Abstract
Management of biliary tract cancer remains challenging. Tumors show high recurrence rates and therapeutic resistance, leading to dismal prognosis and short survival. The cancer stem cell model states that a tumor is a heterogeneous conglomerate of cells, in which a certain subpopulation of cells - the cancer stem cells - possesses stem cell properties. Cancer stem cells have high clinical relevance due to their potential contributions to development, progression and aggressiveness as well as recurrence and metastasis of malignant tumors. Consequently, reliable identification of as well as pharmacological intervention with cancer stem cells is an intensively investigated and promising research field. The involvement of cancer stem cells in biliary tract cancer is likely as a number of studies demonstrated their existence and the obvious clinical relevance of several established cancer stem cell markers in biliary tract cancer models and tissues. In the present article, we review and discuss the currently available literature addressing the role of putative cancer stem cells in biliary tract cancer as well as the connection between known contributors of biliary tract tumorigenesis such as oncogenic signaling pathways, micro-RNAs and the tumor microenvironment with cancer stem cells.
Keywords: Biliary tract cancer, cancer stem cells, Cancer stem cell markers, Tumor microenvironment, Micro-RNAs
Core tip: Using a xenograft model, researchers successfully demonstrated that as few as ten of a specific subpopulation of biliary tract cancer cells had the potency to (serially) establish and recapitulate biliary tract cancer in immunodeficient mice. Furthermore, expression of established cancer stem cell markers, cancer stem cell-related signaling pathways and micro-RNAs was reported in biliary specimens and cell lines - in most cases associated with clinical outcome. Based on these results, the existence of cancer stem cells in biliary tract is well-founded and potentially harbors new options for development of therapeutic strategies.
INTRODUCTION
General aspects of cancer stem cells
In 1994, Lapidot et al[1] identified a subpopulation of cells, characterized by a specific set of surface markers that was able to initiate acute myeloid leukemia in mice. Since then, numerous of such tumor-initiating cells were identified in most solid tumors[2]. These tumor-initiating cells, also referred to as cancer stem cells (TICs or CSCs) have the ability to self-renew as well as to differentiate into different lineages - traits that they share with (adult) stem cells. Further similarities between tumor-initiating cells and normal adult stem cells include the reliance on certain highly conserved embryonic pathways (such as Hedgehog (Hh), Nanog and Wnt), a specialized metabolism (preferential oxidative glycolysis), enhanced protection against DNA damage and oxidative stress, a specific epigenetic profile (e.g., abnormal polycomb repressive complexes activity, see below) as well as the expression of specific surface markers (reviewed in[2]). The specific abilities and profiles of CSC may at least in part explain some of the common clinical problems seen when dealing with cancer. CSCs are slow-cycling cells that often are in a quiescent state. Common chemotherapeutics target proliferating, fast cycling cells, thereby erasing the bulk of the tumor while not affecting CSCs - a phenomenon resulting in tumor recurrence. Moreover, CSCs strongly express drug efflux pumps, contributing to the well-known chemoresistance of these cells[2-5].
Currently, two main models are discussed regarding the origin of CSCs[6]. In the “stochastic model”, each cancer cell is biologically equivalent and unpredictably may acquire a CSC phenotype depending on diverse stochastic events from inside the cells (via genetic and epigenetic changes) as well as from the surrounding environment. The second model called “hierarchic model” states that a tumor is, like solid organs, a hierarchically organized heterogeneous cell conglomerate in which only a small subset of cells - the CSC - have the ability to self-renew and to give rise to daughter cells of various differentiation, whereas the majority of cancer cells that form the bulk of the tumor cannot achieve CSC traits. Besides these two main models, a third possible origin of CSC is currently discussed in the literature, namely de-differentiation of already committed cells - a phenomenon that was observed in different tumor entities and that is likely to play a role in biliary tract cancer (BTC) as discussed later in this article[7-10].
EXPERIMENTAL IDENTIFICATION AND CHARACTERIZATION OF CANCER STEM CELLS
Identification and/or isolation of CSC based on their expression profile (surface markers, signaling pathways) as well as their functional characteristics represent powerful tools in cancer research. The ability of CSCs to form tumors in immunodeficient mice at very low cell numbers surrogates their high tumorigenic potential[2]. Besides in vivo xenograft experiments, also several in vitro techniques are used in CSC research. The clonogenic assay similarly addresses the higher tumorigenic potential of CSC. Here, very few cells (approximate range between 50-200 cells per cm2 - highly cell line-dependent) are seeded in a cell culture receptacle and the tumorigenic potential is evaluated by counting the number of growing colonies, each of them originating from a single cell clone representing a potential CSC[11]. Anchorage-independent growth, i.e., the formation of tumor spheres in a non-adherent environment, is another well-established experimental approach used in CSC research[12]. Likewise, expression of aldehyde-dehydrogenase 1 (ALDH1) is considered as a functional CSC marker[13]. ALDH1 is a detoxifying enzyme that was shown to be up-regulated in cancer and associated with various CSC traits such as enhanced tumor growth and the potential of self-renewal and differentiation[13-16]. Moreover, the so-called side-population phenotype may signify a CSC population. Side-population cells are defined by their ability to excrete fluorescent dyes such as Hoechst 33342 - this characteristic is based on the enhanced expression of efflux pumps in (cancer) stem cells[17,18]. Lastly, potential CSCs are identified and isolated based on their surface markers using fluorescence-activated cell sorting or by analysis of expression of established (cancer) stem cell genes.
BILIARY TRACT CANCER - CLINICAL BACKGROUND
BTC is a heterogeneous malignancy that arises from different locations within the biliary tree. It can be categorized into intrahepatic cholangiocarcinoma (IHC), extrahepatic cholangiocarcinoma (EHC), gallbladder cancer (GBC) and mixed hepatocellular-cholangiocarcinoma (HCC-CC). Although BTC is generally a rare disease, it has high clinical significance due to its dismal outcome and limited therapeutic options[19,20]. Due to late diagnosis, for most patients only palliative treatment is possible; furthermore, the standard chemotherapeutic approach using a combination of cisplatin and gemcitabine results in median survival of approximately one year only[21]. Therefore, advances in understanding the underlying mechanisms of BTC development, progression and aggressiveness are of utmost importance for better management of this disease. BTC is characterized by high recurrence rates, formation of metastasis and high therapeutic resistance towards conventional chemotherapy regimens[19,20]. The involvement of CSC subpopulations in BTC is likely, however, the current literature is sparse. In this article, we discuss current studies on the role and impact of CSCs in BTC. Specifically, we focus on potential CSC markers and signaling pathways in BTC and the clinical consequences of their expression as well as on giving an overview of other aspects of BTC tumorigenesis such as miR expression and tumor microenvironment that can be linked to BTC CSCs.
POTENTIAL ORIGINS OF BILIARY TRACT CANCER CELLS WITH STEM CELL-LIKE CHARACTER
Several hepatic cell types have been suggested to represent the origin of BTC CSC (summarized in Figure 1)[22]. Mature hepatocytes have the ability to de-differentiate into more pluripotent cells through mechanisms of cell plasticity and reprogramming, thereby acting as a population with stem cell traits[23]. IHC is categorized as a primary liver tumor and shows characteristics of both hepatocellular carcinoma and cholangiocarcinoma, suggesting a potential link regarding the cell of origin[24,25]. Evidence for mature hepatocytes being the source and quasi-CSC for IHC comes from two studies that showed that mature hepatocytes are able to transdifferentiate and form IHC[9,10]. In another study, it was shown that loss of tumor suppressor p53 contributes to dedifferentiation of hepatocytes into progenitor cells that can transform into IHC[26]. This is especially interesting, since loss of p53 is a major genetic characteristic of BTC[27]. Cells residing in the Canals of Hering, a hepatic stem cell niche, are another possible source of BTC-initiating cells[28]. Because the Canals of Hering represent the interface between the liver and the biliary system, residing stem cell populations may be (1) hepatic progenitor cells; (2) biliary progenitor cells; or (3) bi-potential progenitor cells, called hepatoblasts in humans and “oval cells” in rodents which have the ability to differentiate into hepatocytes and cholangiocytes[25,29-31]. There is evidence that IHC can originate from hepatic stem cells. Expression of α-fetoprotein (AFP), a protein expressed by fetal hepatocytes and hepatic progenitor cells, was demonstrated in IHC[32-34]. In addition, AFP expression was shown in BTC cell lines and these AFP-expressing BTC cells had characteristics of stem cells[35]. Of note, IHC subtypes that were identified to have a hepatic stem-like gene signature had very poor prognosis, underlining not only the connection between hepatic stem cells and IHC, but also the clinical relevance and consequences[36]. The peribiliary glands were also described as a source of biliary stem cells as they are involved in normal biliary tissue turnover and repair[37,38]. BTC often occurs under (chronic) inflammatory conditions and it was shown that during primary sclerosing cholangitis, a chronic inflammation of the bile ducts, biliary tree stem cells are activated in the peribiliary glands[39]. It can be speculated that due to the chronic inflammation and concomitant constant activation of these normally quiescent stem cells, under these tumor-promoting conditions these cells become exposed to an environment that potentially causes malignant transformation - which is especially relevant taking into account the intrinsic longevity of these cells allowing for accumulation of malignant events which eventually may lead to a tumorigenic CSC phenotype. Moreover, p63, which is a homologue of p53 and also a stem cell marker of prostate gland and squamous cells was aberrantly expressed in IHC arisen from cirrhotic liver[40-42], underlining the connection between (chronic) inflammation and CSC in BTC.
