Abstract
Several composite markers have been proposed for risk assessment in chronic obstructive pulmonary disease (COPD). However, choice of parameters and score complexity restrict clinical applicability. Our aim was to provide and validate a simplified COPD risk index independent of lung function.
The PROMISE study (n=530) was used to develop a novel prognostic index. Index performance was assessed regarding 2-year COPD-related mortality and all-cause mortality. External validity was tested in stable and exacerbated COPD patients in the ProCOLD, COCOMICS and COMIC cohorts (total n=2988).
Using a mixed clinical and statistical approach, body mass index (B), severe acute exacerbations of COPD frequency (AE), modified Medical Research Council dyspnoea severity (D) and copeptin (C) were identified as the most suitable simplified marker combination. 0, 1 or 2 points were assigned to each parameter and totalled to B-AE-D or B-AE-D-C. It was observed that B-AE-D and B-AE-D-C were at least as good as BODE (body mass index, airflow obstruction, dyspnoea, exercise capacity), ADO (age, dyspnoea, airflow obstruction) and DOSE (dyspnoea, obstruction, smoking, exacerbation) indices for predicting 2-year all-cause mortality (c-statistic: 0.74, 0.77, 0.69, 0.72 and 0.63, respectively; Hosmer–Lemeshow test all p>0.05). Both indices were COPD specific (c-statistic for predicting COPD-related 2-year mortality: 0.87 and 0.89, respectively). External validation of B-AE-D was performed in COCOMICS and COMIC (c-statistic for 1-year all-cause mortality: 0.68 and 0.74; c-statistic for 2-year all-cause mortality: 0.65 and 0.67; Hosmer–Lemeshow test all p>0.05).
The B-AE-D index, plus copeptin if available, allows a simple and accurate assessment of COPD-related risk.
Short abstract
The B-AE-D indices allow a simple and accurate assessment of COPD-related risk in the absence of lung function http://ow.ly/XFBox
Introduction
Chronic obstructive pulmonary disease (COPD), one of the leading causes of death and disability, has become a major global epidemic throughout the world [1, 2]. Subsumed under “not fully reversible airway obstruction” COPD is a very heterogeneous disease with numerous molecular and clinical patterns [3]. To overcome the complexity of COPD and the limitations of lung function, a number of composite markers were introduced to assess outcome. Several scores, including the BODE index (body mass index (BMI), airflow obstruction, dyspnoea, exercise capacity), ADO index (age, dyspnoea, airflow obstruction) and DOSE index (dyspnoea, obstruction, smoking, exacerbation) predict COPD-related outcomes better than airflow obstruction alone [4–6]. However, several features of the index parameters are difficult to assess and may restrict routine clinical application. Exercise tests, as well as lung function tests, are associated with a certain test effort and rely on patient motivation. Parameters such as age and most comorbidities cannot be improved and thus do not qualify for serial assessment [7]. Scores incorporating lung function parameters do not allow risk stratification during an acute exacerbation of COPD (AECOPD). However, many patients might not present for follow-up or may exacerbate repeatedly before assessment of stable disease. Perhaps most importantly, simple tools such as the CURB-65 (confusion, urea 7 mmol·L−1, respiratory rate ≥30 breaths·min−1, blood pressure <90 mmHg (systolic), ≤60 mmHg (diastolic), age ≥65 years) are frequently used for risk stratification in pneumonia [8], but many clinicians are very reluctant to use more complicated scores. Novel indices of COPD-related risk, especially if including markers of stress and inflammation, might qualify for simplified risk assessment.
The aim of this study was to provide a novel simplified COPD-specific risk index for clinical application in stable and exacerbated disease and to assess whether excluding lung function parameters limits risk prediction.
Methods
Study design and patients
The investigator-driven PROMISE (Predicting Outcome using Systemic Markers in Severe Exacerbations of Chronic Obstructive Pulmonary Disease; www.isrctn.com ISRCTN99586989) cohort study was employed for the development of a novel risk index. PROMISE was a multi-centre longitudinal COPD study performed at 11 European tertiary respiratory centres evaluating predictors of outcome in COPD, during stable and exacerbated disease [9, 10]. Stable COPD patients in Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade II–IV were included. Index validation was performed in the ProCOLD, COCOMICS and COMIC cohorts. ProCOLD (Procalcitonin guided-antibiotic therapy in acute exacerbations of Chronic Obstructive Lung Disease) was a single centre COPD trial including hospitalised patients with COPD exacerbation [11]. Patients were required to meet the definition of severe AECOPD and spirometric COPD criteria. COCOMICS (Collaborative Cohorts to Assess Multicomponent Indices of COPD in Spain) includes individual patient data of several COPD cohorts in Spain [12]. Seven COCOMICS cohorts including stable COPD patients (Requena [13], Sevilla [14], Tenerife [15], Zaragoza [16]) and exacerbated COPD patients (Terrassa I–III [17, 18]) were used for index validation. COMIC (Cohort of Mortality and Inflammation in COPD) was a single centre study including stable and exacerbated COPD patients from the Netherlands [19]. All studies were approved by the local institutional review boards and all patients gave written informed consent. Detailed study characteristics are described in the supplementary material.
