Abstract
AIM
To explore the effects of omeprazole on chemoradiotherapy efficacy and tumor recurrence in rectal cancer.
METHODS
The medical data of 125 rectal cancer patients who received the same neoadjuvant chemoradiotherapy (CRT) followed by surgery were retrospectively collected. Patients who received omeprazole (OME) orally at a dose of 20 mg at least once daily for six days and/or intravenously at 40 mg a day were recognized as eligible OME users (EOU). Otherwise, patients were regarded as non-eligible OME users (non-EOU). Moreover, a preferred OME dose cut-off of 200 mg on tumor recurrence was obtained by receiver operating characteristic (ROC) curves. Patients were divided into two groups: the effective OME group (EOG, OME ≥ 200 mg) and the non-effective OME group (non-EOG, OME < 200 mg).
RESULTS
The good response rate of CRT efficacy (50.8%) in EOU was significantly increased compared with non-EOU (30.6%) (P = 0.02). The recurrence rate in the EOG was 10.3%, which was significantly lower compared with 31.3% in non-EOG (P = 0.025). The good response rate of CRT efficacy in EOG was 55.2%, which was obviously higher compared with 36.5% in non-EOG, with a significant difference (P = 0.072). Multivariate Cox analysis demonstrated that OME (non-EOG and EOG) was an independent and significant impact factor for DFS (P = 0.048, HR = 0.30, 95%CI: 0.09-0.99).
CONCLUSION
When applied as an adjuvant drug in cancer treatment for relieving common side effects of chemotherapy, omeprazole has a synergetic effect in improving CRT efficacy and decreasing rectal cancer recurrence.
Keywords: Omeprazole, Chemoradiotherapy efficacy, Recurrence, Rectal cancer
Core tip: In in vitro and in vivo studies, proton pump inhibitors (PPIs) induce apoptosis of gastric cancer cells, B-cell tumors and hepatoblastoma cells and promote autophagy in melanoma cells and pancreatic cancer cells. PPIs also sensitize chemo-resistant tumors to cytotoxic drugs and improve the efficacy of T-cell-based cancer immunotherapy. However, whether PPIs affect chemoradiotherapy (CRT) efficacy, decrease tumor recurrence and improve survival in rectal cancer patients remains unclear. In the present study, when used as adjuvant drug in cancer treatment, omeprazole has a synergetic effect in improving CRT efficacy and decreasing recurrences in rectal cancer.
INTRODUCTION
Rectal cancer is one of the worldwide leading causes of cancer related death[1]. Preoperative chemoradiotherapy (CRT) followed by radical surgery is a preferred treatment for patients with advanced rectal cancer for its reduced local recurrence and high sphincter preservation rate[2-4]. However, disease relapse is still a critical factor that affects patient survival[2]. The exploration of factors that affect CRT efficacy and tumor recurrence is important to improve cancer management.
Abnormal pH gradients in the tumor microenvironment are involved in tumorigenesis, tumor progression and drug resistance[5-11]. Vacuolar type H+-ATPases (V-ATPases) are proton pumps expressed on the membrane of endolysosomal organelles and plasma membranes[5], which could modulate the tumor acidic microenvironment[12,13]. V-ATPases are overexpressed in chemo-resistant cancer cells and are induced by cytotoxic drugs[14,15], playing a key role in cancer cells with a multidrug resistance phenotype[16]. Proton pump inhibitors (PPIs), such as omeprazole (OME) and esomeprazole, are used to relieve common side effects of chemotherapy, such as nausea and emesis. In addition to targeting the gastric acid pump, PPIs inhibit the activity of V-ATPases[17-20]. Moreover, PPIs induce apoptosis in gastric cancer cells[21], B-cell tumors[22] and hepatoblastoma cells[23] and promote autophagy in melanoma cells[24] and pancreatic cancer cells[25]. PPIs improve the efficacy of T-cell-based cancer immunotherapy[26-28]. In colorectal cancer, it is reported that PPIs re-sensitize drug-resistant cancer colon adenocarcinomas cell lines to cytotoxic drugs[26]
These study results suggest that the application of PPIs may be helpful in improving cancer treatment. However, whether PPIs could affect CRT efficacy, reduce tumor recurrence and improve survival in rectal cancer patients remain unclear.
MATERIALS AND METHODS
Patients
From May 2008 to March 2016, the medical records of consecutive rectal cancer patients who received the same neoadjuvant CRT followed by radical surgery were retrospectively collected. Neoadjuvant CRT included three-dimensional conformal radiotherapy (3D-CRT) using a total dose of 46 Gy concurrent with two cycles of oxaliplatin plus capecitabine. The disease was diagnosed by a combination of medical history, physical examination, biopsy, and staging examination, including abdominal ultrasound, abdominal-pelvis computed tomography, colonoscopy and endoscopic or trans-rectal ultrasonography. Tumors were staged according to the AJCC (2010 edition). Tumor stages before CRT and after surgery were classified as cTNM and ypTNM, respectively. Patients lacking detailed medical records or those with a second tumor or distant metastasis were excluded. Finally 125 patients met the criteria. The patients were aged 15-78 years, with a mean age of 55.8 ± 12.01 years. The mean body weight and mean height of the patients was 60.1 ± 9.3 kg and 164.1 ± 6.85 cm, respectively. Pre-treatment serum carcinoembryonic antigen (CEA) and CA19-9 data were available in 120 of the 125 patients. The study was approved by the Medical Ethics Committee of Sun Yat-Sen University Cancer Center. Written informed consent was obtained from all patients.
