Abstract
Although gastric tumors have overlapping radiologic appearances, some unusual tumors may present specific imaging features. Using multidetector computed tomography (MDCT), with water as a negative oral contrast agent and intravenous contrast medium, can provide critical information for the diagnosis of gastric diseases. In addition, MDCT can evaluate the involvement of the gastric wall and extragastric extent of the disease, as compared with gastroenteroscopy and double-contrast upper gastrointestinal study. Regarding lesion location and size, enhancing and growth patterns, presence of calcification or fat, and involvement of the gastric wall and adjacent structures, CT may provide useful information. In this review article, we review the relevant literature and discuss the CT features and the histopathologic findings of different types of gastric lesions. The lesions are divided into benign (glomus tumors, schwannomas, leiomyomas, and lipomas), malignant (gastrointestinal stromal tumors, mucinous carcinomas, lymphomas, and carcinoid tumors), and tumor-like lesions (ectopic pancreas and bezoar). Familiarity with imaging appearances and pathologic findings can help physicians make an accurate diagnosis.
Keywords: Multidetector computed tomography, Stomach, Neoplasm, Adenocarcinoma, carcinoid, Lymphoma, Lipoma, Glomus tumor, Heterotopic pancreas, Schwannoma, Gastrointestinal submucosal tumor, Leiomyoma, Bezoar
Core tip: Diagnostic imaging of gastric tumors remains a practical challenge. However, in some cases of uncommon gastric tumors and tumor-like lesions, there are some specific radiographic features. Using the multidetector computed tomography, with water as a negative oral contrast agent and intravenous contrast medium, can provide critical information for the diagnosis of gastric diseases. Familiarity with the computed tomography features of these diseases facilitates accurate diagnosis and further management.
INTRODUCTION
Diagnostic imaging of gastric tumors remains a practical challenge. The most common primary gastric tumors are gastric adenocarcinomas (> 90%)[1].
Esophagogastroduodenoscopy (EGD) is the main test used to find stomach cancer and perform a biopsy, and multidetector computed tomography (MDCT) is used to locate the lesion and determine the extent of the disease. Using MDCT to characterize the disease always leads to a long list of differential diagnoses because many overlapping characteristics have been shown to exist among various gastric tumors (Table 1).
Table 1.
Characteristics of uncommon gastric tumors and tumor-like lesions
| CT features | |
| Benign tumors | |
| Glomus Tumor | Small, solitary, and hypervascular tumor at gastric antrum |
| Schwannoma | Homogeneous attenuated gastric tumor |
| Leiomyoma | Small, endoluminal growth, hypoenhanced tumor at gastric cardia |
| Lipoma | Fat contained tumor |
| Malignant tumors | |
| Adenocarcinoma | Polypoid, or generalized mural thickening, or focal mural thickening with/without ulceration tumor |
| The mucinous type has punctate or miliary calcification within the tumor | |
| GIST | Exophytic hypervascular GI mass arising from submucosa with central ulceration, amorphous calcification |
| Lymphoma | Regional or diffuse wall thickening preserved perigastric fat plane and lymphadenopathy extending below the renal hila |
| Carcinoid | Type I and II, small, polypoid lesion, with marked enhancement. Type III, larger, sporadic, solitary tumor with distant metastasis |
| Tumor-like lesion | |
| Ectopic pancreas | Small solitary lesion at greater curvature of distal antrum with enhancement similar to pancreas |
| Bezoar | Intraluminal gastric filling defect with mottled appearance |
GIST: Gastrointestinal stromal tumor; CT: Computed tomography.
However, in some cases of unusual tumors and tumor-like lesions, familiarity with the most relevant radiologic features with clinical information allows adequate characterization and diagnosis. Using the MDCT, with water as the negative oral contrast agent, can provide useful information regarding lesion location and size, enhancing and growth patterns, presence of calcification or fat, and involvement of the gastric wall and adjacent structures.
In this review article, we review the relevant literature and discuss the CT features and histopathologic findings of different types of gastric lesions. The lesions are divided into benign (glomus tumors, schwannomas, leiomyomas, and lipomas), malignant (gastrointestinal stromal tumors, mucinous carcinomas, lymphomas, and carcinoid tumors), and tumor-like lesions (ectopic pancreas and bezoar).
CT protocol of stomach studies
Dynamic MDCT with stomach distention is optimal for the study of stomach tumors. The stomach is distended with positive or negative oral contrast to avoid overlooking tumors[2]. The traditional positive oral contrast material may not mix uniformly with gastric contents, and mimics pseudotumors. In addition, high-attenuation contrast may mask subtle disease on contrast-enhanced images of the gastric wall[3]. Water and low-concentration barium sulfate are used as a negative oral contrast agent. We preferred to use water as a negative oral contrast agent for optimal assessment. Water is free and well tolerated. Before the CT scan, our patient was fasted for 4 h. After intravenous injection of hyoscine N-butylbromide (Buscopan, Boehringer International, Ingelheim, Germany) for slowing down gastrointestinal movement, the water (500 mL) was administered in a routine procedure to obtain gastric distention before the patient was laid down on the CT table.
The dynamic CT imaging for the gastric lesions was performed in three phases (non-enhanced, arterial, and portovenous). Non-enhanced imaging was obtained to provide a baseline for the degree of lesion enhancement as well as detecting the hemorrhage, calcification, and fat component of the lesion. The arterial phase was obtained 30 seconds after the injection of a dose of 2 mg/kg of nonionic contrast material at a rate of 2.5 mL/s, using an automated power injector. The portovenous phase was obtained 50 s after the contrast injection. The contrast-enhanced phases help in assessing the extent of involvement of the stomach, differentiating mucosal and submucosal tumors, determining the enhancing and growth patterns, and detecting distant metastasis and lymphadenopathy[4,5].