Predictors
Medical history, including the number of AECOPD and severe AECOPD within the previous year, clinical assessment and other predictors of outcome were evaluated at baseline (study inclusion). Lung function tests, 6-min walk distance and the modified Medical Research Council (mMRC) questionnaire were performed as described previously [20–23]. Serum copeptin levels were assessed in PROMISE and ProCOLD. AECOPD was defined as an acute sustained worsening of dyspnoea, cough and/or sputum beyond normal day-to-day variations in a patient with underlying COPD; a severe AECOPD was defined as a COPD exacerbation requiring hospitalisation [24, 25]. If a cause other than COPD was dominant, as assessed by the attending physician, the deterioration was not regarded as an AECOPD. In the COCOMICS cohorts a severe AECOPD was defined as an exacerbation requiring an emergency room visit or hospitalisation. In PROMISE, the 1-year study assessment was used for internal validation. The already established indices BODE, updated BODE (a recalibrated BODE index), ADO and DOSE were calculated as reported previously [4–6].
Outcome
The main outcome was all-cause mortality. In PROMISE, survival was assessed during the 2-year study follow-up. For every death, a physician defined the cause of death as COPD related, COPD unrelated or unknown. A subset of PROMISE patients (all patients included in the study centre in Basel, Switzerland) was additionally contacted 5 years after study inclusion to assess the survival status. Follow-up of the index development and validation cohorts are outlined in figures E1 and E2.
Variable selection
Index variables were selected using a clinical and statistical approach. All parameters had to fulfil four criteria. 1) COPD specificity: parameters were required to be reasonably related to COPD; 2) modifiability: to capture deterioration and improvement during the disease course parameters were required to be modifiable; 3) applicability: to facilitate clinical use parameters had to be easily applicable; 4) to predict outcome independently. Parameters such as age and comorbidities did not qualify due to the missing COPD specificity and non-modifiability. The 6-min walk distance, the lung function test and the St George's Respiratory Questionnaire did not qualify due to limitations in applicability, especially during exacerbations. The performance for predicting outcome was evaluated using univariate and multivariate cox regression analysis (tables E1 and E2). Smoking status and the number of total (moderate plus severe) exacerbations were excluded due to poor performance. BMI, the number of severe AECOPD, mMRC and copeptin remained the clinically most suitable and well-performing parameters. Furthermore, an entirely statistical approach was used to identify the best predictors for 2-year all-cause-mortality out of 58 parameters [26]. 10 parameters were selected including BMI, the number of severe AECOPDs, mMRC and copeptin (data not shown). In a second approach several other predictors were added to the model to test its performance (table E3). When age, sex, forced expiratory volume in 1 s (FEV1), smoking status or 6-min walk distance were added to these parameters the c-statistic of the regression models for predicting 2-year all-cause mortality improved by <0.01, providing only minor additional predictive performance.
Development of simplified and optimised indices
The four parameters BMI (B), severe AECOPD frequency (AE), mMRC dyspnoea severity (D) and copeptin (C) qualified for the novel risk index. Each parameter was stratified into three risk groups. The allocation was largely based on established cut-offs (tables 1 and 2). In two simplified scores (simplified B-AE-D and simplified B-AE-D-C) 0, 1 or 2 points were assigned to each risk group (table 1). Cox proportional hazard survival analysis was used to develop two optimised scores (optimised B-AE-D and optimised B-AE-D-C), with an individual number of points assigned (table 2 and table E4). An increasing number of points indicate an increased risk.
TABLE 1.
Assignment of points for the simplified B-AE-D and B-AE-D-C index
| 0 points | 1 point | 2 points | ||
| B | BMI kg·m−2 | ≥21 | <21 | <18.5 |
| AE | Number of severe AECOPD the previous year | 0 | 1 | ≥2 |
| D | mMRC dyspnoea score (at stable disease) | 0–2 | 3 | 4 |
| C | Copeptin at inclusion pmol·L−1 | <20 | ≥20 | ≥40 |
BMI: body mass index; AECOPD: acute exacerbations of chronic obstructive pulmonary disease; mMRC: modified Medical Research Council.
TABLE 2.
Assignment of points for the optimised B-AE-D and B-AE-D-C index
| 0 points | 3 points | 6 points | 7 points | 9 points | 10 points | ||
| B | BMI kg·m−2 | ≥21 | <21 | <18.5 | |||
| AE | Number of severe AECOPD the previous year | 0 | 1 | ≥2 | |||
| D | mMRC dyspnoea score (at stable disease) | 0–2 | 3 | 4 | |||
| C | Copeptin at inclusion pmol·L−1 | <20 | ≥20 | ≥40 |
BMI: body mass index; AECOPD: acute exacerbations of chronic obstructive pulmonary disease; mMRC: modified Medical Research Council.
Statistics
Discrete variables are expressed as counts (%) and continuous variables as mean±sd. Within group analyses of copeptin levels in survivors and nonsurvivors at baseline and at specified months before death were assessed by the Wilcoxon signed-rank test. Cox regression models were applied for index development, point allocation in optimised indices, assessment of index performance and validation. Performance of predictive models can be assessed by measures of discrimination and calibration. While discrimination describes the discriminative ability, calibration refers to the agreement of observed and predicted outcomes [27]. The c-statistic was used as a measure of discrimination and the Hosmer–Lemeshow goodness-of-fit test was applied for calibration. The latter evaluates whether predicted and observed risk differ across separate risk groups. Time to death was described by Kaplan–Meier survival curves and analysed by the log rank test. All tests were two-tailed and a p-value <0.05 was defined as significant. Data were analysed using SPSS version 20 (IBM, Armonk, NY, USA) and R project (www.r-project.org).