Figure 1.
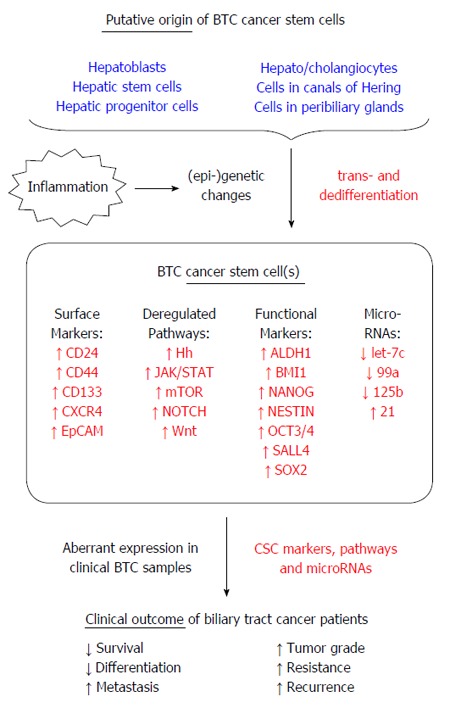
Cancer stem cells in biliary tract cancer. Biliary tract cancer stem cells are thought to originate from various subpopulations of healthy cells that harbor stem cell or stem cell-like traits. Currently available data on clinical biliary tract cancer (BTC) specimens revealed up-regulation of established cancer stem cells (CSC) cell surface and functional markers as well as aberrant activity of signaling pathways and micro-RNA species. Expression of CSC markers and stemness factors in BTC tissues is associated with diverse unfavorable clinico-pathological features and poor prognosis. See text for details.
In general, these studies suggest that there may not be one BTC CSC population but rather several different CSC populations, which in turn mirrors the heterogeneity of BTC and makes targeted and personalized (CSC-based) therapy very challenging and a demanding aim in the future.
CANCER STEM CELL MARKERS IN BILIARY TRACT CANCER - AN OVERVIEW
Limiting dilution cell transplantation assay in immunodeficient mice is a method to determine tumorigenic potential of cancer cells. CSCs, by their nature, harbor high tumorigenic potential, meaning that only few of these cells are able to form a heterogeneous tumor. Raggi et al[43] isolated BTC cells with sphere formation potential from parental BTC cells and demonstrated that these cells retained their potential to form spheres over several passages, indicating self-renewal potential. Furthermore, when injected into immunodeficient mice, as few as ten of these cells were able to generate tumors and this high tumorigenic ability was retained when re-transplanted[43]. Gene expression analysis of these potential BTC CSCs revealed up-regulation of a number of genes including genes responsible for self-renewal and pluripotency (e.g., SOX2, BMI1, NOTCH1), drug resistance (e.g., ABCG2), surface markers (e.g., CD24, CD44, EpCAM) and metastasis[43] that are also expressed in regular stem cell populations. Another study by Wang et al[44] further supports the existence of CSCs in BTC: they demonstrated in xenograft experiments that the subpopulation of CD24+/CD44+/EpCAMhigh cells harbor high tumorigenic potential compared to the CD24-/CD44-/EpCAMlow counterparts. Furthermore, they showed via serial in vivo passaging, that the expression profile of CD24/CD44/EpCAM remained stable comparable with the primary tumor. In addition, tumors resulting from injection of CD24+/CD44+/EpCAMhigh cells contained both, CD24+/CD44+/EpCAMhigh as well as phenotypically different cell populations, demonstrating the ability of CD24+/CD44+/EpCAMhigh to self-renew and to produce heterogeneous daughter cell populations[44].
Expression of several of these established surface and general CSC markers was identified in BTC specimens and cells. As shown in Table 1, expression of these markers was generally associated with disadvantageous clinico-pathological characteristics and shorter disease-free and overall survival. In addition, several in vitro studies investigated downstream targets and processes that are directly connected with the expression of these CSC markers in BTC. Resistance to anti-tumor treatments is a hallmark of cancer and CSCs and caused by up-regulation of genes responsible for drug efflux and DNA repair[4]. Nakashima and colleagues demonstrated an increase of proportion of CD24+/CD44+ cells in gemcitabine-resistant BTC cells and showed that genes of the BRCA/Fanconi repair pathway was over-expressed here, thus connecting the observed chemoresistance in these CSCs with a particular repair pathway[45]. Expression of the drug efflux pump ABCG2 is another mechanism of cells to gain therapeutic resistance and also an established CSC marker[46]. In BTC, ABCG2 was shown to be over-expressed in BTC tumor spheres and in CD44+/CD133+ cells, making it a candidate for pharmacological intervention in putative BTC CSCs[43,47,48].
Table 1.
Surface and functional cancer stem cell markers in biliary tract cancer and their clinical consequences
| Marker | Tissue | M | R | S | TS | Comment | Ref |
| Surface stem cell markers | |||||||
| CD24 | CC, IHC | ↑ | ↑ | ↓ | [68-70] | ||
| CD44 | CC, EHC, IHC, peri-hilar CC, HCC-CC | ↑ | ↓ | ↓differentiation, ↑recurrence | [28,71-75] | ||
| CD133 | CC | ↓ | ↑ | [28,63,71] | |||
| CXCR4 | GBC, IHC | ↑ | ↓ | ↑ | ↑vascular invasion | [52,76] | |
| EpCAM | IHC | ↓ | [28,77] | ||||
| Functional stem cell markers | |||||||
| ALDH1 | EHC, IHC, perihilar CC | ↑ | ↓ | [58,78] | |||
| BMI1 | EHC, HCC-CC, IHC, perihilar CC | ↑in tumor specimens | [71,79,80] | ||||
| NANOG | EHC, IHC, perihilar CC | ||||||
| NESTIN | EHC, IHC, perihilar CC | ||||||
| OCT3/4 | GBC, CC | ↑ | ↓ | ↑ | ↑tumor size | [63,81] | |
| SALL4 | IHC | ↑ | ↓ | ↑vascular and nerve invasion | [82] | ||
| SOX2 | EHC, IHC, perihilar CC | ↑ | ↓ | ↑ | [71,74] | ||
CC: Cholangiocarcinoma; EHC: Extrahepatic cholangiocarcinoma; GBC: Gallbladder cancer; HCC-CC: Combined hepatocellular-cholangiocarcinoma; IHC: Intrahepatic cholangiocarcinoma; M: Metastasis; R: Therapeutic resistance; S: Survival; TS: Tumor stage.
Chemokine receptor 4 (CXCR4) plays an important role in repair and regeneration of tissue in adults and was also identified as a surface marker of (cancer) stem cells[49]. Using a comprehensive gene analysis array, Leelawat et al[50] compared the expression profile of CD24+ and CD24- BTC cells and found enhanced expression of CXCR4 in the CD24+ subpopulation. Drug-based inhibition of CXCR4 using AMD3100 suppressed migration and invasion of BTC cells, and this effect was only observable in the CD24+ CSC subpopulation[50]. Interestingly, AMD3100 treatment also reduced sphere formation potential of BTC cells in another study, further connecting CXCR4 expression with stem cell characteristics[51]. RNA interference-mediated knockdown of CXCR4 in an IHC model inhibited proliferation and colony formation in vitro as well as tumorigenicity in vivo[52].
Cardinale et al[53] demonstrated in xenograft experiments that CD13+/CD90+ BTC spheroids were highly tumorigenic. Interestingly, these two surface molecules are established CSC markers that also play a role in liver cancer, further confirming that hepatocellular carcinoma and BTC may share a common origin or CSC subpopulation[54-57].
