ABSTRACT
Bifidobacterium longum strain JDM301, a widely used commercial strain in China, encodes at least two MazEF-like modules and one RelBE-like toxin-antitoxin (TA) system in its chromosome, designated MazE1F1Bif, MazE2F2Bif, and RelBEBif, respectively. Bacterial TA systems play an important role in several stress responses, but the relationship between these TA systems is largely unknown. In this study, the interactions between MazF1Bif and MazE2Bif or RelBBif were assessed in B. longum strain JDM301. MazF1Bif caused the degradation of tufABif mRNA, and its toxicity was inhibited by forming a protein complex with its cognate antitoxin, MazE1Bif. Notably, MazF1Bif toxicity was also partially neutralized when jointly expressed with noncognate antitoxin MazE2Bif or RelBBif. Our results show that the two noncognate antitoxins also inhibited mRNA degradation caused by MazF1Bif toxin. Furthermore, the physical interplay between MazF1Bif and its noncognate antitoxins was confirmed by immunoprecipitation. These results suggest that MazF1Bif can arrest cell growth and that MazF1Bif toxicity can be neutralized by its cognate and noncognate antitoxins. These results imply that JDM301 uses a sophisticated toxin-antitoxin interaction network to alter its physiology when coping with environmental stress.
IMPORTANCE Although toxin-antitoxin (TA) systems play an important role in several stress responses, the regulatory mechanisms of multiple TA system homologs in the bacterial genome remain largely unclear. In this study, the relationships between MazE1F1Bif and the other two TA systems of Bifidobacterium longum strain JDM301 were explored, and the interactions between MazF1Bif and MazE2Bif or RelBBif were characterized. In addition, the mRNA degradation activity of MazF1Bif was demonstrated. In particular, the interaction of the toxin with noncognate antitoxins was shown, even between different TA families (MazF1Bif toxin and RelBBif antitoxin) in JDM301. This work provides insight into the regulatory mechanisms of TA systems implicated in the stress responses of bifidobacteria.
KEYWORDS: Bifidobacterium longum, toxin-antitoxin system, cross-interaction, mRNA degradation
INTRODUCTION
Type II toxin-antitoxin (TA) systems are ubiquitous in free-living bacteria and consist of adjacent genes encoding a toxin and antitoxin in a single operon (1, 2). The first gene encodes a relatively labile antitoxin and the second gene encodes a stable toxin (2). Bacterial TA systems are considered stress-responsive elements (3, 4). Under normal conditions, antitoxin proteins are abundant, which can neutralize the action of its cognate toxin. The toxin and its cognate antitoxin interact to form an inactive toxin-antitoxin protein complex. The complex or the antitoxin itself acts as a transcriptional autorepressor of the operon (5). However, in response to adverse growth conditions, the amount of antitoxin decreases and the toxin is released, leading to cell death or growth arrest by the toxin acting on its intracellular target (5–7). Transcription is repressed by the antitoxin alone or the toxin-antitoxin complex upon binding to the palindrome upstream of the operon (8). The antitoxin protein is unstable relative to the toxin, since it is susceptible to cleavage by ClpP or/and Lon proteases (9, 10). When stress conditions lead to an increased expression of proteases, the pool of antitoxins is reduced by proteolysis, leading to a relative increase in module transcription, which results in an excess of toxin (5). The free toxin then acts on its target, resulting in transient growth arrest or cell death if antitoxin synthesis does not recover quickly enough (5, 11, 12).
Some TA systems in Escherichia coli are activated under environmental stress, resulting in cell stasis, after which they can recover under favorable conditions (13, 14). The MazEF module (toxin MazF and antitoxin MazE) is a well-characterized TA system of E. coli that is involved in various stress conditions, such as nutritional stress and antibiotic exposure (15–17). Stress conditions lead to the degradation of the antitoxin (MazE) and the release of the free toxin (MazF). The free MazF prevents translation by cleaving RNAs, resulting in cell death or growth arrest (18–20). The RelBE module (toxin RelE and antitoxin RelB) is another TA system in E. coli. Free RelE can induce global inhibition of translation and the arrest of cell growth by cleaving RNAs (21–23). Among them, the tufA (elongation factor Tu) mRNAs are targets of free RelE and HigB (toxin protein of the TA system HigBA) in E. coli (22–24).
Although TA systems are distributed widely in free-living bacteria, which can encode more than one TA system, almost all intracellular bacteria are devoid of TA systems, suggesting that these systems are stress-response elements, which are crucial for bacterial survival in fluctuating environmental conditions (16, 25–27). However, genomes of free-living bacteria usually encode many TA system homologs (28, 29). The relationships between these TA systems in the bacterial genome are largely unknown. Recently, multiple toxin-antitoxin systems were reported to cooperate to increase the persister frequency in E. coli (14). Interactions were also found among three RelB-like TA systems and even between different TA families (MazF toxins and VapB antitoxins) in Mycobacterium tuberculosis (30, 31). Nineteen genes of TA systems belonging to the MazEF and RelBE families were found by an in silico analysis of 36 sequenced genomes from several strains of bifidobacteria (32). The whole genome of Bifidobacterium longum strain JDM301, a widely used commercial strain in China, was completely sequenced (33). A total of 11 putative TA systems were found by bioinformatic analysis of the JDM301 genome (10). The JDM301 genome harbors at least two pairs of functional mazEF-like loci (BLJ_811-BLJ_812 and BLJ_864-BLJ_865) and one pair of functional relBE-like loci (BLJ_989-BLJ_990), designated MazE1F1Bif, MazE2F2Bif, and RelBEBif, respectively (10, 34, 35). In our previous report, we showed that MazE1F1Bif was activated under acid stress (10). However, the roles of these systems in the stress response of JDM301 remain largely unclear. The relationships between MazE1F1Bif and the other two TA systems were explored in this study.
In this study, the physical and functional interplay between toxin MazF1Bif and its noncognate antitoxins was characterized. In addition, the mRNA degradation activity of MazF1Bif was shown. In particular, noncognate interactions were found, even between different TA families (MazF1Bif toxin and RelBBif antitoxin) in B. longum. Interactions with noncognate antitoxins might reduce the toxicity of MazF1Bif in vivo. This work provides insight on the interplay between different TA systems in B. longum, which helps the bacterium adapt to harsh environmental conditions.
RESULTS
MazF1Bif and its cognate antitoxin, MazE1Bif, form a complex.
To show the direct interaction between MazE1Bif and MazF1Bif, the MazE1Bif and MazF1Bif genes were both cloned into a single pET28a expression vector under one promoter in accordance with our previous report (10). Thus, only MazE1Bif was expressed as a His6-tagged fusion protein, while MazF1Bif was expressed as a Myc-tagged fusion protein. The recombinant proteins were expressed and purified from Ni-nitrilotriacetic acid (Ni-NTA) resin. The purified proteins were subjected to Western blot analysis using anti-His6 or anti-Myc monoclonal antibodies. The resulting bands observed corresponded to MazE1Bif (12.6 kDa) with the His6 tag and to MazF1Bif (14.4 kDa) with the Myc tag (Fig. 1). Thus, His6-MazE1Bif and MazF1Bif-Myc were copurified from Ni2+-chelating Sepharose resin to show that MazF1Bif and its cognate antitoxin, MazE1Bif, formed a complex.