Neoadjuvant concurrent CRT
Radiation treatment planning was designed according to the three-dimensional conformal radiation therapy (3D-CRT), with one posterior field and two lateral fields. Patients were treated using a range of 6-15 MV photons. Radiation was delivered at a total dose of 46 Gy (23 fractions with 2 Gy per fraction in 5 wk). Gross tumor volumes (GTVs) included rectal tumors and enlarged lymph nodes. Clinical target volumes (CTVs) included lymphatic drainage areas around the rectum and sacrum. Planning target volume (PTV) included areas with a 0.8-1.0 cm radial margin around the CTV. Patients were treated in the prone position, and a belly board was used to exclude the small bowel out of the radiation field. Oxaliplatin (130 mg/m2) was delivered intravenously over 2 h on the first day of radiation treatment and on day 21. Capecitabine was administered orally twice daily at 1000 mg/m2 on days 1-14 and days 21-34.
Dosage of omeprazole
Omeprazole usage was recorded in detail. Omeprazole was administered orally at 20 mg twice a day (Omeprazole Magnesium Entericcoated Tablets, AstraZeneca AB), 40 mg (Omeprazole Sodium for Injection, AstraZeneca AB) or 60 mg (Omeprazole Sodium for Injection, Changzhou Siyao Pharmaceuticals Co., Ltd.) intravenously one hour before the start of chemotherapy and was continuously administered in the following days if the patients complained of digestive discomfort. The reduction in gastric peak acid secretion after continuous oral administration of 20 mg OME once daily for six days was comparable with the effect of a single intravenous dose of 40 mg OME[29]. Thus, patients who received 20 mg OME orally at least once a day for six days and/or intravenous infusion of 40 mg OME daily were recognized as eligible OME users (EOU); otherwise, the patients were regarded as non-eligible OME users (non-EOU). Among the 125 patients, 63 patients met the criteria as EOU. Moreover, the bioavailability of oral enteric-coated omeprazole granules was initially low (approximately 35%-40%); however, it increased to approximately 65% on repeated dosing[30-33]. Therefore, the oral dose of EOU was multiplied by 65% to convert to a dose comparable with the intravenous dose for the intention of equal drug bioavailability.
Surgery, tumor regression evaluation and adjuvant chemotherapy
Radical surgery was performed 4-6 wk after CRT completion. Primary tumor regression grade (TRG) was determined semiquantitatively according to a modified Dworak scale[34] based on the amount of viable tumor vs the amount of fibrosis as follows: 0, no regression; 1, dominant tumor mass with obvious fibrosis and/or vasculopathy; 2, dominantly fibrotic changes with few tumor cells or groups (easy to find); 3, very few (difficult to find microscopically) tumor cells in fibrotic tissue with or without a mucous-like substance; and 4, no tumor cells and only fibrotic mass (total regression or response). A Dworak grade of 2 or 3 was determined by two experienced pathologists. CRT efficacy was classified as either a “good response” or a “poor response”. Good response cases were those whose tumor regression was classified as TRG 3 or 4; poor response cases were those whose tumor regression was graded as TRG 0, 1 or 2. Patients were advised to undergo four to six cycles of adjuvant chemotherapy that was the same as neoadjuvant chemotherapy 4-6 wk after surgery completion. When patients could not endure the side effects of adjuvant chemotherapy, capecitabine monotherapy was adopted. Finally, 125 patients received 479 cycles of adjuvant chemotherapy.
Follow-up
After completion of combined treatment, patients were followed up every 3 to 6 mo in the first 3 years and every 12 mo thereafter. Patient evaluation included a physical examination, abdominal ultrasonography or computed tomography scan, chest X-ray, and serum CEA and Ca19-9 levels. Diagnosis of recurrence was based on two types of radiologic examination with or without abnormal plasma tumor markers. Histopathological verification was performed when necessary. The survival status was verified by examination of clinical attendance records and direct telecommunication with the patient or their family in March 2016. Survival was censored at the time of the last follow-up on March 1, 2016, with a median follow-up time of 66 mo (range 17-99 mo).
End points and statistical analysis
The study end points were CRT efficacy, recurrence, disease-free survival (DFS) and overall survival (OS). DFS was defined as the interval from surgery to either confirmed recurrence or death, and OS was defined as the time interval between surgery and death.
Continuous variables were expressed as the mean ± SD. Student t test and χ2 tests were used to compare differences between groups. A receiver operating characteristic (ROC) curve was plotted to identify a proper cut-off value. Kaplan-Meier analysis was used to compare survival using the log-rank test. Univariate and multivariate Cox proportional hazard models were used to assess the effect of risk factors on survival. Forward conditional methods were used to establish the multivariate Cox proportional hazards model. A two-tailed P value less than 0.05 was considered statistically significant. Statistical analysis was performed using the SPSS statistical software package (version 22).