BENIGN TUMORS
Glomus tumors
Glomus tumors are modified smooth muscle cells that recapitulate perivascular glomus body cells. They are typically found in peripheral soft tissues, but can occur anywhere in the body[6]. In the gastrointestinal tract, glomus tumors are more common in the stomach as benign vascular submucosal tumors. The clinical symptoms are nonspecific. However, larger lesions are likely to be ulcerated, causing upper gastrointestinal bleeding. Surgical resection is typically curative.
On gastroenteroscopy, the glomus tumors appear as nonspecific submucosal lesions with a smooth surface[7]. Glomus tumors are typically small and solitary, and are commonly located in the gastric antrum. In pre-contrast CT, they are iso-dense to the stomach wall and manifest as solitary hypervascular lesions in the arterial phase, which persist in the portovenous phase in dynamic CT (Figure 1)[8]. Sometimes, they may exhibit a hemangioma-like “central fill-in” enhancement pattern in the delayed phase[9]. By contrast, adenocarcinoma is a relatively poor-enhancing mucosal tumor manifesting as a polypoid lesion with generalized mural thickening or focal mural thickening, with or without ulceration.
Figure 1.
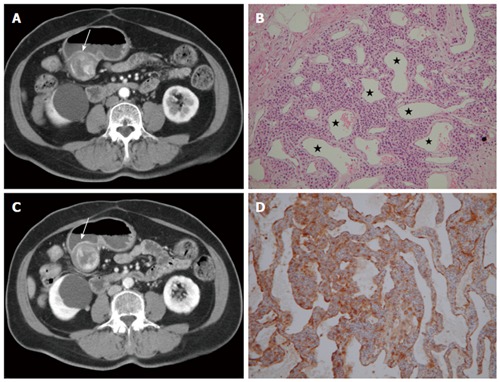
Glomus tumor. A 66-year-old woman presented with epigastric pain for 1 mo. A: Arterial phase showing a submucosal mass at the gastric antrum (arrow) with an exophytic growth pattern. Peripheral nodular enhancement is evident; B: Portovenous phase showing central fill-in enhancement compared with the arterial phase; C: High power photomicrography (original magnification, × 200, HE stain) showing many vessels (star) filled with red blood cells and lined within the tumor cells. The tumor cells were positive for smooth muscle actin (D).
In histologic analysis, glomus tumors are composed of numerous dilated, irregular shaped vessels, covered by numerous, monotonous round cells. The tumor cells are positive for smooth muscle actin (Figure 1)[6]. The prominent vascular structures are responsible for dense contrast enhancement in CT.
Schwannomas
Gastrointestinal schwannomas are considered to be different from conventional schwannomas because of unique different histologic features[10]. Conventional schwannomas are benign neurogenic tumors composed of over-proliferated Schwann cells in the soft tissue or central nervous system. In the gastrointestinal tract, such lesions often display prominent lymphoid cuffing (Figure 2). Gastrointestinal schwannomas are rare in the gastrointestinal tract, with the stomach being the most common site, followed by the colon and rectum. They are believed to arise from the gastrointestinal autonomic nervous system[11]. Gastrointestinal schwannomas account for 4% of all benign gastric neoplasms and the peak incidence occurs is in fourth and fifth decades of life[12]. They are typically asymptomatic, but can present with gastrointestinal bleeding or palpable mass.
Figure 2.
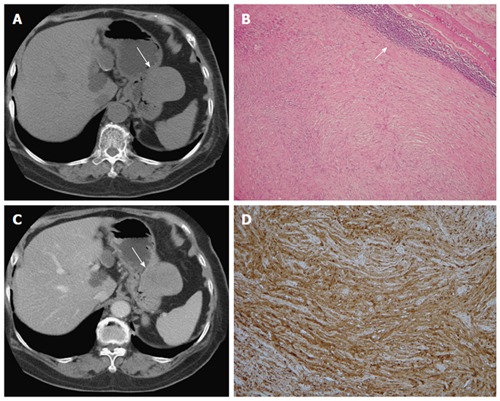
Schwannoma. A 75-year-old woman presented with coffee ground vomitus. A: Pre-contrast transverse computed tomography (CT) showing a homogeneous iso-density tumor in the greater curvature of the stomach (arrow); B: Post-contrast-enhanced CT showing homogeneously moderate enhancement with a mixed (endoluminal and exophytic) growth pattern; C: Low-power photomicrograph (original magnification, × 20; HE stain) showing that the tumor retains its circumscription with lymphoid aggregate cuffing (arrow); D: The vaguely bundled spindle tumor cells were positive for S-100.
Gastric schwannomas are submucosal lesions with endoluminal or exophytic growth patterns and their common CT appearance is homogeneous attenuation in pre-contrast and post-contrast images with moderate enhancement (Figure 2)[10]. Calcification, cystic change, hemorrhage, and necrosis are rarely seen in gastric schwannoma. By contrast, adenocarcinomas typically exhibit ulceration, necrosis, and involvement of the gastric mucosa.
In histologic analysis, gastrointestinal schwannomas consist of focally atypical spindle cells in a microtrabecular-microfascicular pattern with evidence of nerve sheath differentiation (S-100 protein-positive), peripheral lymphoid cuffing, and occasional germinal centers (Figure 2)[11,13].
Leiomyomas
True leiomyomas were not well distinguished from gastrointestinal stromal tumors (GISTs) until the development of immunohistochemical staining techniques. It is clinically important to distinguish GISTs from leiomyomas, because GISTs have a risk of progression and metastasis. True leiomyomas are benign neoplasms and never metastasize; surgery is not required unless obstruction or compression occur.
