanogenesis and the log2 RPKM values from these genes when RNA-seq libraries were mapped against the M. concilii genome. Several proteins from these pathways are composed of multiple subunits, and the range of log2 values reflects the range of expression for all of the subunits. Further details of gene expression are provided in Table S7.
Implications.
The results presented here provide the first evidence, to our knowledge, that DIET may be operative in methanogenic environments other than brewery digesters. The transcriptomic data suggest that Methanothrix spp. were actively reducing carbon dioxide to methane, and electrons derived from DIET are the only known electron donors for carbon dioxide reduction in Methanothrix (4). Geobacter species, which are capable of donating electrons to Methanothrix spp. (4, 10), were also abundant, metabolically active, and transcribed genes coding for e-pilin.
Methanothrix spp. are already thought to be major contributors to global methane production because they are often the most abundant methanogens capable of utilizing acetate in many methanogenic soils and sediments (1, 26, 50–55). The finding that Methanothrix spp. may also produce methane from carbon dioxide by participating in DIET suggests that Methanothrix may play an even more important role in global methane production than previously considered.
The relative contribution of Methanothrix spp. versus other methanogens in the rice paddy soils to carbon dioxide reduction to methane cannot be determined from omics data alone. The metatranscriptomic data suggested that methanogens from other genera, such as Methanobacterium, Methanoculleus, and Methanoregula, which utilize hydrogen as an electron donor for the reduction of carbon dioxide, were also metabolically active in the rice paddy soils. Elucidation of the relative electron flow to carbon dioxide reduction via hydrogen as an intermediary electron carrier and DIET will require much more extensive investigation with novel approaches that can quantify rates of electron flux.
The possibility that Methanothrix is receiving electrons from DIET is an important consideration when attempting to model the growth and activity of Methanothrix in soils and sediments. The growth of Methanothrix harundinacea receiving electrons via DIET as well as acetate in defined coculture with G. metallireducens is much better than the growth of M. harundinacea on acetate alone, suggesting that low-potential electrons that support carbon dioxide reduction enhance growth (4). Therefore, the slow growth attributed to Methanothrix spp. based on laboratory studies with acetate-grown cultures will not be representative for modeling the growth of Methanothrix spp. in terrestrial environments when Methanothrix spp. participate in DIET. More in-depth characterization of the physiology of Methanothrix growing via DIET is warranted.
MATERIALS AND METHODS
Soil incubations.
Rice paddy soil incubations were conducted with soils collected from the Akaogi Farm in Westminster West, VT (http://www.ricenortheasternus.org). Soil samples were placed in sealed mason jars that were completely filled, sealed for transport to the laboratory, and stored at 16°C until use. Surface water was pumped into 5-gallon carboys with a peristaltic pump and stored at 4°C.
A soil slurry with approximately 6 kg of soil mixed with 600 ml of surface water at a ratio of 10:1 was prepared. Six replicate incubations were established in which 500 ml of this slurry and 100 ml of surface water were added to 1-liter bottles in an anaerobic chamber under an N2 atmosphere. No organic or inorganic supplements were added to the incubations. The bottles were sealed with butyl rubber stoppers and incubated in the dark at 25°C for 25 days. When molecular samples were taken from the soil incubations, most of the Fe(III) (92% ± 7%) had been reduced, sulfate concentrations were <10 μM, and ca. 0.3 mmol of methane per liter of soil per day was produced.
Analytical methods.
Fe(II) formation in the soil incubations was monitored over time with a ferrozine assay in a split-beam dual-detector spectrophotometer (Spectronic Genesys 2; Thermo Electron Corp., Mountain View, CA) at an absorbance of 562 nm after a 1-h extraction with 0.5 N HCl (56). The remaining Fe(III) was then converted to Fe(II) with the addition of 0.25 M hydroxylamine, and after an additional hour, Fe(II) was again measured with the ferrozine assay.
Sulfate reduction was monitored with an ion chromatograph (ICS-2100; Dionex, CA) equipped with an AS18 column under isocratic elution with 32 mM KOH as the eluent (57). Methane in the headspace of soil incubations was measured by gas chromatography with a flame ionization detector (GC-8A; Shimadzu).
Extraction of nucleic acids.