Results
530 patients from the PROMISE population with a mean±sd age of 67±10 years, who were predominantly male (70%) and with a mean±sd FEV1 of 49±17% pred were included (figure E1). Baseline characteristics are summarised in table E5. Patients with a follow-up of at least 2 years were more likely to have pulmonary hypertension, previous lung volume reduction surgery and pulmonary rehabilitation (p=0.047, 0.017 and 0.026, respectively). In contrast, patients excluded or lost to follow-up were more likely to smoke, had a higher number of previous exacerbations and required more long-term oxygen therapy (p=0.022, 0.001 and 0.047, respectively). All other baseline characteristics were similar between both groups. The 2-year all-cause mortality was 12% (n=54). The cause of death within 2 years was COPD-related in 46% (n=25), COPD was unrelated in 37% (n=20) and unknown in 17% (n=9). In 231 out of 233 patients from the study centre in Basel a 5-year follow-up was available. The 5-year all-cause mortality was 36% (n=82).
Individual parameters for predicting 2-year all-cause mortality
To assess individual parameters for predicting 2-year all-cause mortality, univariate Cox regression models were performed (table E1). Next to age, the BODE parameters (BMI, FEV1 % pred, mMRC and 6 min walking distance), the number of severe AECOPD, the St George's Respiratory Questionnaire and copeptin predicted 2-year all-cause mortality (all p<0.01). Interestingly, the performance of the GOLD combined assessment groups (A, B, C, D) and the total number of moderate and severe AECOPD in predicting outcome was poor (p=0.076 and 0.055, respectively).
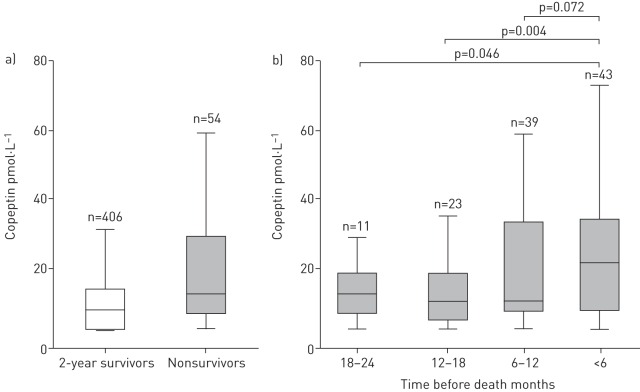
Multivariate Cox regression models were performed to identify independent predictors of all-cause mortality (table E2). FEV1 was not an independent predictor in any score. In contrast, mMRC independently predicted death within all indices. Copeptin was an independent predictor of 2-year all-cause mortality in all investigated models (also when added to parameters of BODE, updated BODE, ADO and DOSE; p≤0.001 in all). Copeptin levels were not different between stable or exacerbated COPD (stable disease versus moderate exacerbation (n=100): mean copeptin difference 1.8±12.5 pmol·L−1, p=0.2; stable disease versus severe exacerbation (n=41): mean copeptin difference 10.7±40.9 pmol·L−1, p=0.10). Interestingly, copeptin levels were highest within 6 months prior to death (figure 1).
FIGURE 1.
Distribution of copeptin levels a) in 2-year survivors and nonsurvivors at baseline and b) at specified time-points before death. Within group analyses were performed using Wilcoxon's signed rank test.
Index performance in predicting all-cause mortality
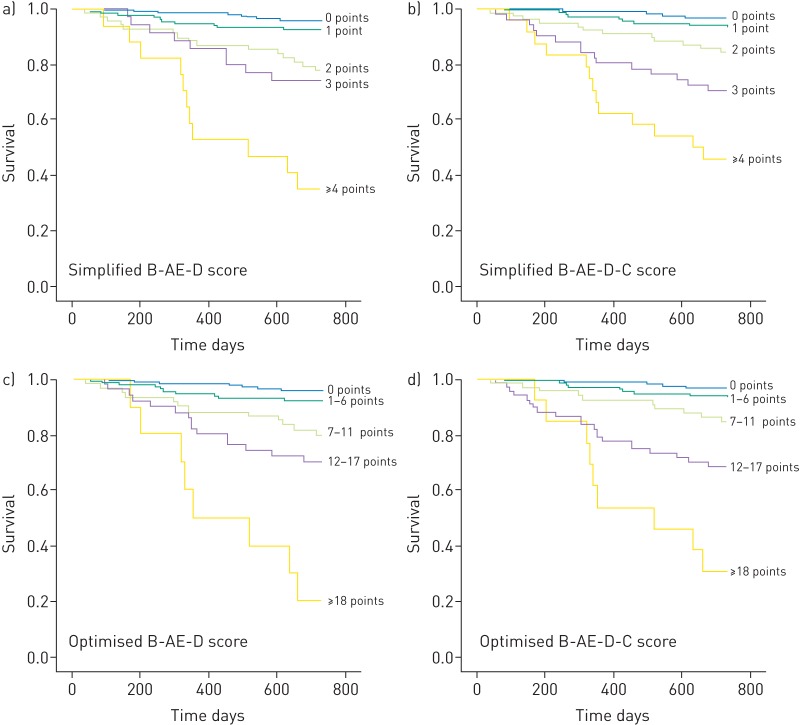
The score performance was assessed using the c-statistic (discrimination) and Hosmer–Lemeshow goodness-of-fit test (calibration). The point assignments of the simplified and optimised B-AE-D and B-AE-D-C indices are described in tables 1 and 2. Among the established indices, the BODE and updated BODE score discriminated best in predicting 1-year all-cause mortality (table 3). In the subgroup of patients with 5 year follow-up the best predictor for 3- and 5-year all-cause mortality was the ADO index (table 4). BODE and ADO were the best predictors of 2-year all-cause mortality. Discrimination declined for predicting 3- and 5-year all-cause mortality. Simplified and optimised B-AE-D and B-AE-D-C indices performed at least as good as other indices in predicting all-cause mortality up to 5 years. Lung function parameters did not improve the performance of B-AE-D or B-AE-D-C in predicting 2-year all-cause mortality (table E3). Calibration was acceptable within all scores (p>0.05). Increasing index points were associated with worse outcome (all indices: log rank p<0.001) (figure 2).
