Abstract
Background and Objectives
The presence of metabolic syndrome (MetS) in childhood is a significant risk factor for later cardiovascular disease (CVD). Recent data showed temporal decreases in a sex- and race/ethnicity-specific MetS severity z-score among U.S. adolescents. Our goal was to characterize the relationship of this MetS z-score with other CVD risk indicators and assess their temporal trends and lifestyle influences.
Methods
We analyzed 4837 participants aged 12–20 years from the National Health and Nutrition Examination Survey by 2-year waves from 1999–2012. We used linear regression to compare MetS z-score and dietary factors with serum levels of low-density lipoprotein (LDL), apolipoprotein-B (ApoB), high-sensitivity C-reactive protein (hsCRP) and uric acid.
Results
MetS severity z-score was positively correlated with LDL, ApoB, hsCRP, and uric acid measurements (p<0.0001 for all). These correlations held true among individual racial/ethnic groups. LDL, ApoB, and hsCRP measurements decreased over time among U.S. adolescents (p=0.002, p<0.0001, p=0.024 respectively). Saturated fat consumption was positively correlated with LDL (p=0.005), ApoB (p=0.012), and inversely related to serum uric acid (p=0.001). Total calorie intake was inversely related to LDL (p=0.003) and serum uric acid (p=0.003). Unsaturated fat, carbohydrate, and protein consumption were not related to LDL, ApoB, hsCRP, or serum uric acid.
Conclusions
There is a positive correlation between MetS severity and all four CVD risk indicators studied. LDL, ApoB, and hsCRP showed favorable temporal trends, which could be related to similar trends in MetS z-score. These data support the importance of considering multiple inter-related factors in clinical CVD risk assessment.
Introduction
The metabolic syndrome (MetS) is a cluster of clinical abnormalities characterized by: increased waist circumference (or increased BMI z-score in pediatrics), elevated blood pressure, hypertriglyceridemia, hyperglycemia, and low HDL cholesterol [1–3]. MetS has traditionally been defined based on abnormalities in at least three of these topics. The presence of MetS in childhood is a significant risk factor for developing cardiovascular disease (CVD) in adulthood [4–6]. The MetS Severity Score (MetS z-score) was developed as a continuous score to assess severity of metabolic derangement and account for sex and racial/ethnic differences in MetS [7–10]. We recently demonstrated that in adults this MetS Severity Score remained a significant predictor of future coronary heart disease, even in models that included all of the individual components of MetS—suggesting that MetS was indeed worth more than the sum of its parts [11].
Childhood manifestations of CVD are rare; however, the pathogenesis can begin in adolescence [12–14]. Thus, it is a priority to characterize possible risk indicators in young patients. It has been previously shown that elevated childhood MetS z-score correlates with increased risk of adulthood CVD and type 2 diabetes mellitus (T2DM)[15–17]. Additionally, the MetS z-score had been shown to correspond with abnormalities in serum uric acid and high-sensitivity C-reactive protein (hsCRP) measurements in adolescents, which are considered CVD risk indicators, though this was only performed in the population sample from which the MetS z-score was derived and has not been further confirmed among additional adolescents [7]. Low-density lipoprotein cholesterol (LDL) and apolipoprotein-B (ApoB) are additional CVD risk indicators [2, 18]; it remains unclear how these relate to the severity of MetS.
We recently reported that the MetS z-score declined among U.S. adolescents from 1999–2012, and this change was correlated with decreasing total calorie consumption, decreasing carbohydrate consumption, and increasing unsaturated fat consumption [19]. The goals of the current project were to 1) assess whether MetS severity correlated with these additional markers of CVD risk, 2) analyze whether these markers exhibited a similar decrease over the same study period and 3) assess for whether these other CVD risk markers were associated with dietary changes, as MetS severity had been. We hypothesized that elevated MetS z-scores would correspond with higher levels of LDL, ApoB, uric acid and hsCRP measurements in adolescents. We subsequently hypothesized that those CVD biochemical risk indicators would also show a decreasing temporal trend and would be related to dietary trends. Such links between CVD risk factors may have implications for the MetS z-score as an overall metabolic health marker and societal trends of other biochemical markers more specifically related to CVD risk.
Material & Methods
We examined participant data from the Centers for Disease Control and Prevention National Health and Nutrition Examination Survey (NHANES 1999–2012). NHANES is a national, stratified, multistage probably, cross-sectional survey conducted in two-year waves, recruiting randomly-selected non-institutionalized U.S. civilians. Racial/ethnic minority groups and those at or below 130% of the federal poverty level were oversampled. Calculated sample weights accounted for this oversampling in addition to correcting for different response rates among groups to ensure nationally representative estimates. The National Center for Health Statistics ethics review board approved this study. Laboratory and clinical measurements were obtained using controlled equipment and protocols [20].