For nucleic acid extraction, 1.5 g of soil was removed from each of the six bottles, suspended in RNAlater, and stored at 4°C overnight. The soil samples were then centrifuged at 20,000 × g for 1 min, the supernatants were discarded, and each 1.5-g sample was divided into three 2-ml tubes, making a total of 18 tubes. Total nucleic acids were then extracted as previously described (58–60). Briefly, the pellets were mixed with equal volumes of glass beads (0.17 to 0.18 mm in diameter) and resuspended in 700 μl of precooled TPM buffer (50 mM Tris-HCl [pH 5.0], 1.7% [wt/vol] polyvinylpyrrolidone, 20 mM MgCl2). Subsequently, the mixture was shaken in a bead beater (FastPrep-24 instrument; MP Biomedicals) at 5.5 ms−1 for 45 s. Soil and cell debris were pelleted by centrifugation at 20,000 × g for 1 min at 4°C, and the supernatant was transferred to a fresh tube. The pellet was suspended in 700 μl of phenol lysis buffer (5 mM Tris-HCl [pH 5.0], 5 mM Na2EDTA, 0.1% [wt/vol] sodium dodecyl sulfate, and 6% [vol/vol] water-saturated phenol), and the lysis procedure was repeated as described above. Supernatants from the two lysis treatments were pooled.
The pooled supernatant was extracted first with water-saturated phenol, second with phenol-chloroform-isoamyl alcohol (25:24:1), and third with chloroform-isoamyl alcohol (24:1), each time using 500 μl of extractant. The resulting aqueous phase was mixed with 0.1 volume of 3 M sodium acetate (pH 5.7) and 1 volume of isopropanol, incubated at −80°C for 1 h, and centrifuged for 30 min at 20,000 × g at 4°C. The nucleic acid pellet was washed with 70% ethanol, air dried, and resuspended in 100 μl of TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]). Total nucleic acids from all three aliquots of soil sample were pooled (making a total of 6 tubes) and used for DNA and mRNA isolation.
DNA isolation and Illumina metagenomic library preparation.
For DNA isolation, 2 μl of RNase (Sigma, USA) was added to 98-μl aliquots of total nucleic acids extracted from six different microcosms and incubated at 37°C for 1 h. Genomic DNA was then precipitated with 100% ethanol. These 6 aliquots were then condensed down to 2 tubes by combining three samples into one. In order to ensure sufficient sequencing coverage, these 2 DNA samples were used to construct two composite multiplex shotgun metagenomic libraries with the TruSeq DNA sample preparation kit (Illumina, USA), according to the manufacturer's directions. Briefly, 1 μg of DNA was fragmented with a nebulizer (Illumina, USA), fragment ends were then repaired, and 3′ ends were adenylated. Indexed paired-end adaptors were then ligated onto the fragments and enriched with a final 10-cycle PCR.
mRNA isolation and Illumina RNA-seq library preparation.
For RNA isolation, 20 μl of DNase I buffer and 5 μl of DNase I (Ambion, USA) were added to six separate tubes containing 175 μl of total nucleic acids and incubated at 37°C for 1 h. Total RNA was further purified with the RNeasy minikit (Qiagen, USA). To ensure that RNA samples were free from DNA contamination, 1 μl of each RNA sample was used as the template for PCR with primers targeting the 16S rRNA gene. The MICROBExpress kit (Ambion) was then used to isolate mRNA from total RNA, and the Experion RNA HiSens kit (Bio-Rad) was used to confirm mRNA enrichment prior to RNA sequencing (RNA-seq) library preparation. The MICROBExpress kit was used for mRNA enrichment because previous studies have shown that it was able to successfully enrich mRNA from rice paddy soil RNA extracts (59, 60).
The TruSeq RNA sample preparation kit (Illumina) was used to prepare RNA-seq libraries, according to the manufacturer's instructions. Briefly, 100 ng of mRNA was chemically fragmented and converted into single-stranded cDNA by random-hexamer priming. Double-stranded cDNA was then synthesized, overhangs were made blunt, and 3′ ends were adenylated. Adenylated products were then ligated with individual adapters containing unique hexameric barcodes and enriched with a final 10-cycle PCR.
Both DNA- and mRNA-based Illumina libraries were assessed and quantified with the Experion DNA HiSens kit (Bio-Rad, USA) prior to sequencing. In total, eight libraries (two from DNA and six from mRNA) containing unique barcodes and representing six different soil incubations were mixed in equimolar concentrations and used for hybridization in an Illumina Hi-Seq 2000 flow cell for paired-end sequencing. Sequencing was conducted by the Deep Sequencing Core Facility at the University of Massachusetts Medical School in Worchester, MA.
Assembly of Illumina reads.