Gastrointestinal leiomyomas are the most frequent mesenchymal tumor of the esophagus[14]. They are relatively rare in the stomach, and are typically located in the gastric cardia. Leiomyomas typically manifest as homogeneous, low attenuation, poorly to moderately enhanced small masses in the gastric cardia (Figure 3). They typically exhibit an endoluminal growth pattern and are relatively small compared with GISTs[15].
Figure 3.
Leiomyoma. A 70-year-old man had no symptoms. A and B: Pre- and post-contrast-enhanced axial computed tomography scans showing the leiomyoma at the gastric cardia (arrow), with an intraluminal growth pattern and homogeneous, poor enhancement. Note the intact enhancing mucosa, indicating the submucosal lesion; C: High-power photomicrograph (original magnification, × 200; HE stain) showing paucicellular spindle cells with low or moderate cellularity, arranged in perpendicularly oriented fascicles; The tumor cells were positive for smooth muscle actin (D) and negative for CD34 and CD117 (not shown).
Histologically, leiomyomas resemble to normal smooth muscle cells. They exhibit hypocellular spindle cells with eosinophilic cytoplasms, arranged in perpendicularly oriented fascicles. The tumor cells are positive for desmin and SMA, and negative for CD34 and CD117 (c-kit) (Figure 3)[16]. In contrast to true leiomyomas, GISTs show higher cellularity, and are positive for CD34 and CD117.
Lipomas
Gastrointestinal lipomas are benign submucosal tumors composed of adipose tissue covered with a fibrous capsule. They are solitary slow-growing tumors and can occur anywhere in the gastrointestinal tract. Approximately 90% to 95% of lipomas are located in the submucosa and the remaining 5% to 10% are subserosal[17]. They are rare in the stomach, and most are located in the gastric antrum with an endoluminal growth pattern[18]. Because lipomas are soft, they may prolapse through the pylorus into the duodenum without gastric outlet obstruction. If the tumor is large enough, it may cause intussusception. CT is the imaging modality of choice for diagnosing of lipoma. A definitive diagnosis is based on a well-circumscribed mass with uniform fat density (-70 to -120 HU) (Figure 4). Soft tissue attenuation may be present in the tumor because of inflammation and ulceration[19].
Figure 4.
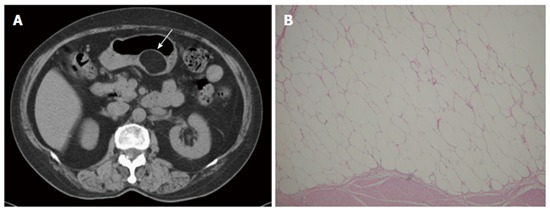
Lipoma. A 69-year-old man presented with abdominal fullness. A: Non-contrast-enhanced computed tomography (CT) showing a round, sharply marginated, uniform fatty mass (arrow) with negative CT numbers (-90 HU) in the greater curvature of the stomach; B: High-power photomicrograph (original magnification, × 200; HE stain) showing that the tumor consists of mature adipocytes.
MALIGNANT TUMORS
Gastrointestinal stromal tumors
GISTs are the most common mesenchymal tumors of the gastrointestinal tract. GISTs can occur anywhere along the gastrointestinal tract, with approximately 60% to 70% occurring in the stomach, and 30% occurring in the small bowel. They arise from the intestinal cell of Cajal in muscularis propria of the gastrointestinal wall. Because they are mesenchymal tumors, they may exhibit exophytic, intraluminal or mixed growth pattern.
Primary GISTs are typically large masses with irregular lobulated margins, mucosal ulceration, central necrosis, hemorrhage, and heterogeneous enhancement (Figure 5). Occasionally, calcifications are observable and are amorphous. Sometimes it is difficult to identify the origin of a mass because of the large size and extraluminal growth pattern[20]. Nearly 50% of patients with GISTs exhibit metastasis, and the liver and peritoneum are the most involved organs[21]. GISTs smaller than 3 cm can be endoluminal and polypoid in appearance. They are typically well-defined, homogeneous, soft-tissue attenuation masses. Sometimes it is difficult to distinguish small GISTs from other benign intramural gastric tumors. Unlike adenocarcinoma, they are submucosal tumors with intact mucosa, prone to exhibiting mixed exophytic and endoluminal growth patterns and amorphous calcification.
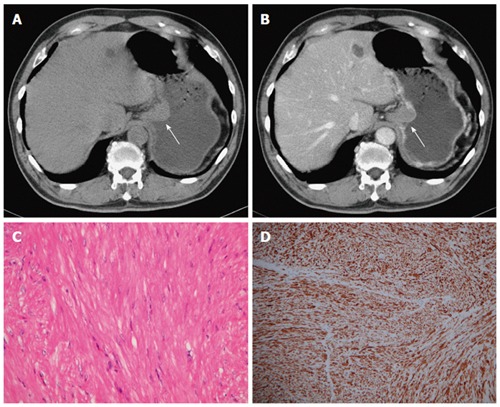
Figure 5.
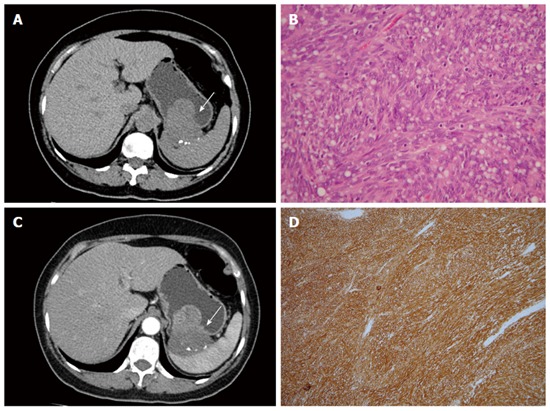
Gastrointestinal stromal tumor. A 58-year-old woman presented with melena and abdominal cramping pain for a year. A: Pre-contrast computed tomography (CT) scan showing amorphous calcifications in a gastric tumor with endoluminal and exophytic growth patterns (arrow); B: Post-contrast-enhanced CT scan showing the intact enhancing mucosa and central necrosis; C: High-power photomicrograph (original magnification, × 100; HE stain) showing spindle cells arranged in lobules; D: The tumor cells were positive for CD117.