RESULTS
Clinicopathological characteristics of patients treated at different doses of OME
Among 63 OME users, 7 patients only received OME orally, 47 patients only received OME intravenously, and 9 patients received OME both orally and intravenously. The detailed information of OME dosage is presented in Table 1. The good response rate (50.8%) in the EOU was significantly increased compared with non-EOU (30.6%) (P = 0.02, OR = 2.336, 95%CI: 1.124-4.856). No significant differences for other clinicopathological factors were found between the EOU and non-EOU groups (all P values > 0.05). The patient characteristics of EOU and non-EOU are summarized in Table 2.
Table 1.
Mean dose and duration of omeprazole administered orally and intravenously
| OME | Cases |
administered dose (mg) |
OME administration (No. of days) |
||||||
| Mean ± SD | 95%CI | Max | Min | Mean ± SD | 95%CI | Max | Min | ||
| Oral1 | 7 | 260.0 ± 143.2 | 127.6-392.4 | 546 | 182 | 11.0 ± 8.0 | 3.6-18.3 | 28 | 7 |
| IV2 | 47 | 217.2 ± 184.8 | 162.8-271.3 | 940 | 40 | 3.8 ± 3.0 | 2.9-4.6 | 16 | 1 |
| IV + Oral3 | 9 | 406.2 ± 184.9 | 264.1-548.4 | 756 | 151 | 13.7 ± 7.0 | 8.2-19.1 | 28 | 7 |
Oral OME multiplied by 65%;
OME received intravenously;
Oral OME multiplied by 65% plus OME received intravenously. OME: Omeprazole.
Table 2.
Differences in the clinicopathological characteristics in eligible omeprazole users and non-eligible omeprazole users
| Characteristics | Total |
EOU |
P value | |
| Non | Yes | |||
| Sex | ||||
| Male | 90 | 46 | 44 | 0.59 |
| Female | 35 | 16 | 19 | |
| Age (yr) | ||||
| < 60 | 73 | 37 | 36 | 0.77 |
| ≥ 60 | 52 | 25 | 27 | |
| BMI | ||||
| < 25 | 100 | 47 | 53 | 0.25 |
| ≥ 25 | 25 | 15 | 10 | |
| Tumor size (cm) | ||||
| ≤ 3 | 49 | 24 | 25 | 0.95 |
| 3-6 | 61 | 30 | 31 | |
| ≥ 6 | 15 | 8 | 7 | |
| Tumor grade | ||||
| 1 | 28 | 14 | 14 | 0.23 |
| 2 | 88 | 46 | 42 | |
| 3 | 9 | 2 | 7 | |
| cTNM | ||||
| II | 39 | 22 | 17 | 0.31 |
| III | 86 | 40 | 46 | |
| CEA (ng/mL) | ||||
| < 5 | 62 | 28 | 34 | 0.47 |
| ≥ 5 | 58 | 30 | 28 | |
| CA19-9 (U/mL) | ||||
| < 35 | 102 | 50 | 52 | 0.72 |
| ≥ 35 | 18 | 8 | 10 | |
| TGR | ||||
| 0 | 39 | 23 | 16 | 0.25 |
| 1 | 15 | 8 | 7 | |
| 2 | 20 | 12 | 8 | |
| 3 | 24 | 9 | 15 | |
| 4 | 27 | 10 | 17 | |
| CRT efficacy | ||||
| Poor | 74 | 43 | 31 | 0.02 |
| Good | 51 | 19 | 32 | |
| ypTNM | ||||
| ypcr | 25 | 9 | 16 | 0.34 |
| I | 26 | 16 | 10 | |
| II | 40 | 20 | 20 | |
| III | 34 | 17 | 17 | |
| Adjuvant CT | ||||
| No | 21 | 9 | 12 | 0.5 |
| Yes | 104 | 53 | 51 | |
| Recurrence | ||||
| No | 92 | 46 | 46 | 0.66 |
| Yes | 33 | 16 | 17 | |
EOU: Eligible OME users; non-EOU: Non-eligible OME users; BMI: Body mass index; TGR: Tumor regression grade; adjuvant CT: Adjuvant chemotherapy.
PPIs inhibit cancer cell proliferation in a dose-dependent manner[25,35]. Therefore, in addition to arbitrarily applying a cut-off that meets the inclusion criterion, a preferred OME dose cut-off for tumor recurrence was investigated by ROC curves. The dose that was closest to the upper left corner (100% sensitivity and 100% specificity) was selected as the cut-off dose. The area under the ROC curve (AUC) was calculated to estimate the discriminatory power of the produced OME dose cut-off of the entire dose range on recurrence. A dose cut-off of 200 mg was identified by ROC as the optimized point that differentiated recurrence from non-recurrence with maximal sensitivity and specificity (Figure 1). The AUC was 0.66 (P = 0.053), and the OME dose of 200 mg differentiated recurrence from non-recurrence with a specificity of 82.4% and a sensitivity of 56.5%. Patients were then divided into the effective OME group (EOG, patients received OME ≥ 200 mg) and non-effective OME group (non-EOG, patients received OME < 200 mg). Non-EOG and EOG patient characteristics are summarized in Table 3.