FIG 1.
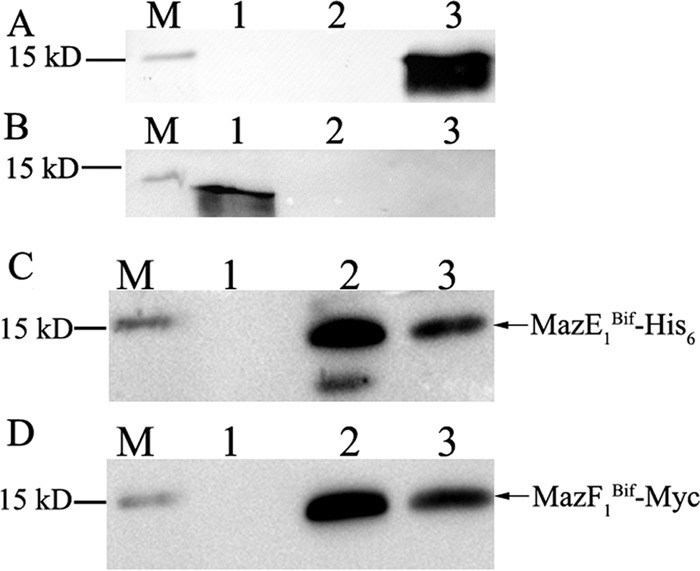
Interaction of Myc-tagged MazF1Bif and His6-tagged MazE1Bif recombinant proteins. Recombinant proteins were expressed and purified using Ni-NTA resin. The purified proteins were detected by Western blotting with anti-His6 (A) and anti-Myc (B) monoclonal antibodies. Recombinant proteins were expressed from IPTG-induced E. coli harboring pET-E1 or pET-F1(Myc). M, molecular mass markers; 1, lysate of E. coli harboring pET-F1(Myc); 2, purified products of E. coli harboring pET-F1(Myc); 3, purified recombinant proteins from E. coli harboring pET-E1. (C) MazE1Bif-His6, including the His6 tag at its N-terminal end. (D) MazF1Bif-Myc, including the Myc tag at its C-terminal end. Recombinant proteins were expressed from IPTG-induced E. coli harboring pET-E1F1(Myc). Both the MazE1Bif-His6 and MazF1Bif-Myc fusion proteins were detected at their expected molecular masses. M, molecular mass markers; 1, eluates of absorbed lysate from uninduced E. coli harboring pET-E1F1(Myc); 2, eluates of absorbed lysate from IPTG-induced E. coli harboring pET-E1F1(Myc); 3, purified recombinant proteins from IPTG-induced E. coli harboring pET-E1F1(Myc).
mRNA degradation by MazF1Bif is antagonized by its cognate antitoxin, MazE1Bif.
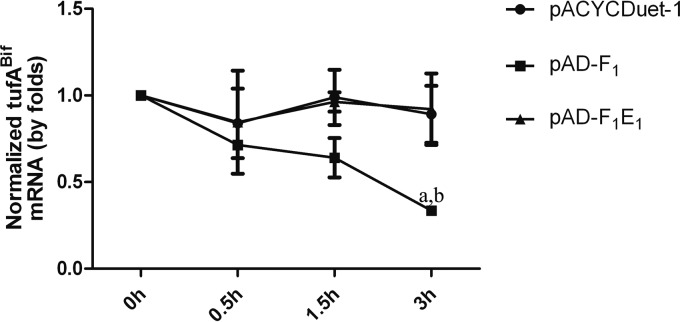
The tufABif gene was cloned into the promoter of the arabinose operon of pBAD/HisB to produce tufABif mRNA in E. coli. pACYCDuet-1, pAD-F1, or pAD-F1E1 was transformed into E. coli with pBA-tufA for the coexpression of MazF1Bif or MazF1Bif and MazE1Bif with tufABif mRNA. Quantitative real-time PCR (qRT-PCR) was used to determine whether MazF1Bif mediates tufA mRNA degradation in strain JDM301 and whether the activity of MazF1Bif is inhibited by MazE1Bif. Our results show that the induction of MazF1Bif in E. coli decreased tufABif mRNA levels compared with levels when tufABif was transcribed alone, while tufABif mRNA levels increased when MazF1Bif was coexpressed with MazE1Bif compared with levels in E. coli expressing only MazF1Bif, indicating that MazE1Bif alleviates the degradation of tufABif mRNA by MazFBif (Fig. 2). These results suggest that MazF1Bif causes the degradation of tufABif mRNA and that the activity of MazF1Bif is alleviated by its cognate, MazE1Bif.
FIG 2.
MazF1Bif is an mRNA interferase that is inhibited by its cognate antitoxin, MazE1Bif. Relative transcript levels of tufABif were determined in E. coli expressing tufABif with pACYCDuet-1, pAD-F1, or pAD-F1E1. The strains were grown with 0.2% arabinose for 2 h to induce tufABif expression. Then, 1 mM IPTG was added to induce MazF1Bif or MazF1Bif and MazE1Bif expression. After 3 h, 200 μg/ml rifampin was added. Samples were collected at the indicated time points after rifampin addition. The levels of tufABif mRNA were monitored by qRT-PCR (normalized to the 16S rRNA transcript level). The values presented are the averages from three independent experiments, and error bars represent the standard deviations. A two-way analysis of variance with Bonferroni posttest was used to obtain P values for each time point: a, P < 0.05 versus pACYCDuet-1; b, P < 0.05 versus pAD-F1E1.
MazF1Bif physically interacts with its noncognate antitoxin protein.
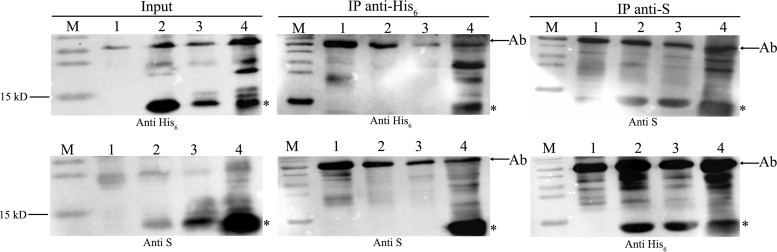
Plasmid pACYCDuet-1, pAD-F1E1, pAD-F1E2, or pAD-F1B was introduced into E. coli to simultaneously express His-tagged MazF1Bif and S-tagged antitoxins (MazE1Bif, MazE2Bif, or RelBBif). Subsequently, coimmunoprecipitation was performed to detect the physical interactions between the toxin MazF1Bif and each of the three antitoxin proteins, including its cognate antitoxin, MazE1Bif, and noncognate antitoxins MazE2Bif and RelBBif. An anti-His antibody against the His-tagged MazF1Bif and an anti-S antibody against the S-tagged antitoxins were used in coimmunoprecipitation experiments. As shown in Fig. 3, noncognate toxin-antitoxin interactions (MazF1Bif with MazE2Bif and MazF1Bif with RelBBif) and a cognate toxin-antitoxin interaction (MazF1Bif with MazE1Bif) were observed by immunoprecipitation. The interaction between the toxin MazF1Bif and the antitoxin MazE2Bif was only observed by immunoprecipitation using the anti-S antibody. The interaction between the toxin MazF1Bif and the antitoxin MazE1Bif was also confirmed by immunoprecipitation using only the anti-S antibody. The reason for this is unclear; however, steric hindrance stemming from the presence of the His tag might be responsible (30). Our results demonstrated that toxin MazF1Bif and its noncognate antitoxins physically interact with each other, indicating that the noncognate antitoxins of MazF1Bif, particularly RelBBif, may act in lieu of its cognate antitoxin, MazE1Bif, to inhibit toxicity.
