Abstract
Background:
From the first recognition of dysplastic nevi as a pathology per se, many debates have been raised and many histological and immunohistological studies have been conducted in order to establish the true significance of these lesions. Therefore, the aim of this study was to establish if there is a correlation between HMB-45, Melan A and Bcl-2 expression and the grade of dysplasia, as well as between the marker’s staining patterns.
Material and Methods:
Ten dysplastic nevi from six female patients were selected and their histological features (size, dysplasia), as well as the immunohistological staining patterns, were studied (HMB-45, Melan A, Bcl-2). The Pearson correlation coefficient and regression was calculated with Windows Excel Data Analysis.
Results:
We demonstrated that there was a notable correlation between the dysplasia and the size of the lesions (r(8)= 0.62 with p-value= 0.052), and also between Melan A and Bcl-2 (a r(6)= 0.73, p<0.05), but we did not obtain a statistically significant correlation between other features (p>0.05).
Conclusions:
We can affirm, at least in our cases, there is a correlation between the grade of dysplasia and the size of the lesion, and also, that there is a correlation between Melan A and Bcl-2 staining, explained by MITF gene. These results were only partial concordant with those in other studies, therefore a larger number of cases is recommended to be further analyzed in order to clearly draw a conclusion.
Keywords:dysplastic nevi, Bcl-2, Melan A, HMB-45, imunohistological stains
BACKGROUND
Dysplastic nevi (DN) represent a controversial pathology due to the uncertainty of evolution and the relationship with melanoma. The clinical and histological diagnosis criteria situate this lesion somewhere between common nevi and melanoma. Clinically, a lesion of 5 mm or more, with “ABCDE” – like criteria is diagnosed as a dysplastic nevus (1). Histologically, dysplastic nevi are considered to be nevi with variable cytological and architectural atypia (2).
The incidence of this pathology in general population ranges between 2-50% in some studies (3), with a prevalence between 2-17% for Caucasian individuals (1,3). The risk for developing melanoma varies depending on the study, with some authors reporting a four to fifth-teen-fold increased risk (4), and others reporting an eightfold greater risk, with twofold risk for the presence of one DN and a twelvefold risk if ten or more DN exists (2). The genetics are still unclear. Alterations of chromosomes 1p36 and 9p21as well as gene mutations like CDKN2A, BRAF, alterations of p16 and p53 proteins or microsatellite instability are only few of the genetic modifications studied so far (5,6). In order to distinguish common melanocytic nevi from dysplastic ones and from early melanoma, lots of studies focused on the different expression of immunohistochemical markers such as Bcl-2, p53 protein, Cyclin D1, HMB-45, Melan A or newer markers such as Mcl-1 (Myeloid leukemia 1) (7), ING-2 (inhibitor of growth family protein) (8), telomerase (9) and biomarkers including Bim, BRG1, Cul1 and ING4 (10). In this paper we study the expression of Bcl-2, Melan A and HMB-45 as well as the histopathological characteristics of dysplastic nevi. The aim of this study, as part of a larger one, was to establish any correlation between different histological and immunohistochemical features (size, dysplasia, Bcl-2, Melan A, HMB-45 staining pattern), in order to better explain the physiopathology of this lesions.
MATERIALS AND METHODS
We selected ten lesions from six female patients which were diagnosed as dysplastic nevi in the Pathology Department of the Emergency University Hospital Bucharest. All lesions examined met both major criteria and at least two minor criteria from the WHO recommendations for dysplastic nevi: basilar proliferations of atypical melanocytes extending to at least three rete ridges beyond the dermal component and organization of proliferation in lentiginous or epithelioid-cell pattern as the two major criteria, lamellar or concentric eosinophilic fibrosis, neovascularization, inflammatory response and fusion of rete ridges as minor criteria.