As mentioned above, CSC can also be identified via functional characteristics such as ALDH1 expression[13]. Using a BTC cell model, Shuang et al[58] demonstrated that, in contrast to ALDH- cells, ALDH+ cells were able to form tumor spheres. In addition, epithelial markers were reduced in ALDH+ cells, whereas mesenchymal markers were strongly expressed - connecting this cell population to epithelial-to-mesenchymal transition (EMT), a process that is closely related to CSC as discussed later in this article[58,59].
It is well established that deregulated epigenetic events play a huge role in transformation of cells towards a malignant phenotype. The polycomb repressive complexes (PRC) 1 and 2 are multi-protein epigenetic regulators which are known to be aberrantly active in cancer and essential for CSC to maintain their stemness character[60]. Several studies indicate a pivotal role of PRC1 and 2 in development and progression of BTC (as reviewed in[61]). BMI1, which was shown to be expressed in BTC patient samples (Table 1), is a core component of the PRC1 and recently it was demonstrated in BTC cells, that pharmacological inhibition of BMI1 resulted in reduction of ALDH+ cells and diminished formation of tumor spheres[62]. Moreover, in another study, BMI1 was found to be significantly higher expressed in BTC cells positive for CD133 and OCT3/4, further suggesting BMI1 as a CSC marker in BTC[63].
Regarding the pluripotency markers NANOG, OCT3/4 and SOX2[64], only few studies investigated their expression and associated outcomes in clinical BTC samples. However, several in vitro and in vivo studies suggest a pivotal role of these pluripotency markers in BTC CSC. In CD133+ spheres derived from GBC cells, OCT4 and NANOG were highly expressed and these cells also showed higher resistance to chemotherapeutics[65]. In line with these findings, two other studies found that in tumor spheres derived from BTC cells, stem cell markers such as CD133, NANOG, SOX2, SALL4 and OCT4 were up-regulated[53,66]. In addition, these spheres over-expressed ABCG2 and were resistant to cisplatin and additionally displayed high tumorigenic potential when injected into nude mice[66]. More evidence for SOX2 being a potentially relevant factor in BTC cells with stem cell character was presented in another study where the authors showed that artificially over-expression of SOX2 enhanced proliferative capacity, apoptosis resistance and migration and invasion potential[67].
RELEVANCE OF STEMNESS PATHWAYS IN BILIARY TRACT CANCER STEM CELLS
Several signaling pathways are involved in generation and maintenance of CSCs, including the embryonic signaling cascades NOTCH, Wnt and Hh as well as the interleukin-6 (IL-6)-JAK/STAT cascade and the mTOR pathway (for detailed pathway descriptions see[5,83,84]). Of note, these embryonic signaling pathways are also involved in basic cholangiocyte differentiation[85].
Several studies found deregulation of these signaling pathways in BTC specimens and corresponding poor clinical outcome parameters (Table 2 and Figure 1). In addition to these findings, in depth experimental approaches as well as experiments on pharmacological intervention of these pathways have shed more light on their roles in putative CSC in BTC. Treatment of cells with the NOTCH pathway inhibitor γ-secretase inhibitor (GSI) IX significantly decreased the CD24+/CD44+ subpopulation in a BTC cell line model[86]. Moreover, single treatment with gemcitabine increased the amount of CD24+/CD44+ BTC cells, whereas combined treatment with gemcitabine and GSI IX mitigated this effect[86]. In an interesting study by Zender et al[87] long-term artificial over-expression of Notch Intracellular Domain (NICD) 1 - an integral factor of the NOTCH signaling cascade - in mouse livers resulted in a cell population that, when injected into immunodeficient mice, was able to form BTC with features of hepatic progenitor cells. This not only demonstrates the involvement of the NOTCH signaling pathway in CSC, but also suggests a role of hepatic progenitor cells in the development of BTC. In the same regard, Ishii and coworkers published that NICD1 was expressed exclusively in BTC cells with CSC characteristics. Furthermore, treatment with GSI DAPT decreased the number of potential BTC CSCs[35]. Combined drug-based Hh and mTOR inhibition reduced viability and proliferation of BTC cells in vitro[88]. Intriguingly, combined treatment also reduced the number of ALDH+ cells as well as the expression of pluripotency factors NANOG and OCT4. Additional in vivo experiments also revealed diminished tumorigenic potential of the treated cells, indicating concerted action of the Hh and mTOR pathway in creation and /or maintenance of a CSC phenotype in BTC[88]. Constitutively β-catenin expression (mimicking active Wnt pathway) promoted self-renewal of hepatic progenitor cells and injection of these cells generated tumors with characteristics of HCC-CC[89].
Table 2.
Overly active pathways associated with cancer stem cell-like phenotype in biliary tract cancer specimens
| Pathway | Component | Tissue | Outcome | Ref. |
| Hh | GLI1 | GBC | ↑ lymph node metastasis | [92,100] |
| SHH | CC, GBC | ↑ grade (by trend) | [92,100,101] | |
| SMO | GBC | [92] | ||
| JAK / STAT | STAT3 | CC, IHC | ↑ tumor size, ↑ metastasis, ↑ vascular invasion, ↓ survival, poor histological differentiation | [102,103] |
| mTOR | mTOR | BTC | ↓ survival | [104] |
| pmTOR1 | GBC | ↓ survival | [105] | |
| NOTCH | NOTCH 1 | EHC | poor histological differentiation, ↓ survival, ↑ tumor grade, ↑ Cyclin E | [86,87] |
| NOTCH 2 | EHC | ↓ survival | [86] | |
| NOTCH 3 | EHC | ↓ survival, ↑ tumor grade, ↑ Cyclin E | [86,87] | |
| NOTCH 4 | EHC | [86] | ||
| HES-1 | EHC | ↓ survival | [86] | |
| Wnt | β-catenin | CC | ↑ metastasis | [91]2 |
Phosphorylated mTor;
In this study, no non-tumor control samples were used as control. CC: Cholangiocarcinoma; EHC: Extrahepatic cholangiocarcinoma; GBC: Gallbladder cancer; IHC: Intrahepatic cholangiocarcinoma.
Cellular plasticity of differentiation is discussed as a characteristic of CSC in BTC[90]. A phenomenon tightly connected to cellular plasticity and therefore to a CSC phenotype is the ability of cells to detach from the primary tumor and to gain access to the lymphatic and/or vascular system, i.e., the cells become invasive. In order to do so, these invasive and potentially stem cell-like cancer cells have to carve through the surrounding extracellular matrix. Matrix metallopeptidases (MMP) are enzymes that are centrally involved in the breakdown of the extracellular matrix. Active Wnt pathway was shown to directly up-regulate MMP expression in BTC cells, giving BTC cells the ability to gain invasive capabilities[91]. More evidence that the Wnt pathway influences the CSC phenotype in BTC comes from Zhao and coworkers: knockdown of the BTC CSC marker CXCR4 (Table 1) also caused inhibition of the Wnt pathway[52]. Direct causality of these events was proven by providing the ligand CXCL12, which resulted in activation the Wnt pathway and the respective downstream targets including CD44[52].
Artificial activation of Hh pathway (via up-regulation of the ligand SHH) enhanced invasiveness of GBC cells and this effect was reversible via shRNA-mediated or drug-based (cyclopamine) intervention[92]. Further experiments showed that regulation of MMP expression via the Hh pathway was a potential underlying molecular mechanism of this observation[92]. In the same study, Hh pathway activation was associated with enhanced colony formation and this effect was reversed by RNA interference-mediated Hh pathway blockage. Finally, GBC cells with active Hh signaling harbored greater tumor-generating capability in vivo, underlining the importance of this pathway in BTC for several aspects connected with CSC characteristics[92].
BTC often develops under inflammatory conditions and in this regard, diverse cytokines such as IL-6 are involved. It was shown that under chronic inflammatory conditions, neoplastic cholangiocytes are able to produce and secrete IL-6 in an autocrine loop, resulting in proliferation and induction of DNA damaging molecules such as reactive oxygen species and nitric oxide[24]. The JAK/STAT signaling cascade is one of the downstream pathways that is activated via inflammation-related cytokines in BTC development[93,94]. In an interesting study using GBC cells, Kong et al[95] isolated side-population cells and compared the functional and molecular characteristics of these potential CSCs with the non-side-population cells. They found enhanced expression of IL-6 and activated (i.e., phosphorylated) STAT3 in the side-population cells. Intriguingly, these cells harbored multiple CSC traits: enhanced tumor sphere and colony formation potential, chemoresistance, the ability to generate both, side-population and non-side-population cells and, finally, high tumorigenic potential in vivo[95]. Drug-based inhibition of STAT3 reduced growth and migration/wound healing potential of BTC cells[94]. Moreover, treatment of cells with the natural compound luteolin suppressed the activation of the IL-6-induced JAK/STAT3 cascade in BTC cells which resulted in diminished migration, wound healing and colony formation potential[96].
mTOR inhibition was directly connected to loss of CSC characteristics in BTC cell lines: treatment with rapamycin decreased migration, invasion as well as sphere formation potential[97]. Similar results were presented by two other studies in GBC cells[98,99]. Of note, activated mTOR pathway was found in highly proliferative and metastatic GBC cells[99].