All of the raw sequencing and QC-filtered data were quality checked with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Initial raw nonfiltered forward and reverse metagenomic and metatranscriptomic sequencing libraries contained an average of 3,913,198 and 3,302,512 reads that were ∼100 bp long (Tables S1A and B). Sequences from all of the libraries were trimmed and filtered with Trimmomatic (61), with the sliding window approach set to trim bases with quality scores lower than 3, strings of 3+Ns, and reads with a mean quality score lower than 20. Bases were also cut from the start and end of reads that fell below a threshold quality of 3, and any reads smaller than 50 bp were eliminated from the library. These parameters yielded an average of 3,318,562 and 2,222,209 quality reads per metagenomic and metatranscriptomic sequencing library, respectively (Tables S1A and B).
All paired-end reads were then merged with FLASH (62), resulting in 306,024 and 1,115,233 reads with an average read length of 154 bp for the metagenomic and metatranscriptomic libraries, respectively (Tables S1A and B). After merging the QC-filtered reads, SortMeRNA (63) was used to separate all rRNA reads from nonribosomal reads in both the metagenomic and metatranscriptomic libraries. Databases used by SortMeRNA to identify all rRNA sequences included Rfam 5.8S Eukarya, Rfam 5S Archaea/Bacteria, SILVA 16S Archaea, SILVA 16S Bacteria, SILVA 23S Bacteria, SILVA 18S Eukarya, and SILVA 28S Eukarya (64, 65).
Contigs were also assembled from QC-filtered and merged metatranscriptomic and nonmerged metagenomic nonribosomal reads with SeqMan NGen (DNAStar) and MegaHit softwares (66), with an overlapping base length of 50 bp and a minimum contig length of 150 bp. Assembly of these reads yielded ∼17,191 and ∼7,432 contigs with average sizes of 455 and 217 bp from the metagenomic and metatranscriptomic libraries, respectively (Table S2). Approximately 22% and 17% of the metagenomic and metatranscriptomic reads, respectively, could be assembled into these contigs. The software program Prodigal (67) was then used to identify open reading frames (ORFs) within these contigs, and this analysis yielded ∼12,473 and ∼4,070 ORFs, respectively.
Taxonomic classification of metagenomic and metatranscriptomic reads.
The phylogenetic composition of the merged QC-filtered reads (∼150 bp long) was determined with 4 different software programs: NCBI BLAST-2.2.31+ standalone software (68), GenomePeek software (69), Kraken (70), and MG-RAST (71). Prior to analysis with NCBI BLAST-2.2.31+ standalone software, protein-coding reads from each of the metagenomic and metatranscriptomic libraries were grouped into clusters with CD-HIT (72) using the BLOSUM62 scoring matrix and an E value of 0.001. All BLASTx output text files were then imported into MEGAN (73) for taxonomic classification.
Merged QC-filtered reads containing both ribosomal and nonribosomal genes and transcripts were also analyzed with GenomePeek (69), Kraken (70), and MG-RAST (71), using default parameters. The minikraken database was used for taxonomic assignment by the Kraken program. Relative abundances of various archaeal and bacterial taxa were determined separately by each of these programs (Tables S3A to D) and then averaged.
The taxonomic distribution of archaeal and bacterial sequences was also determined by analysis of ORFs identified by Prodigal after assembly of metagenomic and metatranscriptomic contigs. Protein sequences were compared to the NR database with the BLASTp algorithm (74). The results obtained from this analysis were similar to those from the analysis of unassembled reads (Table S4).
Analysis and identification of type IV pilin genes.
The e-pili of Geobacter species described to date are composed of a PilA monomer (e-pilin) that is homologous to type IVa PilA proteins found in many bacteria (75, 76). However, the e-pilin genes are phylogenetically distinct from PilA monomers that are the subunits for poorly conductive pili of other microorganisms (75, 77). Therefore, it was possible to differentiate between e-pili and long type IV pilA genes by alignment with previously characterized pilin genes. First, the nhmmer search function (78) was used to scan QC-filtered merged metatranscriptomic and metagenomic reads for the presence of pilA genes by comparison to a pilA nucleotide Hidden Markov Model (HMM) database built by hmmbuild in HMMER 3 (79, 80). The HMM database used for this comparison was built from an alignment of 88 different type IV pilA nucleotide sequences, with 36 e-pilin genes and 52 long pilin genes (Table S5).