The tissue samples were fixed with 10% buffered formalin and were processed by conventional histopathological method using inclusion in paraffin and Hematoxylin–Eosin (HE) staining. We noted the dimensions of the nevi after formalin fixation and graded the dysplasia in mild, moderate and severe on HE staining. Also, immunohistochemical tests were performed using the following antibodies: Bcl-2 (clone 100/D5, dilution 1:100, BioCare), Melan A (clone M2-7C10, M2-9E3, dilution 1:100, BioCare) and HMB 45 (monoclonal, ready to use, Genova). The indirect tristadial Avidin -Biotin complex method was used. The sections were deparaffinased in toluene and alcohol successive baths, rehydrated in alcohol baths with increasing concentrations, washed in phosphate saline buffer and incubated with primary antibody overnight. Next, they were washed in carbonate buffer and developed in 3,3’-diaminobenzidine hydrochloride/hydrogen peroxide nuclear counterstaining with Mayer’s Hematoxylin.
The expressions of the markers were noted as follows: 0 (negative staining), 1 (weak staining), 2 (moderate staining) and 3 (strong staining). Dysplasia was noted as well: 1 - mild dysplasia, 2 - moderate dysplasia and 3 - severe dysplasia. Statistical tests were performed using Windows Excel 2007 - Data Analysis, the Pearson Correlation Coefficient and Regression formulas in order to determine whether there are correlations between different staining patterns and dysplasia.
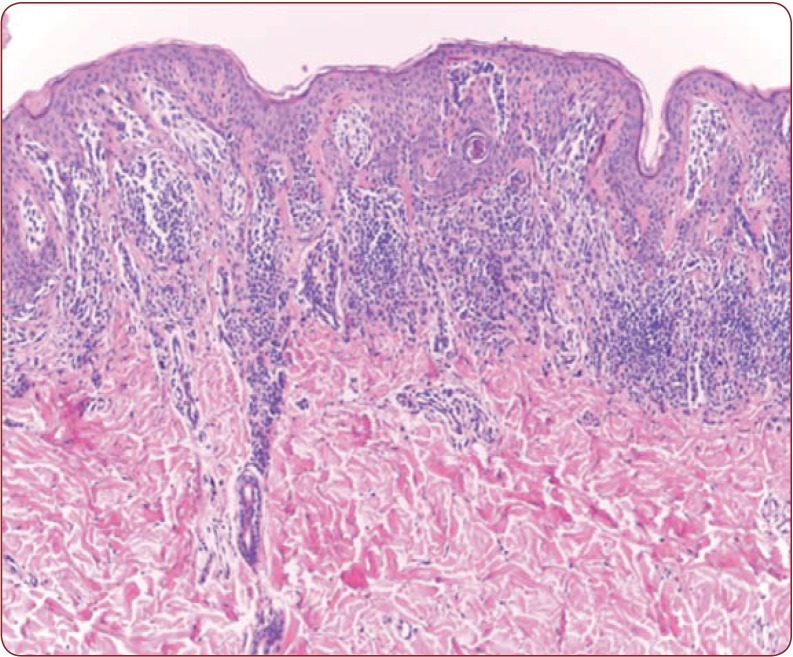
Figure 1.
Figure 1. Dysplastic nevus, moderate dysplasia HE 100x.
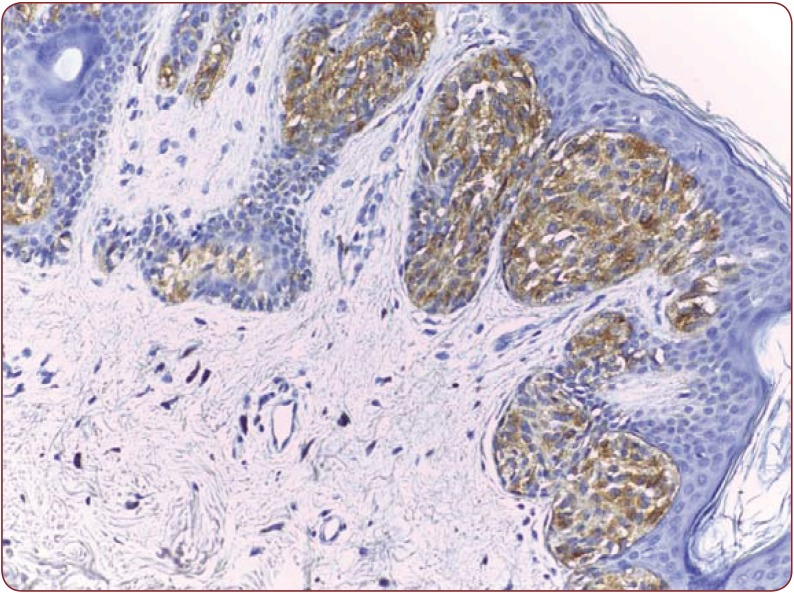
Figure 2.