MIRNAS AND THEIR ROLE IN REGULATION OF BILIARY TRACT CANCER STEM CELL CHARACTERISTICS
Micro-RNAs (miRs) are non-coding RNAs that regulate gene expression by binding and degradation of target mRNAs[106]. As recently reviewed, aberrant miR expression is involved in different aspects of BTC development and progression[107]. However, up to now, only few studies associated deregulated miR expression with CSC characteristics in BTC. The miRs let-7c/99a and 125b originate from the same gene cluster and were shown to be down-regulated in CC patient material, de-facto being tumor suppressor miRs[108]. Interestingly, expression of these miRs was reduced in tumor spheres derived from BTC cells and in addition, enforced expression of let-7c/99a and 125b reduced the expression of CD133 and CD44 in BTC cells as well as the potential to form tumor spheres[108]. Of note, in the same study, the Interleukin 6 (IL-6) pathway, including IL-6 itself, its receptor IL-6R and the downstream transcription factor STAT3 were identified as targets of this particular miR cluster.
MiR21 is a potent oncogenic miR in BTC with several established targets that contributes to disadvantageous clinical outcome[107]. Zhang et al[109] published that miR21 is necessary for survival of CD24+ cells in primary liver cancer, indicating a role of miR21 in the CSC phenotype in BTC.
Although the number of studies regarding miRs and CSC in BTC is limited as of today, the overlap between described deregulated miRs in BTC and the role of these miRs in CSC of other tumor types strongly suggests a role of these miRs also in CSC of BTC. For example, miR200b is down-regulated in BTC samples, resulting in shorter survival[110]. In lung adenocarcinoma, reduced miR200b expression was shown to be a marker of CSC and, interestingly, restoration of miR200b expression resulted in loss of CSC maintenance and chemoresistance[111]. MiR145 is another miR species that was found to be down-regulated in BTC specimens and associated with poor survival[112]. Likewise, in lung adenocarcinoma tissues, miR145 was found to be down-regulated and negatively correlated with expression of OCT4[113]. Moreover, forced expression of miR145 in lung cancer initiating cells markedly reduced CSC features in vitro and in vivo[113]. In prostate cancer, miR34a was down-regulated in the CD44+ CSC subpopulation and the down-regulation of this miR species was also measured in BTC samples and correlated with advanced clinical stage, lymph node metastasis and poor survival[114,115].
BRIEF OUTLOOK ON BILIARY TRACT CANCER STEM CELLS AND TUMOR MICROENVIRONMENT
CSCs are thought to reside in specific environments called stem cell niches. The CSC niche consists of various cell types and structures including immune cells, mesenchymal (stem) cells, fibroblasts, vascular network, soluble factors and extracellular matrix components and has the function to preserve the exclusive features of CSC as well as to protect them from therapeutic intervention[116]. By creating a suitable tumor microenvironment (TME), the CSC niche plays an outstanding role in development and progression of cancer, essentially supporting tumor growth in multiple aspects. On the other hand, CSCs also support their TME for example by inducing the expression of survival genes[116]. Data regarding CSC niches and TME in BTC are very limited, however, the TME likely contributes to angiogenesis, invasion, metastasis, therapeutic resistance, maintenance of CSC niche and survival of CSC also in BTC[117]. In an interesting study, Raggi et al[43] demonstrated the importance of the interaction between BTC cells and macrophages for tumor development. Medium gathered from BTC cells with CSC characteristics (spheres) activated CD14+ macrophages and shifted their phenotype towards CD163+ so-called tumor-associated macrophages (TAM) which harbored high invasive capacity accompanied by expression of the matrix-remodeling gene MMP2. In the same study, CD163+ macrophages were found at the tumor front in BTC samples, suggesting the importance of these immune cells in progression of BTC. Moreover, the levels of TAMs were associated with poor prognosis of BTC patients[118-120]. Evidence that BTC CSCs not only are able to activate TAMs but also to chemo-attract them was shown via chemotaxis experiments, in which CD14+ strongly migrated towards medium derived from BTC spheres[43].
Mesenchymal stem cells (MSCs) are multipotent adult stromal stem cells that have the ability to generate diverse types of connective tissue[121]. They produce a wide range of cytokines and chemokines, thereby strongly communicating with their near and far environment. Several studies demonstrated that MSCs not only support tumor growth and CSC as a part of the TME, but also that MSC actively migrate towards tumor sites[122]. Regarding BTC, media from MSCs increased migration, invasion, proliferation, chemoresistance and colony formation potential of BTC cells in vitro[91]. Moreover, media from MSCs activated Wnt/β-catenin signaling as well as MMP expression. In a xenograft model, the authors also demonstrated that injection of BTC cells together with MSCs resulted in significantly larger tumors[91]. Recently, it was shown that media derived from BTC cells enhanced the migratory potential of MSC as well as the release of IL-6[123]. On the other hand, culture media derived from these “activated” MSCs significantly increased proliferation of BTC cells as well as the amount of pSTAT3. This effect was completely blocked by the addition of an anti-IL-6 antibody. This is especially interesting because of the well-known role of the IL-6/JAK/STAT cascade in CSCs (see above), thus indicating that MSC can directly support CSCs in BTC. Further evidence that MSCs may directly interact with CSC comes from the fact that MSCs secrete CXCL12, the ligand of the putative BTC stem cell surface marker CXCR-4 (Table 1)[121].
Epithelial-to-mesenchymal transition (EMT) is a process in which cells lose their epithelial traits and gain mesenchymal character, allowing them to detach from the primary tumor and subsequently to form secondary tumors. On molecular level, loss of epithelial markers such as E-Cadherin and enhanced expression of mesenchymal markers such as Vimentin can be observed during EMT[124,125]. Key factors that can initiate EMT include Slug, Snail and Twist, which are repressors of E-Cadherin[125]. The process of EMT is closely related to CSC phenotype. For example, several established CSC pathways such as Wnt, NOTCH and mTOR are involved in EMT and forced expression of EMT resulted in enrichment of CSC subpopulation (as reviewed in[126]). For BTC, Shuang et al[58] connected an EMT phenotype with CSC characteristics. They demonstrated that the ALDH+ subpopulation expressed low levels of E-Cadherin and high levels of the mesenchymal markers Vimentin and N-Cadherin. In another study, the authors recognized reduced expression of the ubiquitin ligase FBXW7 in BTC samples and found a correlation with the metastasis status[97]. Interestingly, the authors demonstrated via in vitro and in vivo experiments in BTC cells, that silencing of FBXW7 resulted in both, an EMT and a CSC phenotype: the epithelial marker E-Cadherin was found to be down-regulated, whereas Vimentin was up-regulated. Regarding CSC markers, silencing of FBXW7 increased the expression of OCT4 and NANOG and enhanced tumor sphere formation capability of the tested BTC cells. Conversely, forced expression of FBXW7 reversed both, the EMT and the CSC phenotype[97]. Results from a study conducted by Matsushita and colleagues also suggests a role of the Hh pathway in EMT regulation in BTC: knockdown of the Hh pathway component SMO caused up-regulation of E-Cadherin and down-regulation of Vimentin, accompanied by decreased invasive potential of these cells[92]. Lastly, Kong et al[95] demonstrated an EMT phenotype (high Vimentin and low E-Cadherin protein expression) in side-population CSCs in GBC, further connecting the EMT process with the CSC phenotype.
CONCLUSION
Although several studies suggest the existence of BTC-specific CSC, additional independent studies should verify these results in functional BTC models, especially in xenograft experiments. Furthermore, due to the heterogenic character of BTC, it should be taken into account that multiple CSC subpopulations with potentially different genetic background and surface marker profiles may exist. Involvement of classical stemness pathways and complexes such as Hh, Wnt, NOTCH and the PRCs as well as of diverse miR species in generation and maintenance of CSC in BTC is very likely. However, up to now, only few studies directly associated the ascertained role of these factors for BTC development with CSC characteristics (Table 2). Moreover, future studies should also concentrate on the role of the TME in the creation and maintenance of potential CSC niches in BTC. The CSC status is strongly dependent on the microenvironment, meaning that CSC traits may very well be not a static, but rather a dynamic phenomenon in which the TME and CSC niche play an absolutely pivotal role[127]. In this light, limitations of current in vitro and in vivo models should always be kept in mind, as (artificial) imitation of an environment as complex and as dynamic as the TME seems very challenging. Current clinical trials involving the CSC concept mainly include immunotherapies, use of CSCs as biomarkers, several CSC-targeted therapies (e.g., metformin, Hh or Notch inhibitors with/without concomitant chemotherapy). However, currently no studies are registered at clinicaltrials.gov specifically enrolling patients with BTC. In addition, future studies should also concentrate on the investigation of the role crosstalk between stemness pathways regarding BTC CSC as well as on the role of (chronic) inflammation in the formation and maintenance of a CSC phenotype in BTC, especially due to the fact that in BTC cells positive for CD133 or OCT3/4, enhanced levels of inflammation-related DNA damage was observed[63].
