Abstract
Background
30-day readmissions for hospitalized patients with cirrhosis are common, particularly for patients with hepatic encephalopathy (HE).
Methods
We performed a prospective pre-post study from 2010–2013 to assess the impact of a quality improvement (QI) protocol on 30-day readmissions to a transplant center’s liver unit. The intervention included a yearlong control period, a handheld checklist and an “electronic” phase incorporating checklist items into the electronic provider order entry system. The intervention included goal-directed lactulose therapy and universal rifaximin for overt HE as well as prompts for antibiotic prophylaxis of spontaneous bacterial peritonitis (SBP). 30-day readmission trends were compared to non-cirrhotic patients admitted to hospitalists and patients with decompensated cirrhosis at another center.
Results
824 patients were admitted 1720 times. The average model for end-stage liver disease score on admission was 17.7 ± 7.4. The electronic phase was associated with 40% lower adjusted odds of 30-day readmission compared to control, both overall and for patients with HE. The proportion of admissions for ≥ grade 2 HE that resulted in a readmission fell from 48.9% (66/135) in the control period to 26.0% (27/104) in the electronic phase (p = 0.0003). Rifaximin use for HE and antibiotic secondary prophylaxis of SBP (on discharge) were associated with lower adjusted odds of readmission (respective OR 0.39 and 0.40). The electronic phase was associated with a lower length of stay (beta coefficient −1.34 95% CI: −2.38 – −0.32, p = 0.01).
Conclusion
A QI initiative using electronic decision support reduced 30-day readmissions for patients with cirrhosis.
Keywords: Model for Endstage Liver Disease, Hepatic Encephalopathy, Rifaximin, Lactulose
Introduction
The costs associated with hospitalization for patients with decompensated cirrhosis, particularly those with hepatic encephalopathy (HE), can be tremendous.(1–4) In 2009, complications of chronic liver disease accounted for more than 136,000 hospital discharges in the US, generating an aggregate cost of $2.04 billion dollars (2014 USD).(5) According to the Agency for Healthcare Research and Quality, there were 52,840 discharges with HE as the primary diagnosis in 2012, at an average cost per instance of $10,584.(6) Efforts to reduce these costs by reducing admissions may have a significant clinical and economic impact.
A significant driver of these costs is readmissions within 30-days after discharge. In both the US and Europe, the incidence of readmission within 7 days of discharge for patients with cirrhosis has been reported to be 14% and reaches 30–40% by 30 days.(3, 4, 7) Beyond costs, hospitalization for decompensated cirrhosis is associated with an important negative effect on quality of life.(8) Unsurprisingly, preventable readmissions are an increasing focus for clinicians, patients, payors and regulatory agencies alike.(9)
Given the morbidity, costs and frequency of readmissions, initiatives aimed at reducing the incidence of readmission for patients with cirrhosis are important. Predictors of readmission risk include markers of illness severity (e.g. Model for Endstage liver disease (MELD)), demographics (younger age, white race) and insurance status.(3, 10) Unfortunately, there is little evidence to support specific interventions to reduce readmissions.(11)
We therefore implemented and studied an intervention to reduce 30-day readmissions using checklists and electronic decision support for clinicians caring for inpatients on a dedicated liver unit at tertiary care referral center with a transplant program. Herein, we detail the results of a multi-modal quality improvement (QI) intervention for patients with cirrhosis.