Potential pilin genes were also identified in QC-filtered merged metagenomic and metatranscriptomic libraries with the BLASTx algorithm by comparison to protein databases built from 88 PilA protein sequences with the makeblastdb function using NCBI BLAST-2.2.31+ standalone software (68). In addition, PilFind (81), FlaFind (82), Motif Scan (http://myhits.isb-sib.ch/cgi-bin/motif_scan), and Motif Search (http://www.genome.jp/tools/motif/) were used to scan for the presence of type IV pilin-like motifs. All reads identified by these programs were then further screened by comparison to sequences in the NR database with the BLASTx algorithm.
Mapping mRNA reads.
QC-filtered merged paired-end libraries that had rRNA removed with SortMeRNA software were used for all transcriptomic mapping analyses. For analysis of gene expression by dominant acetoclastic methanogens found in the rice paddy, mRNA reads were mapped against Methanothrix concilii GP-6 (accession no. NC_015416.1). Geobacter gene abundance was determined by mapping against the genome of Geobacter sulfurreducens PCA (accession no. NC_002939.1). Type IV pilin abundance was determined by mapping against a database composed of 88 different type IV pilA nucleotide sequences. The ArrayStar software (DNAStar) was used to map metatranscriptomic reads to M. concilii, G. sulfurreducens, and the pilA database. All mapped reads were normalized with the reads assigned per kilobase of target per million mapped reads (RPKM) method (83). The total numbers of merged QC-filtered metatranscriptomic reads that mapped to merged QC-filtered metagenomic reads, metagenomic-assembled contigs, the G. sulfurreducens genome, the M. concilii genome, or the pilin database were determined with the Bowtie2 software (84) and ArrayStar (Table 1).
Accession number(s).
The metagenomics and metatranscriptomics sequence reads have been submitted to the European Nucleotide Archive (ENA) database under accession no. PRJEB15510.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kristen DeAngelis from the Department of Microbiology at the University of Massachusetts Amherst for her helpful bioinformatics tips.
This research was supported by the Office of Science (BER), U.S. Department of Energy, award no. DE-SC0004485.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00223-17.
REFERENCES
- 1.Smith KS, Ingram-Smith C. 2007. Methanosaeta, the forgotten methanogen? Trends Microbiol 15:150–155. doi: 10.1016/j.tim.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Oren A. 2014. The family Methanotrichaceae, p 297–306. In Rosenberg E. (ed), The prokaryotes–other major lineages of Bacteria and the Archaea. Springer-Verlag, Heidelberg, Germany. [Google Scholar]
- 3.Zhu J, Zheng H, Ai G, Zhang G, Liu D, Liu X, Dong X. 2012. The genome characteristics and predicted function of methyl-group oxidation pathway in the obligate aceticlastic methanogens, Methanosaeta spp. PLoS One 7:e36756. doi: 10.1371/journal.pone.0036756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotaru AE, Shrestha PM, Liu FH, Shrestha M, Shrestha D, Embree M, Zengler K, Wardman C, Nevin KP, Lovley DR. 2014. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ Sci 7:408–415. doi: 10.1039/C3EE42189A. [DOI] [Google Scholar]
- 5.Kamagata Y, Kawasaki H, Oyaizu H, Nakamura K, Mikami E, Endo G, Koga Y, Yamasato K. 1992. Characterization of three thermophilic strains of Methanothrix (“Methanosaeta”) thermophila sp. nov. and rejection of Methanothrix (“Methanosaeta”) thermoacetophila. Int J Syst Bacteriol 42:463–468. doi: 10.1099/00207713-42-3-463. [DOI] [PubMed] [Google Scholar]
- 6.Patel G, Sprott G. 1990. Methanosaeta concilii gen. nov., sp. nov. (“Methanothrix concilii”) and Methanosaeta thermoacetophila nom. rev., comb. nov.? Int J Syst Bacteriol 40:79–82. doi: 10.1099/00207713-40-1-79. [DOI] [Google Scholar]
- 7.Ma K, Liu X, Dong X. 2006. Methanosaeta harundinacea sp. nov., a novel acetate-scavenging methanogen isolated from a UASB reactor. Int J Syst Evol Microbiol 56:127–131. doi: 10.1099/ijs.0.63887-0. [DOI] [PubMed] [Google Scholar]