FIG 3.
Molecular interactions between MazF1Bif and cognate or noncognate antitoxin proteins are confirmed by coimmunoprecipitation assays. Cell lysates or proteins immunoprecipitated with the anti-His6 or anti-S antibodies were analyzed by immunoblotting using anti-His6 or anti-S antibodies. M, molecular mass markers; 1, E. coli carrying pACYCDuet-1 (an empty vector) used as the control; 2, E. coli carrying pAD-F1E1; 3, E. coli carrying pAD-F1E2; 4, E. coli carrying pAD-F1B. Asterisks indicate the bands corresponding to MazF1Bif-His6, MazE1Bif-S, MazE2Bif-S, or RelBBif-S. The bands corresponding to the heavy chains of the anti-His6 or anti-S antibody are indicated by arrows.
MazF1Bif inhibits the growth of E. coli, and the inhibition is alleviated by its noncognate antitoxin proteins.
Several growth curves of E. coli strains carrying pACYCDuet-1, pAD-F1, pAD-F1E1, pAD-F1E2, or pAD-F1B in the presence of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) were plotted to determine whether the toxicity of the MazF1Bif toxin could be inhibited by noncognate antitoxins in vivo. For E. coli strains containing mazF1Bif alone, growth inhibition was observed upon IPTG induction compared with that of the cells containing an empty vector (Fig. 4A). Furthermore, the cells coexpressing MazE1Bif and MazF1Bif grew better than those expressing MazF1Bif alone but worse than those containing the empty vector (Fig. 4A). Notably, when the MazF1Bif toxin was induced in the presence of noncognate antitoxin MazE2Bif or RelEBif, growth inhibition was alleviated (Fig. 4B and C), indicating that cell growth inhibition caused by MazF1Bif can be rescued by the activity of the noncognate antitoxin MazE2Bif or RelEBif. These rescue experiments enabled the detection of interactions that may be less stable in vitro. Our results demonstrated interactions between MazF1Bif and noncognate antitoxins RelEBif and MazE2Bif, which act in lieu of MazE1Bif to inhibit the activity of MazF1Bif.
FIG 4.
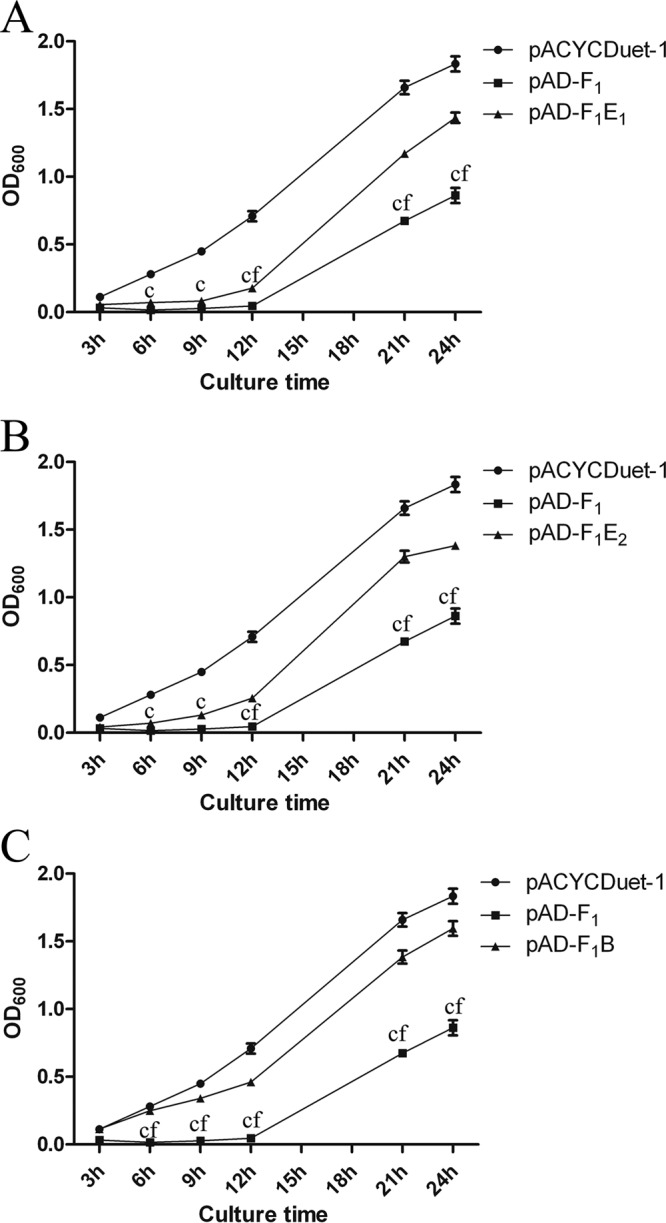
Interactions between MazF1Bif and its cognate antitoxin, MazE1Bif, or noncognate antitoxins affect cell growth. The growth characteristics of E. coli carrying pACYCDuet-1, pAD-F1, and pAD-F1E1 (A), pAD-F1E2 (B), or pAD-F1B (C) were analyzed by measuring absorbance (OD600) following induction with 1 mM IPTG. The values presented are the averages from three independent experiments, and error bars represent the standard deviations. A two-way analysis of variance with Bonferroni posttest was used to obtain P values for each time point: c, P < 0.001 versus pACYCDuet-1; f, P < 0.001 versus pAD-F1E1, pAD-F1E2, or pAD-F1B.
MazF1Bif-induced mRNA degradation is antagonized by noncognate antitoxins.