Figure 1.
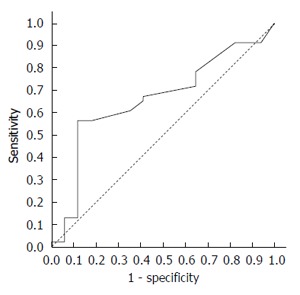
Receiver operating characteristic curve of omeprazole dose for recurrence.
Table 3.
Differences in clinicopathological characteristics of non-eligible omeprazole users and eligible omeprazole users
| Characteristics | Total |
EOG |
P value | |
| Non | Yes | |||
| Sex | ||||
| Male | 90 | 71 | 19 | 0.380 |
| Female | 35 | 25 | 10 | |
| Age(yr) | ||||
| < 60 | 73 | 58 | 15 | 0.410 |
| ≥ 60 | 52 | 38 | 14 | |
| BMI | ||||
| < 25 | 100 | 77 | 23 | 0.920 |
| ≥ 25 | 25 | 19 | 4 | |
| Tumor size (cm) | ||||
| ≤ 3 | 49 | 37 | 12 | 0.940 |
| 3-6 | 61 | 47 | 14 | |
| ≥ 6 | 15 | 12 | 3 | |
| Tumor grade | ||||
| 1 | 28 | 22 | 6 | 0.960 |
| 2 | 88 | 67 | 21 | |
| 3 | 9 | 7 | 2 | |
| cTNM | ||||
| II | 39 | 30 | 9 | 0.980 |
| III | 86 | 66 | 20 | |
| CEA (ng/mL) | ||||
| < 5 | 62 | 45 | 17 | 0.390 |
| ≥ 5 | 58 | 46 | 12 | |
| CA19-9 (U/mL) | ||||
| < 35 | 102 | 76 | 26 | 0.420 |
| ≥ 35 | 18 | 15 | 3 | |
| TGR | ||||
| 0 | 39 | 34 | 5 | 0.330 |
| 1 | 15 | 11 | 4 | |
| 2 | 20 | 16 | 4 | |
| 3 | 24 | 17 | 7 | |
| 4 | 27 | 18 | 9 | |
| CRT efficacy | ||||
| Poor | 74 | 61 | 13 | 0.072 |
| Good | 51 | 35 | 16 | |
| ypTNM | ||||
| ypcr | 25 | 16 | 9 | 0.380 |
| I | 26 | 21 | 5 | |
| II | 40 | 31 | 9 | |
| III | 34 | 28 | 6 | |
| Adjuvant CT | ||||
| No | 21 | 14 | 7 | 0.230 |
| Yes | 104 | 82 | 22 | |
| Recurrence | ||||
| No | 97 | 66 | 26 | 0.025 |
| Yes | 28 | 30 | 3 | |
EOG: Effective OME group; non-EOG: Non-effective OME group; BMI: Body mass index; TGR: Tumor regression grade; adjuvant CT: Adjuvant chemotherapy.
The recurrence rate in EOG was 10.3% (3/29), which was significantly lower than 31.3% (30/96) in non-EOG (P = 0.025, OR = 0.25, 95%CI: 0.07-0.90; Table 3). The response rate of CRT efficacy in EOG was 55.2% (16/29), which was obviously increased compared with 36.5% (35/96) in non-EOG, with a marginally significant difference (P = 0.072, OR = 2.15, 95%CI: 0.93-5.00; Table 3). There was no significant difference in other clinicopathological features between the non-EOG and EOG groups (all P > 0.05, Table 3). Non-EOG received a total of 371 cycles of adjuvant chemotherapy, with a mean value of 3.9 ± 2.2. EOG received 108 cycles, and the mean value was 3.7 ± 2.6. The mean adjuvant chemotherapy cycles were not significantly different (P = 0.77) between the EOG and non-EOG groups.
Survival difference between the non-EOG and EOG
At the end of the study, 96 (76.8%) patients were still alive. The patients who did not survive all died from tumor-related causes, and no patient died of PPI-related severe infection[36] during the CRT treatment. The mean DFS and mean OS of all patients was 62.9 mo ± 25.5 mo, 95%CI: 58.4-67.4) and 66.6 mo ± 21.8 mo, 95%CI: 62.8-70.5), respectively. The 3- and 5-year DFS rates of all patients were 81.6% and 75.1%, respectively. The 3- and 5-year OS rates of all patients were 85.6% and 78.8%, respectively.
A significant difference in DFS was noted between non-EOG and EOG patients (P = 0.032; Figure 2A, Table 4). In addition, a marginally significant difference in OS was also observed (P = 0.092; Figure 2B and Table 4). BMI, ypTNM and CRT efficacy were significantly associated with DFS (P = 0.024, P < 0.005 and P = 0.031, respectively; Table 4), whereas cTNM was a marginally significant factor of DFS (P = 0.067; Table 4). ypTNM was the only significant impact factor of OS (P = 0.003; Table 4), and BMI was a marginally significant factor of OS (P = 0.05; Table 4).
