Abstract
The main hallmarks of Alzheimer's disease (AD) are extracellular deposits of amyloid plaques and intracellular accumulation of hyperphosphorylated neurofibrillary tangles (tau). However, the mechanisms underlying these neuropathological changes remain largely unclear. To date, plenty of studies have shown that microglia-mediated neuroinflammation contributes to the pathogenesis of AD, and the microglia-synapse pathways have been repeatedly identified as the crucial factor in the disease process. In this review, evidences from microglia and synapse studies are presented, and the role of microglia in the pathogenesis of AD, the contributing factors to synapse dysfunction, and the role and mechanisms of microglia-synapse pathways will be discussed.
1. Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder in the elderly that is characterized by progressive cognitive dysfunction [1], synaptic degeneration, neuronal loss, accumulation of amyloid-beta (Aβ) and neurofibrillary tangles (tau), and increased neuroinflammation which are the hallmarks of AD in the brain [2, 3]. During the past 30 years, drug development for AD aimed at various potential targets has experienced tremendous, global setbacks, which have been persistent enough to make the efforts to find anti-AD drugs appear to be an ineffective strategy; for example, the failure rate of anti-Aβ drugs in clinical trials is approximately 100% [4–8]. Discovering new disease mechanisms and investigating the disease network of AD will aid in the identification of the pathogenesis of AD and potential treatments for this disease. Recently, many studies have shown that microglia-mediated neuroinflammation contributes to the pathogenesis of neurodegeneration and AD [9–12], and this neuroinflammation has been identified as a risk factor for AD [13–16], but the underlying mechanisms of neuroinflammation in AD remain unclear. It has been repeatedly shown that microglial activation is associated with synaptic dysfunction, and this response can have harmful consequences for neuronal function that may eventually lead to cognitive and behavioral deficits. Synaptic plasticity is one of the most important factors that regulates the formation of Aβ [17] and the long-term potentiation (LTP) pathways in AD. However, most studies have focused on the relationship between microglia and Aβ and tau, and little is known about microglial activities in early stage AD; microglia-synapse interactions especially need to be clarified. In this review, evidences from microglial studies are presented, and the role of microglia in the AD brain, as well as microglia-synapse pathways, will be discussed.
2. Microglia in Healthy and AD Brains
Microglia are the predominant immune cells in the brain, constantly surveying their microenvironment to detect potential threats and regulating the response to neuroinflammation by rapidly secreting signaling molecules, such as neurotransmitters, cytokines, and extracellular matrix proteins [18, 19]. In contrast to the healthy, young brain, the aging brain contains activated microglia accompanied by elevated levels of chemokines and cytokines, such as interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and Tumor Necrosis Factor-Alpha (TNF-α) [20]. Interestingly, microglia in the brains of aged mice exhibit a distinctly different expression profile and response to lipopolysaccharide (LPS) compared to those of young mice [21]. Upregulation of the major histocompatibility complex II (MHCII) and complement receptor 3 (CR3) in aging brains indicates the aggregation of microglia [22, 23]. The mechanism of these age-dependent changes in microglial function remains elusive. Since similar changes are observed in neurodegenerative disease, the mechanism needs to be studied intensively.
In addition to modulating the inflammatory responses in the brain, microglia also influence neuronal function [24, 25]. Studies have shown that microglia are highly dynamic and make efficient contact with neurons; they can also rapidly cruise throughout the whole brain parenchyma [26]. Microglia have receptors for both neurotransmitters and neuronal modulators, indicating that microglia can respond to neurons [27]. Microglia exert effects upon synapses and neuronal circuits by promoting synapse formation [28], as evidenced by the following findings: genetic depletion of brain-derived neurotrophic factor from microglia largely reduced synaptic spine formation [29] and genetic ablation of microglia decreases spine density, excitatory synapses, and relative connectivity in layer 4 neurons [30], which provides new insights into microglia-mediated neuronal circuit development, with potential implications for the development of brain dysfunction.
Previous research indicates that activated microglia can be categorized into a proinflammatory M1 phenotype and an immunosuppressive M2 phenotype. M2 microglia with Aβ phagocytic capability in APP/PS1 transgenic mice at 6 months of age can switch to M1 phenotypes at 18 months of age, in accordance with the levels of soluble Aβ oligomers [31]. Microglia in AD patients may exhibit mixed activation phenotypes. Colton et al. probed cortical tissue from patients with AD for markers of alternative activation and found increased Arginase-1 (Arg1), Cluster of Differentiation 206 (CD206), Chitinase-3-like protein 1 (Chi3 l1), Chitinase-3-like protein 2 (Chi3 l2), and TNF-α, with unchanged expression of inducible nitric oxide synthase (iNOS) and IL-1β [32]. An early decrease in mitochondrial uncoupling protein-2 (UCP2) was induced by the M1 stimulus LPS and also responds to M2 stimuli, as indicated by its persistent upregulation by IL-4. In UCP2-silenced microglia, IL-4 failed to induce the M2 genes mannose receptor 1 and IL-10 and reduce M1 genes TNF-α and iNOS, indicating that UCP2 is crucial in the microglial activation process with opposing regulation of M1 and M2 responses and the redirection of microglial response toward the protective phenotype in AD [33].
The inflammasome signaling pathway has been described in AD; Aβ can activate the nucleotide-binding and oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome in microglia [34], which is necessary for the secretion of proinflammatory cytokines. Recently, Heneka et al. demonstrated that the activation of the NLRP3 inflammasome plays a critical role in AD pathogenesis by mediating the chronic inflammatory response, while inhibition of NLRP3 almost completely protected against memory impairment and decreased Aβ deposition in APP/PS1/NLRP3−/− transgenic mice [35]. Interestingly, the activation of the cerebral, endogenous NLRP3 inflammasome was restricted to the microglia surrounding the plaques, and the microglial-specific disruption of the NLRP3 inflammasome skewed the microglial phenotypes toward M2, which could potentially reduce Aβ load and protect against cognitive decline [35], suggesting that microglia-specific activation of the NLRP3 inflammasome is important for AD pathogenesis. In addition, using microglia-specific atg7-deficient mice, researchers demonstrated that the Aβ-induced NLRP3 inflammasome is regulated by microglial autophagy associated with Aβ clearance, which suggests that a therapeutic strategy that enhances microglial autophagy could interfere with NLRP3-induced inflammation in AD [36].