TABLE 3.
Discrimination and calibration for predicting all-cause mortality in the PROMISE study
| Indices | 1-year mortality# | 2-year mortality¶ | ||
| C-statistic (95% CI) | Calibration | C-statistic (95% CI) | Calibration | |
| BODE index+ | 0.76 (0.65–0.87) | 0.9 | 0.69 (0.61–0.78) | 0.2 |
| Updated BODE index+ | 0.78 (0.67–0.89) | 0.7 | 0.70 (0.61–0.78) | 0.6 |
| ADO index§ | 0.72 (0.62–0.82) | 0.3 | 0.72 (0.64–0.79) | 0.8 |
| DOSE index§ | 0.64 (0.54–0.73) | 0.9 | 0.63 (0.55–0.70) | 0.8 |
| Simplified B-AE-D index | 0.78 (0.68–0.87) | 0.4 | 0.74 (0.67–0.81) | 0.5 |
| Simplified B-AE-D-C index | 0.80 (0.71–0.90) | 0.7 | 0.77 (0.70–0.85) | 0.9 |
| Optimised B-AE-D index | 0.78 (0.69–0.88) | 0.9 | 0.75 (0.68–0.82) | 0.9 |
| Optimised B-AE-D-C index | 0.81 (0.71–0.91) | 0.2 | 0.78 (0.70–0.85) | 0.2 |
PROMISE: Predicting Outcome using Systemic Markers in Severe Exacerbations of Chronic Obstructive Pulmonary Disease; BODE: body mass index, airflow obstruction, dyspnoea, exercise capacity; ADO: age, dyspnoea, airflow obstruction; DOSE: dyspnoea, obstruction, smoking, exacerbation; B: body mass index; AE: severe acute exacerbation of chronic obstructive pulmonary disease frequency; D: modified Medical Research Council dyspnoea severity; C: copeptin. #: n=530; ¶: n=460; +: missing values in lung function test or 6-min walking distance (1 year: n=488, 2 years: n=416); §: missing values in lung function test (1 year: n=525, 2 years: n=444).
TABLE 4.
Discrimination and calibration for predicting all-cause mortality in the 5-year follow-up subpopulation of the PROMISE study
| Indices | 3-year mortality | 5-year mortality# | ||
| C-statistic (95% CI) | Calibration | C-statistic (95% CI) | Calibration | |
| BODE index¶ | 0.59 (0.50–0.67) | 0.6 | 0.60 (0.54–0.67) | 0.6 |
| Updated BODE index¶ | 0.60 (0.52–0.69) | 0.9 | 0.62 (0.55–0.68) | 0.4 |
| ADO index+ | 0.67 (0.59–0.75) | 0.11 | 0.68 (0.61–0.74) | 0.2 |
| DOSE index+ | 0.58 (0.50–0.65) | 0.7 | 0.58 (0.52–0.64) | 0.2 |
| Simplified B-AE-D index | 0.65 (0.57–0.72) | 0.2 | 0.62 (0.57–0.67) | 0.3 |
| Simplified B-AE-D-C index | 0.71 (0.64–0.79) | 0.7 | 0.68 (0.62–0.74) | 0.4 |
| Optimised B-AE-D index | 0.65 (0.57–0.72) | 0.3 | 0.62 (0.56–0.68) | 0.2 |
| Optimised B-AE-D-C index | 0.71 (0.63–0.78) | 0.10 | 0.67 (0.61–0.73) | 0.07 |
PROMISE: Predicting Outcome using Systemic Markers in Severe Exacerbations of Chronic Obstructive Pulmonary Disease; BODE: body mass index, airflow obstruction, dyspnoea, exercise capacity; ADO: age, dyspnoea, airflow obstruction; DOSE: dyspnoea, obstruction, smoking, exacerbation; B: body mass index; AE: severe acute exacerbation of chronic obstructive pulmonary disease frequency; D: modified Medical Research Council dyspnoea severity; C: copeptin. #: n=231; ¶: 211 missing values in lung function test or 6-min walking distance; +: 229 missing values in lung function test.
FIGURE 2.