Histologically, GISTs exhibit uniform spindle cells or epithelioid cells arranged in lobules. Malignant GISTs are larger, more highly cellular, and more mitotically active than benign GISTs. Most GISTs (90%) are characterized by expression of CD117 (c-kit), which is a tyrosine kinase receptor in the intestinal cells of Cajal (Figure 5). The immunoreactivity of CD117 (c-kit) distinguishes GISTs from true leiomyomas, leiomyosarcomas, schwannomas, and neurofibromas[22].
Mucinous adenocarcinomas
The gastric adenocarcinomas are classified into mucinous, papillary, tubular, signet-ring cell, and undifferentiated types. The prognosis of mucinous carcinoma is poorer than non-mucinous carcinoma. Mucinous carcinoma is characterized by prominent glandular formation and abundant extracellular mucin deposition[23]. Miliary and punctate calcifications are present in the mucin pool, which is a diagnostic clue for mucinous carcinoma (Figure 6)[24]. It is proposed that the alkaline mucin promotes calcium salt deposition[25], but the actual pathogenesis is not entirely clear.
Figure 6.
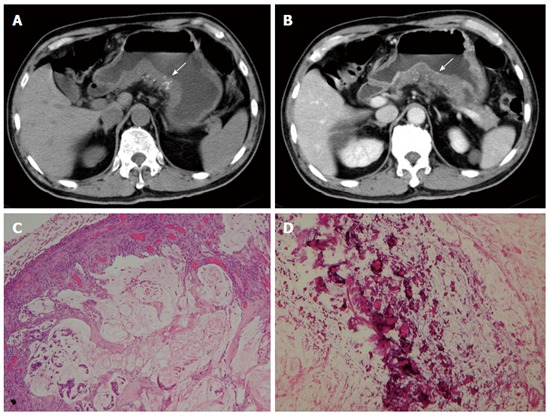
Mucinous adenocarcinoma. A 65-year-old man presented with vomiting and diarrhea for 2 mo. A and B: Pre- and post-contrast-enhanced computed tomography scans shoings a segmental thickening at the posterior wall of the gastric antrum, with poor enhancement and punctate calcification (arrow). Low-power photomicrograph (original magnification, × 20; HE stain) showing abundant extracellular mucin pools (C) with floating tumor cells and calcifications (D).
Mucinous adenocarcinomas exhibit a diffuse thickened gastric wall with relatively low attenuation on pre-contrast-enhanced CT images and poor enhancement after contrast enhancement because of the accumulation of mucin (Figure 6)[26]. Calcifications within the tumor are rarely larger than 3 mm in diameter and are located within the thickened gastric wall. Calcifications in primary untreated gastric cancer are rare, but can sometimes be observed in GISTs and hemangiomas[27]. Calcification is amorphous in GISTs (Figure 5) and manifests as a cluster of phleboliths in hemangiomas, which differs from mucinous adenocarcinomas.
Lymphomas
Primary gastrointestinal lymphomas are the most frequently occurring extranodal lymphomas and are almost exclusively of non-Hodgkin type. The stomach constitutes 50% of all gastrointestinal tract lymphomas and 25% of extranodal lymphomas. Gastric lymphomas are predominantly non-Hodgkin lymphomas of B-cell origin[28,29]. It is believed that primary gastric lymphomas originate from low-grade mucosa-associated lymphoid tissue (MALT), and transform into intermediate or high-grade large cell lymphomas[30].
Gastric lymphomas typically show regional or diffuse gastric wall thickening on CT images (Figure 7). The enhancement is typically homogeneous, with preservation of the underlying gastric rugae, but low-attenuation areas of necrosis may be observed in some cases[31]. The stomach typically remains pliable and distensible, and transpyloric spread of the tumor may occur in 30% of cases[32]. In high-grade gastric lymphomas, involvement of adjacent organs is usually observed, with some perigastric lymph nodes. Low-grade MALT lymphomas frequently result in non-specific findings, such as mucosal nodularity, depressed lesions, and thickened folds. Compared to gastric adenocarcinomas, the perigastric fat plane is more likely to be preserved[33] and lymphadenopathy can typically be observed extending below the renal hilum (Figure 7)[31].
Figure 7.
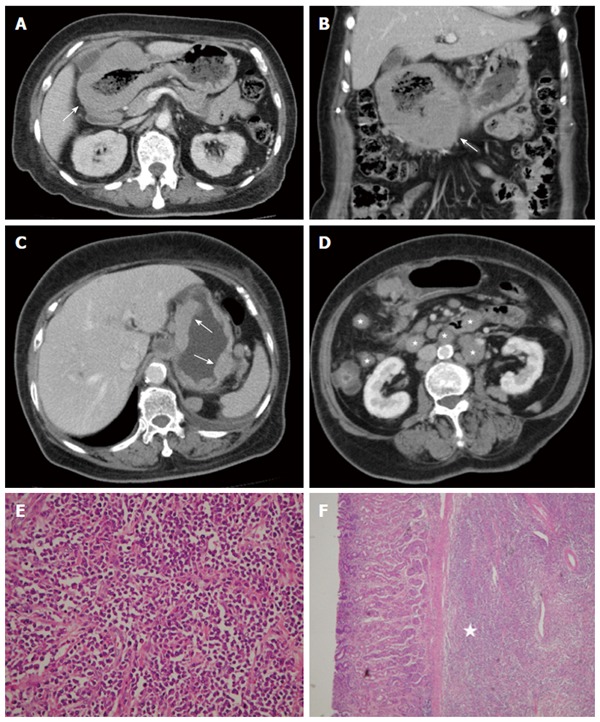
Diffuse large B-cell lymphoma. A 63-year-old woman presented with epigastralgia. A and B: Contrast-enhanced computed tomography (CT) scan showing diffuse, homogeneous gastric wall thickening with a smooth well-defined outer wall (arrow). An 83-year-old woman presented with tarry stool and constipation for a week; C and D: Post-contrast-enhanced CT revealing wall thickness (arrow) at the gastric body and several enlarged lymph nodes in the mesentery and para-aortic retroperitoneum (stars); E: Low-power photomicrograph (original magnification, × 20; HE stain) showing diffuse proliferation of large monomorphic neoplastic cells with abundant cytoplasms; F: The neoplastic cells occupy the full thickness of the submucosa (star).