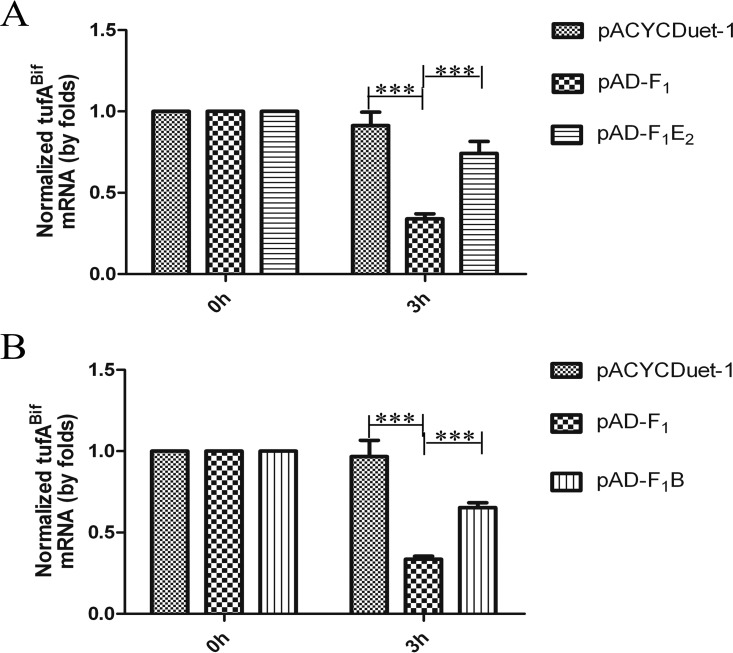
The results above showed that the MazF1Bif toxin associates with the noncognate antitoxin RelEBif or MazE2Bif to alleviate growth inhibition caused by MazF1Bif, implying that the toxic effect of MazF1Bif can be antagonized by noncognate antitoxins. To test this hypothesis, E. coli was transformed with pBA-tufA and pACYCDuet-1 (a blank vector), pBA-tufA and pAD-F1, pBA-tufA and pADuet-F1E2, or pBA-tufA and pAD-F1B. When RelBBif or MazE2Bif was coexpressed with MazF1Bif, the level of tufABif mRNA increased in comparison to the level of tufABif in E. coli expressing MazF1Bif alone (Fig. 5). Our results suggest that MazF1Bif-induced mRNA degradation is inhibited by the noncognate antitoxins RelEBif and MazE2Bif.
FIG 5.
Noncognate antitoxin proteins counteract the mRNA interferase activity of MazF1Bif. Relative transcript levels of tufABif were determined in E. coli expressing tufABif with pACYCDuet-1, pAD-F1, and pAD-F1E2 (A) or pAD-F1B (B). The strains were grown with 0.2% arabinose for 2 h to induce tufABif expression. Then, 1 mM IPTG was added to induce MazF1Bif, MazF1Bif and MazE2Bif, or MazF1Bif and RelBBif expression. After 3 h, 200 μg/ml rifampin was added. Samples were collected at the indicated time points after rifampin addition and the levels of tufABif mRNA were monitored by qRT-PCR (normalized to the 16S rRNA transcript level). The values presented are the averages from three independent experiments, and error bars represent the standard deviations. A two-way analysis of variance with Bonferroni posttest was used to obtain P values for each time point. ***, P < 0.001.
DISCUSSION
The cognate toxin and antitoxin of a TA system are small proteins encoded by two genes organized in one operon (36). The activity of the toxin can be neutralized by forming a protein complex with its cognate antitoxin (30, 37). Our results confirmed that the mazF homologue (BLJ_811) present in the chromosome of the JDM301 strain encodes a toxic protein (MazF1Bif), which forms a complex with its cognate antitoxin, encoded by the adjacent mazE gene (BLJ_812) (10).
To date, toxins of TA systems include ribonucleases, DNA gyrase poisons, phosphotransferases, and protein kinases (2, 38). MazF has been shown to act by cleaving mRNA, resulting in translation inhibition in E. coli, Mycobacterium tuberculosis, Streptococcus mutans, and Clostridium difficile (18, 39–41). Like other toxin proteins from E. coli, M. tuberculosis, Streptococcus mutans, and Clostridium difficile, the MazF1Bif toxin was determined to cause mRNA degradation. Our results suggest that MazF1Bif induces the degradation of tufABif mRNA, which may partially account for the inhibition of protein synthesis and cell growth arrest. Cleavage of elongation factor Tu mRNAs is partly responsible for growth inhibition caused by MazF1Bif. The MazF1Bif toxin was not purified in this study because of its low production level in E. coli. Thus, we performed an in vivo RNase assay with tufABif mRNAs as the substrate. In previous reports, the homologs of tufABif were used to determine the RNase activity of RelE and HigB in E. coli (23, 24). Our previous work showed that MazE1F1Bif is activated through the hydrolysis of MazE1Bif (10). Therefore, it was proposed that in response to adverse conditions, the antitoxin MazE1Bif is degraded, releasing the toxin MazF1Bif to cleave existing transcripts, such as tufABif mRNA. Consequently, cell growth is modulated and stasis may occur, which may help the cells cope with environmental stress (7).
To date, few studies presenting the interaction between different TA modules have been reported. Yang et al. observed that three M. tuberculosis RelE toxins physically interact with the same RelB protein to conditionally regulate RelB binding with promoter DNA (31). Zhu et al. observed noncognate toxin-antitoxin associations, even among different TA families (MazF toxins and VapB antitoxins), in M. tuberculosis (30). Recently, transcriptional cross-activation between toxin-antitoxin systems was found in E. coli (42). Multiple TA systems have been shown to coordinately govern the persister phenotype in E. coli (14). In this study, MazF1Bif was shown to physically interact with the noncognate antitoxin RelEBif, which belongs to another family of TA systems, or to MazE2Bif in strain JDM301. Furthermore, when either antitoxin RelEBif or MazE2Bif was overexpressed in E. coli, cell growth inhibition conferred by the MazF1Bif toxin was alleviated. Thus, the interaction between MazE1F1Bif and RelBEBif or MazE2F2Bif was demonstrated. In addition, RelEBif and MazE2Bif antitoxins were observed to antagonize the degradation of tufABif mRNA by MazF1Bif. Interestingly, the TA system MazE1F1Bif is activated under acid stress (10). In addition, the expression levels of ClpP1Bif and ClpP2Bif proteases responsible for the activation of MazE1F1Bif are also increased significantly during acid stress (10), whereas MazE2F2Bif (data not shown) and RelBEBif are not activated under this adverse condition (35). As a major challenge to bifidobacteria, acid stress might reduce the viability and probiotic effects of these bacteria (43). TA systems have been implicated in the acid stress response of E. coli and Streptococcus mutants. In E. coli, the antitoxin MqsA mediates the general stress response in bacteria, including the acid stress response (27). Furthermore, a mutated strain of Streptococcus devoid of TA systems was shown to be more resistant to changes in pH than the wild-type strain (44).
Generally, there is an excess of antitoxin proteins, since the level of gene expression is often proportional to the gene order in a polycistronic message (i.e., the gene encoding the antitoxin precedes the gene encoding the toxin in the TA operon) (45, 46). In other words, when TA systems are inactivated, the antitoxin proteins exist in excess relative to their cognate toxins (47). Thus, it was speculated that there is an excess of antitoxins (MazE2Bif and RelBBif) in B. longum strain JDM301 under acid stress when TA systems MazEF2Bif and RelBEBif are all inactivated. When strain JDM301 was subjected to acid stress, the activation of MazE1F1Bif led to the release of free MazF1Bif toxin resulting in cell growth inhibition. It is possible that excess noncognate antitoxins MazE2Bif and RelBBif partially abolish the toxicity of MazF1Bif, helping cells to switch more quickly from a state of growth inhibition to one of normal growth when the acid stress is removed. As a common probiotic bacterium, bifidobacteria are added to many types of fermented dairy foods. However, during the industrial process, storage, and passage through the digestive tract of the host, bifidobacteria are subjected to various stresses, such as acid stress. It was implied that the interaction between MazF1Bif and MazE2Bif or RelBBif may facilitate bacterial adaptation to changing environments encountered during the industrial manufacturing process and passage through the digestive tract of the host. However, the industrial environment is probably not an evolutionary driver of TA systems. TA systems in closely related bifidobacterial species show extensive 95% to 100% similarity, which suggests that horizontal gene transport may account for significant portions of the distribution of TA systems among bifidobacteria (45, 48). Given that the gastrointestinal tract (GIT) is a natural environment of Bifidobacterium and a broad variety of bacterial species inhabit the GIT, the main site of horizontal gene transport of bifidobacterial TA modules is the GIT (49, 50). Thus, the harsh conditions in the GIT are the main evolutionary driver of bifidobacterial TA systems. Previously, it was shown that MazEF and RelBE are widely distributed in bifidobacteria (32). Interestingly, among the species, only strain JDM301 and ATCC 15697 have as many TA system genes, while the other bifidobacteria harbor only a few TA systems (48). It was speculated that multiple TA systems may help bifidobacteria to cope with various environmental stresses.