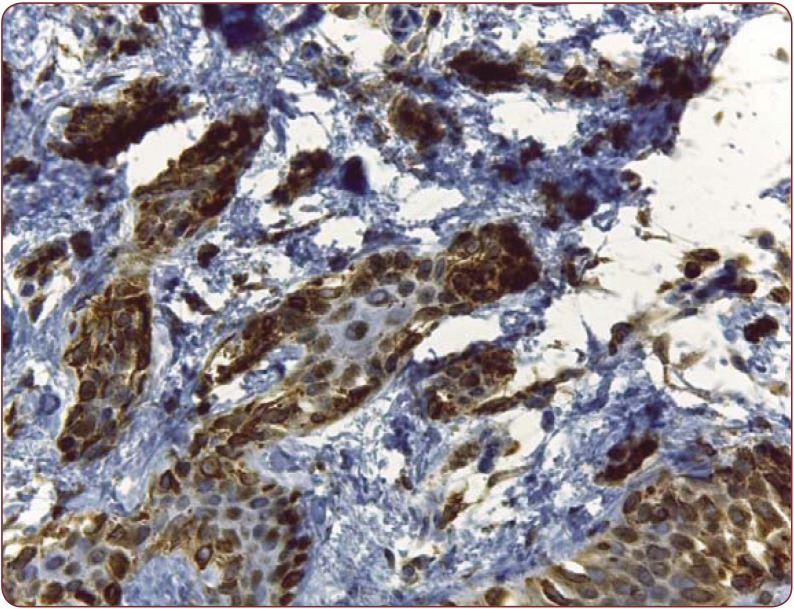
Figure 2. Moderate positivity in a dysplastic nevus, HMB- 45, 200x.
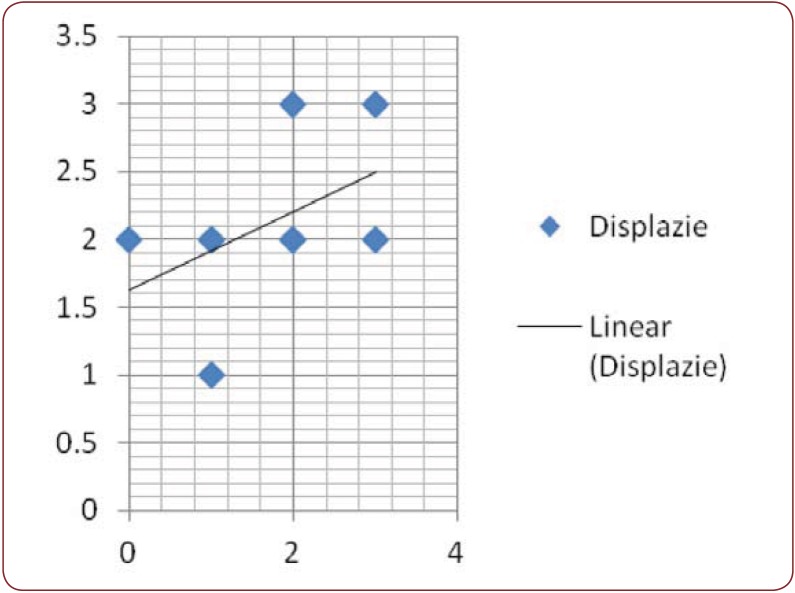
Figure 3.
Figure 3. Linear regression of HMB 45 in association 2, with dysplasia.