Carcinoids
Gastric carcinoids are well-differentiated endocrine neoplasms that originate from enterochromaffin-like cells in the gastric mucosa and are therefore epithelial in origin. Although the stomach is the least common site of gastrointestinal carcinoids, they are clinically important because of the associated carcinoid syndromes. Gastric carcinoids can be divided into three subtypes, each with a distinct pathophysiologic mechanism, resulting in different clinical outcomes and management[34,35].
Type 1 gastric carcinoids are the most common (75%-80% of gastric carcinoids), and are associated with hypergastrinemia and chronic atrophic gastritis. Patients with type 1 gastric carcinoid are typically asymptomatic; tumors are typically encountered during endoscopy for nonspecific symptoms. Type 2 gastric carcinoids are less common (5%-10% of gastric carcinoids) and are associated with Zollinger-Ellison syndrome, with multiple endocrine neoplasms. Approximately 30% of patients with multiple endocrine neoplasia type 1 have gastric carcinoid tumors[36]. In type 2 gastric carcinoids, elevated gastrin levels produce signs and symptoms of hypertrophic, hypersecretory gastritis. Type 1 and type 2 carcinoids are small, circumscribed, mucosal and/or submucosal polypoid tumors in the gastric body and fundus[37,38]. On CT, polypoid lesions appear iso-dense in the pre-contrast phase, with marked enhancement in the arterial phase (Figure 8)[39]. Type 3 gastric carcinoids, which are larger, sporadic, solitary tumors, are not associated with atrophic gastritis or hypergastrinemia; however, they may exhibit ulceration and distant metastases, and the prognosis is poor compared with type 1 and 2 carcinoids[40].
Figure 8.
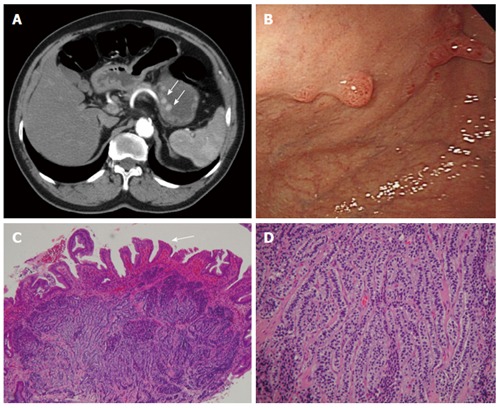
Carcinoid. A 66-year-old man presented with epigastralgia and elevated levels of serum gastrin. A: Contrast-enhanced transverse and coronal computed tomography (CT) scans showing multiple enhancing polypoid lesions (arrows) at the gastric body; B: Endoscopy showing multiple polypoid lesions; C: Low-power photomicrograph (original magnification, × 10; HE stain) showing atrophic gastritis (atrophy in glandular structures, arrow); D: High-power photomicrograph (original magnification, × 100; HE stain) showing uniform cells bearing round nuclei and growing in a festoon or ribbon-like arrangement in the submucosa.
In histologic analysis, carcinoids are composed of small uniform cells arranged in trabecular or nest patterns. The nuclei are round or oval with finely stippled chromatin, infrequent mitoses, and minimal nuclear polymorphism (Figure 8). High-grade sporadic carcinoid tumors may resemble small cell carcinomas, with nuclear pleomorphism, hyperchromasia and higher mitotic activity. These tumors are immunoreactive to chromogranin A and synaptophysin, which are general neuroendocrine markers[38].
TUMOR-LIKE LESIONS
Ectopic pancreas
Ectopic pancreas is a condition whereby pancreatic tissues lack anatomic and vascular connections to the pancreas[41]. The pathogenesis is not clear, but some believe that during normal pancreatic development from evaginations, one or more evaginations may remain in the bowel wall. Others suggest that pancreatic metaplasia of the gastric submucosa may occur during the embryogenesis of endodermal tissues[42]. These lesions typically have a peak incidence in fourth to sixth decades of life, most commonly among men. They are typically discovered incidentally during surgery or autopsy, with an incidence during laparotomy of 0.2%[43]. Although most patients with ectopic pancreas are asymptomatic, some may present with nonspecific abdominal pain, bleeding, and mechanical obstruction[44-46]. Lesions are typically located in the stomach, duodenum, or jejunum. Because they are typically small, slow-growing, and asymptomatic, they might be overlooked in daily practice.
Ectopic pancreas typically manifests as an ill-defined, submucosal mass with endoluminal growth in the stomach, generally located in the greater curvature of the distal antrum[47]. On CT, the enhancement is typically similar to that of the normal pancreas. It has been reported that the enhancement degree depends heavily on the histopathologic composition of the ectopic pancreas[48]. There are three subtypes of ectopic pancreas. The acini-dominant type is more homogeneous and exhibits stronger enhancement than the normal pancreas (Figure 9), the duct-dominant type exhibits lower enhancement than the normal pancreas, and the mixed type exhibits variable CT attenuation values compared with the normal pancreas. Histologically, ectopic pancreas is not a diagnostic problem when pancreatic acini, ducts, islets of Langerhans, and intervening connective tissue are present.