Toll-like receptors (TLRs) are important molecules for initiating immune responses, and the expression of TLR4 has been verified on microglia [37]. TLR4 can mediate microglial activation, such as phagocyte activity, proinflammatory cytokine release, and ROS production, in response to C-terminal-truncated synuclein [38]. Current studies have demonstrated that microglial activation is dependent on TLR4 expression in AD [39]. TLR4 has specifically attracted attention in several inflammatory diseases, including cerebral diseases [40]. Both pharmacological inhibition of TLR4 and TLR4 knock-out in mice induce neuroprotection in experimental stroke [41, 42]. In neuroinflammatory conditions, there is release of endogenous galectin-3 (Gal3), which subsequently binds to and stimulates microglial TLR4 and induces the M1 phenotype in microglia in the brain [43]. Moreover, both activated microglia and Aβ oligomers are implicated in neuronal and cognitive dysfunction in AD. A recent study revealed that the anti-inflammatory drug indomethacin and an IL-1β-receptor antagonist prevented Aβ oligomer-mediated memory impairment. Administration of the TLR4-receptor antagonist or knock-out of the TLR4 gene in mice abolished the effects of Aβ oligomers on cognitive function, providing novel evidence of the crucial role of TLR4 in microglial-associated neuroinflammation and AD pathogenesis [44].
Recently, microRNAs (miRNAs) have emerged as novel, gene-regulatory elements during aging. miRNAs consist of 18–22 nucleotides and are noncoding RNAs that work posttranscriptionally to shape the cellular transcriptome. To date, more than 2500 human miRNAs have been identified, but only highly selective miRNAs appear to be enriched in the CNS. Understanding the functions of miRNAs in the brain will provide further insight into the process of neurodegenerative diseases, including AD. miR-124 is a key regulator of microglia quiescence in brain, and miR-124-knockdown microglia can activate themselves by inhibiting the transcription factor CCAAT/enhancer-binding protein-a (C/EBP-a) and its downstream target PU.1 [45]. Interestingly, a recent study demonstrated that miRNA-146a targets several inflammatory messenger RNAs, including those encoding complement factor-H, a crucial inhibitor of the cerebral inflammatory response [46]. Indeed, upregulation of miR-146a and concomitant downregulation of complement factor-H were observed in the brain of transgenic AD mice [47]. A potential role for miR-181c has been found in the regulation of one of its targets, TNF-α, in which the repressive function of miR-181c is attenuated by microglia-mediated neuronal apoptosis by suppressing TNF-α [48]. miR-27a was also observed to negatively modulate LPS-induced production of inflammatory cytokines such as IL-1β, IL-6, TNF-α, and nitric oxide in microglia, independent of TLR4 and interleukin-1 receptor-associated kinase 4 (IRAK4), suggesting that miR-27a is associated with microglial activation and the inflammatory response [49].
3. Microglia-Mediated Synapse Loss in AD
Most patients with AD have the sporadic form of the disease, which arises from defined genetic and external environmental factors. These factors do not directly affect Aβ formation but destroy the clearance mechanisms instead [50]. Similar to macrophages in CNS, microglia are mainly responsible for phagocytosis and clearance of cellular fragments or misfolded proteins, including Aβ and tau [51, 52], and research shows that microglia are dystrophic rather than activated in the AD-affected CNS [53]. While inflammatory processes regulated by the cerebral immune system are considered to be features of AD pathology, the role of the microglial response in AD has become an attractive area of research. To address the above knowledge gaps, researchers generated APP/PS1 mice deficient for interleukin-10, which is mainly secreted by microglia in central nervous system (CNS), and showed a striking exacerbation of amyloid pathology and cognitive impairment due to impaired microglial motility and phagocyte capacity [54].
Synapse loss is an early event in the course of AD, and the correlation between synapse density and the degree of cognitive impairment, as measured by the Mini Mental State Examination test and verbal fluency test, is well established in patients with AD [55, 56]. Interestingly, spatial learning and memory impairment, an early clinical feature of AD, is caused by synaptic dysfunction rather than neuronal loss in AD transgenic mice [57]. Functional and structural changes of synapses are thought to occur early in the pathogenesis of AD. Studies reported that synapse loss occurs in the dentate gyrus (DG) of the brains of AD patients [58, 59]. Other studies found that the synaptic densities in the DG of Tg2576 transgenic mice have increased [60] or decreased in APP/PS1 mice [61]. In a transgenic mouse model of AD, no changes in synaptophysin expression occur, but the outer molecular layer (OML) of the DG exhibits a significant decrease in synaptic density [61]. In addition, Aβ oligomers cause large dendritic spines to reduce their cytoplasm volume and simultaneously remodel their postsynaptic elements in the neuropil of APP/PS1 mice [62].
In an AD mouse model with Aβ deposits, researchers have noted that the soluble forms of amyloid oligomers have an important role in initiating the disease, possibly by targeting synapses [63]. Using two-photon microscopy (TPM) in vivo imaging, extensive synaptic abnormalities associated with dense core amyloid plaques were observed in mice crossbred from transgenic AD mice and mice expressing green fluorescent protein (GFP) in subsets of neurons [64]. Furthermore, in an AD mouse model of tauopathy, prominent microglial activation coincided with synapse loss in hippocampus and impaired synaptic function prior to the onset of fibrillary tau tangles [65, 66].
Mitochondria, the main source of reactive oxygen species (ROS), play a crucial role in the events leading to ROS release, and mitochondrial dysfunction can be one of the key initiating factors of AD [67]. According to the “mitochondrial cascade” hypothesis [68], decreased ATP synthesis and ROS detoxication result in the overproduction of Aβ and promote tau protein hyperphosphorylation, synapse dysfunction, and apoptosis, effectively exacerbating the neurodegenerative processes. The ensuing consequences of mitochondrial dysfunction are impairment of the cell membrane by free radicals and neuroinflammation, leading to synaptic transmission dysfunction, increased glutamate release from presynaptic terminals, and decreased plasticity in synaptic contacts [20, 69].
Imaging studies have shown that microglia constantly contact synapses and modify synaptic connections in the healthy brain [29, 70–72]. Abnormal microglia, especially with altered expression of immune-related receptors, cause synaptic and wiring dysfunction in the brain during development [71, 72], supporting the role of microglia in pruning synaptic connections. A recent study using the APP/PS1 transgenic mouse model discovered the existence of a new myeloid-cell phenotype, called “dark microglia,” which are distinct from the “normal” microglia described at the ultrastructural level. Dark microglia are rarely present in the healthy brain but are abundant in the brains of APP/PS1 transgenic mice. They strongly engage in the engulfment of dendritic spines, axon, and synapses, which indicates that they participate in the remodeling of neuronal circuits in AD [73]. Dark microglia stain for IBA1 and GFP in the brain tissue of fractalkine receptor 1- (CX3CR1-) GFP mice and strongly express CR3 and microglia-specific 4D4 in the processes that engulf synaptic elements [74]. Identification of key markers of dark microglia is a topic worthy of further investigation, as these cells may mediate synapse degeneration in the pathogenesis of AD.
4. Possible Mechanisms of the Microglia-Synapse Pathways in AD
Microglial interactions with synapses in AD obviously affect the maturation of synapses and neuronal viability; elucidating the possible mechanisms of microglia-synapse pathways may provide further insights into immune system regulation of neuronal circuit development in the AD brain and novel approaches for AD treatment.