Methods
We performed a prospective study of a QI program at Beth Israel Deaconess Medical Center in Boston, MA. All clinical care was provided on the dedicated inpatient hepatology unit reserved for patients with decompensated cirrhosis or liver transplants staffed by housestaff and a hepatologist. (12) The cohort described herein has been examined in studies regarding fall risk(13) and predictors of 90-day mortality.(14) This study was approved by our Institutional Review Board (2012-P-000096/6). The quasi-experimental pre-post design and analysis of this study was performed consistent with the Standards for Quality Improvement Reporting Excellence guidelines.(15)
Development of the intervention
We designed our QI initiative with multiple goals. First, we sought universal use of rifaximin for all patients with HE.(16, 17) Second, we adapted an aggressive protocol of goal-directed therapy for overt HE (adjusting frequency of lactulose dosing to mental status using the Richmond Agitation and Sedation Scale which is familiar to all nurses in our center). (18, 19) Third, we addressed patients with SBP by promoting timely administration of the correct dose of antibiotics and albumin.(1, 20) Finally, we sought to maximize the number of patients who received prophylactic measures - e.g. variceal hemorrhage prophylaxis, subcutaneous heparin for the prevention of venous thrombosis and antibiotics for primary and secondary prophylaxis of SBP.(21, 22)
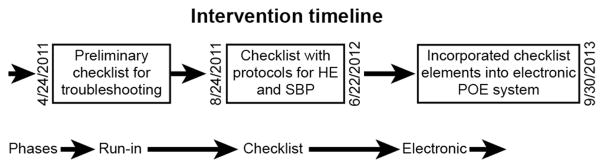
We first developed a hand-held checklist in accordance with published guidelines for checklist development.(23) (Figure 1) We began with a pilot checklist run-in phase in order to ascertain barriers to adherence (April 24th 2011 to August 23rd, 2011). This checklist prompted medication record review and to confirm that candidates for non-selective beta-blockers were on effective doses. The checklist was performed daily during rounds with adherence attested by a physician’s signature on a sheet placed each patient’s chart. We obtained frequent feedback to troubleshoot and refine the mechanics of the intervention and to facilitate adherence. As published elsewhere, we adjusted the intervention to suit clinical workflow and facilitate buy-in from all members of the care team.(12)
Figure 1. Intervention Timeline.
The development, timing and content of our intervention’s phases are described. HE: hepatic encephalopathy, POE: Provider order entry, SBP: spontaneous bacterial peritonitis
Hand-Held Checklist Phase
After the run-in phase, the final version of the paper checklist was deployed. This checklist included the items for the HE protocol, standard SBP treatment and prophylactic measures described above. Finally, throughout the checklist phase, the study coordinator (EBT) provided frequent in-person observation and intervention to promote adherence among the clinicians. This phase lasted from August 24th 2011 through June 22nd, 2012.
Electronic Phase
In the electronic phase (June 23rd, 2012 – Sept 30th, 2013), the checklist items were incorporated into the electronic provider order entry system for the entire hospital using mandatory preset doses and linked medications.(24) Additionally, pre-set templates for admission and progress notes in the electronic health record were modified to include the checklist elements and protocols. Finally, housestaff were given pocket-sized, laminated guides describing the treatment protocols. There was no in-person observation and education during this phase.
Analysis
We designated the pre-implementation period from January 1st 2010 to December 31st 2010 as the baseline control-period. The four-month developmental phase was excluded from analysis. The primary outcome measure was any emergent 30-day readmission to BIDMC. We prespecifed a subgroup analysis among patients with overt HE. A secondary outcome for this subset was length of stay (LOS).
We also sought to compare our experience to local and regional data. First, we acquired data on the number of admissions and 30-day readmissions for patients cared for by BIDMC hospitalists over the same time period. This service cares for an average of 660 general medicine admissions as well as complex pre- and postsurgical patients with significant comorbidities each month. Second, we obtained de-identified data from a neighboring quaternary care institution for patients admitted with decompensated cirrhosis (overt ascites, overt HE, jaundice, or variceal hemorrhage) in which an average of 12 patients (MELD of 24.1 ± 10.2) are admitted with these criteria each month.
Collection of data
All patients admitted to the dedicated liver unit were included. The MELD score was calculated as previously described.(25) The Charlson comorbidity index was calculated using ICD-9 codes.(26) All charts were reviewed to identify the presence of active problems including overt ascites, overt encephalopathy (≥ West Haven grade 2), variceal hemorrhage, hepatocellular carcinoma, SBP, and alcoholic hepatitis. Mortality data was confirmed by search of the US Social Security Death Index and is therefore complete.(27) Nineteen patients were transplanted during their admission and were excluded from the readmissions analysis. Outpatient utilization of rifaximin or SBP prophylaxis was determined by the presence of at least two of the following criteria: a) a prescription recorded in the medical record, b) documentation of utilization by a physician and, where available, c) a record of a filled prescription in an online search of the linked pharmacy database.