Kaplan-Meier estimates of the 2-year survival of simplified and optimised B-AE-D and B-AE-D-C indices. B: body mass index; AE: severe acute exacerbation of chronic obstructive pulmonary disease frequency; D: modified Medical Research Council dyspnoea severity; C: copeptin.
Index performance in predicting COPD-related mortality
To assess whether indices predict COPD-related outcome (COPD-related mortality versus all other outcomes) Cox regression models were used (table 5). Analogous analyses were performed for 2-year COPD-unrelated mortality. B-AE-D and B-AE-D-C performed better in predicting COPD-related mortality than established indices. ADO and B-AE-D-C were the only indices predicting COPD-unrelated survival. Age and the adjusted Charlson comorbidity index were used for control. The performance of both was not better in predicting COPD-related mortality compared to COPD-unrelated mortality.
TABLE 5.
Discrimination for predicting 2-year cause-specific chronic obstructive pulmonary disease (COPD)-related and -unrelated mortality in the PROMISE study#
| Indices | COPD-related 2-year mortality | COPD-unrelated 2-year mortality |
| BODE index¶ | 0.86 (0.72–0.99) | 0.55 (0.42–0.68) |
| Updated BODE index¶ | 0.82 (0.68–0.96) | 0.57 (0.44–0.71) |
| ADO index+ | 0.84 (0.73–0.95) | 0.65 (0.52–0.77) |
| DOSE index+ | 0.78 (0.67–0.89) | 0.52 (0.39–0.64) |
| Simplified B-AE-D index | 0.87 (0.77–0.98) | 0.60 (0.48–0.72) |
| Simplified B-AE-D-C index | 0.89 (0.78–0.99) | 0.64 (0.52–0.76) |
| Optimised B-AE-D index | 0.88 (0.78–0.99) | 0.61 (0.49–0.73) |
| Optimised B-AE-D-C index | 0.90 (0.79–0.99) | 0.65 (0.52–0.77) |
| Age years | 0.59 (0.47–0.70) | 0.64 (0.52–0.77) |
| Adjusted Charlson comorbidity index | 0.62 (0.50–0.73) | 0.62 (0.50–0.74) |
Data are presented as C-statistic (95% CI). PROMISE: Predicting Outcome using Systemic Markers in Severe Exacerbations of Chronic Obstructive Pulmonary Disease; BODE: body mass index, airflow obstruction, dyspnoea, exercise capacity; ADO: age, dyspnoea, airflow obstruction; DOSE: dyspnoea, obstruction, smoking, exacerbation; B: body mass index; AE: severe acute exacerbation of chronic obstructive pulmonary disease frequency; D: modified Medical Research Council dyspnoea severity; C: copeptin.#: n=460; ¶: 416 missing values in lung function test or 6-min walking distance; +: 444 missing values in lung function test.
Internal validation
For internal validation we used all B-AE-D-C parameters 1 year after study inclusion during stable disease rather than a statistical approach. Points were assigned as described above. The score performance regarding 1-year and 4-year outcome was evaluated (n=299 and 185, respectively). Simplified B-AE-D predicted 1- and 4-year all-cause mortality (c-statistic (95% CI) 0.74 (0.63–0.86) and 0.62 (0.54–0.69); Hosmer–Lemeshow test: p=0.7 and 0.3, respectively). When copeptin was included (B-AE-D-C) a performance improvement was observed for predicting 4-year all-cause mortality (c-statistic for 1- and 4-year all-cause mortality: 0.75 (0.62–0.88) and 0.68 (0.61–0.76); Hosmer-Lemeshow test: p=0.2 and 0.9, respectively). Since optimised indices only provided a minor performance improvement at the cost of simplicity we did not validate them.
External validation of B-AE-D-C
Score performance was validated in a different cohort of 160 patients hospitalised for AECOPD, ProCOLD (figure E2). Patients had a mean±sd age of 71±10 years, were mainly male (56%) and had a mean±sd FEV1 of 41±18% pred (table E6). 2-year all-cause mortality was 23% (n=36). Dyspnoea severity (mMRC) was not assessed and therefore not available. Simplified B-AE as well as simplified B-AE-C predicted 2- and 5-year all-cause mortality (2-year all-cause mortality: c-statistic 0.60 (0.52–0.69) and 0.69 (0.60–0.78), respectively; Hosmer–Lemeshow test: p=0.17 and 0.8, respectively; 5-year all-cause mortality: c-statistic 0.59 (0.52–0.66) and 0.65 (0.57–0.72); Hosmer–Lemeshow test: p=0.08 and 0.2, respectively).
External validation of B-AE-D
The B-AE-D index was validated in the COCOMICS cohorts from Spain and the COMIC study from the Netherlands. 2153 patients from seven COCOMICS cohorts were included for B-AE-D validation (figure E2). Patients had a mean±sd age of 66±10 years, were mostly male (93%) and had a mean FEV1 of 52±20% pred (table E6). 2-year all-cause mortality was 15% (n=307). B-AE-D predicted 1-, 2- and 3-year all-cause mortality (c-statistic: 0.68 (0.63–0.72), 0.65 (0.62–0.69) and 0.63 (0.61–0.66), respectively) (table E7). B-AE-D was well calibrated (Hosmer–Lemeshow test: p=0.5, 0.3 and 0.2, respectively). 675 patients of the COMIC study were analysed for further B-AE-D validation (figure E2). Patients of COMIC had a mean±sd age of 67±10 years, were predominately male (60%) and had a mean±sd FEV1 of 53±19% pred (table E6). 2-year all-cause mortality was 12% (n=82). B-AE-D at stable disease predicted 1-, 2- and 3-year all-cause mortality (c-statistic: 0.74 (0.65–0.83), 0.67 (0.61–0.72) and 0.66 (0.61–0.70), respectively). B-AE-D was well calibrated for predicting all-cause mortality (Hosmer–Lemeshow test: p=0.2, 0.5 and 0.5, respectively).