Figure 2.
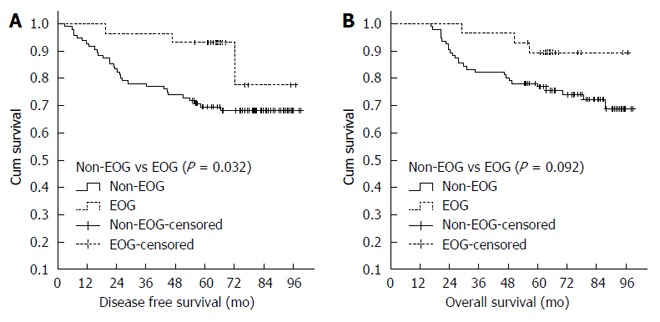
Disease-free survival curves (A) or OS curves (B) of non-omeprazole and omeprazole. EOG: Effective omeprazole.
Table 4.
Univariate analysis of impact of various characteristics on patient survival
| Characteristics | n |
DFS |
P value |
OS |
P value | ||||
| Mean (mo)1 | 3-yr2 | 5- yr2 | Mean (mo)1 | 3- yr2 | 5- yr2 | ||||
| Sex | |||||||||
| Male | 90 | 61.8 ± 25.9 | 81.1% | 74.4% | 0.803 | 65.5 ± 22.2 | 84.4% | 79.9% | 0.855 |
| Female | 35 | 65.6 ± 24.6 | 82.9% | 76.9% | 69.5 ± 20.1 | 88.6% | 79.8% | ||
| Age (yr) | |||||||||
| < 60 | 73 | 63.3 ± 26.3 | 80.1% | 71.1% | 0.533 | 68.4 ± 22.0 | 86.3% | 80.7% | 0.908 |
| ≥ 60 | 52 | 62.4 ± 24.6 | 82.7% | 80.7% | 64.2 ± 21.5 | 84.6% | 78.7% | ||
| Tumor size (cm) | |||||||||
| ≤ 3 | 48 | 62.5 ± 26.1 | 81.2% | 77.0% | 0.571 | 65.0 ± 22.4 | 83.3% | 79.2% | 0.962 |
| > 3 | 77 | 63.2 ± 25.3 | 81.8% | 74.0% | 67.7 ± 21.6 | 87.0% | 80.3% | ||
| BMI | |||||||||
| < 25 | 100 | 60.5 ± 26.8 | 77.0% | 69.9% | 0.024 | 65.3 ± 22.7 | 82.0% | 76.9% | 0.050 |
| ≥ 25 | 25 | 72.7 ± 16.6 | 96.0% | 96.0% | 72.1 ± 17.0 | 96.0% | 92.0% | ||
| Tumor grade | |||||||||
| 1 | 28 | 64.7 ± 28.0 | 78.6% | 75.0% | 0.852 | 69.4 ± 22.5 | 85.7% | 78.6% | 0.931 |
| 2, 3 | 97 | 62.4 ± 25.0 | 82.5% | 75.2% | 65.6 ± 21.7 | 85.6% | 80.2% | ||
| cTNM | |||||||||
| II | 39 | 69.2 ± 23.2 | 87.2% | 84.6% | 0.067 | 71.9 ± 18.8 | 92.3% | 87.2% | 0.137 |
| III | 86 | 60.0 ± 26.2 | 79.1% | 70.7% | 64.2 ± 22.8 | 82.6% | 76.4% | ||
| CEA (ng/mL) | |||||||||
| < 5 | 62 | 77.0 ± 4.1 | 69.2% | 69.2% | 0.789 | 79.6 ± 3.7 | 80.6% | 73.9% | 0.384 |
| ≥ 5 | 58 | 80.4 ± 4.3 | 82.8% | 74.0% | 86.1 ± 3.4 | 89.7% | 84.5% | ||
| CA19-9 (U/mL) | |||||||||
| < 35 | 102 | 81.3 ± 3.1 | 83.3% | 75.4% | 0.174 | 84.2 ± 2.7 | 86.3% | 80.2% | 0.597 |
| ≥ 35 | 18 | 68.1 ± 9.2 | 72.2% | 66.7% | 78.3 ± 7.8 | 77.8% | 72.2% | ||
| CRT efficacy | |||||||||
| Poor | 74 | 60.7 ± 27.2 | 78.4% | 67.5% | 0.031 | 66.2 ± 23.2 | 83.8% | 75.6% | 0.144 |
| Good | 51 | 66.1 ± 23.0 | 90.2% | 86.0% | 67.3 ± 19.9 | 88.2% | 86.1% | ||
| ypTNM | |||||||||
| ypcr,I, II | 91 | 66.1 ± 24.0 | 85.7% | 82.3% | 0.005 | 68.5 ± 20.1 | 89.0% | 84.4% | 0.041 |
| III | 34 | 54.3 ± 28.0 | 70.6% | 55.6% | 61.6 ± 25.5 | 76.5% | 67.6% | ||
| Adjuvant CT | |||||||||
| No | 21 | 60.2 ± 31.5 | 71.4% | 71.4% | 0.385 | 63.5 ± 26.5 | 76.2% | 66.3% | 0.229 |
| Yes | 104 | 63.5 ± 24.3 | 83.7% | 75.8% | 67.3 ± 20.8 | 87.5% | 82.7% | ||
| OME | |||||||||
| Non- EOU | 62 | 70.0 ± 25.8 | 85.5% | 75.6% | 0.658 | 73.9 ± 21.9 | 90.3% | 82.0% | 0.754 |
| EOU | 63 | 55.9 ± 23.5 | 77.8% | 74.6% | 59.5 ± 19.5 | 82.5% | 77.6% | ||
| OME (200 mg) | |||||||||
| Non-EOG | 96 | 62.0 ± 28.2 | 77.1% | 69.6% | 0.032 | 66.9 ± 24.1 | 82.3% | 76.9% | 0.092 |
