Abstract
Objective
To identify the particularities of the clinical phenotype of endothelial dysfunction in a lot of Romanian patients from a reference center and compare it to data reported by international registries.
Material and methods
51 patients were included in a cross-sectional study. The patients were evaluated for the pattern of disease, main visceral involvement, serum markers of disease.
Results
41.2% patients had history of digital ulcers, 27.45% had pulmonary arterial hypertension; cardiovascular involvement also included: diastolic dysfunction in 31.1% of the patients, global systolic dysfunction in 9.8%, rhythm and conduction disturbances in 19.6%, peripheral vascular disease in 19.6%. Scleroderma renal crisis was identified in 2 patients.
Conclusion
Vascular complications are a major cause of morbidity and mortality in systemic sclerosis. Earlier therapeutic intervention demands improved screening and diagnosis in all cases.
INTRODUCTION
Systemic sclerosis or scleroderma (SSc) is one of the most challenging and enigmatic rheumatic disease associated with increased morbidity and mortality. Endothelial dysfunction is probably the primary event in evolution (1). The triggering factors and mechanism of vascular lesions are complex and not fully understood (endothelial cell apoptosis induced by infectious triggers, immune mediated cellular to xicity, antiendothelial antibodies, ischemiareperfusion lesions) (2). Initial functional changes are reversible, but progressive vascular lesions leads to small and medium vessels obliteration with irreversible structural damage (3). Endothelial dysfunction plays a major role in immune activation, triggers coagulation and is an indirect driver of fibrosis (2,3). Clinical aspects of endothelial dysfunction in initial phases includes Raynaud and nailfold capillaroscopy abnormalities. In further stages, major complications such as pulmonary hypertension, heart involvement, scleroderma renal crisis appear (1). Digital ulcers, erectile dysfunction, gastrointestinal involvement are also dependent of endothelial dysfunction (1).
OBJECTIVE
Identifying particularities of clinical phenotype of endothelial dysfunction in Romanian SSc patients from a reference center.
MATERIAL AND METHODS
51 patients were included in a cross-sectional study. The patients were selected according to the American College of Rheumatology criteria for SSc (4). The patients were evaluated for the pattern of disease, Rodnan score, musculoarticular, gastrointestinal, cardiovascular, pulmonary and renal involvement, inflammatory markers, autoantibodies (anti-nuclear, anti- centromere, anti-scleroderma 70), cholesterol and triglyceride levels, nailfold capi llaroscopy. Several questionnaires were completed: European Disease Activity Score (5), MEDSGER severity score (5) and health assessment questionnaire- disability index (HAQ-DI) (6). The subjects’ written consent was obtained according to the Declaration of Helsinki. The study has been approved by a local ethics committee.
All calculations were performed with SPSS Statistics 20.0. All data are presented as mean ± standard deviation (SD) or percentages. Student t-test or Mann-Whitney test were used to evaluate differences for nominal variable. The chi-square test was used to compare categorical variables. Pearson’s bivariate correlation or Spearman’s rank correlation coefficient were used to evaluate the association between evaluated variables. The values of r>0.5 and p@0.05 were considered statistically significant.
RESULTS
27 diffuse and 24 limited SSc, 47 women and 4 men were evaluated. Medium age was 55.65 (12.45) years, medium disease duration was 11.7 (6.9) years. Medium disease activity score was 3.43 (2.14), medium disease severity score was 6.84 (3.15).
Active digital ulcers were identified in 15.68% cases and 41.2% patients had history of digital ulcers. 80.95% patients had recurent digital ulcers and almost a quater of them had critical ischemia with necrosis. Medium disease duration until the appearrence of the first digital ulcer was 4.17 (2.28) years. The difference of the incidence of ulcers comparing subsets (p=0.07) or specific immunologic prophyle (p=0.061) is not significant. The ulcers were correlated with health (p=0.035, r=0.305) and pain (p=0.017, r=0.344) analoque scales included in the HAQ.