Discussion
Herein, we propose a novel, simple and lung function independent tool for risk assessment in COPD. The B-AE-D and B-AE-D-C indices perform as well as established more complicated indices for predicting outcome. Both indices are COPD specific and predict death in stable and exacerbated COPD.
Clinical applicability and significance of included parameters were our priority in index generation. Thus, parameters were primarily selected on clinical features rather than statistical performance. The BMI, severe AECOPD frequency, dyspnoea severity and copeptin were identified as a combination of easy applicable, predictive and relevant COPD parameters. The relevance and predictive properties of all four individual parameters have been reported previously.
Whereas overweight appears to be protective, low body weight is a well-known predictor of mortality [28–30]. Notably, weight change is associated with a change in outcome [31, 32]. Besides the well-established threshold of BMI in COPD (21 kg·m−2) we identified a further increased risk in patients with a BMI <18.5 kg·m−2, the established cut-off for low weight in the normal population [4, 33]. Low in-hospital mortality and high long-term mortality is a familiar phenomenon of severe AECOPD [34]. Several studies reported high 2-year mortality after AECOPD hospitalisation, ranging up to 49% [13, 17, 34, 35]. Interestingly, moderate AECOPD and COPD combined assessment groups, defined by frequent exacerbations only (GOLD C2), were not associated with worse outcome, emphasising exacerbation severity for predicting outcome [13, 36, 37]. This is in line with our finding that the total AECOPD frequency did not predict mortality, whereas severe AECOPD predicted an unfavourable outcome. In the majority of COPD patients, dyspnoea is the predominant and most distressing symptom associated with poor survival not reflected by other parameters [38, 39]. Recently, it was reported that COPD GOLD B patients have a worse survival than group C patients, despite group C patients having a poorer lung function and/or more AECOPD [40]. Consequently, dyspnoea seems to be more relevant than lung function and AECOPD frequency in predicting risk. However, dyspnoea may fluctuate, especially during exacerbations, potentially affecting predictive performance unfavourably. AECOPD frequency and dyspnoea were recently included in the GOLD recommended COPD grading system, further highlighting the importance of both parameters. In contrast to previous COPD risk scores we did not include FEV1 in the novel indices. This was due to the transient reduction of lung volumes during and after AECOPD and the difficulties in performing reliable lung function measurements at exacerbation [41]. Importantly, B-AE-D and B-AE-D-C performed as well as other indices, including airflow obstruction, and there was no further index improvement after the addition of FEV1. Still, lung function is fundamental for COPD diagnosis. However, once a diagnosis is established, practical and intrinsic difficulties of spirometry limit its usefulness for risk prediction, particularly in severe COPD.
Multiple receptors throughout the body sense information and facilitate, if required, subsequent compensatory changes by the autonomic nervous and endocrine system, among others. These well performing systems can be employed for clinical purposes. By this means hypoxaemia, hypercapnia, inflammation and several other stressors cause an increase in arginine vasopressin (AVP) synthesis and release; thus, indicating AVP as a plausible COPD marker [42–44]. However, the short half-life of AVP precludes its clinical application. Copeptin, a glycopeptide comprising the C-terminal part of the AVP prohormone, is a stable, more reliable alternative for AVP measurement [22]. The performance of copeptin in predicting mortality was reported previously and a threshold of 40 pmol·L−1 was suggested [45]. Herein we demonstrate that: 1) copeptin levels are similar in stable and exacerbated COPD; 2) copeptin levels increase within the months before death; and 3) copeptin is an independent well-performing predictor of mortality; all of which are important features of a prognostic marker.
For index point allocation we proposed simplified and optimised indices. However, the performance in predicting outcome was similar. Due to the main objective of simplicity, we propose the simplified B-AE-D index for COPD risk assessment plus copeptin if available. The performance of B-AE-D-C, including copeptin, was similar to B-AE-D for predicting 1- and 2-year all-cause mortality. However, there was a moderate performance improvement after 2 years. Importantly, the additional value of copeptin needs to be better defined in future investigations. Moreover, it is necessary to evaluate whether the performance improvement justifies the additional costs.
The indices are composed of three to four parameters with three easy memorable risk groups. A 0-1-2 allocation is probably the simplest index for risk assessment in COPD to date. Although simplicity was not at the cost of index performance as validated internally and externally. External validation of B-AE-D was performed in the COMICS and COCOMICS cohorts. The large number of patients and variability in COPD severity, phenotypes, and ethnical and social background allowed validation in a broad spectrum of COPD. B-AE-D-C was validated 1 year after inclusion in PROMISE (internal validation) and in ProCOLD without dyspnoea (external validation). Population heterogeneity within and across different cohorts caused variation in index performance but also ensured optimal B-AE-D validation.