| EOG | 29 | 65.9 ± 13.3 | 96.6% | 46.7% | 65.8 ± 12.0 | 96.6% | 89.5% | ||
Mean ± SD (mo);
Three or 5 years survival rate. EOU: Eligible OME users; Non-EOU: Non-eligible OME users; EOG: Effective OME group; Non-EOG: Non-effective OME group; BMI: Body mass index; adjuvant CT: Adjuvant chemotherapy.
Cox proportional hazards model analysis
The univariate Cox analysis revealed that OME (non-EOG and EOG), BMI, CRT efficacy, and ypTNM were significantly associated with DFS (P = 0.044, 0.039, 0.036 and P = 0.006, respectively; Table 5). The cTNM was significantly associated with DFS (P = 0.075; Table 5), and BMI was marginally significantly associated with OS (P = 0.069; Table 5). ypTNM was a significant impact factor for OS (P = 0.045). No other clinicopathological features significantly associated with DFS and OS (all P > 0.05; Table 5).
Table 5.
Univariate Cox analysis of the impact of various characteristics on patient survival
| Characteristics |
DFS |
P value |
OS |
P value | ||
| HR | 95%CI | HR | 95%CI | |||
| Sex | ||||||
| Male vs Female | 0.91 | 0.42-1.65 | 0.800 | 0.93 | 0.41-2.09 | 0.860 |
| Age (yr) | ||||||
| < 60 vs ≥ 60 | 0.80 | 0.40-1.62 | 0.530 | 1.05 | 0.50-2.19 | 0.910 |
| BMI | ||||||
| < 25 vs ≥ 25 | 0.22 | 0.05-0.93 | 0.039 | 0.26 | 0.06-1.11 | 0.069 |
| Tumor size (cm) | ||||||
| ≤ 3 vs > 3 | 1.23 | 0.60-2.51 | 0.570 | 0.98 | 0.46-2.08 | 0.960 |
| Tumor grade | ||||||
| 1 vs 2, 3 | 1.08 | 0.47-2.50 | 0.850 | 0.96 | 0.41-2.26 | 0.930 |
| cTNM | ||||||
| II vs III | 2.23 | 0.92-5.41 | 0.075 | 1.96 | 0.80-4.80 | 0.144 |
| CEA (ng/mL) | ||||||
| < 5 vs ≥ 5 | 0.91 | 0.46-1.81 | 0.790 | 0.72 | 0.34-1.51 | 0.390 |
| CA199 (U/mL) | ||||||
| < 35 vs ≥ 35 | 1.77 | 0.77-4.08 | 0.180 | 1.30 | 0.50-3.40 | 0.600 |
| CRT efficacy | ||||||
| Poor vs good | 0.43 | 0.19-0.95 | 0.036 | 0.55 | 0.24-1.24 | 0.150 |
| ypTNM | ||||||
| ypcr, I,II vs III | 1.61 | 1.14-2.27 | 0.006 | 1.46 | 1.01-2.11 | 0.045 |
| Adjuvant CT | ||||||
| Non vs yes | 0.69 | 0.30-1.60 | 0.390 | 0.60 | 0.25-1.40 | 0.240 |
| EOU | ||||||
| Non vs yes | 1.17 | 0.59-2.31 | 0.660 | 1.13 | 0.54-2.37 | 0.750 |
| EOG | ||||||
| Non vs yes | 0.30 | 0.90-0.97 | 0.044 | 0.37 | 0.11-1.23 | 0.110 |
EOU: Eligible OME users; Non-EOU: Non-eligible OME users; EOG: Effective OME group; Non-EOG: Non-effective OME group; BMI: Body mass index; Adjuvant CT: Adjuvant chemotherapy.
Furthermore, multivariate Cox analysis demonstrated that OME (non-EOG and EOG), BMI and ypTNM were independent and significant predictors of DFS (P = 0.048, HR = 0.30, 95%CI: 0.09-0.99, P = 0.038, HR = 0.22, 95%CI: 0.05-0.92 and P = 0.01, HR = 1.58, 95%CI: 1.12-2.22). ypTNM was also an independent and significant predictor of OS (P = 0.045, HR = 1.46, 95%CI: 1.01-2.11).