Pulmonary arterial hypertension (PAH) was identified in 14 cases (27.45%), 8 diffuse and 6 limited. 35.71% of the patients had mild PAH, 35.71% moderate and 28.57% had severe PAH. PAH seems to be a late complication (66.66% of the diffuse forms and 55.55% of the limited forms developed PAH after 5 years).
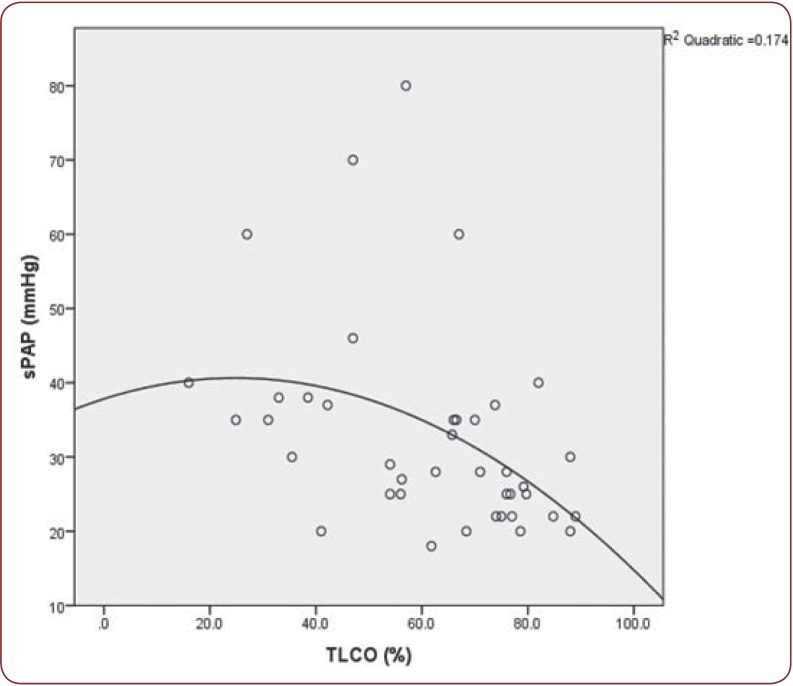
Patients with PAH had increased frequency of dyastolic dysfunction (p@0.001) and arrhythmias (p=0.003), lower diffusing capacity or transfer factor of the lung for carbon monoxide (DLCO)(p@0.001), higher Medsger scores (p@0.001) and late capillaroscopy pattern (p@0.001). The value of systolic pulmonary arterial pressure (sPAP) was correlated with DLCO (p=0.001, r=-0.499) (Figure 1) and FEV1 (p@0.001, r=-0.311), joint contractures (p=0.022, r=0.342), higher HAQ scores (p=0.008, r=0.406), Rodnan scores (p=0.019, r=0.349), activity scores (p=0.033, r=0.318), and severity scores (p=0.001, r=0.461).
The mortality was 3 times higher then the rest of the group (14.28% vs 4.58%). Medium life expectancy after PAH diagnosis was 47.53 months.
Diastolic dysfunction was identified in 14 cases (31.1%) and it was not related to disease subset (p=0.07). Arterial hypertension (p=0.044, r=0.301) and higher severity scores (p=0.042, r=0.303) were correlated with diastolic dysfunction.
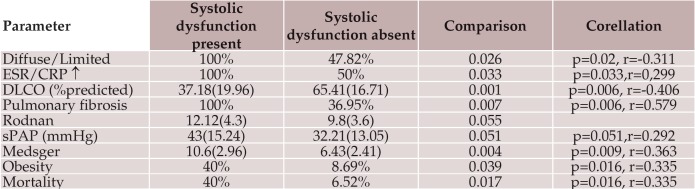
Global systolic dysfunction was identified in 5 patients (9.8%). Patients with altered systolic function had significantly lower lung volumes, higher Rodnan scores, higher sPAP, inflammatory syndrome and higher mortality rate.There was a strong correlation between diffuse form, DLCO, severity scores and mortality rate with systolic dysfunction (Table 1).