Statistical Analyses
Descriptive statistics are displayed as mean ± standard deviation or median [interquartile range] for Gaussian and non-Gaussian distributed variables, respectively unless otherwise indicated. For crude statistical comparisons, we used the Chi-Square test for categorical variables, student t-test for Gaussian distributed continuous variables, and the Kruskal-Wallis test for non-Gaussian distributed variables. For analyses of the odds of readmission within 30 days and death within 90 days of discharge, we used a generalized estimating equation (GEE) approach with a logit link, binomial error structure and a random effect for patient to account for the within-person correlation in the outcome data. This technique allows for adjustment at the patient level to avoid biasing the results, for example, with one patient who was readmitted several times or the practice patterns of a given Hepatologist. Unadjusted associations were examined using a GEE model with intervention phase and provider as fixed effects and patient as a random effect. GEE models were then adjusted for sex, age, ethnicity, payor, admission in preceding 30 days, Charlson index, admission MELD, admission sodium, and admitting Hepatologist. Trends for resource utilization (e.g. rifaximin prescriptions) were assessed with chi-squared tests for trends while the impact of lactulose dosing was assessed the logistic regression, adjusted for West Haven HE grade. Trends for 30-day readmission by month between BIDMC hospital services were analyzed using non-linear regression while trends for readmission between hospital services were analyzed using pairwise two-way analysis of variance. For analyses of length of stay (LOS) for patients with overt HE, negative binomial generalized regression was employed given its right skew. Associations between intervention phases and components (rifaximin and lactulose volume) were assessed separately and adjusted as above. Lactulose was assessed as a dichotomous variable (> or ≤ the median dose in the first 24 hours). A power calculation was performed based on the control data. In order to show a 25% reduction in 30-day readmissions from the control period (37.9%), assuming a power of 80% and an alpha of 0.05, we would need a sample size of 405 patients in each phase. Two-sided P-values are reported. All statistical analyses were performed with SAS Version 9.3 for Windows.
Results
During the study period, 824 unique patients were admitted to the liver unit 1720 times. Of these patients, 485 (58.9%) were admitted once, 268 (32.5%) were admitted 2–4 times and 71 were admitted 5 or more times. 365 admissions were specifically for overt HE. Demographics and clinical characteristics are shown in Table 1. The 90-day all-cause mortality risk following an admission to the liver unit for all patients was 18.9% (325 deaths). The median LOS for all patients was 7 4.0 days (interquartile range: 2.0–8.0). Overall, 253 (14.7%) admissions resulted in a discharge to a rehabilitation hospital.
Table 1.
Admission Characteristics by Intervention Phase
| Intervention Phase | ||||
|---|---|---|---|---|