Identifying genetic interactions in data obtained from genomewide association studies (GWAS) can help develop an understanding of the genetic changes in diseases. In recent years, GWAS have revealed a series of new susceptibility genes for AD, including ABCA7, BIN1, CR1, CD2AP, CD33, CASS4, CELF1, CLU, EPHA1, FERMT2, HLADRB5/HLA-DRB1, INPP5D, MS4A6A, MEF2C, NME8, PICALM, PTK2B, SLC24A4/RIN3, SORL1, TREM2, and ZCWPW1 [9, 10, 13, 75–82]. Among these genes, BIN1, CD33, and TREM2 are microglia-specific genes [83, 84], suggesting that microglia are strongly associated with AD pathogenesis.
BIN1, also known as bridging integrator-1, appears in the microglial signature in GWAS, indicating that it may affect the immune system in AD. Human Bin1/amphiphysin 2 and amphiphysin 1 are members of the amphiphysin family, and amphiphysin 1 and amphiphysin 2 share 50% amino-acid sequence homology. As the second-most prevalent susceptibility gene for late-onset AD, BIN1 has similar domain structure to amphiphysin 1 [85], which is strongly implicated in synaptic vesicle endocytosis [86].
CD33 is a transmembrane protein that regulates innate immunity in CNS. Increased expression of CD33 in microglia has been observed in the AD brain, and the numbers of CD33-positive microglia were positively correlated with insoluble Aβ42 levels, which is reversed in APPSwe/PS1DE9/CD33−/− mice [87], suggesting the potential of CD33 inhibition in microglia as a treatment for AD, but the relationship between CD33 and microglia-synapse interactions remains elusive.
TREM2, also referred to as triggering receptor expressed on myeloid cells 2, is uniquely expressed by microglia in the brain [88] and has been identified as a novel risk target for AD. Although the natural ligands of TREM2 are unknown, TREM2 activation was verified to regulate the activity of microglia, including cytokine secretion and autophagy [89], and to maintain microglial survival [90]. TREM2 overexpression reduced cognitive impairments and ameliorated neuronal and synaptic loss as well as tau hyperphosphorylation in P301S tau transgenic mice. Meanwhile, TREM2 reduced the expression of proinflammatory cytokines as well as the activity of tau kinase [91]. Interestingly, TREM2 overexpression in the brains of wild-type mice did not appear to improve spatial learning and memory, suggesting that the inhibition of neuronal and synaptic loss by TREM2 overexpression was likely caused by the ascending tau hyperphosphorylation pathway. In future studies, the effects of TREM2 on the synaptic pruning pathways in AD should be elucidated.
Elimination of abnormal synapses is critical for neuronal circuit development. A previous study [70] showed that microglia contribute to synaptic pruning during development, and PSD95 (postsynaptic component) or SNAP25 (presynaptic component) was coexpressed in microglia in the hippocampus, indicating that microglia engulf synaptic components. Fractalkine (CX3CL1)/CX3CR1 and the classical complement system are considered mediators of microglia interactions with synapses [70]. CX3CR1, specifically expressed on the surface of microglia, appears to play a crucial role in synaptic pruning. Fuhrmann et al. [92] crossed AD mice with transgenic mice (3XTg, CX3CR1-GFP, and Thy1-YFP) lacking the CX3CR1 receptor (3XTg:CX3CR1−/−:Thy1-YFP) and showed significant synapse loss in addition to attenuated microglial migratory velocity, indicating a critical role for the CX3CR1 chemokine receptor in microglia-mediated neuronal circuits in AD.
The classical complement proteins C1q and C3 localize to the synapse and mediate synapse elimination by microglia [93, 94]. C1q, the initiating protein of the classical complement cascade, was increased and associated with synapses before obvious plaque deposition in hAPP transgenic mice. Inhibition of C1q, C3, or the microglial CR3 reduces the number of microglia, as well as the extent of synapse loss in those mice at 3 to 4 months of age. Moreover, microglia in adult brains engulf synaptic material in a CR3-dependent process when exposed to soluble Aβ oligomers [25]. These findings suggest that the complement-dependent pathway and microglia (which prune synapses during development) mediate synapse loss in AD.
A very early loss of synaptic plasticity in vivo was reported in APP/PS1 transgenic mice; these mice exhibit defective ocular dominance plasticity (ODP) in visual cortex during the critical period in development [95]. This observation directly contrasts with results from mice lacking paired immunoglobulin-like receptor B (PirB) associated with synapses, in which ODP is enhanced during the development of the visual cortex [96]. A recent study showed that human PirB and its ortholog leukocyte immunoglobulin-like receptor B2 (LilrB2) are receptors for Aβ oligomers. Cofilin is recruited and activated by PirB in an Aβ-dependent manner in vivo and in vitro and is altered in the human AD frontal cortex. In mice, the deleterious effect of Aβ oligomers on long-term potentiation in hippocampus required PirB, and, in the APP/PS1 transgenic model of AD, PirB not only contributed to the memory deficits present in adult mice but also mediated loss of synaptic plasticity in the juvenile visual cortex [97]. PirB is also expressed in neurons [96] and modulates neurite outgrowth and neuronal plasticity by interacting with three axonal outgrowth inhibitors, Nogo, OMgp, and MAG. It was also shown that genetic deletion of PirB can dampen the inhibitory effects of myelin-associated inhibitory proteins (MAIs) [98]. Additionally, PirB mutant mice showed greater visual cortical plasticity following injury compared with control mice [99]. These findings suggest that PirB may play an inhibitory role in neurite growth following CNS injury. Therefore, it may be possible to enhance axonal regeneration, synaptic plasticity, and subsequent motor recovery by antagonizing PirB.
Mitochondrial dysfunction is one of the early features in the AD brain [100, 101]. Studies have highlighted the role of mitochondrial Aβ accumulation in the pathogenesis of AD. Accumulation of Aβ in the mitochondria precedes the extracellular Aβ deposition in the AD brain and increases with age and is also associated with early onset of loss of synapses, synaptic damage, and mitochondrial oxidative damage [102–104]. CcO is a key enzyme involved in complex IV of the mitochondrial respiratory electron transport chain. CcO reduction is well documented at various stages of AD, including the early mild cognitive-impairment stage [105, 106]. A recent study found a significant reduction in CcO activity in transgenic mAPP mice at the age of 8-9 months compared to wild-type mice. Similarly, ATP levels were significantly reduced in transgenic mAPP mice, indicating dysfunctional mitochondria and energy metabolism, and mitochondrial dysfunction also exacerbates learning and memory deficits, as demonstrated by mAPP mice with impairment of spatial learning and memory [107].
5. Microglia-Synapse Interactions in the Early Stages of AD
Synapse degeneration is an early, important event in AD; however, whether microglia participate in these early stages of AD pathogenesis and interact with synaptic dysfunction remains unclear. Hong et al. have demonstrated that resident microglia in the adult CNS phagocytose synapses when challenged by oligomeric Aβ. Interestingly, depending on microglial CR3 expression, microglia act as early mediators of synapse degeneration or loss that occur in AD mice at 3 to 4 months of age, prior to plaque deposition, providing new evidence for how synapses are lost in early stage of AD. Microglia could be a potential early therapeutic target in AD involving synaptic loss and cognitive impairment.