Rhythm and condunction disturbances were frecquent (19.6%) and associated with specific disease features as: joint crepitus (p=0.006, r=0.377), elevated creatinkinase (p=0.016, r=0.335), Medsger score (p=0.047, r=0.279) and lower lung lung volumes [forced vital capacity (FVC) p=0.026, r=-0.336 and forced expiratory volume (FEV) p=0.031, r= - 0.325)].
Scleroderma renal crisis was identified in 2 patients, both males with diffuse skin disease. Both of them had higher activity (p=0.005) and severity scores (p=0.008).
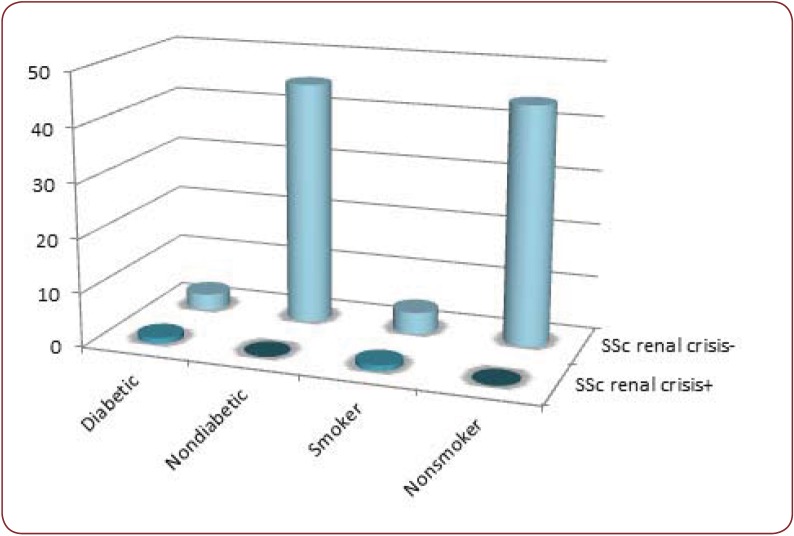
Cardiovascular risk factors: the studied group included 4 patients with type II diabetes mellitus, 5 smokers, 9 patients with dyslipidemia, 8 with arterial hypertension and 6 obese patients. Both diabetes and smoking was correlated with increased incidence of digital ulcers (p=0.013 for diabetes, p=0.005 for smoking) and for scleroderma renal crisis (p=0.025 for diabetes, p=0.051 for smoking) (Figure 2). Patients with dyslipidemia and obesity associated in a higher percentage lung fibrosis (p=0.021 for dyslipidemia, p=0.034 for obesity). A negative correlation was identified between lung volumes and the level of dyslipidemia (FVC - p=0.032, r=-0.324, FEV - p=0.026, r=-0.282). Patients with dyslipidemia developed more often cardiac rhythm and conduction distubances (p=0.039).
Reported macrovascular events were: stroke-1 case, peripheral vascular disease-10 cases, stable coronary heart disease-6 cases. Scleroderma patients with coronary heart disease had higher activity and severity scores then the others (p=0.027, respectivly 0.046). Patients with peripheral vascular disease used more frequently corticosteroids (p=0.04).
Figure 1.
Figure 1. Correlation between sPAP (systolic pulmonary artery pressure) and DLCO (diffusing capacity for carbon monoxide) (p=0.001, r=-0.499).
Table 1.
Table 1. Differences between patients with and without systolic dysfunction. Data are presented as mean values (standard deviation) or percentages for each parameter, p values for differences, r - Pearson’s bivariate correlation/ Spearman’s rank correlation coefficient for the association between evaluated variable.
ESR-erythrocytes sedimentation rate, CRP-C reactive protein, DLCO-diffusing capacity for carbon monoxide, sPAPsystolic pulmonary artery pressure.
Figure 2.
Figure 2. Diabetes mellitus and smoking are associated with increased risk of scleroderma renal crisis (p=0.025, respectively p=0.051).
DISCUSSION
Digital ulcers are a frequent complication of systemic sclerosis, between 8 and 31% (7), with an important impact on the quality of life, especially due to pain and esthetic problems (8). In our group they are an early disease complication (first 5 years), although there was no correlation between digital ulcers and disease duration, pointing to the fact that ulcers are related not only to primary endothelial dysfunction in the earlier stages of disease but also to fibrosis and vascular remodeling. Due to high incidence, recurency, possible evolution to severe ischemia, higher pain and disease activity scales they have an important morbidity impact. Their appearence is independent of the immunological prophyle (p=0.07) and disease subset (p=0.06), as most of the registries report (8,9).