| Control (n=626) | Checklist (n=470) | Electronic (n=624) | p-value | |
| Age - Mean (SD) | 55.8 (11.2) | 56.1 (11.3) | 58.3 (11.3) | 0.009 |
| Female Sex - % | 36.3% | 42.8% | 36.9% | 0.61 |
| Non-White Ethnicity - % | 20.1% | 31.9% | 29.7% | 0.029 |
| Charlson Comorbidity Index - Mean (SD) | 4.23 (2.63) | 4.0 (2.6) | 4.4 (2.7) | 0.093 |
| Model for End-Stage Liver Disease Score - Mean (SD) | 17.7 (7.08) | 17.7 (7.1) | 17.4 (7.5) | 0.46 |
| Admission Sodium Level (meq/L)- Mean (SD) | 134.4 (5.65) | 134.5 (5.8) | 134.3 (5.9) | 0.89 |
| Admission in Past 30 days - % | 22.7% | 22.1% | 16.7% | 0.041 |
| Payor= Medicare or Private Insurance - % | 70.6% | 73.0% | 72.9% | 0.82 |
| Primary Etiology of Liver Disease (%) (%, does not sum to 100) | ||||
| Alcoholic Liver Disease | 34.0% | 30.0% | 29.3% | 0.39 |
| Hepatitis C | 44.9% | 40.4% | 40.2% | 0.50 |
| Hepatitis B | 5.4% | 4.47 | 4.3% | 0.64 |
| Biliary Cirrhosis | 1.8% | 4.5% | 2.4% | 0.63 |
| Active Problems during hospitalization (%, does not sum to 100) | ||||
| Gastrointestinal Bleeding | 28.3% | 29.8% | 31.9% | 0.59 |
| Ascites | 43.6% | 43.8% | 43.6% | 1.0 |
| Hepatic Encephalopathy | 47.8% | 43.8% | 47.4% | 0.72 |
| Spontaneous Bacterial Peritonitis | 6.9% | 6.6% | 9.1% | 0.25 |
| Alcoholic Hepatitis | 7.8% | 9.2% | 5.6% | 0.11 |
| Hepatocellular Carcinoma | 3.4% | 2.5% | 5.3% | 0.32 |
| Previous Transplant | 19.3% | 22.8% | 21.3% | 0.63 |
SD = standard deviation
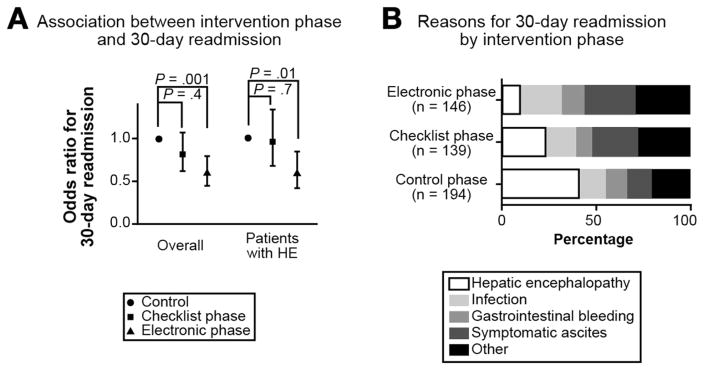
We observed statistically significant variation in 30-day readmissions across the QI intervention phases (Table 2). In pairwise analyses (Figure 2A), 30-day readmissions were statistically significantly lower during the electronic and checklist phases compared to the control phase. Furthermore, 30-day readmissions were significantly lower in the electronic than checklist phase. Table 3 shows that after accounting for relevant clinical confounders, the electronic phase was associated with 40% and 38% lower odds of readmission within 30 days compared to the control and checklist phases (both overall and for patients with HE). There were no statistically significant differences in 90-day all-cause mortality across any of the intervention phases.
Table 2.
Unadjusted Associations between QI Intervention Phase and Clinical Outcomes
| 30 day readmission (%) | 90 day mortality (%) | |||
|---|---|---|---|---|
|
| ||||
| All Admissions (N = 1720) | Among Admissions for patients with HE (N=1036) | All Admissions (N = 1720) | Among Admissions for patients with HE (N=1036) | |
| Overall | 32.9 | 35.6 | 18.9 | 26.7 |
| Intervention phase | ||||
| Control | 37.9 | 39.2 | 20.1 | 25.4 |
| Checklist Phase | 34.7 | 40.9 | 15.1 | 22.7 |
| Electronic Phase | 26.6 | 27.6 | 20.5 | 31.2 |
| P-value | 0.004 | 0.01 | 0.12 | 0.14 |
HE = hepatic encephalopathy
Figure 2. Differences in Outcome by Intervention Phase.
A: The association of each phase with 30-day readmissions is depicted overall and for patients with hepatic encephalopathy (HE). * denotes a p value < 0.05.
B: The reasons for a 30-day readmission are described by intervention phase.
Table 3.