Increased microglial activation and neuroinflammation appear to accompany both Aβ and tau pathology [108, 109]. Since TREM2 is prominently involved in the activation of microglia, soluble TREM2 (sTREM2), which can be detected in cerebrospinal fluid (CSF), is a potential candidate marker for tracking the progression of AD. Some studies [110, 111] measured CSF sTREM2 in AD patients and found that sTREM2 is slightly increased. Suárez-Calvet et al. [112] measured the level of CSF TREM2 in subjects with preclinical AD, mild cognitive impairment due to AD (MCI-AD), and AD case-controls and found that the levels of CSF sTREM2 dynamically change during the AD process and peak at MCI-AD patients. Similarly, Matarin et al. [113] found that the gene expression of TREM2, accompanied by microglia activation, is significantly increased at 18 months in AD mice with tau pathology. Another interesting finding is that the increased CSF sTREM2 levels in these tauopathy mice were associated with higher CSF phospho-tau, which significantly contributed to synaptic degeneration [114]. Based on these findings, it can be anticipated that sTREM2 probably reflects a corresponding change in microglial function in response to synaptic degeneration in the early stage of AD.
It will be very helpful to learn whether microglia principally participate in the plaque formation or tau-related pathology of AD; however, it is generally confirmed that microglia-associated neuroinflammation may directly contribute to the initiation of synaptic and AD-like cognitive impairment. Therefore, it is time to focus on the investigation of the early stage AD processes and mechanisms of the microglia-synapse pathway that contribute to the pathogenesis of AD.
6. Microglia-Synapse Pathways for AD Therapy
The mechanisms underlying the neuropathology of AD remain unclear. Current therapeutic strategies for AD are mainly limited to ineffective symptomatic treatments, but some of the available disease-modifying drugs also perform poorly. Among the disease-modifying drugs, most of the trials involve passive immunosuppression. However, so far, nearly all of the clinical trials have failed, which leads to the initiation of clinical trials targeting patients at the presymptomatic or early stages of AD. Increasingly, microglia-synapse pathways have been confirmed as a promising strategy for early stage AD treatment.
It has been demonstrated that depletion of NLRP3 could significantly suppress amyloidosis and neuropathology, as well as improve cognitive function in AD mice [35], indicating that the NLRP3 inflammasome is a possible molecular target for neuroprotection and therapeutic intervention in AD. Agents for handling the activation of the NLRP3 inflammasome in microglia may offer considerable promise to block neuroinflammation and slow the progression of AD. The type 2 diabetes drug glyburide also prevents activation of the NLRP3 inflammasome [115]. More importantly, the antimalarial drug artemisinin was recently shown to exert protective effects in AD pathology via inhibiting the activation of NF-κB and the NLRP3 inflammasome in AD mice [116], further verifying that targeting the NLRP3 inflammasome may offer a crucial intervention for AD progression.
Mdivi-1, a noncompetitive inhibitor of Drp1 GTPase activity that attenuates Drp1-mediated mitochondrial-fission and responds to the stimulation of proapoptotic signaling [117], has been developed as a therapeutic agent for AD. Mdivi-1 also partially rescues the mitochondrial damage due to inactivation of PTEN-induced putative kinase 1 (PINK1)/Parkin pathway, which modulates mitophagy [118]. AD-related mitochondrial stress effectively triggers Parkin-dependent mitophagy, which establishes the need for further investigation of the regulation of mitophagy to potentially ameliorate mitochondrial dysfunction within synapses in AD. Future research should focus on targeting therapeutics toward preserving mitochondrial function as a treatment for AD.
A recent study showed that synaptic dysfunction in cognitively normal AD mice was due to proinflammatory factors [119]. It has also been reported that maze performance and dendritic spine density improved following the removal of most microglia [120] or one subset of dysfunctional microglia [121] in AD mice by pharmacological inhibition of colony-stimulating factor 1 receptor (CSFR1) for 1 month. Since elimination of microglia was shown to impair learning [29], inducing normal microglia may be a more effective therapeutic strategy. Microglia can self-renew without peripheral aid [122], and the repopulated microglial niche may be functional and could potentially provide further benefit in AD brains, especially if the microglia have never encountered Aβ, tau, or other AD-related factors. Moreover, improving microglial phagocytosis via PPAR-receptor agonism-dependent induction of CD36 was found to stimulate Aβ clearance and enhance spatial memory [123]. However, not all phagocytosis is inherently good or bad, and it is important to simultaneously prevent C1qa-dependent synapse loss while promoting Aβ phagocytosis [25].
There is still no available therapy that can successfully reverse AD, but the current evidence suggests that the best treatment for AD may consist of a therapeutic strategy combined with anti-inflammatory factors. Therapy for AD faces many challenges with respect to timing and efficacy, and targeting the proinflammatory mediators may not be effective. However, early stage intervention targeting dysregulated proinflammatory mediators might be therapeutically beneficial. A small molecule named MW-151 has been tested during two distinct therapeutic temporal windows at the early stage of the disease in APP/PS1 transgenic mice. MW-151 treatment attenuates microglial activation and the production of proinflammatory cytokines in the cortex, which protects against the synaptic dysfunction implicated in learning and memory deficits [124]. Targeting the balance of M1/M2 phenotypes in microglia depends on the temporal window, since the stages of AD are associated with changes in the microglial activation states. Anti-inflammatory drugs will need the ability to access the CNS, and the chemicals fasudil [125] and minocycline [126] both have the ability to effectively cross the blood-brain barrier, which was demonstrated to enhance the anti-inflammatory abilities of M2 microglia. Recently, replacement of diseased neurons with new neurons derived from embryonic stem cells or pluripotent stem cells was shown to have a beneficial clinical outcome.
Promisingly, nervous system growth factors can prevent neuronal loss in AD animal models. Previous studies revealed that nerve growth factor (NGF) can stimulate cholinergic neurons associated with cognition and prevent their impairment in early stage AD. However, systemic administration of NGF can cause adverse side effects, requiring a targeted delivery strategy to control the localization and spread in the brain. In a phase I trial, NGF was verified to provide beneficial effects over a two-year observation period. Another phase I clinical trial enrolled 10 patients who received AAV2-NGF. NGF induced axonal sprouting toward the NGF, lowered the rate of cognitive decline, and increased cortical glucose uptake. Glial cell-derived neurotrophic factor (GDNF) is a neurotrophic factor with therapeutic potential for AD. It was reported that 3xTg-AD mice showed preserved learning and memory with 6 months of GDNF overexpression. GDNF therapy did not significantly reduce amyloid or tau pathology, but it upregulated the expression of BDNF and induced neuroprotection. In mice with Atg7-deficient microglia, the loss of autophagy in early developmental microglia impaired synaptic pruning and increased dendritic spine density, suggesting that microglial autophagy has an important role in regulating synaptic homeostasis and neuropsychological behaviors. IL-4 has also been characterized as a potential modulator of neuronal activities in the brain. IL-4 receptors are expressed in the hippocampus, and downregulation of IL-4 causes aging-related deficits in hippocampal LTP. Anti-inflammatory cytokines such as IL-10 may also have significant therapeutic potential for the treatment of AD. Neuronal expression of the mouse IL-10 gene ameliorates cognitive dysfunction in APP/PS1 transgenic mice [127].