Taken together, CSCs are promising and attractive targets for pharmacological intervention. Therefore, regarding BTC, after identification and validation of putative CSC populations, screening and testing of anti-CSC-targeting compounds in BTC models will be of great importance in order to develop new therapeutic strategies and approaches.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Ocker M is an employee of Bayer AG, Berlin. Matthias Ocker owns shares of Bayer Pharma AG, Berlin. The other authors have no conflicts of interests.
Peer-review started: February 1, 2017
First decision: February 10, 2017
Article in press: March 21, 2017
P- Reviewer: Ishikawa T, Vaccarezza M, Saeki K, Tanabe S S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 2.S Franco S, Szczesna K, Iliou MS, Al-Qahtani M, Mobasheri A, Kobolák J, Dinnyés A. In vitro models of cancer stem cells and clinical applications. BMC Cancer. 2016;16:738. doi: 10.1186/s12885-016-2774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Deshmukh A, Deshpande K, Arfuso F, Newsholme P, Dharmarajan A. Cancer stem cell metabolism: a potential target for cancer therapy. Mol Cancer. 2016;15:69. doi: 10.1186/s12943-016-0555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oren O, Smith BD. Eliminating Cancer Stem Cells by Targeting Embryonic Signaling Pathways. Stem Cell Rev. 2017;13:17–23. doi: 10.1007/s12015-016-9691-3. [DOI] [PubMed] [Google Scholar]
- 6.Dick JE. Looking ahead in cancer stem cell research. Nat Biotechnol. 2009;27:44–46. doi: 10.1038/nbt0109-44. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz P, Iliou MS, Esteller M. Epigenetic alterations involved in cancer stem cell reprogramming. Mol Oncol. 2012;6:620–636. doi: 10.1016/j.molonc.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- 9.Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–2915. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–3918. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafehi H, Orlowski C, Georgiadis GT, Ververis K, El-Osta A, Karagiannis TC. Clonogenic assay: adherent cells. J Vis Exp. 2011;(49):pii: 2573. doi: 10.3791/2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schatton T, Frank NY, Frank MH. Identification and targeting of cancer stem cells. Bioessays. 2009;31:1038–1049. doi: 10.1002/bies.200900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douville J, Beaulieu R, Balicki D. ALDH1 as a functional marker of cancer stem and progenitor cells. Stem Cells Dev. 2009;18:17–25. doi: 10.1089/scd.2008.0055. [DOI] [PubMed] [Google Scholar]
- 14.Charafe-Jauffret E, Ginestier C, Bertucci F, Cabaud O, Wicinski J, Finetti P, Josselin E, Adelaide J, Nguyen TT, Monville F, et al. ALDH1-positive cancer stem cells predict engraftment of primary breast tumors and are governed by a common stem cell program. Cancer Res. 2013;73:7290–7300. doi: 10.1158/0008-5472.CAN-12-4704. [DOI] [PubMed] [Google Scholar]
- 15.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu A, Luo W, Zhang Q, Yang Z, Zhang G, Li S, Yao K. Aldehyde dehydrogenase 1, a functional marker for identifying cancer stem cells in human nasopharyngeal carcinoma. Cancer Lett. 2013;330:181–189. doi: 10.1016/j.canlet.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 17.Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312:3701–3710. doi: 10.1016/j.yexcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Wu C, Alman BA. Side population cells in human cancers. Cancer Lett. 2008;268:1–9. doi: 10.1016/j.canlet.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 19.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel T. Cholangiocarcinoma--controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189–200. doi: 10.1038/nrgastro.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, Bridgewater J, Okusaka T. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25:391–398. doi: 10.1093/annonc/mdt540. [DOI] [PubMed] [Google Scholar]
- 22.Kokuryo T, Yokoyama Y, Nagino M. Recent advances in cancer stem cell research for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:606–613. doi: 10.1007/s00534-012-0542-6. [DOI] [PubMed] [Google Scholar]
- 23.Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, Grompe M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei M, Lü L, Lin P, Chen Z, Quan Z, Tang Z. Multiple cellular origins and molecular evolution of intrahepatic cholangiocarcinoma. Cancer Lett. 2016;379:253–261. doi: 10.1016/j.canlet.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 25.Joo I, Kim H, Lee JM. Cancer stem cells in primary liver cancers: pathological concepts and imaging findings. Korean J Radiol. 2015;16:50–68. doi: 10.3348/kjr.2015.16.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tschaharganeh DF, Xue W, Calvisi DF, Evert M, Michurina TV, Dow LE, Banito A, Katz SF, Kastenhuber ER, Weissmueller S, et al. p53-Dependent Nestin Regulation Links Tumor Suppression to Cellular Plasticity in Liver Cancer. Cell. 2016;165:1546–1547. doi: 10.1016/j.cell.2016.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guest RV, Boulter L, Kendall TJ, Minnis-Lyons SE, Walker R, Wigmore SJ, Sansom OJ, Forbes SJ. Cell lineage tracing reveals a biliary origin of intrahepatic cholangiocarcinoma. Cancer Res. 2014;74:1005–1010. doi: 10.1158/0008-5472.CAN-13-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwahashi S, Utsunomiya T, Shimada M, Saito Y, Morine Y, Imura S, Ikemoto T, Mori H, Hanaoka J, Bando Y. High expression of cancer stem cell markers in cholangiolocellular carcinoma. Surg Today. 2013;43:654–660. doi: 10.1007/s00595-012-0437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena R, Theise N. Canals of Hering: recent insights and current knowledge. Semin Liver Dis. 2004;24:43–48. doi: 10.1055/s-2004-823100. [DOI] [PubMed] [Google Scholar]
- 30.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 31.Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene. 2006;25:3818–3822. doi: 10.1038/sj.onc.1209558. [DOI] [PubMed] [Google Scholar]
- 32.Gitlin D, Perricelli A, Gitlin GM. Synthesis of -fetoprotein by liver, yolk sac, and gastrointestinal tract of the human conceptus. Cancer Res. 1972;32:979–982. [PubMed] [Google Scholar]
- 33.Shiojiri N, Lemire JM, Fausto N. Cell lineages and oval cell progenitors in rat liver development. Cancer Res. 1991;51:2611–2620. [PubMed] [Google Scholar]
- 34.Ishikawa K, Sasaki A, Haraguchi N, Yoshikawa Y, Mori M. A case of an alpha-fetoprotein-producing intrahepatic cholangiocarcinoma suggests probable cancer stem cell origin. Oncologist. 2007;12:320–324. doi: 10.1634/theoncologist.12-3-320. [DOI] [PubMed] [Google Scholar]