Adjusted Associations between QI Intervention Phase and Clinical Outcomes
| 30 day readmissions | 90 day mortality | |||
|---|---|---|---|---|
|
|
|
|||
| All Admissions (N = 1720) | Among Admissions for patients with HE (N=1036) | All Admissions (N = 1720) | Among Admissions for patients with HE (N=1036) | |
|
|
|
|||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Intervention phase | ||||
| Electronic vs Checklist | 0.74 (0.55–1.00) | 0.62 (0.42–0.92) | 1.40 (0.90–2.17) | 1.39 (0.86–2.24) |
| Electronic vs. Control | 0.60 (0.45–0.79) | 0.60 (0.42–0.85) | 0.93 (0.61–1.40) | 1.03 (0.66–1.59) |
| Checklist vs. Control | 0.81 (0.62–1.07) | 0.96 (0.68–1.34) | 0.66 (0.43–1.04) | 0.74 (0.45–1.22) |
Generalized estimating equation models were adjusted for sex, age, ethnicity, payor, admission in past 30 days, Charlson index, baseline MELD, sodium and a random effect for each patient.
HE = hepatic encephalopathy, OR=Odds Ratio; 95% C.I.= 95% Confidence Interval
Among patients initially admitted with overt HE (≥ grade 2), the proportion readmitted within 30 days was significantly lower in the electronic phase (27 out of 104, 26.0%) compared with the checklist (34 out of 76, 44.7%) and control phases (66 out of 135, 48.9%), p<0.001 and p=0.002 respectively. Figure 2B shows that the proportion of readmissions attributable to HE was lower in the electronic phase (14 out of 146, 9.6%) than during the checklist (32 out of 139, 23.0%) and control phases (79 out of 194, 40.7%), p=0.002 and p<0.001 respectively. Additionally for patients with overt HE, the electronic phase was associated with a lower adjusted LOS (beta coefficient −1.34 95% CI: −2.38 – −0.32, p = 0.01).
We assessed the effect of protocol adherence on readmissions. First, rifaximin utilization for patients with any HE rose progressively from the control through the checklist and electronic phases: 78.1%, 89.4%, 96.3%-p-trend = 0.0002. The use of rifaximin for patients admitted for overt HE was associated with lower adjusted odds of 30-day readmission (odds ratio (OR) 0.39 (95% CI: 0.16 – 0.87), p = 0.02). Of the 362 patients admitted specifically for overt HE who neither died nor were transplanted during their admission, outpatient rifaximin utilization was documented in 245 (67.7%) patients and was associated with lower adjusted odds of 30-day readmission (OR 0.33 (95% CI: 0.21 – 0.53), p < 0.0001). Second, while the increase secondary SBP prophylaxis by phase was not significant (42.2%, 42.4%, 50.0%, p-trend 0.85), of the 224 patients with a history of or index admission for SBP, 94 (42.0%) received secondary antibiotic prophylaxis which was associated with a lower adjusted odds of 30-day readmission (OR 0.40 (95% CI: 0.21 – 0.75), p = 0.004). After further adjustment for the effect of rifaximin adherence, the association with readmission for those taking SBP prophylaxis was still significant (OR 0.51 95% CI: 0.31 – 0.83, p 0.007).
We also suggested higher dose/frequency lactulose for the treatment of overt HE. Overall, during the control phase, 6209 20-cc doses of lactulose were delivered. This increased to 8738 and 8858 doses during the checklist and electronic phases, respectively. Among the 365 patients admitted with overt HE, the median dose of lactulose on the day of admission was 6 cups (IQR: 4 to 10). Patients with overt HE who received 6 or more cups of lactulose on the day of their admission experienced a significantly reduced adjusted LOS (−2.36 95%CI:−3.40 – −1.31), p < 0.0001). After adjusting for the grade of HE, we found that the odds of readmission within 30 days was 28.8% (95% CI: 5.3% to 52.7%, p=0.02) lower among patients receiving this high dose.