The pathogenesis of digital ulcers is complex: vascular dysfunction, skin atrophy and thethering, calcinosis, digital contractures, local microtrauma (10). For patients with active digital ulcers correlations with cutaneous thinning scores (p=0.031) digital contractures p=0.012).
The incidence of cardiac disease in SSc is hard to estimate due to late and polymorph clinical symptoms, lack of specificity of the usual evaluation procedures (electrocardiography, echocardiography) (11).
The reported frequency of systolic dysfunction of 5.4%, but up to 30% of the patients can have one or multiple hipokinetic regions (12). Advanced age, male sex, diffuse skin involvement, long disease duration, prevalent digital ulcers, renal, pulmonary and muscular involvement have been associated with left ventricular dysfunction (13). In our group, global systolic dysfunction seems to be more frequent than registries data. It was associated with the diffuse subset (p=0.026, r=-0.311) and had a correlation trend with the Rodnan score (p=0.055). It can be considered a negative prognostic factor due to higher incidence of concomitant severe visceral involement (lung fibrosis, pulmonary hypertension), higher severity scores and mortality rates (Table 1).
Diastolic dysfunction is common in scleroderma patients, up to 52% of the patients (13). The causes are complex: decreased coronary reserve related to coronary microcirculation abnormalities, fibrotic remodeling, ischemiareperfusion lesions (13,14). For our group, the incidence of diastolic dysfunction is lower (31.1%) possibly related to the lack of accesibility to tissue Doppler, with higher sensitivity and specificity in early detection. Though diastolic dysfunction is frecquently related to diffuse forms (14), in our group there was no difference when comparing the two subsets (p=0.84). The close relationship between diastolic dysfunction and age is well known. Even using common diagnostic techniques, we could correlate dyastolic dysfunction with Medsger score, sPAP (p=0.06, r=0.283) and disease duration (p=0.059, r=-0.269), One recent review of myocadial involvement in systemic sclerosis states that diastolic dysfunction is related to disease duration and is an independent predictor of death (15).
Rhythm and conduction disturbances were associated with lower respiratory volumes, dyslipidemia and coronary heart disease suggesting that primary miocardial involvement was not their cause. Correlations with specific features of disease as joint crepitus (p=0.006, r=0.377) and elevated creatinkinase (p=0.016, r=0.335) support the idea that are secondary to excitoconductor tissue fibrosis (16).
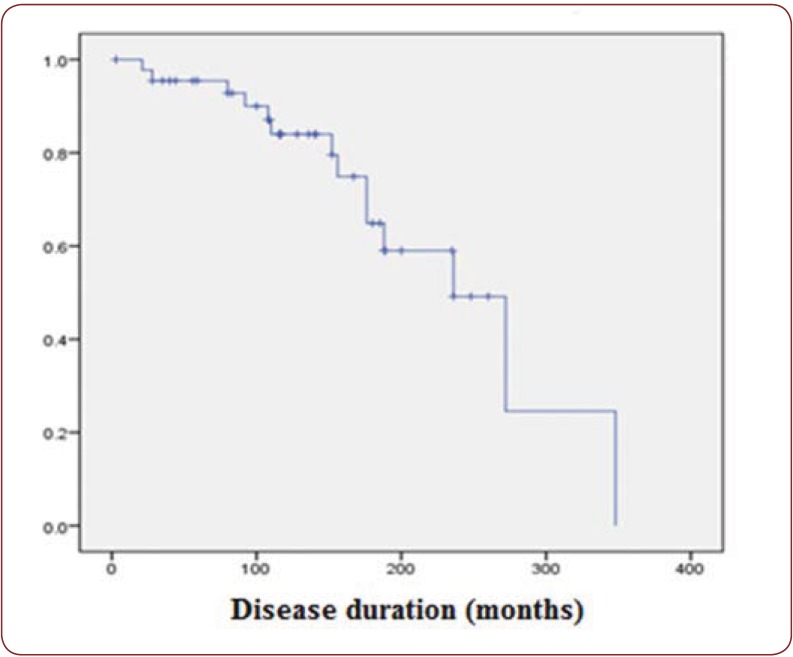
The prevalence of pulmonary hypertension in our group was a little higher compared to registries (17.45% compared to 8-12%) (17), possibly related to the limited acces to right heart catherization, while echocardiography usually overestimates the values sPAP. We have also to consider the bias related to the evaluation of the patients in a reference center. Although there is no correlation with disease duration, PAH seems to be a late complication (66.66% of the diffuse forms, 55.55% of the limited forms develop PAH after first 5 years) (Figure 3).