- 35.Ishii T, Yasuchika K, Suemori H, Nakatsuji N, Ikai I, Uemoto S. Alpha-fetoprotein producing cells act as cancer progenitor cells in human cholangiocarcinoma. Cancer Lett. 2010;294:25–34. doi: 10.1016/j.canlet.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Oishi N, Kumar MR, Roessler S, Ji J, Forgues M, Budhu A, Zhao X, Andersen JB, Ye QH, Jia HL, et al. Transcriptomic profiling reveals hepatic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology. 2012;56:1792–1803. doi: 10.1002/hep.25890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carpino G, Cardinale V, Onori P, Franchitto A, Berloco PB, Rossi M, Wang Y, Semeraro R, Anceschi M, Brunelli R, et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat. 2012;220:186–199. doi: 10.1111/j.1469-7580.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, Berloco PB, Cantafora A, Wauthier E, Furth ME, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 39.Carpino G, Cardinale V, Renzi A, Hov JR, Berloco PB, Rossi M, Karlsen TH, Alvaro D, Gaudio E. Activation of biliary tree stem cells within peribiliary glands in primary sclerosing cholangitis. J Hepatol. 2015;63:1220–1228. doi: 10.1016/j.jhep.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 40.Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, Yang A, Montironi R, McKeon F, Loda M. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157:1769–1775. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nomoto K, Tsuneyama K, Cheng C, Takahashi H, Hori R, Murai Y, Takano Y. Intrahepatic cholangiocarcinoma arising in cirrhotic liver frequently expressed p63-positive basal/stem-cell phenotype. Pathol Res Pract. 2006;202:71–76. doi: 10.1016/j.prp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Raggi C, Correnti M, Sica A, Andersen JB, Cardinale V, Alvaro D, Chiorino G, Forti E, Glaser S, Alpini G, et al. Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. J Hepatol. 2017;66:102–115. doi: 10.1016/j.jhep.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Xiao J, Shen M, Yahong Y, Tian R, Zhu F, Jiang J, Du Z, Hu J, Liu W, et al. Isolation and characterization of tumorigenic extrahepatic cholangiocarcinoma cells with stem cell-like properties. Int J Cancer. 2011;128:72–81. doi: 10.1002/ijc.25317. [DOI] [PubMed] [Google Scholar]
- 45.Nakashima S, Kobayashi S, Nagano H, Tomokuni A, Tomimaru Y, Asaoka T, Hama N, Wada H, Kawamoto K, Marubashi S, et al. BRCA/Fanconi anemia pathway implicates chemoresistance to gemcitabine in biliary tract cancer. Cancer Sci. 2015;106:584–591. doi: 10.1111/cas.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakanishi T, Ross DD. Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression. Chin J Cancer. 2012;31:73–99. doi: 10.5732/cjc.011.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayr C, Wagner A, Stoecklinger A, Jakab M, Illig R, Berr F, Pichler M, Di Fazio P, Ocker M, Neureiter D, et al. 3-Deazaneplanocin A May Directly Target Putative Cancer Stem Cells in Biliary Tract Cancer. Anticancer Res. 2015;35:4697–4705. [PubMed] [Google Scholar]
- 48.Shi C, Tian R, Wang M, Wang X, Jiang J, Zhang Z, Li X, He Z, Gong W, Qin R. CD44+ CD133+ population exhibits cancer stem cell-like characteristics in human gallbladder carcinoma. Cancer Biol Ther. 2010;10:1182–1190. doi: 10.4161/cbt.10.11.13664. [DOI] [PubMed] [Google Scholar]
- 49.Furusato B, Mohamed A, Uhlén M, Rhim JS. CXCR4 and cancer. Pathol Int. 2010;60:497–505. doi: 10.1111/j.1440-1827.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- 50.Leelawat K, Keeratichamroen S, Leelawat S, Tohtong R. CD24 induces the invasion of cholangiocarcinoma cells by upregulating CXCR4 and increasing the phosphorylation of ERK1/2. Oncol Lett. 2013;6:1439–1446. doi: 10.3892/ol.2013.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayr C, Neureiter D, Pichler M, Berr F, Wagner A, Kiesslich T, Namberger K. Cytotoxic effects of chemokine receptor 4 inhibition by AMD3100 in biliary tract cancer cells: Potential drug synergism with gemcitabine. Mol Med Rep. 2015;12:2247–2252. doi: 10.3892/mmr.2015.3589. [DOI] [PubMed] [Google Scholar]
- 52.Zhao S, Wang J, Qin C. Blockade of CXCL12/CXCR4 signaling inhibits intrahepatic cholangiocarcinoma progression and metastasis via inactivation of canonical Wnt pathway. J Exp Clin Cancer Res. 2014;33:103. doi: 10.1186/s13046-014-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cardinale V, Renzi A, Carpino G, Torrice A, Bragazzi MC, Giuliante F, DeRose AM, Fraveto A, Onori P, Napoletano C, et al. Profiles of cancer stem cell subpopulations in cholangiocarcinomas. Am J Pathol. 2015;185:1724–1739. doi: 10.1016/j.ajpath.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sukowati CH, Anfuso B, Torre G, Francalanci P, Crocè LS, Tiribelli C. The expression of CD90/Thy-1 in hepatocellular carcinoma: an in vivo and in vitro study. PLoS One. 2013;8:e76830. doi: 10.1371/journal.pone.0076830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Röcken C, Licht J, Roessner A, Carl-McGrath S. Canalicular immunostaining of aminopeptidase N (CD13) as a diagnostic marker for hepatocellular carcinoma. J Clin Pathol. 2005;58:1069–1075. doi: 10.1136/jcp.2005.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagano H, Ishii H, Marubashi S, Haraguchi N, Eguchi H, Doki Y, Mori M. Novel therapeutic target for cancer stem cells in hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2012;19:600–605. doi: 10.1007/s00534-012-0543-5. [DOI] [PubMed] [Google Scholar]
- 57.Shaikh MV, Kala M, Nivsarkar M. CD90 a potential cancer stem cell marker and a therapeutic target. Cancer Biomark. 2016;16:301–307. doi: 10.3233/CBM-160590. [DOI] [PubMed] [Google Scholar]
- 58.Shuang ZY, Wu WC, Xu J, Lin G, Liu YC, Lao XM, Zheng L, Li S. Transforming growth factor-β1-induced epithelial-mesenchymal transition generates ALDH-positive cells with stem cell properties in cholangiocarcinoma. Cancer Lett. 2014;354:320–328. doi: 10.1016/j.canlet.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 59.Ishiwata T. Cancer stem cells and epithelial-mesenchymal transition: Novel therapeutic targets for cancer. Pathol Int. 2016;66:601–608. doi: 10.1111/pin.12447. [DOI] [PubMed] [Google Scholar]
- 60.Sauvageau M, Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell. 2010;7:299–313. doi: 10.1016/j.stem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayr C, Neureiter D, Wagner A, Pichler M, Kiesslich T. The role of polycomb repressive complexes in biliary tract cancer. Expert Opin Ther Targets. 2015;19:363–375. doi: 10.1517/14728222.2014.986460. [DOI] [PubMed] [Google Scholar]
- 62.Mayr C, Wagner A, Loeffelberger M, Bruckner D, Jakab M, Berr F, Di Fazio P, Ocker M, Neureiter D, Pichler M, et al. The BMI1 inhibitor PTC-209 is a potential compound to halt cellular growth in biliary tract cancer cells. Oncotarget. 2016;7:745–758. doi: 10.18632/oncotarget.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thanan R, Pairojkul C, Pinlaor S, Khuntikeo N, Wongkham C, Sripa B, Ma N, Vaeteewoottacharn K, Furukawa A, Kobayashi H, et al. Inflammation-related DNA damage and expression of CD133 and Oct3/4 in cholangiocarcinoma patients with poor prognosis. Free Radic Biol Med. 2013;65:1464–1472. doi: 10.1016/j.freeradbiomed.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 64.Ruiz-Vela A, Aguilar-Gallardo C, Simón C. Building a framework for embryonic microenvironments and cancer stem cells. Stem Cell Rev. 2009;5:319–327. doi: 10.1007/s12015-009-9096-7. [DOI] [PubMed] [Google Scholar]
- 65.Shi CJ, Gao J, Wang M, Wang X, Tian R, Zhu F, Shen M, Qin RY. CD133(+) gallbladder carcinoma cells exhibit self-renewal ability and tumorigenicity. World J Gastroenterol. 2011;17:2965–2971. doi: 10.3748/wjg.v17.i24.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yin BB, Wu SJ, Zong HJ, Ma BJ, Cai D. Preliminary screening and identification of stem cell-like sphere clones in a gallbladder cancer cell line GBC-SD. J Zhejiang Univ Sci B. 2011;12:256–263. doi: 10.1631/jzus.B1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun Q, Li J, Wang G, Xie Y. Role of the embryonic protein SOX2 in cholangiocarcinoma. Cell Biochem Biophys. 2014;70:1311–1316. doi: 10.1007/s12013-014-0056-8. [DOI] [PubMed] [Google Scholar]
- 68.Su MC, Hsu C, Kao HL, Jeng YM. CD24 expression is a prognostic factor in intrahepatic cholangiocarcinoma. Cancer Lett. 2006;235:34–39. doi: 10.1016/j.canlet.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 69.Agrawal S, Kuvshinoff BW, Khoury T, Yu J, Javle MM, LeVea C, Groth J, Coignet LJ, Gibbs JF. CD24 expression is an independent prognostic marker in cholangiocarcinoma. J Gastrointest Surg. 2007;11:445–451. doi: 10.1007/s11605-007-0091-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keeratichamroen S, Leelawat K, Thongtawee T, Narong S, Aegem U, Tujinda S, Praditphol N, Tohtong R. Expression of CD24 in cholangiocarcinoma cells is associated with disease progression and reduced patient survival. Int J Oncol. 2011;39:873–881. doi: 10.3892/ijo.2011.1088. [DOI] [PubMed] [Google Scholar]