Given that the codon bias and GC content of B. longum and E. coli are different and that chromosomally encoded MazF1Bif and antitoxin proteins are heterologously produced by the double promoter plasmid, the heterologous expression of toxin and antitoxin proteins may be influenced. Similarly, in our previous reports, MazE1Bif did not thoroughly abolish the toxicity of MazF1Bif when MazE1Bif and MazF1Bif were jointly expressed under the control of one promoter, while MazE2Bif and RelEBif completely neutralized their cognate toxins MazF2Bif and RelEBif, respectively (10, 34, 35). We speculate that in the original host, MazF1Bif is inhibited by the compensatory actions of noncognate antitoxins under normal conditions, as MazE1Bif did not completely abolish the toxicity of MazF1Bif. On the other hand, apart from a species-specific pattern in codon usage, there are also considerable differences among genes in many species (51). Our results may be due to a larger deviation in codon bias between B. longum and E. coli, leading to a disproportionately lower translation efficiency of MazE1Bif than of MazF1Bif. Further studies are needed to gain a deeper insight into the role of interactions among TA system families in regulating B. longum cell growth. Overall, these results provide molecular insight regarding the interactions of TA systems implicated in the bifidobacterial stress response and can serve as a foundation for future studies of TA systems in their natural host, B. longum.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A summary of the bacterial strains used in this study is shown in Table 1. JDM301 was cultured anaerobically in MRS (Difco) supplemented with 0.05% (wt/vol) l-cysteine-HCl at 37°C for 14 to 16 h. The DH5α and BL21(DE3) strains of E. coli were each cultured aerobically in LB medium on a rotary shaker (220 rpm) at 37°C or cultured on LB agar plates. When needed, the culture medium was supplemented with 50 μg/ml kanamycin, 35 μg/ml chloramphenicol, 100 μg/ml ampicillin, or 200 μg/ml rifampin for E. coli. IPTG was added at a final concentration of 0.5 mM or 1 mM to induce the expression of toxin and antitoxin proteins in E. coli. Additionally, 0.2% (wt/vol) arabinose was added to induce the transcription of tufABif mRNA driven by the promoter of the arabinose operon (PBAD).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| E. coli BL21(DE3) | General expression strain | Tiangen Biotech |
| B. longum JDM301 | Commercial strain | 33 |
| pET28a | Expression vector with strong T7 promoter, His tag | Novagen (USA) |
| pET-E1 | pET28a derivative carrying the mazE1Bif gene, His tag | This study |
| pET-F1(Myc) | pET28a derivative carrying the mazE1F1Bif gene, Myc tag | This study |
| pET-E1F1(Myc) | pET28a derivative carrying the mazE1F1Bif gene, His tag and Myc tag | 10 |
| pBAD/HisB | Expression vector with PBAD promoter, His tag | Invitrogen |
| pBAD-tufA | pBAD/HisB derivative carrying the tufABif gene, His tag | This study |
| pACYCDuet-1 | Expression vector for coexpressing two target genes with T7 promoter, His tag and S tag | Novagen |
| pAD-F1 | pACYCDuet-1 derivative carrying the mazF1Bif gene | This study |
| pAD-F1E1 | pACYCDuet-1 derivative carrying the mazF1Bif gene and mazE1Bif gene | This study |
| pAD-F1E2 | pACYCDuet-1 derivative carrying the mazF1Bif gene and mazE2Bif gene | This study |
| pAD-F1B | pACYCDuet-1 derivative carrying the relBBif gene and mazF1Bif gene | This study |
Construction of plasmids.
All plasmids used in this study are listed in Table 1. PCR primers and restriction sites used are shown in Table 2. As previously reported (10), the intact TA locus (mazE1F1Bif) was amplified and cloned into pET28a to yield pET-E1F1(Myc), which encodes an N-terminal His6-tagged MazE1Bif and a Myc-tagged MazF1Bif under the control of one promoter. Thus, the native mazEBif-mazFBif gene organization was kept intact to coexpress MazF1Bif and MazE1Bif under the control of one promoter. The full-length tufABif gene was amplified and cloned into pBAD/HisB to yield pBA-tufA (35). The mazF1Bif gene was placed under the control of the T7 promoter-1 of pACYCDuet-1, to yield pAD-F1. The mazE1Bif, mazE2Bif, and relBBif genes were amplified and subcloned under the control of the T7 promoter-2 of pAD-F1, resulting in pAD-F1E1, pAD-F1E2, and pAD-F1B, respectively. All the genes were amplified using JDM301 genomic DNA as the template, which was extracted from mid-log-phase cultures grown in MRS broth.
TABLE 2.
Oligonucleotide primers used in PCR and qRT-PCR
| Gene | Assay | Direction | Primer sequencea | Length (bp) | Source or reference |
|---|---|---|---|---|---|
| mazF1Bif | PCR | Sense | CCGGAATTCAATGAAACGAGGTGAGATT | 324 | This work |
| Antisense | CCCAAGCTTTCACAGCAAACCTAGAAC | ||||
| mazF1Bif (primer mazF1Bif-2) | PCR | Sense | TTAAGAAGGAGATATACCATGGAAATGAAACGAGGTGAGATTCG | 354 | This work |
| Antisense | CTCGAGTGCGGCCGCAAGCTTTTACAGATCCTCTTCTGAGATGAGTTTTTGTTCCAGCAAACCTAGAACCCG | ||||
| mazE1Bif | PCR | Sense | GGAAGATCTAATGAGCATACAGATTGCCA | 246 | This work |
| Antisense | CCGCTCGAGCCTCGTTTCATCGGACAT | ||||
| mazE1Bif (primer mazE1Bif-2) | PCR | Sense | GGATCCATGAGCATACAGATTGCCA | 246 | This work |
| Antisense | CTCGAGCCTCGTTTCATCGGACAT | ||||
| mazE2Bif | PCR | Sense | GGAAGATCTTATGGCTATCAAGGAGAAGG | 294 | This work |
| Antisense | CCGCTCGAGGTCTTCATCATCGTCCCA | ||||
| relBBif | PCR | Sense | GGAAGATCTATTGTCTTACGTAATGATGTTGG | 300 | This work |
| Antisense | CCGCTCGAGGATTCCCAACGAATCGAA | ||||
| mazEBif | qRT-PCR | Sense | GCTGCGTTGTTTAAGGAGAC | 94 | 10 |
| Antisense | ACCCACGGTAATCATTGAAC | ||||
| tufABif | qRT-PCR | Sense | ATCCGTCCGACCCAGACC | 123 | 54 |
| Antisense | CTCGACATCCTCACGGCC | ||||
| 16S | qRT-PCR | Sense | TACGGGAGGCAGCAG | 191 | 55 |
| Antisense | ATTACCGCGGCTGCTGG |
Restriction sites for XhoI, EcoRI, BglII, and HindIII incorporated into the primers are in boldface, and the sequence for the Myc tag is underlined.