The protocols for obtaining measurements for MetS assessment have been previously cited [19, 21, 22]. Fasting blood samples were obtained from participants who attended the morning session at CDC NHANES mobile examination centers. HDL was measured by direct immunoassay. Fasting glucose was measured using an enzyme linked hexokinase method. Triglycerides measurement used a timed-endpoint method. BMI z-scores were calculated in accordance with the U.S. CDC 2000 growth reference adjusting for age and sex [23]. Elevated blood pressure (BP) was defined as systolic or diastolic BP exceeding the 90th percentile for sex, age, and height [24]. MetS z-score was calculated using the Pediatric MetS z-score (http://mets.health-outcomes-policy.ufl.edu/calculator/) [7].
The biochemical CVD markers of interest were measured in accordance with CDC NHANES protocol [20]. LDL was calculated according to the Friedewald calculation. ApoB was measured with an immunochemical light spectrometry method. Serum uric acid was measured with a timed-endpoint method. High-sensitivity CRP was quantified with latex-enhanced nephelometry; levels of hsCRP were only available for 1999–2010.
Dietary intake and physical activity were determined as previously cited [19]. Dietary intake was determined from a 24-hour food recall administered by trained dietary interviewers using a 4-step multi-pass approach on examination day at the mobile examination centers. Data was processed and coded based on individual foods and portion sizes to determine specific nutrient intake in accordance with the U.S. Department of Agriculture’s National Nutrient Database for Standard Reference [25]. Food group consumption was reported as percentage of total calories accounted for by specific food group [26]. The equations were: % total energy from carbohydrates = (4 * grams of carbohydrates)/total calories, % total energy from fats (saturated or unsaturated) = (9 * grams of fat)/total calories, and % total energy from protein = (7 * grams of protein)/total calories.
Additionally, we used Food Frequency Questionnaire data from 2003–2006 and 2009–2010 (years for which data was made available) to assess intake of high fructose foods. NHANES participants were questioned on how often they ate a specific food. High fructose foods included juices, sugar sweetened beverages, and desserts such as cookies and ice cream [27]. Notably this questionnaire did not include information about serving sizes. These data were compiled to create a daily frequency of high fructose foods consumption. This value was then log transformed given the skewed distribution.
From 2007–2012, physical activity was assessed as weekly minutes of moderate to vigorous physical recreational activity. Participants were initially asked if they participated in any moderate to vigorous-intensity sports, fitness, or recreational activities. If they answered yes, they were further questioned how many minutes per typical day they do these activities.
Participants were excluded due to the following conditions that may introduce confounding factors in correlating their MetS z-score with biochemical CVD markers: non-fasting status (n=993), pregnancy (n=242), active hepatitis-B infection (n=8), physician diagnosed diabetes (n=66), or current use of antidiabetic or antihyperlipidemic medication (n=42). Participants taking antihypertensives (n=69) were not excluded, but instead classed as having elevated BP.
Statistical significance was defined as P<0.05. Statistical analysis was performed using SAS (version 9.4, Cary, NC). Survey procedures were used to account for the NHANES survey design and obtain population-based estimates. Linear regression was used to create models correlating the different CVD indicators with MetS z-score. Initial comparison of MetS and CVD factors was unadjusted to generate Pearson’s r for these relationships. Subsequent linear regression analysis adjusted for age, sex, and race/ethnicity. To compare between CVD risk factors for associations with MetS severity, each factor was standardized before performing linear regression. Natural log transformation of hsCRP levels was performed to achieve normality. Linear regression was used to estimate quantitative measurements means across each NHANES period and assess temporal linear trends. Analyses of dietary factors with risk of CVD indicators were unadjusted to yield Pearson’s R values for each relationship.
Results
We examined data from 4837 adolescent NHANES participants age 12–20 years who met the inclusion/exclusion criteria described above. Of these, there were 1428 non-Hispanic whites, 1483 non-Hispanic blacks, and 1895 Hispanics. The sample was 51.7% male. Mean age was 16.0 years. Detailed breakdown of participant demographics across each NHANES wave is reported in Table 1.
Table 1. Participant characteristics –
numbers of participants in each racial/ethnic category are reported along with population weighted percentages.