- 71.Kemmerling R, Alinger B, Dietze O, Bösmüller HC, Ocker M, Wolkersdörfer GW, Berr F, Neureiter D, Kiesslich T. Association of stem cell marker expression pattern and survival in human biliary tract cancer. Int J Oncol. 2012;41:511–522. doi: 10.3892/ijo.2012.1477. [DOI] [PubMed] [Google Scholar]
- 72.Thanee M, Loilome W, Techasen A, Sugihara E, Okazaki S, Abe S, Ueda S, Masuko T, Namwat N, Khuntikeo N, et al. CD44 variant-dependent redox status regulation in liver fluke-associated cholangiocarcinoma: A target for cholangiocarcinoma treatment. Cancer Sci. 2016;107:991–1000. doi: 10.1111/cas.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kunlabut K, Vaeteewoottacharn K, Wongkham C, Khuntikeo N, Waraasawapati S, Pairojkul C, Wongkham S. Aberrant expression of CD44 in bile duct cancer correlates with poor prognosis. Asian Pac J Cancer Prev. 2012;13 Suppl:95–99. [PubMed] [Google Scholar]
- 74.Gu MJ, Jang BI. Clinicopathologic significance of Sox2, CD44 and CD44v6 expression in intrahepatic cholangiocarcinoma. Pathol Oncol Res. 2014;20:655–660. doi: 10.1007/s12253-014-9745-2. [DOI] [PubMed] [Google Scholar]
- 75.Kim R, Kim SB, Cho EH, Park SH, Park SB, Hong SK, Chae G. CD44 expression in patients with combined hepatocellular cholangiocarcinoma. Ann Surg Treat Res. 2015;89:9–16. doi: 10.4174/astr.2015.89.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao X, Zhou L, Han S, Chen Y. High expression of CXCR4 and CXCR7 predicts poor survival in gallbladder cancer. J Int Med Res. 2011;39:1253–1264. doi: 10.1177/147323001103900413. [DOI] [PubMed] [Google Scholar]
- 77.Sulpice L, Rayar M, Turlin B, Boucher E, Bellaud P, Desille M, Meunier B, Clément B, Boudjema K, Coulouarn C. Epithelial cell adhesion molecule is a prognosis marker for intrahepatic cholangiocarcinoma. J Surg Res. 2014;192:117–123. doi: 10.1016/j.jss.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 78.Chen MH, Weng JJ, Cheng CT, Wu RC, Huang SC, Wu CE, Chung YH, Liu CY, Chang MH, Chen MH, et al. ALDH1A3, the Major Aldehyde Dehydrogenase Isoform in Human Cholangiocarcinoma Cells, Affects Prognosis and Gemcitabine Resistance in Cholangiocarcinoma Patients. Clin Cancer Res. 2016;22:4225–4235. doi: 10.1158/1078-0432.CCR-15-1800. [DOI] [PubMed] [Google Scholar]
- 79.Sasaki M, Ikeda H, Itatsu K, Yamaguchi J, Sawada S, Minato H, Ohta T, Nakanuma Y. The overexpression of polycomb group proteins Bmi1 and EZH2 is associated with the progression and aggressive biological behavior of hepatocellular carcinoma. Lab Invest. 2008;88:873–882. doi: 10.1038/labinvest.2008.52. [DOI] [PubMed] [Google Scholar]
- 80.Sasaki M, Yamaguchi J, Ikeda H, Itatsu K, Nakanuma Y. Polycomb group protein Bmi1 is overexpressed and essential in anchorage-independent colony formation, cell proliferation and repression of cellular senescence in cholangiocarcinoma: tissue and culture studies. Hum Pathol. 2009;40:1723–1730. doi: 10.1016/j.humpath.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 81.Zou Q, Yang L, Yang Z, Huang J, Fu X. PSCA and Oct-4 expression in the benign and malignant lesions of gallbladder: implication for carcinogenesis, progression, and prognosis of gallbladder adenocarcinoma. Biomed Res Int. 2013;2013:648420. doi: 10.1155/2013/648420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deng G, Zhu L, Huang F, Nie W, Huang W, Xu H, Zheng S, Yi Z, Wan T. SALL4 is a novel therapeutic target in intrahepatic cholangiocarcinoma. Oncotarget. 2015;6:27416–27426. doi: 10.18632/oncotarget.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pires BR, DE Amorim ÍS, Souza LD, Rodrigues JA, Mencalha AL. Targeting Cellular Signaling Pathways in Breast Cancer Stem Cells and its Implication for Cancer Treatment. Anticancer Res. 2016;36:5681–5691. doi: 10.21873/anticanres.11151. [DOI] [PubMed] [Google Scholar]
- 85.Liu WH, Ren LN, Chen T, Liu LY, Tang LJ. Stages based molecular mechanisms for generating cholangiocytes from liver stem/progenitor cells. World J Gastroenterol. 2013;19:7032–7041. doi: 10.3748/wjg.v19.i41.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aoki S, Mizuma M, Takahashi Y, Haji Y, Okada R, Abe T, Karasawa H, Tamai K, Okada T, Morikawa T, et al. Aberrant activation of Notch signaling in extrahepatic cholangiocarcinoma: clinicopathological features and therapeutic potential for cancer stem cell-like properties. BMC Cancer. 2016;16:854. doi: 10.1186/s12885-016-2919-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zender S, Nickeleit I, Wuestefeld T, Sörensen I, Dauch D, Bozko P, El-Khatib M, Geffers R, Bektas H, Manns MP, et al. A Critical Role for Notch Signaling in the Formation of Cholangiocellular Carcinomas. Cancer Cell. 2016;30:353–356. doi: 10.1016/j.ccell.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 88.Zuo M, Rashid A, Churi C, Vauthey JN, Chang P, Li Y, Hung MC, Li D, Javle M. Novel therapeutic strategy targeting the Hedgehog signalling and mTOR pathways in biliary tract cancer. Br J Cancer. 2015;112:1042–1051. doi: 10.1038/bjc.2014.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chiba T, Zheng YW, Kita K, Yokosuka O, Saisho H, Onodera M, Miyoshi H, Nakano M, Zen Y, Nakanuma Y, et al. Enhanced self-renewal capability in hepatic stem/progenitor cells drives cancer initiation. Gastroenterology. 2007;133:937–950. doi: 10.1053/j.gastro.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 90.Oikawa T. Cancer Stem cells and their cellular origins in primary liver and biliary tract cancers. Hepatology. 2016;64:645–651. doi: 10.1002/hep.28485. [DOI] [PubMed] [Google Scholar]
- 91.Wang W, Zhong W, Yuan J, Yan C, Hu S, Tong Y, Mao Y, Hu T, Zhang B, Song G. Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma. Oncotarget. 2015;6:42276–42289. doi: 10.18632/oncotarget.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsushita S, Onishi H, Nakano K, Nagamatsu I, Imaizumi A, Hattori M, Oda Y, Tanaka M, Katano M. Hedgehog signaling pathway is a potential therapeutic target for gallbladder cancer. Cancer Sci. 2014;105:272–280. doi: 10.1111/cas.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maemura K, Natsugoe S, Takao S. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat Sci. 2014;21:754–760. doi: 10.1002/jhbp.126. [DOI] [PubMed] [Google Scholar]
- 94.Senggunprai L, Kukongviriyapan V, Prawan A, Kukongviriyapan U. Quercetin and EGCG exhibit chemopreventive effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phytother Res. 2014;28:841–848. doi: 10.1002/ptr.5061. [DOI] [PubMed] [Google Scholar]
- 95.Kong X, Ma MZ, Zhang Y, Weng MZ, Gong W, Guo LQ, Zhang JX, Wang GD, Su Q, Quan ZW, et al. Differentiation therapy: sesamin as an effective agent in targeting cancer stem-like side population cells of human gallbladder carcinoma. BMC Complement Altern Med. 2014;14:254. doi: 10.1186/1472-6882-14-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aneknan P, Kukongviriyapan V, Prawan A, Kongpetch S, Sripa B, Senggunprai L. Luteolin arrests cell cycling, induces apoptosis and inhibits the JAK/STAT3 pathway in human cholangiocarcinoma cells. Asian Pac J Cancer Prev. 2014;15:5071–5076. doi: 10.7314/apjcp.2014.15.12.5071. [DOI] [PubMed] [Google Scholar]