DISCUSSION
Neoadjuvant CRT could greatly improve the anus save rate and decrease local recurrence rate in advanced rectal cancer patients[2-4,37]. However, results addressing whether neoadjuvant CRT could improve survival are inconsistent[2,37]. The results of the present study showed that CRT efficacy is a significant clinicopathological factor associated with DFS (P = 0.031) and exhibits a favorable trend with OS (P = 0.144), indicating that CRT could decrease recurrence and potentially benefit OS. The results of the present study suggest that CRT efficacy is a significant clinicopathological factor associated with DFS, and this result is consistent with previous studies[2-4]. The present study results suggest that CRT has a potential benefit in OS, but is not a significant predictor. These results were consistent with the study by Sauer et al[37] but not with the study of Calogero Cammà et al[2]. As a potential chemotherapeutic agent[27,38-42], PPIs are safe to humans at high doses and with long-term treatment[37,38]. The mechanisms by which PPIs affect cancer include inhibiting V-ATPase activity[17,18], inducing apoptosis[21-23], promoting autophagy[24,25] and stimulating caspase-dependent cell death[35]. PPIs could sensitize chemo-resistant tumors to cytotoxic drugs[26] and could improve the efficacy of T-cell-based cancer immunotherapy[27,28], suggesting that PPIs may improve cancer treatment efficacy. In the present study, we found a good response rate (50.8%) in the EOU group that was significantly increased compared with the non-EOU group (30.6%) (P = 0.02), suggesting that OME could enhance the sensitivity of rectal cancer to concurrent CRT. We noticed that after the OME dose cut-off was increased, the good response rate of CRT efficacy between EOG (55.2%) and non-EOG (36.5%) patients exhibited a marginally significant difference (P = 0.072). This result was likely caused by an elevated cut-off that resulted in a decreased EOG sample size, which would reduce statistical power. To the best of our knowledge, this study is the first to investigate the effect of PPIs on CRT efficacy.
Abnormal extracellular acidic pH could enhance the invasive capacity and metastatic behavior of cancer cells[43-46]. V-ATPase is involved in pH-dependent degradation of the extracellular matrix and promotion of tumor invasion and metastasis[39,47], suggesting that inhibition of V-ATPase may prevent metastasis. Consistent with these studies, the present study results showed that the recurrence rate in EOG patients was 10.3%, which was significantly lower compared with 31.3% in non-EOG patients (P = 0.025). In addition, a significant difference in DFS was noted between non-EOG and EOG patients (P = 0.032), and a marginally significant difference in OS was noted (P = 0.092). Further multivariate Cox analysis demonstrated that OME (non-EOG and EOG) is an independent and significant predictor of DFS (P = 0.048). These results suggest that when administered as an adjuvant chemoradiotherapy drug, OME may exert synergistic effects with concurrent CRT to reduce tumor recurrence.
Whether the plasma concentration of the including criteria for dosage of OME in the present study could affect cancer cell vitality should be further discussed. The oral intake of 20 mg OME could produce a maximal plasma concentration of 2.5 mg/mL after two hours in patients[48]. The minimum OME dosage for the inclusion criteria in the present study was 40 mg intravenously administered, achieving a plasma concentration of 5 mg/mL. In in vitro studies, OME dissolved in normal saline at a concentration of 1 mg/mL induces apoptosis in B-cell cancers[22] and re-sensitizes drug-resistant cancer cell lines (22 melanomas, 2 colon adenocarcinomas, 2 breast cancers and 2 ovarian carcinomas) to cytotoxic drugs[26]. In in vivo studies, 0.4 mg/kg OME co-administered with dichloroacetate and tamoxifen exhibit a synergistically anti-proliferative effect on cholangiocarcinoma[49]. In addition, 2 mg/kg OME combined with dichloroacetate exhibited an antitumor effect on HT1080 fibrosarcoma cells inoculated in mice[50]. ESOM (2.5 mg/kg) reduced tumor growth in SCID mice engrafted with human melanoma[35]. In the present study, the minimum OME dose per kilogram of body weight was approximately 0.67 mg/kg (40 mg/60 kg), and the mean dose per kilogram of body weight was 3.6 mg/kg (217.2 mg/60.0 kg), which were higher than the least functional dosage reported above[49].
BMI was significantly associated with DFS (P = 0.024) and was a marginally significant factor associated with OS (P = 0.05). Further multivariate Cox analysis demonstrated that BMI was an independent and significant predictor of DFS (P = 0.038), which was consistent with a previous study[51].
Our study has several limitations. Although consecutive patients were included, it is a retrospective study. In addition, the patient sample of the study was relatively small. However, the effects of OME on CRT efficacy, tumor recurrence and patient survival were first investigated in the present study, which would be helpful for randomized and controlled trials in the future.
In conclusion, when used as an adjuvant drug in cancer treatment, omeprazole has a synergetic effect on improving CRT efficacy and decreasing rectal cancer recurrence.