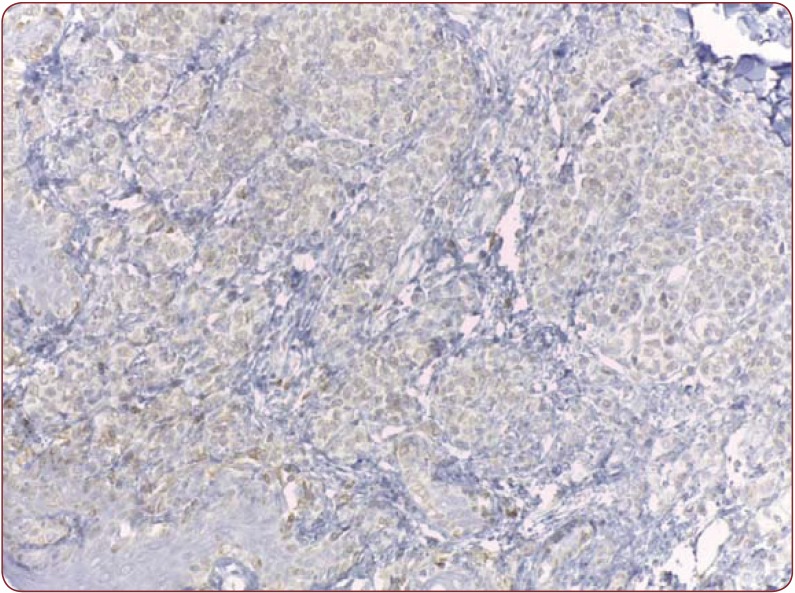
Figure 4.
Figure 4. Weak positivity in a dysplastic nevus, Bcl-200X.
RESULTS
All six patients were of female gender and had ages between 24 and 33 years, with an average age of 28.6. Nine of ten lesions were located on the posterior thorax, only one was close to the umbilicus. On macroscopic examination, nine nevi had more than 5 mm in greatest diameter and one lesion had 3/4 mm in size. The mean indicative was 7.7/5.7 mm with standard deviation of 2.9, respectively 1.41. The colors varied from dark brown to grey, all of them presenting a slight grade of asymmetry. On HE staining, two lesions presented with severe dysplasia, seven had moderate dysplasia (Figure 1) and one presented mild dysplasia. All of them presented lamellar or concentric fibrosis, fusion of rete ridges and dusty melanin, and in five lesions (50%) the inflammatory infiltrate was minimal or absent, with one dysplastic nevus presenting severe dysplasia and no inflammatory infiltrate. A correlation between the greatest diameter and dysplasia was made, reveling a r (8)= 0.62 with p-value= 0.052, which represents a rather moderate association between the two, with a confidence level of 94.8%.
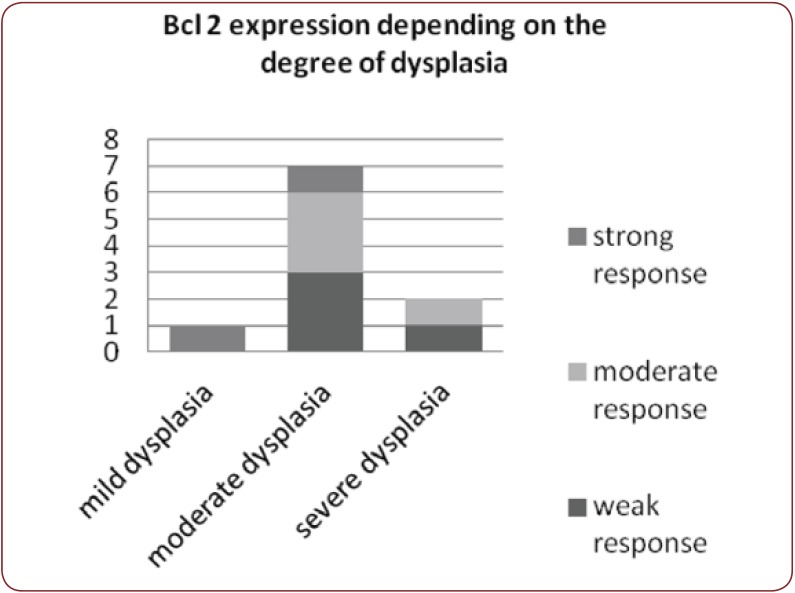
Nine dysplastic nevi stained positive (cytoplasmatic) with HMB 45, with four of them presenting a weak and diffuse positivity, two of them strong positivity and the rest presenting moderate positivity (Figure 2). One case presented artifacts and could not be assessed. A moderate correlation (r(7) = 0.55, p<0.12) (Figure 3) between the grade of dysplasia and the pattern of staining was observed, although a stronger positivity was seen in nevi with moderate and severe dysplasia in comparison with those with mild dysplasia. The positivity of the marker was stronger in the nevus cells of the dysplastic nests than in the deeper cells which presented a variable grade of maturation. Pearson coefficient presented a p value>0.05, thus the correlation is not statistically significant. Melan A also stained positive in eight nevi (80%), three of which had a strong staining pattern. Two nevi could not be processed for Melan A due to small dimensions and tissue depletion. For Bcl-2 staining, four nevi presented moderate positivity and other four a weak and diffuse response (Figure 4), whereas two of them had a strong Bcl-2 expression (Figure 5). A clear correlation between the immunohistochemical stains and histological aspects could not be made. The correlation coefficient between the HMB 45 staining and Bcl-2 was r(7)=0.61, p=0.07, with a confidence level of 93% whereas the correlation coefficient between the grade of dysplasia and Bcl-2 expression r(8)= 0.44 with a p value <0.2 (Figure 6). A relevant correlation between Melan A and Bcl-2 was obtained, with a r(6)= 0.73, p<0.05.
DISCUSSION