- 97.Yang H, Lu X, Liu Z, Chen L, Xu Y, Wang Y, Wei G, Chen Y. FBXW7 suppresses epithelial-mesenchymal transition, stemness and metastatic potential of cholangiocarcinoma cells. Oncotarget. 2015;6:6310–6325. doi: 10.18632/oncotarget.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zong H, Yin B, Zhou H, Cai D, Ma B, Xiang Y. Inhibition of mTOR pathway attenuates migration and invasion of gallbladder cancer via EMT inhibition. Mol Biol Rep. 2014;41:4507–4512. doi: 10.1007/s11033-014-3321-4. [DOI] [PubMed] [Google Scholar]
- 99.Cao Y, Liu X, Lu W, Chen Y, Wu X, Li M, Wang XA, Zhang F, Jiang L, Zhang Y, et al. Fibronectin promotes cell proliferation and invasion through mTOR signaling pathway activation in gallbladder cancer. Cancer Lett. 2015;360:141–150. doi: 10.1016/j.canlet.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 100.Kiesslich T, Mayr C, Wachter J, Bach D, Fuereder J, Wagner A, Alinger B, Pichler M, Di Fazio P, Ocker M, et al. Activated hedgehog pathway is a potential target for pharmacological intervention in biliary tract cancer. Mol Cell Biochem. 2014;396:257–268. doi: 10.1007/s11010-014-2161-9. [DOI] [PubMed] [Google Scholar]
- 101.Al-Bahrani R, Nagamori S, Leng R, Petryk A, Sergi C. Differential Expression of Sonic Hedgehog Protein in Human Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Pathol Oncol Res. 2015;21:901–908. doi: 10.1007/s12253-015-9918-7. [DOI] [PubMed] [Google Scholar]
- 102.Yang XW, Li L, Hou GJ, Yan XZ, Xu QG, Chen L, Zhang BH, Shen F. STAT3 overexpression promotes metastasis in intrahepatic cholangiocarcinoma and correlates negatively with surgical outcome. Oncotarget. 2017;8:7710–7721. doi: 10.18632/oncotarget.13846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dokduang H, Techasen A, Namwat N, Khuntikeo N, Pairojkul C, Murakami Y, Loilome W, Yongvanit P. STATs profiling reveals predominantly-activated STAT3 in cholangiocarcinoma genesis and progression. J Hepatobiliary Pancreat Sci. 2014;21:767–776. doi: 10.1002/jhbp.131. [DOI] [PubMed] [Google Scholar]
- 104.Herberger B, Puhalla H, Lehnert M, Wrba F, Novak S, Brandstetter A, Gruenberger B, Gruenberger T, Pirker R, Filipits M. Activated mammalian target of rapamycin is an adverse prognostic factor in patients with biliary tract adenocarcinoma. Clin Cancer Res. 2007;13:4795–4799. doi: 10.1158/1078-0432.CCR-07-0738. [DOI] [PubMed] [Google Scholar]
- 105.Leal P, García P, Sandoval A, Letelier P, Brebi P, Ili C, Álvarez H, Tapia O, Roa JC. Immunohistochemical expression of phospho-mTOR is associated with poor prognosis in patients with gallbladder adenocarcinoma. Arch Pathol Lab Med. 2013;137:552–557. doi: 10.5858/arpa.2012-0032-OA. [DOI] [PubMed] [Google Scholar]
- 106.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663–4670. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mayr C, Beyreis M, Wagner A, Pichler M, Neureiter D, Kiesslich T. Deregulated MicroRNAs in Biliary Tract Cancer: Functional Targets and Potential Biomarkers. Biomed Res Int. 2016;2016:4805270. doi: 10.1155/2016/4805270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin KY, Ye H, Han BW, Wang WT, Wei PP, He B, Li XJ, Chen YQ. Genome-wide screen identified let-7c/miR-99a/miR-125b regulating tumor progression and stem-like properties in cholangiocarcinoma. Oncogene. 2016;35:3376–3386. doi: 10.1038/onc.2015.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang J, Jiao J, Cermelli S, Muir K, Jung KH, Zou R, Rashid A, Gagea M, Zabludoff S, Kalluri R, et al. miR-21 Inhibition Reduces Liver Fibrosis and Prevents Tumor Development by Inducing Apoptosis of CD24+ Progenitor Cells. Cancer Res. 2015;75:1859–1867. doi: 10.1158/0008-5472.CAN-14-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Urbas R, Mayr C, Klieser E, Fuereder J, Bach D, Stättner S, Primavesi F, Jaeger T, Stanzer S, Ress AL, et al. Relevance of MicroRNA200 Family and MicroRNA205 for Epithelial to Mesenchymal Transition and Clinical Outcome in Biliary Tract Cancer Patients. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17122053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen DQ, Huang JY, Feng B, Pan BZ, De W, Wang R, Chen LB. Histone deacetylase 1/Sp1/microRNA-200b signaling accounts for maintenance of cancer stem-like cells in human lung adenocarcinoma. PLoS One. 2014;9:e109578. doi: 10.1371/journal.pone.0109578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhan M, Zhao X, Wang H, Chen W, Xu S, Wang W, Shen H, Huang S, Wang J. miR-145 sensitizes gallbladder cancer to cisplatin by regulating multidrug resistance associated protein 1. Tumour Biol. 2016;37:10553–10562. doi: 10.1007/s13277-016-4957-6. [DOI] [PubMed] [Google Scholar]
- 113.Hu J, Qiu M, Jiang F, Zhang S, Yang X, Wang J, Xu L, Yin R. MiR-145 regulates cancer stem-like properties and epithelial-to-mesenchymal transition in lung adenocarcinoma-initiating cells. Tumour Biol. 2014;35:8953–8961. doi: 10.1007/s13277-014-2158-8. [DOI] [PubMed] [Google Scholar]
- 114.Jin K, Xiang Y, Tang J, Wu G, Li J, Xiao H, Li C, Chen Y, Zhao J. miR-34 is associated with poor prognosis of patients with gallbladder cancer through regulating telomere length in tumor stem cells. Tumour Biol. 2014;35:1503–1510. doi: 10.1007/s13277-013-1207-z. [DOI] [PubMed] [Google Scholar]
- 115.Qiao P, Li G, Bi W, Yang L, Yao L, Wu D. microRNA-34a inhibits epithelial mesenchymal transition in human cholangiocarcinoma by targeting Smad4 through transforming growth factor-beta/Smad pathway. BMC Cancer. 2015;15:469. doi: 10.1186/s12885-015-1359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Borovski T, De Sousa E Melo F, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–639. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 117.Romano M, De Francesco F, Gringeri E, Giordano A, Ferraro GA, Di Domenico M, Cillo U. Tumor Microenvironment Versus Cancer Stem Cells in Cholangiocarcinoma: Synergistic Effects? J Cell Physiol. 2016;231:768–776. doi: 10.1002/jcp.25190. [DOI] [PubMed] [Google Scholar]
- 118.Subimerb C, Pinlaor S, Lulitanond V, Khuntikeo N, Okada S, McGrath MS, Wongkham S. Circulating CD14(+) CD16(+) monocyte levels predict tissue invasive character of cholangiocarcinoma. Clin Exp Immunol. 2010;161:471–479. doi: 10.1111/j.1365-2249.2010.04200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Subimerb C, Pinlaor S, Khuntikeo N, Leelayuwat C, Morris A, McGrath MS, Wongkham S. Tissue invasive macrophage density is correlated with prognosis in cholangiocarcinoma. Mol Med Rep. 2010;3:597–605. doi: 10.3892/mmr_00000303. [DOI] [PubMed] [Google Scholar]
- 120.Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE, Folseraas T, Forbes SJ, Fouassier L, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA) Nat Rev Gastroenterol Hepatol. 2016;13:261–280. doi: 10.1038/nrgastro.2016.51. [DOI] [PubMed] [Google Scholar]
- 121.Sherman LS, Shaker M, Mariotti V, Rameshwar P. Mesenchymal stromal/stem cells in drug therapy: New perspective. Cytotherapy. 2017;19:19–27. doi: 10.1016/j.jcyt.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Norozi F, Ahmadzadeh A, Shahrabi S, Vosoughi T, Saki N. Mesenchymal stem cells as a double-edged sword in suppression or progression of solid tumor cells. Tumour Biol. 2016;37:11679–11689. doi: 10.1007/s13277-016-5187-7. [DOI] [PubMed] [Google Scholar]
- 123.Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles. 2015;4:24900. doi: 10.3402/jev.v4.24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kiesslich T, Pichler M, Neureiter D. Epigenetic control of epithelial-mesenchymal-transition in human cancer. Mol Clin Oncol. 2013;1:3–11. doi: 10.3892/mco.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mladinich M, Ruan D, Chan CH. Tackling Cancer Stem Cells via Inhibition of EMT Transcription Factors. Stem Cells Int. 2016;2016:5285892. doi: 10.1155/2016/5285892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol. 2010;222:268–277. doi: 10.1002/jcp.21940. [DOI] [PubMed] [Google Scholar]