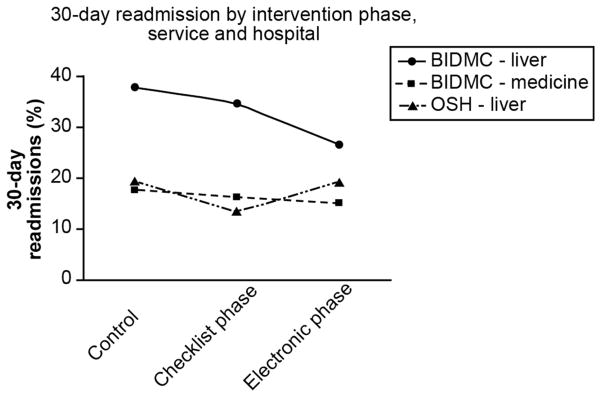
Figure 3 demonstrates the secular trends in the proportion of patients readmitted within 30 days. During the timeframe coinciding with the electronic phase, the slope of the decline was significantly steeper on our liver service compared with patients cared for by BIDMC hospitalists and patients with decompensated cirrhosis cared for at a neighboring institution (p < 0.0001).
Figure 3. Secular trends in readmissions.
The 30-day readmission rate for the periods covered by our intervention phases are depicted for the liver and medicine services as well as for all patients with decompensated cirrhosis admitted to a neighboring institution. The trend is significantly different for our liver service compared to the others (p < 0.0001) during the electronic phase’s timeframe. BIDMC = Beth Israel Deaconess Medical Center, OSH = outside hospital
Discussion
Readmissions are an important target for quality improvement (QI) as they are associated with tremendous costs and morbidity for patients with cirrhosis. This study of a QI intervention on an inpatient liver unit showed that care protocols supported by electronic decision support in the provider order entry system were associated with a 40% reduction in 30-day readmissions, likely driven by a decrease in readmissions for HE. Furthermore, this trend was unique when compared to control populations. We also show that intensified therapy for overt HE can reduce LOS.
The efficacy of many elements of our intervention are known: rifaximin decreases readmission for patients with HE(16, 17); high dose lactulose is associated with rapid mental status normalization and decreased intensive care utilization (18); and appropriate dosing of ceftriaxone for SBP is associated with a less complex admission.(20) Some elements require further study. Reduced readmissions were likely mediated in large part by the ability to obtain the medication as an outpatient, however the role of rifaximin co-therapy during an admission for overt HE should be explored further. First, even short-term exposure may modify gut ammoniagenesis and could reduce early readmissions. Second, starting a medication during hospitalization may increase the rate of prescription and facilitate prior-authorization at the time of discharge. Similarly it must be confirmed whether high-dose lactulose by itself is associated with lower LOS and 30-day readmission rates. While it is known that secondary prophylaxis of SBP is beneficial,(1) and we show that it is associated with reduced odds of 30-day readmission, it is unclear from these data whether it independently reduces readmissions. Finally, there were no significant changes in 90-day mortality over the study phases indicating both that decreased readmissions were not achieved at the expense of and that readmissions may not independently contribute to an increased risk of death.
Our study advances the current literature on QI for patients with cirrhosis by presenting an inexpensive, easy to implement and generalizable approach. Three prior studies addressed readmission interventions in this population.(2, 28, 29) An Italian group analyzed the impact of a specialized post-acute care pathway among 100 patients with cirrhosis and ascites and found a significant reduction in 30-day readmission rates, compared to a well matched control group (42.4% to 15.4%).(2) To do so, they created a specialized outpatient unit staffed with dedicated hepatologists and nurses for rapid post-discharge follow-up visits where same-day endoscopy and liver ultrasound was available. An Australian group also studied a post-acute care program for patients with decompensated cirrhosis.(28) Using dedicated nurses and regular home visits, they were able to demonstrate improved medication adherence and rates of clinical follow up, albeit without significant reductions in hospital-days or readmissions. The drawback of these programs is that, unlike our intervention, they require costly infrastructure, expertise and significant institutional commitments.
A third study from Wisconsin demonstrated that a combination of standardized hand-written order sets and educational conferences resulted in increased compliance with standard measures for gastrointestinal bleeding and decreased readmissions from 41% (24/59) to 13% (7/55).(29) This program demonstrates the power of standard checklists and education. Our study extends these results by showing that outcomes improve further when checklist items are hard-wired into the ordering system.