PAH was not correlated with the disease duration (p=0.9) but with the age of the patients (p=0.019, r=0.366). Advanced age at diagnosis was associated with early onset of PAH during scleroderma evolution, poor hemodynamics, worse prognosis, higher mortality rate, probably related to comorbidities (18).
Literature reports that scleroderma PAH is associated with myocardial fibrosis (19), heart failure with normal outflow (20), unlike idiopathic PAH. PAH in our group also was associated with higher prevalence of diastolic dysfunction, rhythm and conduction disturbances.
Although the incidence of pulmonary fibrosis was not different for patients with PAH and for those without, the DLCO was significantly lower for patients with PAH. (p=0.001) with a negative correlation between the two parameters (p=0.014, r=-0.385), confiming the prognostic value of decreased TLCO (below 55%) (21).
Patients with PAH had more frequently associated articular involvement: muscle weakness (p=0.01) and digital contractures (p=0.022). The impact of musculoskeletal problems on scleroderma PAH is not well established, but it couId be an important impediment for the validation of 5 minutes walking tests for PAH associated with SSc (22).
Dinamic follow-up of nailfold capillaroscopy can be predictive for PAH, capillaroscopy score>1 being considered a risk factor for PAH (23). Most of the studied patients with PAH had a late capillaroscopy pattern (p@0.001).
Due to multivisceral involvement associated, higher HAQ scores we can consider PAH an element of disease severity with important morbidity and mortality.
Classic cardiovascular risk factors can worsen endothelial dysfunction for scleroderma patients. Diabetic and smoking patients developed more frequent digital ulcers (p=0.013, respectively p=0.005) and scleroderma renal crisis (p=0.007, respectively p=0.024). Smoking has been associated with tripling the risk for digital ulcers in scleroderma (24). The predictive value is so high that smoking has been included together with disease duration, digital contractures and early onset in a composite score for digital ulcers prediction (24). Micro and macrovascular involvement in diabetes mellitus explains the increased risk for digital ulcers for scleroderma patients with preexisting endothelial dysfunction.
Dyslipidemia and obesity have been associated with significantly lower lung volumes (p=0.021, respectiv p=0.034). A recent study reports also association of dyslipidemia and the android and ginoid obesity rate with FVC no matter of sex, age and disease duration, suggesting that further studies are needed to establish if loosing weight would improve lung parameters (25).
Macrovascular involvement in systemic sclerosis is still a matter of debate. A recent metanalysis (26) concludes that scleroderma patients have high atherosclerotic risk. Most of the studies report increased incidence of peripheral vascular disease in upper and lower limbs, but no differnece regarding coronary and cerebral involvement (26-28). Our group had also increased incidence of peripheral vascular disease of lower limbs (19.6%) wihich was associated with corticosteroids use (p=0.028) and disease severity (p=0.046), rather then traditional cardiovascular risk factors. Patients with coronary heart disease had higher activity scores (p=0.027).
The incidence of scleroderma renal crisis was 3.92%, lower then that reported in regisitries 10% (29). No precipitanting factors could be identified except cold exposure. Rapid cutaneous involvement had been identified to be an important risk factor for renal crisis (30). Still, this association has not been identified for our patients (p=0.07), as the design of the study did not monitor the dinamic changes of Rodnan score. Male sex and history of digital ulcers were correlated with scleroderma renal crisis (p=0.007, r=0.378, respectively p=0.088, r=0.241).