| Overall n | Non-Hispanic- White n (%) | Non-Hispanic- Black n (%) | Hispanic n (%) | Mean Age, years (95% CI) | Male (%) | |
|---|---|---|---|---|---|---|
| 1999–2000 | 878 | 203 (62.6) | 232 (15.0) | 441 (22.4) | 15.9 (15.6, 16.3) | 54.3 |
| 2001–2002 | 887 | 292 (69.8) | 272 (14.8) | 321 (15.4) | 16.0 (15.6, 16.3) | 46.5 |
| 2003–2004 | 872 | 243 (66.9) | 330 (16.5) | 290 (16.6) | 16.1 (15.8, 16.5) | 52.7 |
| 2005–2006 | 815 | 222 (68.8) | 274 (15.2) | 315 (16.1) | 16.0 (15.8, 16.2) | 53.9 |
| 2007–2008 | 420 | 148 (66.5) | 100 (14.4) | 172 (19.2) | 16.1 (15.8, 16.3) | 51.5 |
| 2009–2010 | 526 | 182 (62.7) | 108 (16.1) | 226 (21.2) | 15.8 (15.6, 16.1) | 51.5 |
| 2011–2012 | 439 | 138 (61.0) | 167 (17.0) | 130 (22.) | 15.9 (15.5, 16.3) | 52.4 |
| Total | 4837 | 1435 (65.6) | 1491 (15.6) | 1911 (18.8) | 16.0 (15.9, 16.1) | 51.7 |
CVD risk factors: correlation with MetS z-score and time
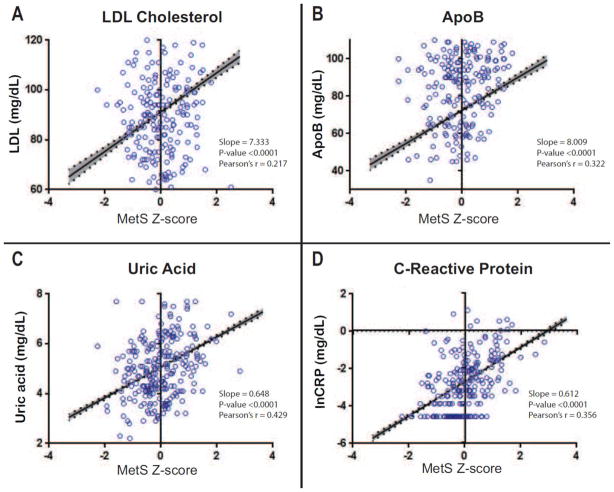
Linear regression analysis showed that the MetS z-score was significantly positively correlated for all 4 biochemical CVD risk markers analyzed: LDL, ApoB, CRP, and uric acid (Figure 1). This was true in unadjusted analyses (Figure 1), as well as following for adjustment for age, sex, and race/ethnicity (Table 2), and was true in each of the three specific racial/ethnic groups analyzed individually (Table 2). There was a significant MetS – race/ethnicity interaction for both ApoB and LDL, with levels highest in Hispanic and non-Hispanic white participants.
Figure 1. The MetS Z-score is significantly correlated with CVD risk indicators in U.S. adolescents.
Regression line is shown with 95% confidence bands shaded in. Scatterplots shown were generated from a random sub-sample of the analytic cohort for a more interpretable visual representation. The MetS Z-score was significantly correlated with all 4 biochemical markers analyzed.
Table 2. Racial/ethnic variations in the correlations between the MetS Z-score and biochemical markers.
The MetS Z-score was significantly correlated with all 4 biochemical markers analyzed in non-Hispanic white, non-Hispanic black, and Hispanic adolescents.
| Correlation of MetS Z-score to biochemical health markers | ||||
|---|---|---|---|---|
| Number | Standardized Slope (95% CI) | R-squared | ||
| Overall | Apolipoprotein-B † | 2200 | 8.20 (6.64, 9.76)* | 0.142 |
| Log (C-Reactive Protein) | 4394 | 0.65 (0.59, 0.70)* | 0.199 | |
| Uric Acid | 4819 | 0.55 (0.49, 0.61)* | 0.392 | |
| LDL † | 4786 | 7.77 (6.21, 9.32)* | 0.064 | |
| Non-Hispanic White | Apolipoprotein-B | 696 | 7.81 (5.37, 10.25)* | 0.089 |
| Log (C-Reactive Protein) | 1297 | 0.63 (0.54, 0.72)* | 0.203 | |
| Uric Acid | 1432 | 0.71 (0.62, 0.81)* | 0.213 | |
| LDL | 1416 | 7.51 (5.12, 9.90)* | 0.046 | |
| Non-Hispanic Black | Apolipoprotein-B | 653 | 4.82 (2.83, 6.81)* | 0.041 |
| Log (C-Reactive Protein) | 1318 | 0.75 (0.63, 0.86)* | 0.226 | |
| Uric Acid | 1482 | 0.47 (0.39, 0.56)* | 0.107 | |
| LDL | 1477 | 5.42 (3.30, 7.54)* | 0.025 | |
| Hispanic | Apolipoprotein-B | 851 | 10.8 (8.7, 13.0)* | 0.230 |
| Log (C-Reactive Protein) | 1779 | 0.08 (0.06, 0.10)* | 0.021 | |
| Uric Acid | 1905 | 0.57 (0.48, 0.66)* | 0.160 | |
| LDL | 1893 | 8.80 (7.14, 10.46)* | 0.085 | |
P<0.001
Significant MetS × race/ethnicity interaction
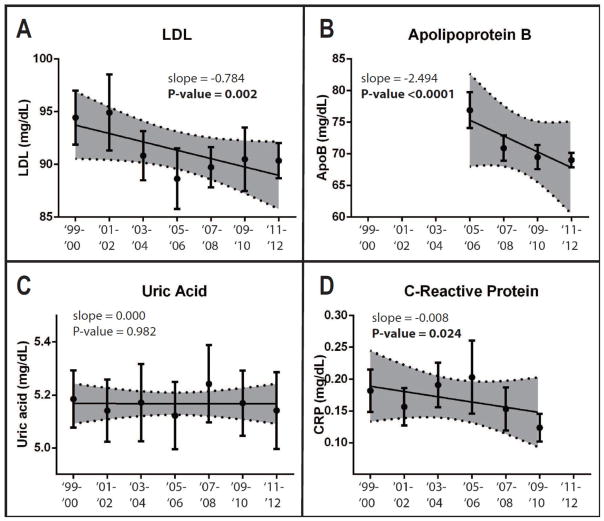