- 8.Mori K, Iino T, Suzuki K, Yamaguchi K, Kamagata Y. 2012. Aceticlastic and NaCl-requiring methanogen “Methanosaeta pelagica” sp. nov., isolated from marine tidal flat sediment. Appl Environ Microbiol 78:3416–3423. doi: 10.1128/AEM.07484-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber RD, Zhang L, Harnack M, Olson MV, Kaul R, Ingram-Smith C, Smith KS. 2011. Complete genome sequence of Methanosaeta concilii, a specialist in aceticlastic methanogenesis. J Bacteriol 193:3668–3669. doi: 10.1128/JB.05031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang LY, Nevin KP, Woodard TL, Mu BZ, Lovley DR. 2016. Expanding the diet for DIET: electron donors supporting direct interspecies electron transfer (DIET) in defined co-cultures. Front Microbiol 7:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita M, Malvankar NS, Franks AE, Summers ZM, Giloteaux L, Rotaru AE, Rotaru C, Lovley DR. 2011. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. mBio 2(4):e00159-11. doi: 10.1128/mBio.00159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrestha P, Malvankar N, Werner J, Franks A, Rotaryu A, Shrestha M, Liu F, Nevin K, Angenent L, Lovley D. 2014. Correlation between microbial community and granule conductivity in anaerobic bioreactors for brewery wastewater treatment. Bioresour Technol 174:306–310. doi: 10.1016/j.biortech.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Lovley D, Klug M. 1982. Intermediary metabolism of organic carbon in the sediment of a eutrophic lake. Appl Environ Microbiol 43:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tveit AT, Urich T, Frenzel P, Svenning MM. 2015. Metabolic and trophic interactions modulate methane production by Arctic peat microbiota in response to warming. Proc Natl Acad Sci U S A 112:E2507–2516. doi: 10.1073/pnas.1420797112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotaru AE, Shrestha PM, Liu F, Markovaite B, Chen S, Nevin KP, Lovley DR. 2014. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl Environ Microbiol 80:4599–4605. doi: 10.1128/AEM.00895-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Summers ZM, Fogarty HE, Leang C, Franks AE, Malvankar NS, Lovley DR. 2010. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330:1413–1415. doi: 10.1126/science.1196526. [DOI] [PubMed] [Google Scholar]
- 17.Shrestha PM, Rotaru AE, Aklujkar M, Liu F, Shrestha M, Summers ZM, Malvankar N, Flores DC, Lovley DR. 2013. Syntrophic growth with direct interspecies electron transfer as the primary mechanism for energy exchange. Environ Microbiol Rep 5:904–910. doi: 10.1111/1758-2229.12093. [DOI] [PubMed] [Google Scholar]
- 18.Shrestha PM, Rotaru AE, Summers ZM, Shrestha M, Liu F, Lovley DR. 2013. Transcriptomic and genetic analysis of direct interspecies electron transfer. Appl Environ Microbiol 79:2397–2404. doi: 10.1128/AEM.03837-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Rotaru A, Shrestha P, Malvankar N, Nevin K, Lovley D. 2012. Promoting direct interspecies electron transfer with activated carbon. Energy Environ Sci 5:8982–8989. doi: 10.1039/c2ee22459c. [DOI] [Google Scholar]
- 20.Chen S, Rotaru AE, Liu F, Philips J, Woodard TL, Nevin KP, Lovley DR. 2014. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresour Technol 173:82–86. doi: 10.1016/j.biortech.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Rotaru AE, Shrestha PM, Malvankar NS, Liu F, Fan W, Nevin KP, Lovley DR. 2014. Promoting interspecies electron transfer with biochar. Sci Rep 4:5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato S, Hashimoto K, Watanabe K. 2012. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ Microbiol 14:1646–1654. doi: 10.1111/j.1462-2920.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Rotaru AE, Shrestha PM, Malvankar NS, Nevin KP, Lovley DR. 2015. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ Microbiol 17:648–655. doi: 10.1111/1462-2920.12485. [DOI] [PubMed] [Google Scholar]
- 24.Hori T, Muller A, Igarashi Y, Conrad R, Friedrich MW. 2010. Identification of iron-reducing microorganisms in anoxic rice paddy soil by 13C-acetate probing. ISME J 4:267–278. doi: 10.1038/ismej.2009.100. [DOI] [PubMed] [Google Scholar]