HMB 45 is a mouse monoclonal antigen which recognizes the gp 100 protein from early melanosomes (11) and stains the cytoplasm of immature and activated melanocytes (12). Common dermal nevi are HMB 45 negative whereas dysplastic nevi and melanomas stain positive (11). Also, intraepidermal nevoid cells and junctional melanocytes stain positive, HMB 45 demonstrating the maturation of the melanocytes within a nevus (11), aspect that is also seen in our cases. As in other studies, our dysplastic nevi stained positive too, although weak and diffuse in some cases. None of the dysplastic nevi with severe dysplasia had a different staining pattern in comparison with the ones with moderate dysplasia, hence the degree of dysplasia is not, at least in our cases, a relevant factor influencing this marker. Although a correlation has been made, revealing a slight association between the two of them, a relevant P-value was not obtained and a statistically correct result could not be assessed. As it was demonstrated since 1989 (13), dysplastic nevi express low levels of HMB 45 in dermal nevoid cells in the same way residual nevus cells adjacent to melanoma do, whereas melanoma expresses a strong staining. The role of the HMB 45 staining is not of discriminating value in differentiating and identifying the risk of promoted malignancy (14) but it represents a helpful marker in assessing dysplastic melanocytic lesions.
Melan A or Melanoma Antigen Recognized by T cells-1 (MART 1), on the other hand, is a marker for melanocytic differentiation, staining all benign and malignant melanocytic tumors. No different patterns of staining were observed in association with the grade of dysplasia or other macroscopically or microscopically characteristics. Melan A is usually used for differentiating melanocytic nevi from other lesions with resembling HE aspects, although some authors claim that MART-1 could be used in assessing the prognostic of the patients with melanoma (15).
Bcl-2 is a member of the family of proteins that are key regulators of apoptosis (16) and it is considered to have a protective role against apoptosis of the melanocytes (16). The marker is a rather new one in this field of pathology, especially in studying the grade of dysplasia in melanocytic nevi with cyto-arhitectural disorders. Some studies reveled a correlation between the Bcl-2 staining and progression of dysplasia, whereas others showed that Bcl-2 stains less in melanoma in comparison with common and dysplastic nevi (17,18). As ananti-apoptotic protein, Bcl-2 expression in melanomas could justify the progression and resistance of this malignancy, while the lack of Bcl-2 can be explained by the well documented life span of the melanocytic nevi which is longer than in malignant lesions. In our study, Bcl-2 was positive in all nevi, with a weak positivity in 3 cases and strong response in the other two. One nevus with severe dysplasia stained weak and diffuse and the other one presented a moderate expression whereas the nevus with mild dysplasia stained stronger. We could not demonstrate a real correlation between the degree of dysplasia and the staining pattern (r(8)= 0.44, p value <0.2), and also, the correlation between HMB-45 and Bcl-2 staining is a rather moderate one (r(7)=0.61, p=0.07), although more cases would be required in order to draw a correct conclusion.