Figure 9.
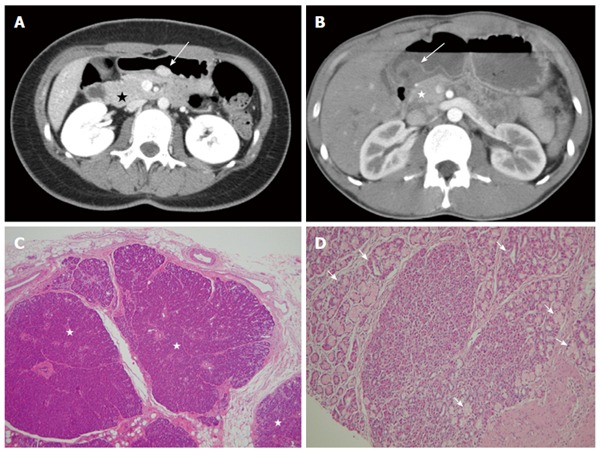
Ectopic pancreas. A 26-year-old woman presented with postprandial epigastric pain for 2 years. A: Transverse computed tomography (CT) scan showing a small round submucosal lesion with well-defined margins in the wall of the antrum (arrow). Note the contrast material enhancement is higher than that of the normal pancreas (star); B: Low-power photomicrograph (original magnification, × 20; HE stain) showing that pancreas tissue (star) is predominant in the acinar tissue. A 20-year-old man presented with intermittent epigastralgia for 2 mo; C: Transverse CT scan showing a submucosal round mass (arrow) with necrosis at the gastric antrum. Note the poorly enhancing nodular mass, as compared with the markedly enhancing adjacent normal pancreas (star); D: Low-power photomicrograph (original magnification, × 200; HE stain) showing ectopic pancreatic tissue, composed primarily of pancreatic ducts (arrow) in the gastric mucosal layer.
Bezoar
Bezoars, mimicking gastric neoplasms, consist of ingested foreign materials that accumulate within the gastrointestinal tract. They include trichobezoars, which are composed of hair; phytobezoars, which are composed of fruit or vegetable matter; lactobezoars, which are undigested milk concretions; and pharmacobezoars, which are composed of medications[49]. They are typically confined to the stomach but can extend through the pylorus into the jejunum, ileum and even up to the colon (known as Rapunzel syndrome).
Most bezoar cases are diagnosed using plain films or a barium meal, but CT may be requested for patients who present with abdominal masses. A bezoar in the stomach presents as a mobile intraluminal gastric filling defect, with a mottled appearance caused by air bubbles retained in interstices of the mass. (Figure 10)[50]. Clinical history is crucial for accurate diagnosis.
Figure 10.
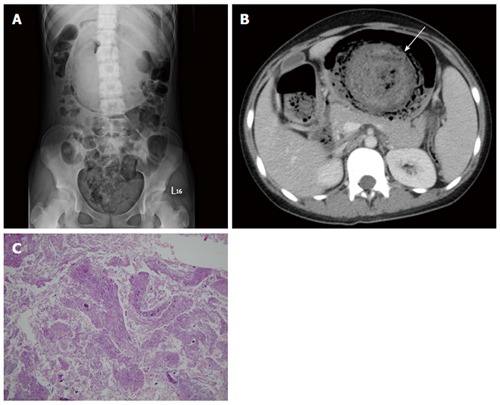
Trichobezoar. A 13-year-old girl presented with intermittent fever. She exhibited obsessive and compulsive hair pulling. A: Plain film revealing a bezoar outlined by air in the stomach; B: Transverse computed tomography image showing an inhomogeneous mass with a mottled gas pattern in the distended stomach (arrow); C: Low-power photomicrograph (original magnification, × 10; HE stain) showing hair tissue with inflammatory exudate.
CONCLUSION
It is often difficult to determine the etiology of gastric lesions based on the basis of clinical findings. Using MDCT with water as a negative oral contrast can provide useful information for the diagnosis of unusual gastric tumors or tumor-like lesions. Familiarity with imaging appearances and pathologic findings can help physicians make an accurate diagnosis.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare that they have no conflict of interest or source of external funding.