Assessment of toxin and antitoxin activities in E. coli.
E. coli strains carrying pACYCDuet-1 (a blank vector), pAD-F1, pAD-F1E1, pAD-F1E2, or pAD-F1B were grown in LB broth with IPTG (1 mM) to induce gene expression. Growth curves of the E. coli carrying the corresponding vectors were determined by measuring the OD values at 600 nm (OD600) to assess the effects of the toxin and antitoxin on cell growth. Cultures of the E. coli strains were initially grown in LB overnight with 35 μg/ml chloramphenicol and then transferred into fresh LB using 1% inoculum in the presence of 1 mM IPTG (IPTG was added to the LB at 0 h). Samples were taken at different time points, and the optical density at 600 nm was determined for each.
In vivo cleavage of the tufABif mRNA.
pBA-tufABif and pACYCDuet-1 (a blank vector), pBA-tufABif and pAD-F1, pBA-tufA and pAD-F1E1, pBA-tufA and pAD-F1E2, or pBA-tufA and pAD-F1B were transformed into E. coli to determine whether MazF1Bif causes the degradation of tufABif mRNA and whether MazF1Bif toxicity is inhibited by its cognate antitoxin, MazE1Bif, or noncognate antitoxins MazE2Bif and RelBBif. The plasmids used are listed in Table 1, and primers for qRT-PCR of tufABif mRNA are listed in Table 2. Cells transformed with the corresponding plasmids were grown at 37°C on a rotary shaker. When the cultures reached an OD600 of 0.5, 0.2% l-arabinose was added to the medium to induce the transcription of tufABif mRNA. After incubating at 28°C for 2 h, 1 mM IPTG was added to induce the expression of toxin (and antitoxin) proteins for 3 h. Then, 200 μg/ml rifampin was added to halt transcription. At different time points, 2-ml aliquots were taken. qRT-PCR was performed to determine the level of tufABif mRNA. The transcription of 16S rRNA genes in E. coli was evaluated as an internal control.
Quantitative real-time PCR.
Total RNA was extracted from E. coli strains carrying pBA-tufA and pACYCDuet-1 (a blank vector), pBA-tufA and pAD-F1, pBA-tufA and pADuet-F1E1, pBA-tufA and pAD-F1E2, or pBA-tufA and pAD-F1B and was treated with DNase (Roche, Basel, Switzerland). For use in qRT-PCR, cDNA was generated from total RNA (2 μg) using a Superscript III first strand synthesis RT-PCR kit (Invitrogen, Carlsbad, California, USA) according to the manufacturer's protocol. Primers targeting tufABif were designed for detecting mRNA expression by qRT-PCR. The primers used are shown in Table 2. The reaction was performed with an ABI 7500 system (Applied Biosystems, Branchburg, New Jersey, USA) using the following conditions: 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Calculations were performed using the 16S rDNA gene as an internal standard. The 2-ΔΔCT method was used to determine the relative gene expression (52).
Protein purification and Western blot analysis.
pET-E1, pET-F1(Myc), and pET-E1F1(Myc) were transferred into E. coli to express the His-tagged MazE1Bif and/or Myc-tagged MazF1Bif recombinant proteins. Cells transformed with the corresponding plasmids were grown at 37°C on a rotary shaker. When the cultures reached an OD600 of 0.5, IPTG (at a final concentration of 0.5 mM) was added to induce the expression of recombinant fusion proteins (His-tagged MazE1Bif and Myc-tagged MazF1Bif) for 5 h. The cells were pelleted and disrupted on ice by sonication, and the soluble or insoluble fraction was recovered by centrifugation. The recombinant proteins were purified by affinity chromatography using Ni-NTA resin (Qiagen, Hilden, Germany) according to the manufacturer's protocol. The purified proteins were fractionated by 15% SDS-PAGE and transferred to a nitrocellulose membrane for detection by Western blot analysis. Anti-His and anti-Myc monoclonal antibodies were used to detect His-tagged MazE1Bif and Myc-tagged MazF1Bif, respectively.
Coimmunoprecipitation assay.
E. coli strains carrying pACYCDuet-1 (a blank vector), pAD-F1E1, pAD-F1E2, or pAD-F1B were grown in LB until an OD600 of 0.5 was reached. After that, IPTG (at a final concentration of 1 mM) was added to the medium to induce protein expression for 5 h at 28°C. The cells were pelleted, and the proteins were isolated and immunoprecipitated as described previously (53) with a few modifications. Protein extracts (400 μg) were incubated with anti-His (His-probe [G-18], sc-804; Santa Cruz Biotechnology) or anti-S (MB2016; Bioworld Technology) antibodies on a rotator overnight at 4°C. The immunoprecipitated proteins were incubated with protein A/G agarose (P2012; Beyotime Biotechnology) for 4 h at 4°C. The immunoprecipitated complexes were dissolved in 2× SDS gel loading buffer (at a final concentration of 2% [wt/vol] SDS, 0.1% [wt/vol] bromophenol blue, 10% glycerol, and 50 mM Tris/HCl, pH 6.8). Then, the samples were incubated at 95°C for 5 min and subjected to SDS-PAGE. Extracted proteins and immunocomplexes were analyzed by immunoblotting using anti-His or anti-S monoclonal antibodies. A horseradish peroxidase-labeled goat anti-rabbit IgG antibody was used as a secondary antibody (sc-2054; Santa Cruz Biotechnology). Chemiluminescence signals were visualized with an enhanced chemiluminescence (ECL) reagent (Thermo Scientific, Rockford, Illinois, USA) and were exposed to film.
ACKNOWLEDGMENTS
This work was funded by the National Natural Science Foundation of China (31300029), the Natural Science Foundation of Jiangsu Province, China (BK20130213), the Scientific Research Foundation for the Talents of Xuzhou Medical University (D2012014), the Program for Youth Science and Technology Innovative Research Team of Xuzhou Medical University, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Scientific Research Innovation Project for College Graduates of Jiangsu Province (KYZZ15_0389) and was sponsored by the Qing Lan Project of Jiangsu Province, China.