CONCLUSION
Vascular complications dominate the clinical picture in scleroderma. They are not specific to a certain period of disease evolution, most of them being independent of disease subset and immunologic abnormalities. They often are associated with other severe visceral involvement with high morbidity and mortality. Some of them have a long subclinical evolution, so screening is needed for early detection. Classic cardiovascular risk factors, especially diabetes and smoking can worsen endothelial dysfunction.
Figure 3.
Figure 3. The incidence of pulmonary hypertension in relation with disease duration.
Conflict of interests: none declared.
Financial support: none declared.
Contributor Information
Laura Groseanu, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
Florian Berghea, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
Andra Balanescu, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
Denisa Predeteanu, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
Violeta Bojinca, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
Ioana Saulescu, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
Cosmin Constantinescu, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
Daniela Opris, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
Mihai Abobului, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
Andreea Borangiu, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
Maria-Magdalena Negru, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
Violeta Vlad, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
Ruxandra Ionescu, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Sfanta Maria” Clinical Hospital, Bucharest, Romania.
REFERENCES
- Wigley F - Overview: Cardiovascular manifestation and managment. In: Varga J., Denton C., Wigley F. Scleroderma from pathogenesis to comprehensive management. Springer Science+Business Media. 2012:311–313. [Google Scholar]
- Geyer M, Muller-Ladner U - The pathogenesis of systemic sclerosis revisited. Clin Rev Allergy Immunol. 2011;40:92–103. doi: 10.1007/s12016-009-8193-3. [DOI] [PubMed] [Google Scholar]
- Pattanaik D, Brown M, Postlethwaite AE - Vascular involvement in systemic sclerosis (scleroderma) Journal of Inflammation Research. 2011;4:105–125. doi: 10.2147/JIR.S18145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Rheumatism Association Diagnostic and Therapeutic Criteria Committee - Preliminary criteria for the classification of systemic sclerosis. Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- Hudson M, Steele R - Canadian Scleroderma Research Group (CSRG) Baron M. Update on indices of disease activity in systemic sclerosis. Semin Arthritis Rheum. 2007;37:93–8. doi: 10.1016/j.semarthrit.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Johnson SR, Lee P - The HAQ disability index in scleroderma. Rheumatology. 2004;43:1200–1201. doi: 10.1093/rheumatology/keh288. [DOI] [PubMed] [Google Scholar]
- Galluccio F, Matucci-Cerinic M - Registry Evaluation of Digital Ulcers in Systemic Sclerosis Hindawi Publishing Corporation International. Journal of Rheumatology. 2010 doi: 10.1155/2010/363679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouthon L, Mestre-Stanislas C, Berezne A, et al. - Impact of digital ulcers on disability and health-related quality of life in systemic sclerosis. Ann Rheum Dis. 2010;69:214–217. doi: 10.1136/ard.2008.094193. [DOI] [PubMed] [Google Scholar]
- Tyndal AJ, Banert B, Vonk M - Causes and risk factor for death in systemic sclerosis a study from EULAR Scleroderma trial and Research group database (EUSTAR). Ann of Rheum Dis. 2010;69:1809–15. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- Amanzi L, Braschi F, Fiori G, et al. - Digital ulcers in scleroderma: staging, characteristics and sub-setting through observation of 1614 digital lesions. Rheumatology. 2010;49:1374–1382. doi: 10.1093/rheumatology/keq097. [DOI] [PubMed] [Google Scholar]
- Allanore Y, Meune C - Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol. 2010;28(5 Suppl 62):S48–53. [PubMed] [Google Scholar]