Peer-review started: December 28, 2016
First decision: January 19, 2017
Article in press: March 15, 2017
P- Reviewer: Garcia-Olmo D, Huang LY, Li YZ S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
References
- 1.Fishman EK, Urban BA, Hruban RH. CT of the stomach: spectrum of disease. Radiographics. 1996;16:1035–1054. doi: 10.1148/radiographics.16.5.8888389. [DOI] [PubMed] [Google Scholar]
- 2.Springer P, Dessl A, Giacomuzzi SM, Buchberger W, Stöger A, Oberwalder M, Jaschke W. Virtual computed tomography gastroscopy: a new technique. Endoscopy. 1997;29:632–634. doi: 10.1055/s-2007-1004269. [DOI] [PubMed] [Google Scholar]
- 3.Horton KM, Eng J, Fishman EK. Normal enhancement of the small bowel: evaluation with spiral CT. J Comput Assist Tomogr. 2000;24:67–71. doi: 10.1097/00004728-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Horton KM, Fishman EK. Current role of CT in imaging of the stomach. Radiographics. 2003;23:75–87. doi: 10.1148/rg.231025071. [DOI] [PubMed] [Google Scholar]
- 5.Kang HC, Menias CO, Gaballah AH, Shroff S, Taggart MW, Garg N, Elsayes KM. Beyond the GIST: mesenchymal tumors of the stomach. Radiographics. 2013;33:1673–1690. doi: 10.1148/rg.336135507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol. 2002;26:301–311. doi: 10.1097/00000478-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Zhou P, Xu M, Chen W, Li Q, Ji Y, Yao L. Endoscopic diagnosis and treatment of gastric glomus tumors. Gastrointest Endosc. 2011;73:371–375. doi: 10.1016/j.gie.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Haque S, Modlin IM, West AB. Multiple glomus tumors of the stomach with intravascular spread. Am J Surg Pathol. 1992;16:291–299. doi: 10.1097/00000478-199203000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Hur BY, Kim SH, Choi JY, Rha SE, Lee MW, Kim SY, Han JK, Choi BI. Gastroduodenal glomus tumors: differentiation from other subepithelial lesions based on dynamic contrast-enhanced CT findings. AJR Am J Roentgenol. 2011;197:1351–1359. doi: 10.2214/AJR.10.6360. [DOI] [PubMed] [Google Scholar]
- 10.Levy AD, Quiles AM, Miettinen M, Sobin LH. Gastrointestinal schwannomas: CT features with clinicopathologic correlation. AJR Am J Roentgenol. 2005;184:797–802. doi: 10.2214/ajr.184.3.01840797. [DOI] [PubMed] [Google Scholar]
- 11.Sarlomo-Rikala M, Miettinen M. Gastric schwannoma--a clinicopathological analysis of six cases. Histopathology. 1995;27:355–360. doi: 10.1111/j.1365-2559.1995.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 12.Melvin WS, Wilkinson MG. Gastric schwannoma. Clinical and pathologic considerations. Am Surg. 1993;59:293–296. [PubMed] [Google Scholar]
- 13.Voltaggio L, Murray R, Lasota J, Miettinen M. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol. 2012;43:650–659. doi: 10.1016/j.humpath.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 15.Lee MJ, Lim JS, Kwon JE, Kim H, Hyung WJ, Park MS, Kim MJ, Kim KW. Gastric true leiomyoma: computed tomographic findings and pathological correlation. J Comput Assist Tomogr. 2007;31:204–208. doi: 10.1097/01.rct.0000237812.95875.bd. [DOI] [PubMed] [Google Scholar]
- 16.Miettinen M, Sarlomo-Rikala M, Sobin LH, Lasota J. Esophageal stromal tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol. 2000;24:211–222. doi: 10.1097/00000478-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez MJ, Davis RP, Nora PF. Gastrointestinal lipomas. Arch Surg. 1983;118:1081–1083. doi: 10.1001/archsurg.1983.01390090065015. [DOI] [PubMed] [Google Scholar]
- 18.Taylor AJ, Stewart ET, Dodds WJ. Gastrointestinal lipomas: a radiologic and pathologic review. AJR Am J Roentgenol. 1990;155:1205–1210. doi: 10.2214/ajr.155.6.2122666. [DOI] [PubMed] [Google Scholar]
- 19.Thompson WM, Kende AI, Levy AD. Imaging characteristics of gastric lipomas in 16 adult and pediatric patients. AJR Am J Roentgenol. 2003;181:981–985. doi: 10.2214/ajr.181.4.1810981. [DOI] [PubMed] [Google Scholar]
- 20.Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283–304, 456; quiz 532. doi: 10.1148/rg.232025146. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson B, Bümming P, Meis-Kindblom JM, Odén A, Dortok A, Gustavsson B, Sablinska K, Kindblom LG. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 22.Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478–483. doi: 10.1053/hupa.2002.124123. [DOI] [PubMed] [Google Scholar]
- 23.Park SH, Han JK, Kim TK, Lee JW, Kim SH, Kim YI, Choi BI, Yeon KM, Han MC. Unusual gastric tumors: radiologic-pathologic correlation. Radiographics. 1999;19:1435–1446. doi: 10.1148/radiographics.19.6.g99no051435. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura K, Togashi K, Tohdo G, Dodo Y, Tanada S, Nakano Y, Torizuka K. Computed tomography of calcified gastric carcinoma. J Comput Assist Tomogr. 1984;8:1010–1011. doi: 10.1097/00004728-198410000-00044. [DOI] [PubMed] [Google Scholar]
- 25.Balestreri L, Canzonieri V, Morassut S. Calcified gastric cancer--CT findings before and after chemotherapy. Case report and discussion of the pathogenesis of this type of calcification. Clin Imaging. 1997;21:122–125. doi: 10.1016/s0899-7071(96)00010-1. [DOI] [PubMed] [Google Scholar]
- 26.Miyake H, Maeda H, Kurauchi S, Watanabe H, Kawaguchi M, Tsuji K. Thickened gastric walls showing diffuse low attenuation on CT. J Comput Assist Tomogr. 1989;13:253–255. doi: 10.1097/00004728-198903000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Ghahremani GG, Meyers MA, Port RB. Calcified primary tumors of the gastrointestinal tract. Gastrointest Radiol. 1978;2:331–339. doi: 10.1007/BF02256516. [DOI] [PubMed] [Google Scholar]