- 25.Hori T, Noll M, Igarashi Y, Friedrich MW, Conrad R. 2007. Identification of acetate-assimilating microorganisms under methanogenic conditions in anoxic rice field soil by comparative stable isotope probing of RNA. Appl Environ Microbiol 73:101–109. doi: 10.1128/AEM.01676-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y, Liesack W. 2015. Differential assemblage of functional units in paddy soil microbiomes. PLoS One 10:e0122221. doi: 10.1371/journal.pone.0122221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan Y, Adhikari RY, Malvankar NS, Ward JE, Nevin KP, Woodard TL, Smith JA, Snoeyenbos-West OL, Franks AE, Tuominen MT, Lovley DR. 2016. The low conductivity of Geobacter uraniireducens pili suggests a diversity of extracellular electron transfer mechanisms in the genus Geobacter. Front Microbiol 7:980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotaru AE, Woodard TL, Nevin KP, Lovley DR. 2015. Link between capacity for current production and syntrophic growth in Geobacter species. Front Microbiol 6:744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikaloff Fletcher S, Tans P, Bruhwiler L, Miller J, Heimann M. 2004. CH4 sources estimated from atmospheric observations of CH4 and its 13C/12C isotopic ratios: 1. Inverse modeling of source processes. Glob Biogeochem Cycles 18:1944–9224. [Google Scholar]
- 30.Wuebbles D, Hayhoe K. 2002. Atmospheric methane and global change. Earth Sci Rev 57:177–210. doi: 10.1016/S0012-8252(01)00062-9. [DOI] [Google Scholar]
- 31.Lovley DR, Roden EE, Phillips EJP, Woodward JC. 1993. Enzymatic iron and uranium reduction by sulfate-reducing bacteria. Mar Geol 113:41–53. doi: 10.1016/0025-3227(93)90148-O. [DOI] [Google Scholar]
- 32.Greene AC, Patel BKC, Sheehy AJ. 1997. Deferribacter thermophilus gen. nov., sp. nov., a novel thermophilic manganese- and iron-reducing bacterium isolated from a petroleum reservoir. Int J Syst Bacteriol 47:505–509. doi: 10.1099/00207713-47-2-505. [DOI] [PubMed] [Google Scholar]
- 33.Miroshnichenko ML, Slobodkin AI, Kostrikina NA, L'Haridon S, Nercessian O, Spring S, Stackebrandt E, Bonch-Osmolovskaya EA, Jeanthon C. 2003. Deferribacter abyssi sp. nov., an anaerobic thermophile from deep-sea hydrothermal vents of the Mid-Atlantic Ridge. Int J Syst Evol Microbiol 53:1637–1641. doi: 10.1099/ijs.0.02673-0. [DOI] [PubMed] [Google Scholar]
- 34.Slobodkina GB, Kolganova TV, Chernyh NA, Querellou J, Bonch-Osmolovskaya EA, Slobodkin AI. 2009. Deferribacter autotrophicus sp. nov., an iron(III)-reducing bacterium from a deep-sea hydrothermal vent. Int J Syst Evol Microbiol 59:1508–1512. doi: 10.1099/ijs.0.006767-0. [DOI] [PubMed] [Google Scholar]
- 35.Zavarzina DG, Kolganova TV, Bulygina ES, Kostrikina NA, Turova TP, Zavarzin GA. 2006. Geoalkalibacter ferrihydriticus gen. nov., sp. nov., the first alkaliphilic representative of the family Geobacteraceae, isolated from a soda lake. Mikrobiologiia 75:673–682. (In Russian.) [PubMed] [Google Scholar]
- 36.Zavarzina D, Kevbrin V, Zhilina T, Chistyakova N, Shapkin A, Zavarzin G. 2011. Reduction of synthetic ferrihydrite by a binary anaerobic culture of Anaerobacillus alkalilacustris and Geoalkalibacter ferrihydriticus grown on mannitol at pH 9.5. Microbiology 80:743–757. doi: 10.1134/S0026261711060233. [DOI] [PubMed] [Google Scholar]
- 37.Greene AC, Patel BK, Yacob S. 2009. Geoalkalibacter subterraneus sp. nov., an anaerobic Fe(III)- and Mn(IV)-reducing bacterium from a petroleum reservoir, and emended descriptions of the family Desulfuromonadaceae and the genus Geoalkalibacter. Int J Syst Evol Microbiol 59:781–785. doi: 10.1099/ijs.0.001537-0. [DOI] [PubMed] [Google Scholar]
- 38.Mathis BJ, Marshall CW, Milliken CE, Makkar RS, Creager SE, May HD. 2008. Electricity generation by thermophilic microorganisms from marine sediment. Appl Microbiol Biotechnol 78:147–155. doi: 10.1007/s00253-007-1266-4. [DOI] [PubMed] [Google Scholar]
- 39.Jong BC, Kim BH, Chang IS, Liew PW, Choo YF, Kang GS. 2006. Enrichment, performance, and microbial diversity of a thermophilic mediatorless microbial fuel cell. Environ Sci Technol 40:6449–6454. doi: 10.1021/es0613512. [DOI] [PubMed] [Google Scholar]
- 40.Badalamenti JP, Krajmalnik-Brown R, Torres CI. 2013. Generation of high current densities by pure cultures of anode-respiring Geoalkalibacter spp. under alkaline and saline conditions in microbial electrochemical cells. mBio 4(3):e00144-13. doi: 10.1128/mBio.00144-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoho RA, Popat SC, Rago L, Guisasola A, Torres CI. 2015. Anode biofilms of Geoalkalibacter ferrihydriticus exhibit electrochemical signatures of multiple electron transport pathways. Langmuir 31:12552–12559. doi: 10.1021/acs.langmuir.5b02953. [DOI] [PubMed] [Google Scholar]