Temporal trends in LDL, ApoB, CRP, and uric acid are reported in Figure 2. Mean LDL measurements were 94.44 mg/dL in 1999–2000 and 90.36 mg/dL in 2011–2012. Linear regression analysis showed LDL measurements decreased from 1999–2012 (p=0.002). ApoB data was available starting with the 2005–2006 wave of NHANES. Mean ApoB measurements were 76.91 mg/dL in 2005–2006 and 69.02 mg/dL in 2011–2012. Linear regression analysis showed ApoB measurements decreased from 2005–2012 (p<0.0001). hsCRP data for the 2011–2012 wave of NHANES were not yet available. Mean hsCRP measurements were 0.18 mg/dL in 1999–2000 and 0.12 mg/dL in in 2009–2010. Linear regression analysis showed hsCRP measurements decreased from 1999–2010 (p=0.024). There were no significant temporal linear trends in serum uric acid measurements. Racial/ethnic variations in these temporal trends are reported in Table 3.
Figure 2. Metabolic health marker measurements show varying temporal trends from 1999–2012.
Individual means and 95% confidence interval bars are shown for each sampling period. Regression lines are shown with 95% confidence bands shaded. P-values are reported for non-zero trends. For prevalence values, slope is reported as change in percentage per sampling period. For measurements, slope is reported as change in measurement unit per sampling period. There were significant decreasing trends of low HDL and hypertriglyceridemia prevalence. There were significant decreasing trends in MetS Z-score and fasting plasma triglycerides. There were significant increasing trends in BMI Z-score and HDL.
Table 3. Racial/ethnic variations in the temporal linear trends of biochemical markers.
There were racial/ethnic variations in the temporal trends of the different biochemical markers.
| Temporal trends in biochemical markers from 1999–2012 | |||
|---|---|---|---|
| Estimated Slope (95% CI) | P-value | ||
| Non-Hispanic White | Apolipoprotein-B* | −2.82 (−4.22, −1.42) | 0.0002 |
| C-Reactive Protein** | −0.01 (−0.02, 0.00) | 0.200 | |
| Uric Acid | 0.02 (−0.020, 0.06) | 0.336 | |
| LDL | −0.58 (−1.27, 0.11) | 0.097 | |
| Non-Hispanic Black | Apolipoprotein-B* | −2.00 (−3.57, −0.43) | 0.013 |
| C-Reactive Protein** | −0.02 (−0.03, 0.00) | 0.018 | |
| Uric Acid | −0.01 (−0.04, 0.03) | 0.791 | |
| LDL | −1.21 (−2.01, −0.41) | 0.004 | |
| Hispanic | Apolipoprotein-B* | −2.50 (−3.92, −1.12) | 0.0006 |
| C-Reactive Protein** | 0.00 (−0.02, 0.01) | 0.776 | |
| Uric Acid | −0.01 (−0.05, 0.03) | 0.610 | |
| LDL | −0.52 (−1.25, 0.22) | 0.166 | |
Data were available from 2005–2012.
Data were available from 1999–2010.
CVD risk factors: correlation with lifestyle variables
We subsequently investigated if these trends in CVD risk indicators could be related to our previously-reported findings on temporal nutrition trends. We had previously reported trends of decreasing total calorie consumption, decreasing carbohydrate consumption, increasing unsaturated fat consumption, increasing protein consumption, no trends in saturated fat consumption, and no trends in weekly minutes of moderate to vigorous physical recreational activity [19].
While LDL was negatively correlated with total calorie consumption (kCal) (p=0.003) and positively correlated with saturated fat consumption (p=0.005) the Pearson’s R values for these relationships were low (Pearson’s R: −0.136 and 0.125, respectively), demonstrating a weak association. LDL was not significantly correlated with unsaturated fat consumption, carbohydrate consumption, protein consumption, and total weekly minutes of recreational physical activity (data not shown).
ApoB was significantly correlated with saturated fat consumption (Pearson’s R: 0.201, p=0.012). ApoB was not significantly correlated with total calorie consumption, unsaturated fat consumption, carbohydrate consumption, protein consumption, and total weekly minutes of recreational physical activity (data not shown).