We also demonstrate that B-AE-D and B-AE-D-C predicted COPD-related mortality. Accordingly, the main determinant of low BMI, severe AECOPD, dyspnoea severity and high copeptin levels is the baseline severity of COPD; i.e. the greater the severity of COPD the greater the likeliness of low BMI, severe AECOPD, severe dyspnoea, high copeptin and also death.
Comorbidities are common in COPD and have important therapeutic and prognostic implications [46, 47]. Moreover comorbidities, in particular cardiac disease, may increase the number of severe AECOPDs and bias dyspnoea assessment. In line with previous findings several comorbidities had an effect on AECOPD frequency and outcome. But importantly, comorbidities did not restrict the performance of B-AE-D or B-AE-D-C in predicting outcome (data not shown). Furthermore, phenotypes might affect index performance. In a previous study BODE and ADO were only poorly associated with COPD phenotypes [48]. In PROMISE, several markers reflecting COPD phenotypes (more than two COPD exacerbations per year, chronic cough, oxygen saturation at rest and during exercise) had no effect on B-AE-D performance (data not shown).
We acknowledge several limitations. First, we stratified dyspnoea by using the mMRC dyspnoea scale. Despite its frequent use and reasonably good validation, more precise tools for dyspnoea assessment are available and may further improve predictive performance [6, 49]. Secondly, dyspnoea was not assessed in the ProCOLD validation cohort; consequently, B-AE-C was evaluated rather than B-AE-D-C. Dyspnoea may further increase the predictive performance of B-AE-C, however, this cannot be proved. Thirdly, due to different inclusion criteria and study locations, patient characteristics across the cohorts are diverse and index performance is variable; although population heterogeneity may reflect real-life conditions. Finally, ethnicity and differences in dyspnoea perception possibly affect B-AE-D point allocation and potentially performance. Thus, further validation across a range of disease severities and different populations outside Europe is required.
However, a large sample size with a sufficient number of events, follow-up time of 5 years, assessment of both all-cause and COPD-related mortality, as well as internal and extensive external validation are strengths of the current study, mostly not provided in reports of other novel COPD indices. This study allowed us to establish a very simple lung function independent index of risk in COPD. B-AE-D is easy to obtain, COPD-specific and performs at stable and exacerbated COPD. Furthermore, B-AE-D is assessed at almost no cost, therefore, accessible in high- and low-income countries.
To conclude, the B-AE-D index, plus copeptin where available, allows a simple and accurate assessment of COPD-related risk in the absence of lung function.
Acknowledgements
A. Schötzau performed data management of PROMISE for which he received financial compensation.
Footnotes
Editorial comment in Eur Respir J 2016; 47: 1601–1605.
This article has supplementary material available from erj.ersjournals.com
Support statement: Thermo Scientific Biomarkers (Hennigsdorf, Germany) provided all the reagents for copeptin measurements. Funding information for this article has been deposited with FundRef
Conflict of interest: None declared.
References
- 1.Rosenbaum L, Lamas D. Facing a “slow-motion disaster” – the UN Meeting on noncommunicable diseases. N Engl J Med 2011; 365: 2345–2348. [DOI] [PubMed] [Google Scholar]
- 2.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370: 765–773. [DOI] [PubMed] [Google Scholar]
- 3.Taraseviciene-Stewart L, Voelkel NF. Molecular pathogenesis of emphysema. J Clin Invest 2008; 118: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celli BR, Cote CG, Marín JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 5.Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet 2009; 374: 704–711. [DOI] [PubMed] [Google Scholar]
- 6.Jones RC, Donaldson GC, Chavannes NH, et al. Derivation and validation of a composite index of severity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 180: 1189–1195. [DOI] [PubMed] [Google Scholar]
- 7.Celli BR, Marin JM, Cote CG, et al. Prognostic assessment of patients with COPD. Lancet 2009; 374: 1885–1887. [DOI] [PubMed] [Google Scholar]
- 8.Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolz D, Kostikas K, Blasi F, et al. Adrenomedullin refines mortality prediction by the BODE index in COPD: the “BODE-A” index. Eur Respir J 2014; 43: 397–408. [DOI] [PubMed] [Google Scholar]
- 10.Stolz D, Meyer A, Rakic J, et al. Mortality risk prediction in COPD by a prognostic biomarker panel. Eur Respir J 2014; 44: 1557–1570. [DOI] [PubMed] [Google Scholar]
- 11.Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD. Chest 2007; 131: 9–19. [DOI] [PubMed] [Google Scholar]
- 12.Marín JM, Alfageme I, Almagro P, et al. Multicomponent indices to predict survival in COPD: the COCOMICS study. Eur Respir J 2013; 42: 323–332. [DOI] [PubMed] [Google Scholar]
- 13.Soler-Cataluña JJ, Martínez-García MA, Román, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfageme I, Reyes N, Merino M, et al. The effect of airflow limitation on the cause of death in patients with COPD. Chronic Respir Dis 2010; 7: 135–145. [DOI] [PubMed] [Google Scholar]