7. Perspectives
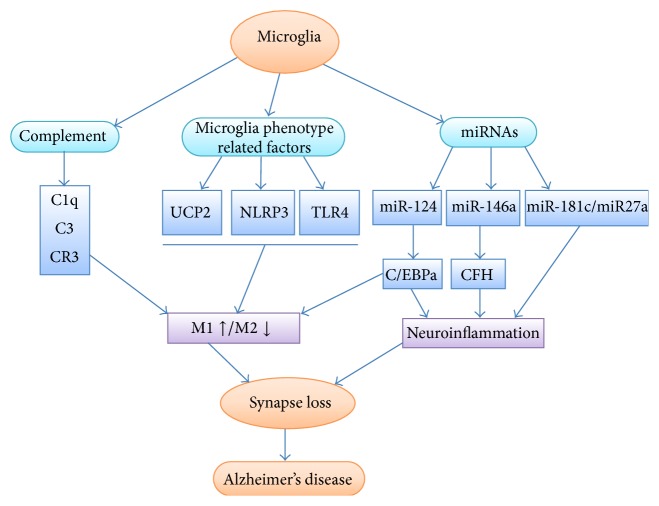
Recently, in the AD etiology literature, considerable attention has been paid to the emerging specialized functions of microglia in regulating synaptic development and degeneration, as synapse loss is the most directly relevant pathology to the development of cognitive deficits in AD. As shown in Figure 1, many studies have repeatedly identified the microglia-synapse pathway as the crucial factor in AD pathogenesis. In future studies, how microglia respond to their environment and switch their phenotypes following the early stages of an insult should be determined in both the infant and aging brain, because current evidence shows that microglia with normal cellular homeostasis become aberrant and dysregulated during the aging process of the CNS, resulting in an increased susceptibility to subsequent immune challenges. In addition, genetic approaches should be applied to explore key targets contributing to the connections or activation of the microglia-synapse pathways to achieve early prevention and a potential cure for AD.
Figure 1.
Schematic representation of possible mechanisms of the microglia-synapse pathways in AD. Microglial phenotype could switch from an immunosuppressive M2 phenotype into a proinflammatory M1 phenotype through complement pathways and several crucial factors, such as UCP2, NLRP3, and TLR4. The phenotype transition and neuroinflammation by related miRNAs induce synapse loss in AD pathogenesis.
Acknowledgments
This study was supported by grants from the Natural Science Foundation of Guangdong Province, China (no. 2015A030310038; no. 2014A030310106).
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Jingdun Xie and Haitao Wang contributed equally to this work.
References
- 1.Sloane P. D., Zimmerman S., Suchindran C., et al. The public health impact of Alzheimer's disease, 2000–2050: potential implication of treatment advances. Annual Review of Public Health. 2002;23:213–231. doi: 10.1146/annurev.publhealth.23.100901.140525. [DOI] [PubMed] [Google Scholar]
- 2.Querfurth H. W., LaFerla F. M. Alzheimer's disease. New England Journal of Medicine. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 3.Rubio-Perez J. M., Morillas-Ruiz J. M. A review: inflammatory process in Alzheimer's disease, role of cytokines. The Scientific World Journal. 2012;2012:15. doi: 10.1100/2012/756357.756357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemere C. A., Masliah E. Can Alzheimer disease be prevented by amyloid-Β immunotherapy? Nature Reviews Neurology. 2010;6(2):108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicoll J., Boche D., Holmes C. Amyloid-β vaccination for Alzheimer's dementia. The Lancet. 2008;372(9647):1381–1382. doi: 10.1016/s0140-6736(08)61580-9. [DOI] [Google Scholar]
- 6.Vellas B. Tarenflurbil for Alzheimer's disease: a ‘shot on goal’ that missed. The Lancet Neurology. 2010;9(3):235–237. doi: 10.1016/s1474-4422(10)70030-2. [DOI] [PubMed] [Google Scholar]
- 7.Salloway S., Sperling R., Fox N. C., et al. Two phase 3 trials of Bapineuzumab in mild-to-moderate Alzheimer's disease. New England Journal of Medicine. 2014;370(4):322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doody R. S., Thomas R. G., Farlow M., et al. Phase 3 trials of solanezumab for mild-to-moderate alzheimer's disease. New England Journal of Medicine. 2014;370(4):311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. GLIA. 2013;61(1):71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 10.Bodea L.-G., Wang Y., Linnartz-Gerlach B., et al. Neurodegeneration by activation of the microglial complement-phagosome pathway. Journal of Neuroscience. 2014;34(25):8546–8556. doi: 10.1523/JNEUROSCI.5002-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mhatre S. D., Tsai C. A., Rubin A. J., James M. L., Andreasson K. I. Microglial malfunction: the third rail in the development of Alzheimer's disease. Trends in Neurosciences. 2015;38(10):621–636. doi: 10.1016/j.tins.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J. H., Cheng X. R., Zhang X. R., et al. Neuroendocrine immunomodulation network dysfunction in SAMP8 mice and PrP-hAbetaPPswe/PS1DeltaE9 mice: potential mechanism underlying cognitive impairment. Oncotarget. 2016;7(17):22988–23005. doi: 10.18632/oncotarget.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert J. C., Heath S., Even G., et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nature Genetics. 2009;41(10):1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 14.Hollingworth P., Harold D., Sims R., et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nature Genetics. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naj A. C., Jun G., Beecham G. W., et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nature Genetics. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonsson T., Stefansson H., Steinberg S., et al. Variant of TREM2 associated with the risk of Alzheimer's disease. The New England Journal of Medicine. 2013;368(2):107–116. doi: 10.1056/nejmoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng X., Wu J., Geng M., Xiong J. The role of synaptic activity in the regulation of amyloid beta levels in Alzheimer's disease. Neurobiology of Aging. 2014;35(6):1217–1232. doi: 10.1016/j.neurobiolaging.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Nimmerjahn A., Kirchhoff F., Helmchen F. Neuroscience: resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 19.Norden D. M., Godbout J. P. Review: Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathology and Applied Neurobiology. 2013;39(1):19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sierra A., Gottfried-Blackmore A. C., Mcewen B. S., Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55(4):412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- 21.Holtman I. R., Raj D. D., Miller J. A., et al. Induction of a common microglia gene expression signature by aging and neurodegenerative conditions: a co-expression meta-analysis. Acta Neuropathologica Communications. 2015;3(1):p. 31. doi: 10.1186/s40478-015-0203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrientos R. M., Higgins E. A., Biedenkapp J. C., et al. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiology of Aging. 2006;27(5):723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 23.VanGuilder H. D., Bixler G. V., Brucklacher R. M., et al. Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. Journal of Neuroinflammation. 2011;8, article 138 doi: 10.1186/1742-2094-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heppner F. L., Ransohoff R. M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nature Reviews Neuroscience. 2015;16(6):358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 25.Hong S., Beja-Glasser V. F., Nfonoyim B. M., et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schafer D. P., Lehrman E. K., Stevens B. The ‘quad-partite’ synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61(1):24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kettenmann H., Hanisch U.-K., Noda M., Verkhratsky A. Physiology of microglia. Physiological Reviews. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 28.Ueno M., Yamashita T. Bidirectional tuning of microglia in the developing brain: from neurogenesis to neural circuit formation. Current Opinion in Neurobiology. 2014;27:8–15. doi: 10.1016/j.conb.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Parkhurst C. N., Yang G., Ninan I., et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyamoto A., Wake H., Ishikawa A. W., et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nature Communications. 2016;7, article 12540 doi: 10.1038/ncomms12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jimenez S., Baglietto-Vargas D., Caballero C., et al. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer's disease: age-dependent switch in the microglial phenotype from alternative to classic. The Journal of Neuroscience. 2008;28(45):11650–11661. doi: 10.1523/jneurosci.3024-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colton C. A., Mott R. T., Sharpe H., Xu Q., Van Nostrand W. E., Vitek M. P. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. Journal of Neuroinflammation. 2006;3, article no. 27 doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Simone R., Ajmone-Cat M. A., Pandolfi M., et al. The mitochondrial uncoupling protein-2 is a master regulator of both M1 and M2 microglial responses. Journal of Neurochemistry. 2015;135(1):147–156. doi: 10.1111/jnc.13244. [DOI] [PubMed] [Google Scholar]