Serum uric acid was significantly inversely correlated with total calorie consumption (Pearson’s R: −0.522, p=0.003) and saturated fat consumption (Pearson’s R: −0.523, p=0.001). Serum uric acid was not correlated with unsaturated fat consumption, carbohydrate consumption, protein consumption, and total weekly minutes of recreational physical activity (data now shown).
hsCRP was not correlated with total calorie consumption, unsaturated fat consumption, saturated fat consumption, carbohydrate consumption, protein consumption, and total weekly minutes of recreational physical activity (data not shown).
Frequency of high fructose food consumption was able to be calculated for 1116 adolescent NHANES participants for whom food frequency questionnaire data were available. Frequency of high fructose food consumption was not associated with any of the CVD risk markers when controlling for race/ethnicity, age, sex, and total caloric intake (data now shown).
Discussion
MetS has been associated with a variety of negative health outcomes such as T2DM and CVD [4–6]. Recent studies showed that patients who developed CVD and T2DM earlier in adulthood had higher childhood MetS z-scores than individuals who developed disease later or not at all [15, 16, 28], and these z-scores are associated with coronary heart disease independent of the individual MetS components [11]. Given that mechanisms of CVD pathogenesis can begin in adolescence [12–14], it is an important priority to characterize and assess future-CVD risk factors in pediatric patients. The recently-developed MetS z-score is a promising tool for assessing and tracking CVD risk longitudinally [7–9].
MetS is thought to be related to inflammatory processes and oxidative stress that are linked to underlying adipocyte cellular dysfunction [29, 30]. Thus, it is unsurprising that our data shows that the MetS z-score is correlated with serum uric acid and hsCRP measurements, which is consistent with previous studies [7, 8]. LDL and ApoB are significant CVD risk indicators with clear lifestyle influences and other associations with adipocyte abnormalities [31, 32], but have not been considered part of MetS largely in part due to their genetic variability [7, 33–35]. There has been a calling for the use of multiple indices to assess CVD risk, including ApoB, which has been proposed as a more sensitive and reliable index of CVD risk than LDL [2, 31, 36–38]. We showed that the MetS z-score was correlated with both of these indices of CVD risk—potentially because of overlapping underlying pathogenesis[39]. In addition, we showed that the correlations of the MetS z-score with all 4 of these measurements were true across multiple racial/ethnic groups: non-Hispanic white, non-Hispanic black, and Hispanic. Previous criteria for defining MetS did not account for racial/ethnic differences in MetS and resulted in under-diagnosis in non-Hispanic blacks [7, 40–45]. This, along with the limitations of using a binary definition of MetS, limited the usefulness of MetS as a health risk indicator [7, 46].
Our findings contribute to our hypothesis that the MetS z-score could be useful clinically to assess CVD risk in adolescents across different racial/ethnic groups. LDL screening is currently recommended for all children, largely to detect those with familial hypercholesterolemia [47]. Even though it is rare for adolescents to present clinical symptoms of CVD, elevations in MetS z-score could additionaly indicate the need to evaluate CVD risk indices such as ApoB, hsCRP, and serum uric acid. It was previously shown that childhood elevations in MetS z-score correlated with development of CVD and T2DM in adulthood [15, 16]. Thus, the MetS z-score not only reflects present clinical CVD risk, it also correlates with longitudinal risk. The MetS z-score could be a valuable tool for clinicians to track the severity of metabolic derangement, serve as an indicator for the need to assess additional CVD risk indices, and understand future adverse-outcome risk in young patients.
We were interested in how lifestyle factors associated with LDL, ApoB, hsCRP, and serum uric acid correlated with their temporal trends. We had previously shown that the MetS z-score declined in U.S. adolescents from 1999–2012 [19]. This appeared to be related to changing dietary patterns, specifically with decreased intake of carbohydrate and increased intake of unsaturated fat [19]. Given that we showed that LDL, ApoB, CRP, and serum uric acid were all correlated with the MetS z-score, we investigated if these measures showed corresponding trends. Previous studies had shown that LDL levels had decreased between 1999 and 2012 among U.S. adolescents [48]. Our data were consistent with those previous findings. We also showed that in conjunction with the decreasing LDL levels, ApoB and hsCRP measurements were decreasing among U.S. adolescents. While trends in these factors may have been due to lower MetS z-scores over time, we recognized that these improvements may also have been related to the changing dietary patterns. Nevertheless, dietary recall carries lower accuracy than observed ingestion or diary documentation [49], leaving uncertain whether these relationships were representative of the US population. LDL and ApoB were both positively correlated with saturated fat consumption. However, hsCRP was not associated with any of the dietary factors. This suggests that the decreasing trend in hsCRP could be more strongly related to the declining severity of metabolic derangement in U.S. adolescents.