- 42.Holmes DE, Nevin KP, O'Neil RA, Ward JE, Adams LA, Woodard TL, Vrionis HA, Lovley DR. 2005. Potential for quantifying expression of the Geobacteraceae citrate synthase gene to assess the activity of Geobacteraceae in the subsurface and on current-harvesting electrodes. Appl Environ Microbiol 71:6870–6877. doi: 10.1128/AEM.71.11.6870-6877.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilkins MJ, Callister SJ, Miletto M, Williams KH, Nicora CD, Lovley DR, Long PE, Lipton MS. 2011. Development of a biomarker for Geobacter activity and strain composition; proteogenomic analysis of the citrate synthase protein during bioremediation of U(VI). Microb Biotechnol 4:55–63. doi: 10.1111/j.1751-7915.2010.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yun J, Ueki T, Miletto M, Lovley DR. 2011. Monitoring the metabolic status of Geobacter species in contaminated groundwater by quantifying key metabolic proteins with Geobacter-specific antibodies. Appl Environ Microbiol 77:4597–4602. doi: 10.1128/AEM.00114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holmes DE, Giloteaux L, Barlett M, Chavan MA, Smith JA, Williams KH, Wilkins M, Long P, Lovley DR. 2013. Molecular analysis of the in situ growth rates of subsurface Geobacter species. Appl Environ Microbiol 79:1646–1653. doi: 10.1128/AEM.03263-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leang C, Qian X, Mester T, Lovley DR. 2010. Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens. Appl Environ Microbiol 76:4080–4084. doi: 10.1128/AEM.00023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HJ, Jeong SE, Kim PJ, Madsen EL, Jeon CO. 2015. High resolution depth distribution of Bacteria, Archaea, methanotrophs, and methanogens in the bulk and rhizosphere soils of a flooded rice paddy. Front Microbiol 6:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liesack W, Schnell S, Revsbech NP. 2000. Microbiology of flooded rice paddies. FEMS Microbiol Rev 24:625–645. doi: 10.1111/j.1574-6976.2000.tb00563.x. [DOI] [PubMed] [Google Scholar]
- 49.Boone D, Mah R. 2015. Methanosarcina, p 1–15. In Boone DR, Mah RA (ed), Bergey's manual of systematics of Archaea and Bacteria. John Wiley & Sons, Ltd., New York, NY. [Google Scholar]
- 50.Glissman K, Chin KJ, Casper P, Conrad R. 2004. Methanogenic pathway and archaeal community structure in the sediment of eutrophic Lake Dagow: effect of temperature. Microb Ecol 48:389–399. doi: 10.1007/s00248-003-2027-2. [DOI] [PubMed] [Google Scholar]
- 51.Grosskopf R, Janssen PH, Liesack W. 1998. Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl Environ Microbiol 64:960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan OC, Claus P, Casper P, Ulrich A, Lueders T, Conrad R. 2005. Vertical distribution of structure and function of the methanogenic archaeal community in Lake Dagow sediment. Environ Microbiol 7:1139–1149. doi: 10.1111/j.1462-2920.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 53.Cadillo-Quiroz H, Yashiro E, Yavitt JB, Zinder SH. 2008. Characterization of the archaeal community in a minerotrophic fen and terminal restriction fragment length polymorphism-directed isolation of a novel hydrogenotrophic methanogen. Appl Environ Microbiol 74:2059–2068. doi: 10.1128/AEM.02222-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma K, Conrad R, Lu YH. 2012. Responses of methanogen mcrA genes and their transcripts to an alternate dry/wet cycle of paddy field soil. Appl Environ Microbiol 78:445–454. doi: 10.1128/AEM.06934-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang GH, Watanabe T, Jin JA, Liu XB, Kimura M, Asakawa S. 2010. Methanogenic archaeal communities in paddy field soils in north-east China as evaluated by PCR-DGGE, sequencing and real-time PCR analyses. Soil Sci Plant Nutr 56:831–838. doi: 10.1111/j.1747-0765.2010.00521.x. [DOI] [Google Scholar]
- 56.Lovley DR, Phillips EJ. 1987. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol 53:1536–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holmes DE, Giloteaux L, Orellana R, Williams KH, Robbins MJ, Lovley DR. 2014. Methane production from protozoan endosymbionts following stimulation of microbial metabolism within subsurface sediments. Front Microbiol 5:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noll M, Matthies D, Frenzel P, Derakshani M, Liesack W. 2005. Succession of bacterial community structure and diversity in a paddy soil oxygen gradient. Environ Microbiol 7:382–395. doi: 10.1111/j.1462-2920.2005.00700.x. [DOI] [PubMed] [Google Scholar]