The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Buts LLJ, Dao-Thi MH, Wyns L, Loris R. 2005. Toxin-antitoxin modules as bacterial metabolic stress managers. Trends Biochem Sci 30:672–679. doi: 10.1016/j.tibs.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Van Melderen L, Saavedra De Bast M. 2009. Bacterial toxin-antitoxin systems: more than selfish entities? PLoS Genet 5:e1000437. doi: 10.1371/journal.pgen.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerdes K, Christensen SK, Lobner-Olesen A. 2005. Prokaryotic toxin-antitoxin stress response loci. Nat Rev Microbiol 3:371–382. doi: 10.1038/nrmicro1147. [DOI] [PubMed] [Google Scholar]
- 4.Sala A, Calderon V, Bordes P, Genevaux P. 2013. TAC from Mycobacterium tuberculosis: a paradigm for stress-responsive toxin-antitoxin systems controlled by SecB-like chaperones. Cell Stress Chaperones 18:129–135. doi: 10.1007/s12192-012-0396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sofos N, Xu K, Dedic E, Brodersen DE. 2015. Cut to the chase–regulating translation through RNA cleavage. Biochimie 114:10–17. doi: 10.1016/j.biochi.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Ramage HR, Connolly LE, Cox JS. 2009. Comprehensive functional analysis of Mycobacterium tuberculosis toxin-antitoxin systems: implications for pathogenesis, stress responses, and evolution. PLoS Genet 5:e1000767. doi: 10.1371/journal.pgen.1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maisonneuve E, Shakespeare LJ, Jorgensen MG, Gerdes K. 2011. Bacterial persistence by RNA endonucleases. Proc Natl Acad Sci U S A 108:13206–13211. doi: 10.1073/pnas.1100186108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Zorzini V, Buts L, Schrank E, Sterckx YG, Respondek M, Engelberg-Kulka H, Loris R, Zangger K, van Nuland NA. 2015. Escherichia coli antitoxin MazE as transcription factor: insights into MazE-DNA binding. Nucleic Acids Res 43:1241–1256. doi: 10.1093/nar/gku1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donegan NP, Thompson ET, Fu Z, Cheung AL. 2010. Proteolytic regulation of toxin-antitoxin systems by ClpPC in Staphylococcus aureus. J Bacteriol 192:1416–1422. doi: 10.1128/JB.00233-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei YX, Ye L, Liu DB, Zhang ZY, Liu C, Guo XK. 2015. Activation of the chromosomally encoded mazEF(Bif) locus of Bifidobacterium longum under acid stress. Int J Food Microbiol 207:16–22. doi: 10.1016/j.ijfoodmicro.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Schifano JM, Woychik NA. 2014. 23S rRNA as an a-Maz-ing new bacterial toxin target. RNA Biol 11:101–105. doi: 10.4161/rna.27949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amitai S, Yassin Y, Engelberg-Kulka H. 2004. MazF-mediated cell death in Escherichia coli: a point of no return. J Bacteriol 186:8295–8300. doi: 10.1128/JB.186.24.8295-8300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islam S, Benedik MJ, Wood TK. 2015. Orphan toxin OrtT (YdcX) of Escherichia coli reduces growth during the stringent response. Toxins (Basel) 7:299–321. doi: 10.3390/toxins7020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fasani RA, Savageau MA. 2013. Molecular mechanisms of multiple toxin-antitoxin systems are coordinated to govern the persister phenotype. Proc Natl Acad Sci U S A 110:E2528–E2537. doi: 10.1073/pnas.1301023110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwan BW, Lord DM, Peti W, Page R, Benedik MJ, Wood TK. 2014. The MqsR/MqsA toxin/antitoxin system protects Escherichia coli during bile acid stress. Environ Microbiol 17:3168–3181. doi: 10.1111/1462-2920.12749. [DOI] [PubMed] [Google Scholar]
- 16.Norton JP, Mulvey MA. 2012. Toxin-antitoxin systems are important for niche-specific colonization and stress resistance of uropathogenic Escherichia coli. PLoS Pathog 8:e1002954. doi: 10.1371/journal.ppat.1002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazan RSB, Engelberg-Kulka H. 2004. Escherichia coli mazEF-mediated cell death is triggered by various stressful conditions. J Bacteriol 186:3663–3669. doi: 10.1128/JB.186.11.3663-3669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothenbacher FP, Suzuki M, Hurley JM, Montville TJ, Kirn TJ, Ouyang M, Woychik NA. 2012. Clostridium difficile MazF toxin exhibits selective, not global, mRNA cleavage. J Bacteriol 194:3464–3474. doi: 10.1128/JB.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang J, Hoeflich KP, Ikura M, Qing G, Inouye M. 2003. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol Cell 12:913–923. doi: 10.1016/S1097-2765(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Wood TK. 2011. Toxin/antitoxin systems influence biofilm and persister cell formation and the general stress response. Appl Environ Microbiol 77:5577–5583. doi: 10.1128/AEM.05068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheverton AM, Gollan B, Przydacz M, Wong CT, Mylona A, Hare SA, Helaine S. 2016. A Salmonella toxin promotes persister formation through acetylation of tRNA. Mol Cell 63:86–96. doi: 10.1016/j.molcel.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christensen SK, Gerdes K. 2003. RelE toxins from bacteria and Archaea cleave mRNAs on translating ribosomes, which are rescued by tmRNA. Mol Microbiol 48:1389–1400. doi: 10.1046/j.1365-2958.2003.03512.x. [DOI] [PubMed] [Google Scholar]
- 23.Hurley JM, Cruz JW, Ouyang M, Woychik NA. 2011. Bacterial toxin RelE mediates frequent codon-independent mRNA cleavage from the 5′ end of coding regions in vivo. J Biol Chem 286:14770–14778. doi: 10.1074/jbc.M110.108969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurley JM, Woychik NA. 2009. Bacterial toxin HigB associates with ribosomes and mediates translation-dependent mRNA cleavage at A-rich sites. J Biol Chem 284:18605–18613. doi: 10.1074/jbc.M109.008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makarova KS, Wolf YI, Koonin EV. 2009. Comprehensive comparative-genomic analysis of type 2 toxin-antitoxin systems and related mobile stress response systems in prokaryotes. Biol Direct 4:19. doi: 10.1186/1745-6150-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frampton R, Aggio RB, Villas-Boas SG, Arcus VL, Cook GM. 2012. Toxin-antitoxin systems of Mycobacterium smegmatis are essential for cell survival. J Biol Chem 287:5340–5356. doi: 10.1074/jbc.M111.286856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Kim Y, Hong SH, Ma Q, Brown BL, Pu M, Tarone AM, Benedik MJ, Peti W, Page R, Wood TK. 2011. Antitoxin MqsA helps mediate the bacterial general stress response. Nat Chem Biol 7:359–366. doi: 10.1038/nchembio.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey DP, Gerdes K. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res 33:966–976. doi: 10.1093/nar/gki201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiedoruk K, Daniluk T, Swiecicka I, Sciepuk M, Leszczynska K. 2015. Type II toxin-antitoxin systems are unevenly distributed among Escherichia coli phylogroups. Microbiology 161:158–167. doi: 10.1099/mic.0.082883-0. [DOI] [PubMed] [Google Scholar]