Despite longstanding evidence of higher rates of CVD in countries consuming higher saturated fat, recent studies suggested a lack of conclusive evidence associating dietary saturated fat consumption with increased CVD risk [50, 51]. However, there exists a plethora of data showing replacement of dietary saturated fat with unsaturated fat results in favorable lipid profile changes [52–54]. We previously showed that there were no temporal trends in saturated fat intake but an increase in unsaturated fat intake among U.S. adolescents from 1999–2012 [19]. LDL, ApoB, and CRP measurements showed decreasing trends in the last decade while not being correlated with unsaturated fat intake. Overall, this raises the possibility that these temporal changes may be related less to dietary changes and more to the temporal improvements in MetS z-score—or potentially to additional unmeasured confounders.
The finding that serum uric acid and saturated fat consumption are inversely related was surprising and the reason for this is unclear. Previous data suggested that serum uric acid was more directly related to purine-rich foods intake [55]. Another interesting finding is that serum uric acid and LDL are inversely related to total calorie consumption. This may be due to the phenomenon of reverse causality [19, 56, 57]. Participants conscious of their increased risk for metabolic abnormalities may be more conscious of their total calorie intake. These data are also subject to potential recall or response bias such as under-reporting total calorie intake. It should be noted that while we did not find associations between physical activity levels and these factors, our ability to assess physical activity was also limited, given that NHANES did not have a consistent means of estimating physical activity from the entire study period 1999–2012. Moreover, the physical activity data to which we did have access are subject to the same bias and limitations as the dietary recall data [58].
We noted overall low R-squared values in correlating MetS z-score with the CVD risk markers. It is important to consider that there are many factors contributing to the different CVD risk markers in addition to the MetS z-score. This is especially true with LDL, as genetics play an important role in variations in serum levels [33–35]. However, the strong positive correlation of the MetS z-score with multiple CVD risk factors and longitudinal data corresponding MetS z-score to CVD development [16] warrants the consideration of the MetS z-score for clinical use in CVD risk assessment, for example through a calculator as part of the electronic medical record.
This study was limited in our ability to assess correlations of the changes in CVD risk indices with physical activity measures. We had previously reported on the limitations of NHANES physical activity data, notably that a consistent measurement was only initiated in 2007 [19]. We were additionally limited in by the following measures of CVD risk: 1) ApoB data was not collected until starting in 2005; 2) hsCRP data for 2011–2012 was not yet available; 3) LDL particle size was not assessed; 4) other important CVD risk markers were not measured, including steatosis and vascular plaque burden. Dietary pattern assessment was limited by its dependence on participant recall accuracy and by a lack of differentiation between sub-types of nutritional foods (e.g., mono- vs. poly-unsaturated fats and whether these came from fish vs. plant sources). High fructose food intake frequency was unassociated with CVD risk markers in this study, but high fructose intake has been previously associated with CVD risk factors [27, 59]. Because the NHANES questionnaire did not assess serving size, we were limited in that we were unable to estimate total fructose intake, which may have revealed associations with CVD risk markers. We were unable to account for some medications (e.g. non-steroidal anti-inflammatory drugs and oral contraceptive pills) that may have affected how MetS interacts with CVD risk. We also lacked data regarding pubertal stage, which is likely important given that during adolescence, advancing pubertal stage is associated with a transient decrease in LDL level but an increase in insulin resistance [60]. Nevertheless, all of our analyses were adjusted for age to help account for these changes. Additionally, NHANES is a cross-sectional study and we were not able to assess causality between the relations between MetS severity, dietary factors, and the CVD indices of interest.
Conclusions
We showed that elevations in the MetS severity correlated with higher LDL, ApoB, hsCRP, and uric acid measurements in adolescents. This supports the potential for this MetS z-score to be integrated into clinical practice as an additional measure for tracking overall health and CVD risk in adolescent patients. LDL, ApoB, and hsCRP also showed decreasing temporal trends, which were correlated with concurrent improvements in MetS z-score. This suggests improvements in metabolic health among U.S. adolescents, which might be attributable to increased health consciousness and public health efforts. Overall, these findings support the need for continued efforts to investigate use of MetS severity as a potential health marker, along with continued efforts to combat the obesity epidemic and its associated adverse outcomes.
Supplementary Material
Highlights.
The severity of the metabolic syndrome was highly correlated with LDL, ApoB, uric acid and CRP.
The relationship between MetS and LDL and ApoB was strongest in Hispanic adolescents.
LDL, ApoB and CRP levels in US adolescents have improved overall since 1999.
Regarding dietary influence on CVD risk factors, LDL and ApoB were related to saturated fat intake.
Acknowledgments
This work was supported by National Institutes of Health grant 1R01HL120960 (MJG and MDD).
Footnotes
Conflicts of interest: none.