The correlation between Melan A and Bcl-2 was a statistically significant one (r(6)= 0.73, p<0.05), demonstrating, thus, that the antiapoptotic protein could be a relevant factor in better understanding melanocytes and could be associated with the life-span of this cells. The linkage between MART 1 and Bcl-2 is the MITF gene, which regulates both of them and plays a critical role in the differentiation of the melanocytes on one side, being considered a central regulator of melanogenesis, and the cell survival on the other, regulating Bcl-2 protein (19). MITF has been extensively discussed in melanoma, but few studies regarding its role in dysplastic nevi were found. At this moment, there are still many debates concerning the role of MITF, Bcl-2 and even MART-1 in the progression of melanoma and the physiopathology of melanocytes and melanocytic lesions.
A statistically correct correlation was made between the greatest diameter and the grade of dysplasia, leading to the idea that the larger of the lesions are, the greater is the probability and grade of the dysplasia. The staining patterns of the various markers used in this study did not fail to demonstrate, nor did they confirm an association between the dysplasia and the imunohisdstochemical expressions observed, issues long debated in the literature.
Figure 5.
Figure 5. Strong positivity in a dysplastic nevus, Bcl-2, degree of 400x.
Figure 6.
Figure 6. Bcl-2 expression depending on the dysplasia.
CONCLUSIONS
Correlations between dysplasia and different markers used in this study were not statistically significant, but a real question is raised concerning the association between HMB-45, Bcl-2 and dysplasia, as well as the physiopathology of the dysplastic nevi as demonstrated by the expression of the markers. Nevertheless, we managed to establish a connection between the lesion’s size and dysplasia and, also, between Melan A, HMB-45 and Bcl-2. Therefore we can affirm that at least in our cases, the grade of dysplasia correlates with the size of the lesion, and also, that there is a correlation between Melan A and Bcl-2 staining, explained by MITF gene. In order to upgrade our knowledge about dysplastic nevi and to define their position between common nevi and melanoma, more studies and more cases should be further analyzed.
Conflict of interests: none declared.
Financial support: This work received financial support through the project entitled: “CERO – Career profile: Romanian Researcher”, grant number POSDRU/159/1.5/S/135760, cofinanced by the European Social Fund for Sectorial Operational Program Human Resources Development 2007-2013.
Contributor Information
Oana Maria Patrascu, Department of Pathology, Emergency University Hospital, Bucharest, Romania.
Mariana Costache, Department of Pathology, Emergency University Hospital, Bucharest, Romania; Department of Pathology, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Adrian Vasile Dumitru, Department of Pathology, Emergency University Hospital, Bucharest, Romania.
Corina Nicoleta Mehotin, Department of Pathology, Emergency University Hospital, Bucharest, Romania.