- Meune C, Vignaux O, Kahan C, et al. - Heart involvement in systemic sclerosis: evolving concepts and diagnostic methodologies. Arch Cardiovasc Dis. 2010;103:46–52. doi: 10.1016/j.acvd.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Kahan A, Coghlan G, McLaughlin V - Cardiac complications of systemic sclerosis. Rheumatology (Oxford). 2009;48 Suppl 3:45–8. doi: 10.1093/rheumatology/kep110. [DOI] [PubMed] [Google Scholar]
- Champion HC - The heart in scleroderma. Rheum Dis Clin North Amer. 2008;34:181–90. doi: 10.1016/j.rdc.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allanore Y, Meune C, Kahan A - Systemic sclerosis and cardiac dysfunction:evolving cooncepts and diagnostic methodologies. Curr Opin Rheumatol. 2008;20:697–702. doi: 10.1097/BOR.0b013e328313bcf1. [DOI] [PubMed] [Google Scholar]
- Desai CS, Lee DC, Shah SJ - Systemic sclerosis and the heart: current diagnosis and management . Curr Opin Rheumatol. 2011;23:545–554. doi: 10.1097/BOR.0b013e32834b8975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avouac J, Airo P, Meune C, et al. - Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasian patients and metaanaylis of 5 studies. J Rheumatol. 2010;37:2290, 8. doi: 10.3899/jrheum.100245. [DOI] [PubMed] [Google Scholar]
- Le Pavec J, Humbert M, Mouthon L - Hassoun- Systemic Sclerosis-associated Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2010;181:1285–93. doi: 10.1164/rccm.200909-1331PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badesch DB, Champion HC, Sanchez MA, et al. - Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S55–66. doi: 10.1016/j.jacc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Hachulla E, Denton CP - Early intervention in pulmonary arterial hypertension associated with systemic sclerosis: an essential component of disease management. Eur Respir Rev. 2010;19:314–20. doi: 10.1183/09059180.00007810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan JG, Denton CP, Grünig E, et al. - Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis. 2014;73:1340–1349. doi: 10.1136/annrheumdis-2013-203301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galie N and the Task Force for Diagnosis and treatment of Pulmonary Hypertension of European Society of Cardiology (ESC), European respiratory Society (ERS), International Society of Heart and Lung transplantation (ISHLT) - Guidelines for the diagnosis and treatment of pulmonary arterial hypertension. Eur Rspir J. 2009;34:1219–1263. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- Smith V, Riccieri V, Pizzorni C, et al. - Nailfold capillaroscopy for prediction of novel future severe organ involvement in systemic. J Rheumatol. 2013;40:2023–8. doi: 10.3899/jrheum.130528. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Silman AJ, Hider SL, et al. - Cigarette smoking as a significant risk factor for digital vascular disease in patients with systemic sclerosis. Arthritis Rheum. 2002;46:3312–6. doi: 10.1002/art.10685. [DOI] [PubMed] [Google Scholar]
- Caramaschi P, Biasi D, Caimmi C, et al. - Relationship between body composition and both cardiovascular risk factors and lung function in systemic sclerosis. Clin Rheumatol. 2014;33:77–82. doi: 10.1007/s10067-013-2388-y. [DOI] [PubMed] [Google Scholar]
- Nordin A, Jensen-Urstad K, Björnådal L, et al. - Ischemic arterial events and atherosclerosis in patients with systemic sclerosis: a population-based case-control study. Arthritis Res Ther. 2013;15:R87. doi: 10.1186/ar4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Li M, Xu D, et al. - Macrovascular involvement in systemic sclerosis: evidence of correlation with disease activity. Clin Exp Rheumatol. 2012;30(2 Suppl 71):S76–80. [PubMed] [Google Scholar]
- Nussinovitch U, Shoenfeld Y - Atherosclerosis and macrovascular involvement in systemic sclerosis: myth or reality. Autoimmun Rev. 2011;10:259–66. doi: 10.1016/j.autrev.2010.09.014. [DOI] [PubMed] [Google Scholar]
- Sabir O, Younas H, Tanvir I, et al. - Scleroderma renal crises: case report and review of literature. J Pak Med Assoc. 2013;63:916–8. [PubMed] [Google Scholar]
- Teixeira L, Mouthon L, Mahr A, et al. - Mortality and risk factors for scleroderma renal crisis a french retrospective study of 50 patients. Ann Rheum Dis. 2008;67:110–6. doi: 10.1136/ard.2006.066985. [DOI] [PubMed] [Google Scholar]