Contributors: AML designed the analysis, performed research and wrote the manuscript. MJG designed the analysis and performed research. MDD designed the analysis, performed research and wrote the manuscript. All authors have approved the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, et al. Diagnosis and management of the metabolic syndrome - An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173:309–314. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110:1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 7.Gurka MJ, Ice CL, Sun SS, DeBoer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovascular Diabetology. 2012:11. doi: 10.1186/1475-2840-11-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurka MJ, Lilly CL, Norman OM, DeBoer MD. An Examination of Sex and Racial/Ethnic Differences in the Metabolic Syndrome among Adults: A Confirmatory Factor Analysis and a Resulting Continuous Severity Score. Metabolism. 2014;63:218–225. doi: 10.1016/j.metabol.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee AM, Gurka MJ, DeBoer MD. A metabolic syndrome severity score to estimate risk in adolescents and adults: current evidence and future potential. Expert Rev Cardiovasc Ther. 2016;14:411–413. doi: 10.1586/14779072.2016.1143360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBoer MD. Underdiagnosis of Metabolic Syndrome in Non-Hispanic Black Adolescents: A Call for Ethnic-Specific Criteria. Curr Cardiovasc Risk Rep. 2010;4:302–310. doi: 10.1007/s12170-010-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBoer MD, Gurka MJ, Hill Golden S, Musani SK, Sims M, Vishnu A, Guo Y, Pearson TA. Independent Associations between Metabolic Syndrome Severity & Future Coronary Heart Disease by Sex and Race. J Am Coll Card. 2016 doi: 10.1016/j.jacc.2016.10.088. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 13.McGill HC, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr. 2000;72:1307S–1315S. doi: 10.1093/ajcn/72.5.1307s. [DOI] [PubMed] [Google Scholar]
- 14.Hudson LD, Rapala A, Khan T, Williams B, Viner RM. Evidence for contemporary arterial stiffening in obese children and adolescents using pulse wave velocity: A systematic review and meta-analysis. Atherosclerosis. 2015;241:376–386. doi: 10.1016/j.atherosclerosis.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 15.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the Princeton Lipid Research Cohort Study. Diabetologia. 2015;58:2745–2752. doi: 10.1007/s00125-015-3759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of Metabolic Syndrome as a Predictor of Cardiovascular Disease Between Childhood and Adulthood: The Princeton Lipid Research Cohort Study. J Amer Coll Card. 2015;66:755–757. doi: 10.1016/j.jacc.2015.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeBoer MD, Gurka MJ, Morrison JA, Woo JG. Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int J Obes (Lond) 2016;40:1353–1359. doi: 10.1038/ijo.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 19.Lee AM, Gurka MJ, DeBoer MD. Trends in Metabolic Syndrome Severity and Lifestyle Factors Among Adolescents. Pediatrics. 2016;137:1–9. doi: 10.1542/peds.2015-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National_Center_for_Health_Statistics C. [Access date, 7 December 2016];National Health and Nutrition Examination Survey Protocol. http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 21.Beltrán-Sánchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol. 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Obesity and Trends in Body Mass Index Among US Children and Adolescents, 1999–2010. Jama-Journal of the American Medical Association. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 24.Kaplan N. Kaplan’s clinical hypertension. Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 25.National Nutrient Database for Standard Reference. http://ars.usda.gov/ba/bhncr/ndl.
- 26.Reedy J, Krebs-Smith SM. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J Am Diet Assoc. 2010;110:1477–1484. doi: 10.1016/j.jada.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jalal DI, Smits G, Johnson RJ, Chonchol M. Increased fructose associates with elevated blood pressure. J Am Soc Nephrol. 2010;21:1543–1549. doi: 10.1681/ASN.2009111111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DDM, Gurka MJ, Morrison JA, Woo JG. Inter-relationships between the Severity of Metabolic Syndrome, Insulin and Adiponectin and their Relationship to Future Type 2 Diabetes and Cardiovascular Disease. International Journal of Obesity. 2016 doi: 10.1038/ijo.2016.81. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeBoer MD. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: A need for screening tools to target interventions. Nutrition. 2013;29:379–386. doi: 10.1016/j.nut.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62:1543–1552. doi: 10.1016/j.metabol.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidson MH. Apolipoprotein measurements: is more widespread use clinically indicated? Clin Cardiol. 2009;32:482–486. doi: 10.1002/clc.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko BJ, Park KH, Shin S, Zaichenko L, Davis CR, Crowell JA, Joung H, Mantzoros CS. Diet quality and diet patterns in relation to circulating cardiometabolic biomarkers. Clin Nutr. 2016;35:484–490. doi: 10.1016/j.clnu.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1:445–466. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- 34.Yue P, Averna M, Lin X, Schonfeld G. The c. 43_44insCTG variation in PCSK9 is associated with low plasma LDL-cholesterol in a Caucasian population. Hum Mutat. 2006;27:460–466. doi: 10.1002/humu.20316. [DOI] [PubMed] [Google Scholar]