- 34.Halle A., Hornung V., Petzold G. C., et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nature Immunology. 2008;9(8):857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heneka M. T., Kummer M. P., Stutz A., et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho M.-H., Cho K., Kang H.-J., et al. Autophagy in microglia degrades extracellular β-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy. 2014;10(10):1761–1775. doi: 10.4161/auto.29647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lehnardt S., Massillon L., Follett P., et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fellner L., Irschick R., Schanda K., et al. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. GLIA. 2013;61(3):349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song M., Jin J., Lim J.-E., et al. TLR4 mutation reduces microglial activation, increases Aβ deposits and exacerbates cognitive deficits in a mouse model of Alzheimer's disease. Journal of Neuroinflammation. 2011;8, article 92 doi: 10.1186/1742-2094-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buchanan M. M., Hutchinson M., Watkins L. R., Yin H. Toll-like receptor 4 in CNS pathologies. Journal of Neurochemistry. 2010;114(1):13–27. doi: 10.1111/j.1471-4159.2010.06736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hyakkoku K., Hamanaka J., Tsuruma K., et al. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171(1):258–267. doi: 10.1016/j.neuroscience.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki Y., Hattori K., Hamanaka J., et al. Pharmacological inhibition of TLR4-NOX4 signal protects against neuronal death in transient focal ischemia. Scientific Reports. 2012;2, article 896 doi: 10.1038/srep00896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burguillos M., Svensson M., Schulte T., et al. Microglia-secreted galectin-3 acts as a toll-like receptor 4 ligand and contributes to microglial activation. Cell Reports. 2015;10(9):1626–1638. doi: 10.1016/j.celrep.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Balducci C., Frasca A., Zotti M., et al. Toll-like receptor 4-dependent glial cell activation mediates the impairment in memory establishment induced by β-amyloid oligomers in an acute mouse model of Alzheimer's disease. Brain, Behavior, and Immunity. 2017;60:188–197. doi: 10.1016/j.bbi.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Ponomarev E. D., Veremeyko T., Barteneva N., Krichevsky A. M., Weiner H. L. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nature Medicine. 2011;17(1):64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y. Y., Cui J. G., Dua P., Pogue A. I., Bhattacharjee S., Lukiw W. J. Differential expression of miRNA-146a-regulated inflammatory genes in human primary neural, astroglial and microglial cells. Neuroscience Letters. 2011;499(2):109–113. doi: 10.1016/j.neulet.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y. Y., Cui J. G., Hill J. M., Bhattacharjee S., Zhao Y., Lukiw W. J. Increased expression of miRNA-146a in Alzheimer's disease transgenic mouse models. Neuroscience Letters. 2011;487(1):94–98. doi: 10.1016/j.neulet.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L., Dong L.-Y., Li Y.-J., Hong Z., Wei W.-S. The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor. Journal of Neuroinflammation. 2012;9, article 211 doi: 10.1186/1742-2094-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lv Y.-N., Ou-yang A.-J., Fu L.-S. MicroRNA-27a negatively modulates the inflammatory response in lipopolysaccharide-stimulated microglia by targeting TLR4 and IRAK4. Cellular and Molecular Neurobiology. 2016:1–16. doi: 10.1007/s10571-016-0361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mawuenyega K. G., Sigurdson W., Ovod V., et al. Decreased clearance of CNS β-amyloid in Alzheimer's disease. Science. 2010;330(6012):p. 1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grathwohl S. A., Kälin R. E., Bolmont T., et al. Formation and maintenance of Alzheimer's disease β-amyloid plaques in the absence of microglia. Nature Neuroscience. 2009;12(11):1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shibuya Y., Niu Z., Bryleva E. Y., et al. Acyl-coenzyme A: CHolesterol acyltransferase 1 blockage enhances autophagy in the neurons of triple transgenic Alzheimer's disease mouse and reduces human P301L-tau content at the presymptomatic stage. Neurobiology of Aging. 2015;36(7):2248–2259. doi: 10.1016/j.neurobiolaging.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Streit W. J., Braak H., Xue Q.-S., Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathologica. 2009;118(4):475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guillot-Sestier M.-V., Doty K. R., Gate D., et al. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron. 2015;85(3):534–548. doi: 10.1016/j.neuron.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheff S. W., Price D. A., Schmitt F. A., Scheff M. A., Mufson E. J. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer's disease. Journal of Alzheimer's Disease. 2011;24(3):547–557. doi: 10.3233/JAD-2011-101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheff S. W., Price D. A., Ansari M. A., et al. Synaptic change in the posterior cingulate gyrus in the progression of Alzheimer's disease. Journal of Alzheimer's Disease. 2015;43(3):1073–1090. doi: 10.3233/JAD-141518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chapman P. F., White G. L., Jones M. W., et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nature Neuroscience. 1999;2(3):271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 58.Scheff S. W., Sparks D. L., Price D. A. Quantitative assessment of synaptic density in the outer molecular layer of the hippocampal dentate gyrus in Alzheimer's disease. Dementia. 1996;7(4):226–232. doi: 10.1159/000106884. [DOI] [PubMed] [Google Scholar]
- 59.Masliah E., Crews L., Hansen L. Synaptic remodeling during aging and in Alzheimer's disease. Journal of Alzheimer's Disease. 2006;9(3):91–99. doi: 10.3233/jad-2006-9s311. [DOI] [PubMed] [Google Scholar]
- 60.King D. L., Arendash G. W. Maintained synaptophysin immunoreactivity in Tg2576 transgenic mice during aging: correlations with cognitive impairment. Brain Research. 2002;926(1-2):58–68. doi: 10.1016/s0006-8993(01)03294-2. [DOI] [PubMed] [Google Scholar]