- 30.Zhu LSJ, Kobayashi H, Woychik NA, Inouye M. 2010. Noncognate mycobacterium tuberculosis toxin-antitoxins can physically and functionally interact. J Biol Chem 285:39732–39738. doi: 10.1074/jbc.M110.163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M, Gao C, Wang Y, Zhang H, He ZG. 2010. Characterization of the interaction and cross-regulation of three Mycobacterium tuberculosis RelBE modules. PLoS One 5:e10672. doi: 10.1371/journal.pone.0010672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Averina OV, Alekseeva MG, Abilev SK, Il'in VK, Danilenko VN. 2013. Distribution of genes of toxin-antitoxin systems of mazEF and relBE families in bifidobacteria from human intestinal microbiota. Genetika 49:315–327. (In Russian.) [DOI] [PubMed] [Google Scholar]
- 33.Wei YX, Zhang ZY, Liu C, Zhu YZ, Zhu YQ, Zheng H, Zhao GP, Wang S, Guo XK. 2010. Complete genome sequence of Bifidobacterium longum JDM301. J Bacteriol 192:4076–4077. doi: 10.1128/JB.00538-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei YX, Ye L, Liu DB, Guo XK. 2014. A type II toxin-antitoxin system encoded by BLJ_864 and BLJ_865 in the chromosome of Bifidobacterium longum. J Shanghai Jiaotong Univ Med Sci 34:1319–1324. [Google Scholar]
- 35.Wei YX, Ye L, Li Y, Yang F, Liu DB, Guo XK, Tang RX, Liu C. 2016. Functional characterization of RelBE toxin-antitoxin system in probiotic Bifidobacterium longum JDM301. Acta Biochim Biophys Sin (Shanghai) 48:741–749. doi: 10.1093/abbs/gmw056. [DOI] [PubMed] [Google Scholar]
- 36.Barbosa LC, Garrido SS, Marchetto R. 2015. BtoxDB: a comprehensive database of protein structural data on toxin-antitoxin systems. Comput Biol Med 58:146–153. doi: 10.1016/j.compbiomed.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Lee HJ, Jin HM, Park MS, Park W, Madsen EL, Jeon CO. 2015. Recovery of plasmid pEMB1, whose toxin-antitoxin system stabilizes an ampicillin resistance-conferring β-lactamase gene in Escherichia coli, from natural environments. Appl Environ Microbiol 81:40–47. doi: 10.1128/AEM.02691-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaspy I, Rotem E, Weiss N, Ronin I, Balaban NQ, Glaser G. 2013. HipA-mediated antibiotic persistence via phosphorylation of the glutamyl-tRNA-synthetase. Nat Commun 4:3001. doi: 10.1038/ncomms4001. [DOI] [PubMed] [Google Scholar]
- 39.Tiwari P, Arora G, Singh M, Kidwai S, Narayan OP, Singh R. 2015. MazF ribonucleases promote Mycobacterium tuberculosis drug tolerance and virulence in guinea pigs. Nat Commun 6:6059. doi: 10.1038/ncomms7059. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Zhang J, Hara H, Kato I, Inouye M. 2005. Insights into the mRNA cleavage mechanism by MazF, an mRNA interferase. J Biol Chem 280:3143–3150. doi: 10.1074/jbc.M411811200. [DOI] [PubMed] [Google Scholar]
- 41.Syed MA, Koyanagi S, Sharma E, Jobin MC, Yakunin AF, Levesque CM. 2011. The chromosomally-encoded mazEFSmu locus of Streptococcus mutans encodes a functional type ii toxin-antitoxin addiction system. J Bacteriol 193:1122–1130. doi: 10.1128/JB.01114-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasari V, Mets T, Tenson T, Kaldalu N. 2013. Transcriptional cross-activation between toxin-antitoxin systems of Escherichia coli. BMC Microbiol 13:45. doi: 10.1186/1471-2180-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin J, Qin Q, Guo H, Liu S, Ge S, Zhang H, Cui J, Ren F. 2015. Effect of pre-stressing on the acid-stress response in Bifidobacterium revealed using proteomic and physiological approaches. PLoS One 10:e0117702. doi: 10.1371/journal.pone.0117702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemos JA, Brown TA Jr, Abranches J, Burne RA. 2005. Characteristics of Streptococcus mutans strains lacking the MazEF and RelBE toxin-antitoxin modules. FEMS Microbiol Lett 253:251–257. doi: 10.1016/j.femsle.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 45.Leplae R, Geeraerts D, Hallez R, Guglielmini J, Dreze P, Van Melderen L. 2011. Diversity of bacterial type II toxin-antitoxin systems: a comprehensive search and functional analysis of novel families. Nucleic Acids Res 39:5513–5525. doi: 10.1093/nar/gkr131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchi Y, Inouye M. 2011. Regulation of growth and death in Escherichia coli by toxin-antitoxin systems. Nat Rev Microbiol 9:779–790. doi: 10.1038/nrmicro2651. [DOI] [PubMed] [Google Scholar]
- 47.Heaton BE, Herrou J, Blackwell AE, Wysocki VH, Crosson S. 2012. Molecular structure and function of the novel BrnT/BrnA toxin-antitoxin system of Brucella abortus. J Biol Chem 287:12098–12110. doi: 10.1074/jbc.M111.332163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Averina O, Alekseeva M, Shkoporov A, Danilenko V. 2015. Functional analysis of the type II toxin-antitoxin systems of the MazEF and RelBE families in Bifidobacterium longum subsp. infantis ATCC 15697. Anaerobe 35:59–67. doi: 10.1016/j.anaerobe.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Huddleston JR. 2014. Horizontal gene transfer in the human gastrointestinal tract: potential spread of antibiotic resistance genes. Infect Drug Resist 7:167–176. doi: 10.2147/IDR.S48820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayes F, Van Melderen L. 2011. Toxins-antitoxins: diversity, evolution and function. Crit Rev Biochem Mol Biol 46:386–408. doi: 10.3109/10409238.2011.600437. [DOI] [PubMed] [Google Scholar]
- 51.Sharp PM, Cowe E, Higgins DG, Shields DC, Wolfe KH, Wright F. 1988. Codon usage patterns in Escherichia coli, Bacillus subtilis, Saccharomyces cerevisiae, Schizosaccharomyces pombe, Drosophila melanogaster and Homo sapiens; a review of the considerable within-species diversity. Nucleic Acids Res 16:8207–8211. doi: 10.1093/nar/16.17.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C[T]) method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 53.Akache B, MacPherson S, Sylvain MA, Turcotte B. 2004. Complex interplay among regulators of drug resistance genes in Saccharomyces cerevisiae. J Biol Chem 279:27855–27860. doi: 10.1074/jbc.M403487200. [DOI] [PubMed] [Google Scholar]
- 54.Sheu SJ, Hwang WZ, Chiang YC, Lin WH, Chen HC, Tsen HY. 2010. Use of tuf gene-based primers for the PCR detection of probiotic Bifidobacterium species and enumeration of bifidobacteria in fermented milk by cultural and quantitative real-time PCR methods. J Food Sci 75:M521–527. doi: 10.1111/j.1750-3841.2010.01816.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Y, Lin P, Li Q, Han L, Zheng H, Wei Y, Cui Z, Ni Y, Guo X. 2010. Analysis of the microbiota of sputum samples from patients with lower respiratory tract infections. Acta Biochim Biophys Sin (Shanghai) 42:754–761. doi: 10.1093/abbs/gmq081. [DOI] [PubMed] [Google Scholar]