Our results must be interpreted in the context of the study design. First, as is common in QI, multiple interventions were deployed simultaneously in an unblinded fashion. Second, readmissions, while costly and likely associated with decreased quality of life for patients, are surrogate markers of morbidity and quality care. Third, thought we adjusted for all physician-level and patient-level effects including multiple readmissions, residual confounding by unaccounted differences between periods cannot be excluded. Fourth, while general practice within Boston is to refer patients with complex disease to the hospital from which they were recently discharged, we cannot be sure to have captured all readmissions. Fifth, the comparison with the hospitalist and outside hospital liver services are only intended to show that our observed changes in readmission rate were not the result of a local or regional change in policy or reporting. Importantly, we demonstrate that a QI initiative reduced our readmissions while readmission rates were relatively constant elsewhere.
In summary, a QI program which integrated multiple interventions of proven benefit for patients with advanced liver disease results in a significant reduction in the 30-day readmission rate, particularly for those with HE. The readmission rate for HE appears to be highly modifiable and therefore efforts to intensify therapy for these patients should be encouraged and further studied.
Acknowledgments
The authors would like to thank Dr. J. Thomas Lamont and Dr. Gary Horowitz for their input and generous mentorship on this manuscript.
Funding: This work was supported by a grant from the Carl J. Shapiro Institute for Education and Research. This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Abbreviations
- BIDMC
Beth Israel Deaconess Medical Center
- GEE
generalized estimating equation
- HE
hepatic encephalopathy
- IQR
Interquartile range
- MELD
Model for Endstage liver disease
- OR
odds ratio
- POE
Provider order entry
- QI
quality improvement
- SBP
spontaneous bacterial peritonitis
Footnotes
Conflicts of interest: No author has any conflicts of interested to disclose, financial or otherwise
Contributions: Tapper (study concept, design, analysis, writing, funding, supervision); Finkelstein (analysis, review), Mittleman (analysis, review), Piatkowski (data acquisition, review), Chang (data acquisition), Lai (design, review)
References
- 1.Kanwal F, Kramer J, Asch SM, El-Serag H, Spiegel BM, Edmundowicz S, Sanyal AJ, et al. An explicit quality indicator set for measurement of quality of care in patients with cirrhosis. Clin Gastroenterol Hepatol. 2010;8:709–717. doi: 10.1016/j.cgh.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 2.Morando F, Maresio G, Piano S, Fasolato S, Cavallin M, Romano A, Rosi S, et al. How to improve care in outpatients with cirrhosis and ascites: a new model of care coordination by consultant hepatologists. J Hepatol. 2013;59:257–264. doi: 10.1016/j.jhep.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Singal AG, Rahimi RS, Clark C, Ma Y, Cuthbert JA, Rockey DC, Amarasingham R. An automated model using electronic medical record data identifies patients with cirrhosis at high risk for readmission. Clin Gastroenterol Hepatol. 2013;11:1335–1341. e1331. doi: 10.1016/j.cgh.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247–252. doi: 10.1038/ajg.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, Gangarosa LM, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187. e1173. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Statistics for Hepatic Encephalopathy Discharges. 2012. [Google Scholar]
- 7.Ghaoui R, Friderici J, Visintainer P, PKL, Lagu T, Desilets D. Measurement of the quality of care of patients admitted with decompensated cirrhosis. Liver Int. 2014;34:204–210. doi: 10.1111/liv.12225. [DOI] [PubMed] [Google Scholar]
- 8.Arguedas MR, DeLawrence TG, McGuire BM. Influence of hepatic encephalopathy on health-related quality of life in patients with cirrhosis. Digestive diseases and sciences. 2003;48:1622–1626. doi: 10.1023/a:1024784327783. [DOI] [PubMed] [Google Scholar]