- 61.Dong H., Martin M. V., Chambers S., Csernansky J. G. Spatial relationship between synapse loss and β-amyloid deposition in Tg2576 mice. Journal of Comparative Neurology. 2007;500(2):311–321. doi: 10.1002/cne.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alonso-Nanclares L., Merino-Serrais P., Gonzalez S., DeFelipe J. Synaptic changes in the dentate gyrus of APP/PS1 transgenic mice revealed by electron microscopy. Journal of Neuropathology and Experimental Neurology. 2013;72(5):386–395. doi: 10.1097/nen.0b013e31828d41ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lacor P. N., Buniel M. C., Chang L., et al. Synaptic targeting by Alzheimer's-related amyloid β oligomers. Journal of Neuroscience. 2004;24(45):10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spires T. L., Meyer-Luehmann M., Stern E. A., et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. Journal of Neuroscience. 2005;25(31):7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshiyama Y., Higuchi M., Zhang B., et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53(3):337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 66.Ittner L. M., Ke Y. D., Delerue F., et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 67.Ridge P. G., Ebbert M. T. W., Kauwe J. S. K. Genetics of Alzheimer's disease. BioMed Research International. 2013;2013:13. doi: 10.1155/2013/254954.254954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swerdlow R. H., Burns J. M., Khan S. M. The Alzheimer's disease mitochondrial cascade hypothesis: progress and perspectives. Biochimica et Biophysica Acta—Molecular Basis of Disease. 2014;1842(8):1219–1231. doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kilbride S. M., Telford J. E., Tipton K. F., Davey G. P. Partial inhibition of complex I activity increases Ca2+-independent glutamate release rates from depolarized synaptosomes. Journal of Neurochemistry. 2008;106(2):826–834. doi: 10.1111/j.1471-4159.2008.05441.x. [DOI] [PubMed] [Google Scholar]
- 70.Paolicelli R. C., Bolasco G., Pagani F., et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 71.Ueno M., Fujita Y., Tanaka T., et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nature Neuroscience. 2013;16(5):543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- 72.Squarzoni P., Oller G., Hoeffel G., et al. Microglia modulate wiring of the embryonic forebrain. Cell Reports. 2014;8(5):1271–1279. doi: 10.1016/j.celrep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 73.Audoy-Rémus J., Bozoyan L., Dumas A., et al. GPR84 deficiency reduces microgliosis, but accelerates dendritic degeneration and cognitive decline in a mouse model of Alzheimer's disease. Brain, Behavior, and Immunity. 2015;46:112–120. doi: 10.1016/j.bbi.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 74.Bisht K., Sharma K. P., Lecours C., et al. Dark microglia: a new phenotype predominantly associated with pathological states. Glia. 2016;64(5):826–839. doi: 10.1002/glia.22966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seshadri S., Fitzpatrick A. L., Ikram M. A., et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. The Journal of the American Medical Association. 2010;303(18):1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lambert J. C., Ibrahim-Verbaas C. A., Harold D., et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nature Genetics. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benitez B. A., Cooper B., Pastor P., et al. TREM2 is associated with the risk of Alzheimer's disease in Spanish population. Neurobiology of Aging. 2013;34(6):1711.e15–1711.e17. doi: 10.1016/j.neurobiolaging.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giraldo M., Lopera F., Siniard A. L., et al. Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer's disease. Neurobiology of Aging. 2013;34(8):2077.e11–2077.e18. doi: 10.1016/j.neurobiolaging.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonzalez Murcia J. D., Schmutz C., Munger C., et al. Assessment of Trem2 Rs75932628 association with Alzheimer's disease in a population-based sample: the cache county study. Neurobiology of Aging. 2013;34(12):e2811–2883. doi: 10.1016/j.neurobiolaging.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guerreiro R., Wojtas A., Bras J., et al. TREM2 variants in Alzheimer's disease. New England Journal of Medicine. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pottier C., Wallon D., Rousseau S., et al. TREM2 R47H variant as a risk factor for early-onset Alzheimer's disease. Journal of Alzheimer's Disease. 2013;35(1):45–49. doi: 10.3233/jad-122311. [DOI] [PubMed] [Google Scholar]
- 82.Cuyvers E., Bettens K., Philtjens S., et al. Investigating the role of rare heterozygous TREM2 variants in Alzheimer's disease and frontotemporal dementia. Neurobiology of Aging. 2014;35(3):726.e11–726.e19. doi: 10.1016/j.neurobiolaging.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 83.Hickman S. E., Kingery N. D., Ohsumi T. K., et al. The microglial sensome revealed by direct RNA sequencing. Nature Neuroscience. 2013;16(12):1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Butovsky O., Jedrychowski M. P., Moore C. S., et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nature Neuroscience. 2014;17(1):131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Owen D. J., Wigge P., Vallis Y., Moore J. D. A., Evans P. R., McMahon H. T. Crystal structure of the amphiphysin-2 SH3 domain and its role in the prevention of dynamin ring formation. The EMBO Journal. 1998;17(18):5273–5285. doi: 10.1093/emboj/17.18.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prokic I., Cowling B. S., Laporte J. Amphiphysin 2 (BIN1) in physiology and diseases. Journal of Molecular Medicine. 2014;92(5):453–463. doi: 10.1007/s00109-014-1138-1. [DOI] [PubMed] [Google Scholar]
- 87.Griciuc A., Serrano-Pozo A., Parrado A. R., et al. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78(4):631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frank S., Burbach G. J., Bonin M., et al. TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia. 2008;56(13):1438–1447. doi: 10.1002/glia.20710. [DOI] [PubMed] [Google Scholar]
- 89.Zhong L., Chen X.-F., Zhang Z.-L., et al. DAP12 stabilizes the C-terminal fragment of the triggering receptor expressed on myeloid cells-2 (TREM2) and protects against LPS-induced pro-inflammatory response. Journal of Biological Chemistry. 2015;290(25):15866–15877. doi: 10.1074/jbc.M115.645986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y., Cella M., Mallinson K., et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell. 2015;160(6):1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang T., Zhang Y.-D., Chen Q., et al. TREM2 modifies microglial phenotype and provides neuroprotection in P301S tau transgenic mice. Neuropharmacology. 2016;105:196–206. doi: 10.1016/j.neuropharm.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 92.Fuhrmann M., Bittner T., Jung C. K. E., et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nature Neuroscience. 2010;13(4):411–413. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schafer D. P., Lehrman E. K., Kautzman A. G., et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bialas A. R., Stevens B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nature Neuroscience. 2013;16(12):1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.William C. M., Andermann M. L., Goldey G. J., et al. Synaptic plasticity defect following visual deprivation in Alzheimer's disease model transgenic mice. Journal of Neuroscience. 2012;32(23):8004–8011. doi: 10.1523/JNEUROSCI.5369-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Syken J., GrandPre T., Kanold P. O., Shatz C. J. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313(5794):1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 97.Kim T., Vidal G. S., Djurisic M., et al. Human LilrB2 is a β-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer's model. Science. 2013;341(6152):1399–1404. doi: 10.1126/science.1242077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Atwal J. K., Pinkston-Gosse J., Syken J., et al. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322(5903):967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 99.Datwani A., McConnell M. J., Kanold P. O., et al. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64(4):463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin M. T., Beal M. F. Alzheimer's APP mangles mitochondria. Nature Medicine. 2006;12(11):1241–1243. doi: 10.1038/nm1106-1241. [DOI] [PubMed] [Google Scholar]