- 59.Mettel C, Kim Y, Shrestha PM, Liesack W. 2010. Extraction of mRNA from soil. Appl Environ Microbiol 76:5995–6000. doi: 10.1128/AEM.03047-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shrestha PM, Kube M, Reinhardt R, Liesack W. 2009. Transcriptional activity of paddy soil bacterial communities. Environ Microbiol 11:960–970. doi: 10.1111/j.1462-2920.2008.01821.x. [DOI] [PubMed] [Google Scholar]
- 61.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magoč T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kopylova E, Noe L, Touzet H. 2012. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 28:3211–3217. doi: 10.1093/bioinformatics/bts611. [DOI] [PubMed] [Google Scholar]
- 64.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and Web-based tools. Nucleic Acids Res 41:D590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burge SW, Daub J, Eberhardt R, Tate J, Barquist L, Nawrocki EP, Eddy SR, Gardner PP, Bateman A. 2013. Rfam 11.0: 10 years of RNA families. Nucleic Acids Res 41:D226–D232. doi: 10.1093/nar/gks1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li D, Liu CM, Luo R, Sadakane K, Lam TW. 2015. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- 67.Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McNair K, Edwards RA. 2015. GenomePeek-an online tool for prokaryotic genome and metagenome analysis. PeerJ 3:e1025. doi: 10.7717/peerj.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wood DE, Salzberg SL. 2014. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15:R46. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meyer F, Paarmann D, D'Souza M, Olson R, Glass EM, Kubal M, Paczian T, Rodriguez A, Stevens R, Wilke A, Wilkening J, Edwards RA. 2008. The metagenomics RAST server–a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics 9:386. doi: 10.1186/1471-2105-9-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu L, Niu B, Zhu Z, Wu S, Li W. 2012. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics 28:3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huson DH, Auch AF, Qi J, Schuster SC. 2007. MEGAN analysis of metagenomic data. Genome Res 17:377–386. doi: 10.1101/gr.5969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- 76.Tan Y, Adhikari RY, Malvankar NS, Ward JE, Woodard TL, Nevin KP, Lovley DR. 2017. Expressing the Geobacter metallireducens PilA in Geobacter sulfurreducens yields pili with exceptional conductivity. mBio 8(1):e02203-16. doi: 10.1128/mBio.02203-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holmes D, Dang Y, Walker D, Lovley D. 2016. The electrically conductive pili of Geobacter species are a recently evolved feature for extracellular electron transfer. Microb Genom 11:52. doi: 10.1099/mgen.0.000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wheeler TJ, Eddy SR. 2013. nhmmer: DNA homology search with profile HMMs. Bioinformatics 29:2487–2489. doi: 10.1093/bioinformatics/btt403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eddy SR. 2008. A probabilistic model of local sequence alignment that simplifies statistical significance estimation. PLoS Comput Biol 4:e1000069. doi: 10.1371/journal.pcbi.1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eddy SR. 2011. Accelerated profile HMM searches. PLoS Comput Biol 7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Imam S, Chen Z, Roos DS, Pohlschroder M. 2011. Identification of surprisingly diverse type IV pili, across a broad range of Gram-positive bacteria. PLoS One 6:e28919. doi: 10.1371/journal.pone.0028919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Szabó Z, Stahl AO, Albers SV, Kissinger JC, Driessen AJ, Pohlschroder M. 2007. Identification of diverse archaeal proteins with class III signal peptides cleaved by distinct archaeal prepilin peptidases. J Bacteriol 189:772–778. doi: 10.1128/JB.01547-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. 2008. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 84.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.