Maria Sajin, Department of Pathology, Emergency University Hospital, Bucharest, Romania; Department of Pathology, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Anca Mihaela Lazaroiu, Department of Pathology, Emergency University Hospital, Bucharest, Romania; Department of Pathology, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
REFERENCES
- Silva JH, de Sa BC, Avila ARL, et al. - Atypical dyplastic nevi: identification of population at risk for developing melanoma – review article. Clinics. 2011;66:493–499. doi: 10.1590/S1807-59322011000300023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein MR - Melanocytic Dysplastic naevioccupy the middle ground between beningn melanocytic naevi and malignant melanomas: emerging clues. J Clin Pathol. 2005;58:453–456. doi: 10.1136/jcp.2004.019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic M, Janjic Z, Nikolic J - Dysplastic nevus – a risk factor for developing skin melanoma. Clinical and epidemiological study with retrospective study of literature. . Med Pregl. 2011;64:315–318. doi: 10.2298/mpns1106315m. [DOI] [PubMed] [Google Scholar]
- Duffy K, Grossman D - The dysplastic nevus: from historical perspective to management in the modern era. Historical, histologic, and clinical aspects. J Am Acad Dermatol. 2012;64:1.e1–18. doi: 10.1016/j.jaad.2012.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy K, Grossman D - The dysplastic nevus: from historical perspective to management in the modern era. Molecular aspects and clinical management. J Am Acad Dermatol. 2012;67:19.e1–12. doi: 10.1016/j.jaad.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein MR, Wood GS - J Mol Diagn. Endocr Rev. 2002;4:71–80. doi: 10.1016/S1525-1578(10)60684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RP, Khosravi S, Martinka M, et al. - Myeloid leukemia-1 expression in benign and malignant melanocytic lesions. Oncol Rep. 2008;19:933–7. [PubMed] [Google Scholar]
- Lu F, Dai DL, Martinka M, et al. - Nuclear ING2 expression is reduced in human cutaneous melanomas. Br J Cancer. 2006;95:80–86. doi: 10.1038/sj.bjc.6603205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batinac T, Hadzisejdic I, Brumini G, et al. - Expression of cell cycle and apoptosis regulatory proteins and telomerase in melanocitic lesions. Coll Antropol. 2007;31Suppl 1:17–22. [PubMed] [Google Scholar]
- Zhang G, Li G - Novel Multiple Markers to Distinguish Melanoma from Dysplastic Nevi. PLoS ONE. 2012;7:e45037. doi: 10.1371/journal.pone.0045037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg BG, Moeder C, Kluger H, et al. - Nuclear to non-nuclear Pmel17/ gp100 expression (HMB45 staining) as a discriminator between benign and malignant melanocytic lesions. Modern Pathology. 2008;21:1121–1129. doi: 10.1038/modpathol.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-González S, Reventós A, Pedragosa R, et al. - Estudio inmunohistoquimico en dos hermanas gemelas. Patologia. 1996;29:209–215. [Google Scholar]
- Smoller BR, McNutt NS, Hsu A - HMB-45 staining of dysplastic nevi. Support for a spectrum of progression toward melanoma. Am J Surg Pathol. 1989;13:680–4. doi: 10.1097/00000478-198908000-00006. [DOI] [PubMed] [Google Scholar]
- Ahmed I, Piepkorn M, Goldgar DE, et al. - HMB-45 staining of dysplastic melanocytic nevi in melanoma risk groups. J Cutan Pathol. 1991;18:257–260. doi: 10.1111/j.1600-0560.1991.tb01232.x. [DOI] [PubMed] [Google Scholar]
- Berset, M, Cerottini JP, Guggisberg D, et al. - Expression of melan-a/MART-1 antigen as a prognostic factor in primary cutaneous melanoma. Int J Cancer. 2001;95:73–77. doi: 10.1002/1097-0215(20010120)95:1<73::aid-ijc1013>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Lee CS, Scolyer RA, et al. - Mcl-1, Bcl-XL and Stat3 expression are associated with progression of melanoma whereas Bcl-2, AP-2 and MITF levels decrease during progression of melanoma. Mod Pathol. 2007;20:416–26. doi: 10.1038/modpathol.3800750. [DOI] [PubMed] [Google Scholar]
- Tron VA, Krajewski S, Klein-Parker H, et al. - Immunohistochemical analysis of Bcl-2 protein regulation in cutaneous melanoma. Am J Pathol. 1995;146:643, 650. [PMC free article] [PubMed] [Google Scholar]
- Bush J, Li G - The role of Bcl-2 family members in the progression of cutaneous melanoma. Clin Exp Metastasis. 2003;20:531–539. doi: 10.1023/a:1025874502181. [DOI] [PubMed] [Google Scholar]
- Levy C, Khaled M, Fisher DE - MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12:406–14. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]