- 35.Polisecki E, Muallem H, Maeda N, Peter I, Robertson M, McMahon AD, Ford I, Packard C, Shepherd J, Jukema JW, et al. Genetic variation at the LDL receptor and HMG-CoA reductase gene loci, lipid levels, statin response, and cardiovascular disease incidence in PROSPER. Atherosclerosis. 2008;200:109–114. doi: 10.1016/j.atherosclerosis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barter PJ, Ballantyne CM, Carmena R, Castro Cabezas M, Chapman MJ, Couture P, de Graaf J, Durrington PN, Faergeman O, Frohlich J, et al. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. 2006;259:247–258. doi: 10.1111/j.1365-2796.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 37.Bachorik PS, Lovejoy KL, Carroll MD, Johnson CL. Apolipoprotein B and AI distributions in the United States, 1988–1991: results of the National Health and Nutrition Examination Survey III (NHANES III) Clin Chem. 1997;43:2364–2378. [PubMed] [Google Scholar]
- 38.Contois JH, McNamara JR, Lammi-Keefe CJ, Wilson PW, Massov T, Schaefer EJ. Reference intervals for plasma apolipoprotein B determined with a standardized commercial immunoturbidimetric assay: results from the Framingham Offspring Study. Clin Chem. 1996;42:515–523. [PubMed] [Google Scholar]
- 39.DeBoer MD, Gurka MJ. Ability among adolescents for the metabolic syndrome to predict elevations in factors associated with type 2 diabetes and cardiovascular disease: data from the national health and nutrition examination survey 1999–2006. Metab Syndr Relat Disord. 2010;8:343–353. doi: 10.1089/met.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sumner AE. Ethnic Differences in Triglyceride Levels and High-Density Lipoprotein Lead to Underdiagnosis of the Metabolic Syndrome in Black Children and Adults. Journal of Pediatrics. 2009;155:e7–e11. doi: 10.1016/j.jpeds.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the Metabolic Syndrome Is Associated With Disproportionately High Levels of High-Sensitivity C-Reactive Protein in Non-Hispanic Black Adolescents: An analysis of NHANES 1999–2008. Diabetes Care. 2011;34:734–740. doi: 10.2337/dc10-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeBoer MD, Dong L, Gurka MJ. Racial/Ethnic and Sex Differences in the Ability of Metabolic Syndrome Criteria to Predict Elevations in Fasting Insulin Levels in Adolescents. Journal of Pediatrics. 2011;159:975–981. doi: 10.1016/j.jpeds.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and sex differences in the relationship between uric acid and metabolic syndrome in adolescents: an analysis of National Health and Nutrition Survey 1999–2006. Metabolism. 2012;61:554–561. doi: 10.1016/j.metabol.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeBoer MD, Gurka MJ. Low sensitivity for the metabolic syndrome to detect uric acid elevations in females and non-Hispanic-black male adolescents: An analysis of NHANES 1999–2006. Atherosclerosis. 2012;220:575–580. doi: 10.1016/j.atherosclerosis.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.05.006. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 47.Adolescents EPoIGfCHaRRiCa, National Heart Ln, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr. 2015;169:272–279. doi: 10.1001/jamapediatrics.2014.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bingham SA. Limitations of the various methods for collecting dietary intake data. Ann Nutr Metab. 1991;35:117–127. doi: 10.1159/000177635. [DOI] [PubMed] [Google Scholar]
- 50.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Astrup A, Dyerberg J, Elwood P, Hermansen K, Hu FB, Jakobsen MU, Kok FJ, Krauss RM, Lecerf JM, LeGrand P, et al. The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: where does the evidence stand in 2010? Am J Clin Nutr. 2011;93:684–688. doi: 10.3945/ajcn.110.004622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leren P. The Oslo diet-heart study. Eleven-year report. Circulation. 1970;42:935–942. doi: 10.1161/01.cir.42.5.935. [DOI] [PubMed] [Google Scholar]
- 53.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 54.Turpeinen O, Karvonen MJ, Pekkarinen M, Miettinen M, Elosuo R, Paavilainen E. Dietary prevention of coronary heart disease: the Finnish Mental Hospital Study. Int J Epidemiol. 1979;8:99–118. doi: 10.1093/ije/8.2.99. [DOI] [PubMed] [Google Scholar]
- 55.Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52:283–289. doi: 10.1002/art.20761. [DOI] [PubMed] [Google Scholar]
- 56.Freedman LS, Commins JM, Moler JE, Willett W, Tinker LF, Subar AF, Spiegelman D, Rhodes D, Potischman N, Neuhouser ML, et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for potassium and sodium intake. Am J Epidemiol. 2015;181:473–487. doi: 10.1093/aje/kwu325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mackenzie T, Brooks B, O’Connor G. Beverage intake, diabetes, and glucose control of adults in America. Ann Epidemiol. 2006;16:688–691. doi: 10.1016/j.annepidem.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 58.Ford ES, Li C, Zhao G, Pearson WS, Tsai J, Churilla JR. Sedentary behavior, physical activity, and concentrations of insulin among US adults. Metabolism. 2010;59:1268–1275. doi: 10.1016/j.metabol.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 59.Wang DD, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Cozma AI, Mirrahimi A, Yu ME, Carleton AJ, Di Buono M, et al. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J Nutr. 2012;142:916–923. doi: 10.3945/jn.111.151951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J Pediatr. 2008;152:165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.