- 28.Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract: a study of 117 cases presenting with gastrointestinal disease. Cancer. 1978;42:693–707. doi: 10.1002/1097-0142(197808)42:2<693::aid-cncr2820420241>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 29.Koh PK, Horsman JM, Radstone CR, Hancock H, Goepel JR, Hancock BW. Localised extranodal non-Hodgkin’s lymphoma of the gastrointestinal tract: Sheffield Lymphoma Group experience (1989-1998) Int J Oncol. 2001;18:743–748. doi: 10.3892/ijo.18.4.743. [DOI] [PubMed] [Google Scholar]
- 30.Yoo CC, Levine MS, Furth EE, Salhany KE, Rubesin SE, Laufer I, Herlinger H. Gastric mucosa-associated lymphoid tissue lymphoma: radiographic findings in six patients. Radiology. 1998;208:239–243. doi: 10.1148/radiology.208.1.9646819. [DOI] [PubMed] [Google Scholar]
- 31.Buy JN, Moss AA. Computed tomography of gastric lymphoma. AJR Am J Roentgenol. 1982;138:859–865. doi: 10.2214/ajr.138.5.859. [DOI] [PubMed] [Google Scholar]
- 32.Park MS, Kim KW, Yu JS, Park C, Kim JK, Yoon SW, Lee KH, Ryu YH, Kim H, Kim MJ, et al. Radiographic findings of primary B-cell lymphoma of the stomach: low-grade versus high-grade malignancy in relation to the mucosa-associated lymphoid tissue concept. AJR Am J Roentgenol. 2002;179:1297–1304. doi: 10.2214/ajr.179.5.1791297. [DOI] [PubMed] [Google Scholar]
- 33.Miller FH, Kochman ML, Talamonti MS, Ghahremani GG, Gore RM. Gastric cancer. Radiologic staging. Radiol Clin North Am. 1997;35:331–349. [PubMed] [Google Scholar]
- 34.Mulkeen A, Cha C. Gastric carcinoid. Curr Opin Oncol. 2005;17:1–6. doi: 10.1097/01.cco.0000147899.04701.c6. [DOI] [PubMed] [Google Scholar]
- 35.Ruszniewski P, Delle Fave G, Cadiot G, Komminoth P, Chung D, Kos-Kudla B, Kianmanesh R, Hochhauser D, Arnold R, Ahlman H, et al. Well-differentiated gastric tumors/carcinomas. Neuroendocrinology. 2006;84:158–164. doi: 10.1159/000098007. [DOI] [PubMed] [Google Scholar]
- 36.Cadiot G, Cattan D, Mignon M. Diagnosis and treatment of ECL cell tumors. Yale J Biol Med. 1998;71:311–323. [PMC free article] [PubMed] [Google Scholar]
- 37.Lehy T, Cadiot G, Mignon M, Ruszniewski P, Bonfils S. Influence of multiple endocrine neoplasia type 1 on gastric endocrine cells in patients with the Zollinger-Ellison syndrome. Gut. 1992;33:1275–1279. doi: 10.1136/gut.33.9.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168–172. doi: 10.1007/s002689900026. [DOI] [PubMed] [Google Scholar]
- 39.Berger MW, Stephens DH. Gastric carcinoid tumors associated with chronic hypergastrinemia in a patient with Zollinger-Ellison syndrome. Radiology. 1996;201:371–373. doi: 10.1148/radiology.201.2.8888225. [DOI] [PubMed] [Google Scholar]
- 40.Ba-Ssalamah A, Prokop M, Uffmann M, Pokieser P, Teleky B, Lechner G. Dedicated multidetector CT of the stomach: spectrum of diseases. Radiographics. 2003;23:625–644. doi: 10.1148/rg.233025127. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa O, Ishiguro S, Ohhigashi H, Sasaki Y, Yasuda T, Imaoka S, Iwanaga T, Nakaizumi A, Fujita M, Wada A. Solid and papillary neoplasm arising from an ectopic pancreas in the mesocolon. Am J Gastroenterol. 1990;85:597–601. [PubMed] [Google Scholar]
- 42.Chandan VS, Wang W. Pancreatic heterotopia in the gastric antrum. Arch Pathol Lab Med. 2004;128:111–112. doi: 10.5858/2004-128-111-PHITGA. [DOI] [PubMed] [Google Scholar]
- 43.Ura H, Denno R, Hirata K, Saeki A, Hirata K, Natori H. Carcinoma arising from ectopic pancreas in the stomach: endosonographic detection of malignant change. J Clin Ultrasound. 1998;26:265–268. doi: 10.1002/(sici)1097-0096(199806)26:5<265::aid-jcu7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 44.Bethel CA, Luquette MH, Besner GE. Cystic degeneration of heterotopic pancreas. Pediatr Surg Int. 1998;13:428–430. doi: 10.1007/s003830050358. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu M, Matsumoto T, Sakurai T, Ohmoto K, Moriya T, Hirokawa M, Manabe T. Acute terminal pancreatitis occurring in jejunal heterotopic pancreas. Int J Pancreatol. 1998;23:171–173. doi: 10.1385/IJGC:23:2:171. [DOI] [PubMed] [Google Scholar]
- 46.Jeong HY, Yang HW, Seo SW, Seong JK, Na BK, Lee BS, Song GS, Park HS, Lee HY. Adenocarcinoma arising from an ectopic pancreas in the stomach. Endoscopy. 2002;34:1014–1017. doi: 10.1055/s-2002-35836. [DOI] [PubMed] [Google Scholar]
- 47.Wei R, Wang QB, Chen QH, Liu JS, Zhang B. Upper gastrointestinal tract heterotopic pancreas: findings from CT and endoscopic imaging with histopathologic correlation. Clin Imaging. 2011;35:353–359. doi: 10.1016/j.clinimag.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Kim JY, Lee JM, Kim KW, Park HS, Choi JY, Kim SH, Kim MA, Lee JY, Han JK, Choi BI. Ectopic pancreas: CT findings with emphasis on differentiation from small gastrointestinal stromal tumor and leiomyoma. Radiology. 2009;252:92–100. doi: 10.1148/radiol.2521081441. [DOI] [PubMed] [Google Scholar]
- 49.Gorter RR, Kneepkens CM, Mattens EC, Aronson DC, Heij HA. Management of trichobezoar: case report and literature review. Pediatr Surg Int. 2010;26:457–463. doi: 10.1007/s00383-010-2570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ripollés T, García-Aguayo J, Martínez MJ, Gil P. Gastrointestinal bezoars: sonographic and CT characteristics. AJR Am J Roentgenol. 2001;177:65–69. doi: 10.2214/ajr.177.1.1770065. [DOI] [PubMed] [Google Scholar]