- 101.Reddy P. H. Mitochondrial medicine for aging and neurodegenerative diseases. NeuroMolecular Medicine. 2008;10(4):291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Du H., Guo L., Yan S., Sosunov A. A., McKhann G. M., Yan S. S. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(43):18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lustbader J. W., Cirilli M., Lin C., et al. ABAD directly links Aβ to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304(5669):448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 104.Oddo S., Caccamo A., Shepherd J. D., et al. Triple-transgenic model of Alzheimer's Disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 105.Alikhani N., Guo L., Yan S., et al. Decreased proteolytic activity of the mitochondrial amyloid-β degrading enzyme, PreP peptidasome, in alzheimer's disease brain mitochondria. Journal of Alzheimer's Disease. 2011;27(1):75–87. doi: 10.3233/JAD-2011-101716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valla J., Schneider L., Niedzielko T., et al. Impaired platelet mitochondrial activity in Alzheimer's disease and mild cognitive impairment. Mitochondrion. 2006;6(6):323–330. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fang D., Zhang Z., Li H., et al. Increased electron paramagnetic resonance signal correlates with mitochondrial dysfunction and oxidative stress in an Alzheimer's disease mouse brain. Journal of Alzheimer's Disease. 2016;51(2):571–580. doi: 10.3233/JAD-150917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heneka M. T., Carson M. J., Khoury J. E., et al. Neuroinflammation in Alzheimer's disease. The Lancet Neurology. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mosher K. I., Wyss-Coray T. Microglial dysfunction in brain aging and Alzheimer's disease. Biochemical Pharmacology. 2014;88(4):594–604. doi: 10.1016/j.bcp.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Heslegrave A., Heywood W., Paterson R., et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer's disease. Molecular Neurodegeneration. 2016;11(1, article 3) doi: 10.1186/s13024-016-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Piccio L., Deming Y., Del-Águila J. L., et al. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathologica. 2016;131(6):925–933. doi: 10.1007/s00401-016-1533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suárez-Calvet M., Kleinberger G., Araque Caballero M. Á., et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer's disease and associate with neuronal injury markers. EMBO Molecular Medicine. 2016;8(5):466–476. doi: 10.15252/emmm.201506123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matarin M., Salih D. A., Yasvoina M., et al. A Genome-wide gene-expression analysis and database in transgenic mice during development of amyloid or tau pathology. Cell Reports. 2015;10(4):633–645. doi: 10.1016/j.celrep.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 114.Kambe T., Motoi Y., Inoue R., et al. Differential regional distribution of phosphorylated tau and synapse loss in the nucleus accumbens in tauopathy model mice. Neurobiology of Disease. 2011;42(3):404–414. doi: 10.1016/j.nbd.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 115.Lamkanfi M., Mueller J. L., Vitari A. C., et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. Journal of Cell Biology. 2009;187(1):61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shi J.-Q., Zhang C.-C., Sun X.-L., et al. Antimalarial drug artemisinin extenuates amyloidogenesis and neuroinflammation in APPswe/PS1dE9 transgenic mice via inhibition of nuclear factor-κB and NLRP3 inflammasome activation. CNS Neuroscience and Therapeutics. 2013;19(4):262–268. doi: 10.1111/cns.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cassidy-Stone A., Chipuk J. E., Ingerman E., et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Developmental Cell. 2008;14(2):193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cui M., Tang X., Christian W. V., Yoon Y., Tieu K. Perturbations in mitochondrial dynamics induced by human mutant PINK1 can be rescued by the mitochondrial division inhibitor mdivi-1. Journal of Biological Chemistry. 2010;285(15):11740–11752. doi: 10.1074/jbc.M109.066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zou C., Shi Y., Ohli J., Schüller U., Dorostkar M. M., Herms J. Neuroinflammation impairs adaptive structural plasticity of dendritic spines in a preclinical model of Alzheimer’s disease. Acta Neuropathologica. 2016;131(2):235–246. doi: 10.1007/s00401-015-1527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spangenberg E. E., Lee R. J., Najafi A. R., et al. Eliminating microglia in Alzheimer's mice prevents neuronal loss without modulating amyloid-β pathology. Brain. 2016;139(4):1265–1281. doi: 10.1093/brain/aww016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Olmos-Alonso A., Schetters S. T. T., Sri S., et al. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer's-like pathology. Brain. 2016;139(3):891–907. doi: 10.1093/brain/awv379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bruttger J., Karram K., Wörtge S., et al. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity. 2015;43(1):92–107. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 123.Yamanaka M., Ishikawa T., Griep A., Axt D., Kummer M. P., Heneka M. T. PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. The Journal of Neuroscience. 2012;32(48):17321–17331. doi: 10.1523/jneurosci.1569-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bachstetter A. D., Norris C. M., Sompol P., et al. Early stage drug treatment that normalizes proinflammatory cytokine production attenuates synaptic dysfunction in a mouse model that exhibits age-dependent progression of alzheimer's disease-related pathology. Journal of Neuroscience. 2012;32(30):10201–10210. doi: 10.1523/JNEUROSCI.1496-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang H., Li Y., Yu J., et al. Rho kinase inhibitor fasudil regulates microglia polarization and function. NeuroImmunoModulation. 2013;20(6):313–322. doi: 10.1159/000351221. [DOI] [PubMed] [Google Scholar]
- 126.Kobayashi K., Imagama S., Ohgomori T., et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death and Disease. 2013;4(3, article e525) doi: 10.1038/cddis.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rossignol D. A., Frye R. E. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Frontiers in Physiology. 2014;5, article 150 doi: 10.3389/fphys.2014.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]