- 15.Casanova C, Cote C, de Torres JP, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171: 591–597. [DOI] [PubMed] [Google Scholar]
- 16.Marín JM, Soriano JB, Carrizo SJ, et al. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. Am J Respir Crit Care Med 2010; 182: 325–331. [DOI] [PubMed] [Google Scholar]
- 17.Almagro P, Calbo E, Ochoa de Echagüen A, et al. Mortality after hospitalization for COPD. Chest 2002; 121: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 18.Almagro P, Salvadó M, Garcia-Vidal C, et al. Recent improvement in long-term survival after a COPD hospitalisation. Thorax 2010; 65: 298–302. [DOI] [PubMed] [Google Scholar]
- 19.Zuur-Telgen MC, Brusse-Keizer MG, Vandervalk PD, et al. Stable state MR-proadrenomedullin level is a strong predictor of mortality in COPD patients. Chest 2013; 145: 534–541. [DOI] [PubMed] [Google Scholar]
- 20.Crapo RO, Casaburi R, Coates AL, et al. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 21.Brusasco V, Crapo R, Viegi G, et al. Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J 2005; 26: 1–2. [DOI] [PubMed] [Google Scholar]
- 22.Morgenthaler NG, Morgenthaler NG, Struck J, et al. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 2006; 52: 112–119. [DOI] [PubMed] [Google Scholar]
- 23.Morgenthaler NG, Struck J, Jochberger S, et al. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metabol 2008; 19: 43–49. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000; 117: Suppl. 2, 398S–401S. [DOI] [PubMed] [Google Scholar]
- 25.Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J 2008; 31: 416–469. [DOI] [PubMed] [Google Scholar]
- 26.Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol 1996; 58: 267–288. [Google Scholar]
- 27.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models. Epidemiology 2010; 21: 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landbo C, Prescott E, Lange P, et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 160: 1856–1861. [DOI] [PubMed] [Google Scholar]
- 29.Hallin R, Gudmundsson G, Suppli Ulrik C, et al. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD). Respir Med 2007; 101: 1954–1960. [DOI] [PubMed] [Google Scholar]
- 30.Rutten EPA, Calverley PMA, Casaburi R, et al. Changes in body composition in patients with chronic obstructive pulmonary disease: do they influence patient-related outcomes. Ann Nutr Metab 2013; 63: 239–247. [DOI] [PubMed] [Google Scholar]
- 31.Schols A, Slangen J, Volovics L, et al. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 157: 1791–1797. [DOI] [PubMed] [Google Scholar]
- 32.Prescott E, Almdal T, Mikkelsen KL, et al. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J 2002; 20: 539–544. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation (World Health Organization technical report series 894) WHO, Geneva, 2000. Available from: www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ [Google Scholar]
- 34.Connors AF, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. Am J Respir Crit Care Med 1996; 154: 959–967. [DOI] [PubMed] [Google Scholar]
- 35.Groenewegen KH, Schols A, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003; 124: 459–467. [DOI] [PubMed] [Google Scholar]
- 36.Agusti A, Edwards LD, Celli B, et al. Characteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohort. Eur Respir J 2013; 42: 636–646. [DOI] [PubMed] [Google Scholar]
- 37.Soler-Cataluña JJ, Martínez-García MA, Sánchez LS, et al. Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med 2009; 103: 692–699. [DOI] [PubMed] [Google Scholar]
- 38.Nishimura K, Izumi T, Tsukino M, et al. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002; 121: 1434–1440. [DOI] [PubMed] [Google Scholar]
- 39.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54: 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification. Am J Respir Crit Care Med 2012; 186: 975–981. [DOI] [PubMed] [Google Scholar]
- 41.Cote CG, Dordelly LJ, Celli BR. Impact of COPD exacerbations on patient-centered outcomes. Chest 2007; 131: 696–704. [DOI] [PubMed] [Google Scholar]
- 42.Raff H, Shinsako J, Keil LC, et al. Vasopressin, ACTH, and corticosteroids during hypercapnia and graded hypoxia in dogs. Am J Physiol 1983; 244: E453–E458. [DOI] [PubMed] [Google Scholar]
- 43.Schrier RW, Berl T, Anderson RJ. Osmotic and nonosmotic control of vasopressin release. Am J Physiol 1979; 236: F321–F332. [DOI] [PubMed] [Google Scholar]
- 44.Raff H, Kane CW, Wood CE. Arginine vasopressin responses to hypoxia and hypercapnia in late-gestation fetal sheep. Am J Physiol 1991; 260: R1077–R1081. [DOI] [PubMed] [Google Scholar]
- 45.Stolz D, Christ-Crain M, Morgenthaler NG, et al. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest 2007; 131: 1058–1067. [DOI] [PubMed] [Google Scholar]
- 46.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009; 33: 1165–1185. [DOI] [PubMed] [Google Scholar]
- 47.Fabbri LM, Luppi F, Beghé B, et al. Complex chronic comorbidities of COPD. Eur Respir J 2008; 31: 204–212. [DOI] [PubMed] [Google Scholar]
- 48.Camiciottoli G, Bigazzi F, Bartolucci M, et al. BODE-index, modified BODE-index and ADO-score in chronic obstructive pulmonary disease: relationship with COPD phenotypes and CT lung density changes. COPD 2012; 9: 297–304. [DOI] [PubMed] [Google Scholar]
- 49.Han MK, Muellerova H, Curran-Everett D, et al. GOLD 2011 disease severity classification in COPDGene: a prospective cohort study. Lancet Respir Med 2013; 1: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]