- 9.Song Z, Safran DG, Landon BE, He Y, Ellis RP, Mechanic RE, Day MP, et al. Health care spending and quality in year 1 of the alternative quality contract. New England Journal of Medicine. 2011;365:909–918. doi: 10.1056/NEJMsa1101416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganesh S, Rogal SS, Yadav D, Humar A, Behari J. Risk factors for frequent readmissions and barriers to transplantation in patients with cirrhosis. PLoS One. 2013;8:e55140. doi: 10.1371/journal.pone.0055140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanwal F. Coordinating Care in Patients With Cirrhosis. Clinical Gastroenterology and Hepatology. 2013;11:859–861. doi: 10.1016/j.cgh.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Tapper EB, Lai M. Factors affecting adherence to a quality improvement checklist on an inpatient hepatology service. Proc (Bayl Univ Med Cent) 2014;27:100–102. doi: 10.1080/08998280.2014.11929069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tapper EB, Risech-Neyman Y, Sengupta N. Psychoactive medications increase the risk of falls and fall-related injuries in hospitalized patients with cirrhosis. Clinical Gastroenterology and Hepatology. 2015 doi: 10.1016/j.cgh.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Tapper EB, Finkelstein D, Mittleman MA, Piatkowski G, Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015 doi: 10.1002/hep.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clancy CM, Berwick DM. The science of safety improvement: learning while doing. Ann Intern Med. 2011;154:699–701. doi: 10.7326/0003-4819-154-10-201105170-00013. [DOI] [PubMed] [Google Scholar]
- 16.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 17.Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458–1463. doi: 10.1038/ajg.2013.219. [DOI] [PubMed] [Google Scholar]
- 18.Al-Osaimi AQB, Melo I, Romeo LM, Berg CL, Caldwell SH, et al. In-patient hepatic encephalopathy protocol improves in-patient outcome measures: Interim analysis. Hepatology. 2009;50:446A. [Google Scholar]
- 19.Tapper EB, Jiang ZG, Patwardhan VR. Refining the Ammonia Hypothesis: A Physiology-Driven Approach to the Treatment of Hepatic Encephalopathy. Mayo Clinic Proceedings; 2015; Elsevier; 2015. [DOI] [PubMed] [Google Scholar]
- 20.Mazer LTE, Piatkowski G, Lai M. The need for antibiotic stewardship and treatment standardization in the care of cirrhotic patients with spontaneous bacterial peritonitis – a retrospective cohort study examining the effect of ceftriaxone dosing. F1000Research. 2014;3:57. doi: 10.12688/f1000research.3-57.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rimola A, Garcia-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club J Hepatol. 2000;32:142–153. doi: 10.1016/s0168-8278(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 22.Sogaard KK, Horvath-Puho E, Gronbaek H, Jepsen P, Vilstrup H, Sorensen HT. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol. 2009;104:96–101. doi: 10.1038/ajg.2008.34. [DOI] [PubMed] [Google Scholar]
- 23.McCoy AB, Waitman LR, Lewis JB, Wright JA, Choma DP, Miller RA, Peterson JF. A framework for evaluating the appropriateness of clinical decision support alerts and responses. J Am Med Inform Assoc. 2012;19:346–352. doi: 10.1136/amiajnl-2011-000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teich JM, Merchia PR, Schmiz JL, Kuperman GJ, Spurr CD, Bates DW. Effects of computerized physician order entry on prescribing practices. Arch Intern Med. 2000;160:2741–2747. doi: 10.1001/archinte.160.18.2741. [DOI] [PubMed] [Google Scholar]
- 25.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 26.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 27.Hauser TH, Ho KK. Accuracy of on-line databases in determining vital status. J Clin Epidemiol. 2001;54:1267–1270. doi: 10.1016/s0895-4356(01)00421-8. [DOI] [PubMed] [Google Scholar]
- 28.Wigg AJ, McCormick R, Wundke R, Woodman RJ. Efficacy of a chronic disease management model for patients with chronic liver failure. Clinical Gastroenterology and Hepatology. 2013;11:850–858. e854. doi: 10.1016/j.cgh.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Johnson E, Spier B, Leff J, Lucey M, Said A. Optimising the care of patients with cirrhosis and gastrointestinal haemorrhage: a quality improvement study. Alimentary pharmacology & therapeutics. 2011;34:76–82. doi: 10.1111/j.1365-2036.2011.04692.x. [DOI] [PubMed] [Google Scholar]