COMMENTS
Background
Abnormal pH gradients of tumor microenvironment are involved in tumorigenesis, tumor progression and drug resistance. Vacuolar type H+-ATPases (V-ATPases) are proton pumps expressed on the membrane of endolysosomal organelles and the plasma membrane, which could modulate the tumor acidic microenvironment. Proton pump inhibitors (PPIs), such as omeprazole (OME) and esomeprazole, are used to relieve common side effects of chemotherapy, such as nausea and emesis. In addition to targeting the gastric acid pump, PPIs inhibit the activity of V-ATPases. Moreover, PPIs induce apoptosis in multiple cancer cells and promotes cancer cell autophagy. PPIs also sensitize chemo-resistant tumors to cytotoxic drugs and improve the efficacy of T-cell-based cancer immunotherapy. These study results suggest that application of PPIs may be helpful to improve cancer treatment. However, whether PPIs affect CRT efficacy, reduce tumor recurrence and improve survival in rectal cancer patients remain unclear.
Research frontiers
The present study investigates whether omeprazole used as an adjuvant drug in cancer treatment could improve cancer treatment efficacy.
Innovations and breakthroughs
In contrast with previous in vitro and in vivo studies, the present study clinically revealed that when used as an adjuvant drug in cancer treatment, omeprazole has synergetic effects on improving CRT efficacy and reducing rectal cancer recurrence.
Applications
When used as an adjuvant drug in cancer treatment, omeprazole has a synergetic effect on improving CRT efficacy and reducing rectal cancer recurrence and is helpful in improving cancer treatment efficacy.
Peer-review
Zhang et al retrospectively reviewed a series of 125 patients with rectal cancer and demonstrated that omeprazole users had better prognosis in term of response and recurrence rates and disease-free survival.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The IRB of SYSUCC has approved the protocol of this study.
Informed consent statement: All study participants or their legal guardian provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare no conflicts of interest.
Data sharing statement: No additional data are available.
Peer-review started: December 11, 2016
First decision: December 29, 2016
Article in press: February 16, 2017
P- Reviewer: Ierardi E S- Editor: Ma YJ L- Editor: Ma JY E- Editor: Wang CH
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Cammà C, Giunta M, Fiorica F, Pagliaro L, Craxì A, Cottone M. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA. 2000;284:1008–1015. doi: 10.1001/jama.284.8.1008. [DOI] [PubMed] [Google Scholar]
- 3.Colorectal CCG. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001;358:1291–1304. doi: 10.1016/S0140-6736(01)06409-1. [DOI] [PubMed] [Google Scholar]
- 4.Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, Bonnetain F, Bosset JF, Bujko K, Cionini L, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29:3163–3172. doi: 10.1200/JCO.2010.33.1595. [DOI] [PubMed] [Google Scholar]
- 5.Nishi T, Forgac M. The vacuolar (H+)-ATPases--nature’s most versatile proton pumps. Nat Rev Mol Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 6.Sennoune SR, Luo D, Martínez-Zaguilán R. Plasmalemmal vacuolar-type H+-ATPase in cancer biology. Cell Biochem Biophys. 2004;40:185–206. doi: 10.1385/CBB:40:2:185. [DOI] [PubMed] [Google Scholar]
- 7.De Milito A, Fais S. Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol. 2005;1:779–786. doi: 10.2217/14796694.1.6.779. [DOI] [PubMed] [Google Scholar]
- 8.Gerweck LE. Tumor pH: implications for treatment and novel drug design. Semin Radiat Oncol. 1998;8:176–182. doi: 10.1016/s1053-4296(98)80043-x. [DOI] [PubMed] [Google Scholar]
- 9.Altan N, Chen Y, Schindler M, Simon SM. Defective acidification in human breast tumor cells and implications for chemotherapy. J Exp Med. 1998;187:1583–1598. doi: 10.1084/jem.187.10.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerweck LE, Vijayappa S, Kozin S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther. 2006;5:1275–1279. doi: 10.1158/1535-7163.MCT-06-0024. [DOI] [PubMed] [Google Scholar]
- 11.Raghunand N, Gillies RJ. pH and drug resistance in tumors. Drug Resist Updat. 2000;3:39–47. doi: 10.1054/drup.2000.0119. [DOI] [PubMed] [Google Scholar]
- 12.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 13.Pérez-Sayáns M, Somoza-Martín JM, Barros-Angueira F, Rey JM, García-García A. V-ATPase inhibitors and implication in cancer treatment. Cancer Treat Rev. 2009;35:707–713. doi: 10.1016/j.ctrv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Murakami T, Shibuya I, Ise T, Chen ZS, Akiyama S, Nakagawa M, Izumi H, Nakamura T, Matsuo K, Yamada Y, et al. Elevated expression of vacuolar proton pump genes and cellular PH in cisplatin resistance. Int J Cancer. 2001;93:869–874. doi: 10.1002/ijc.1418. [DOI] [PubMed] [Google Scholar]
- 15.Torigoe T, Izumi H, Ishiguchi H, Uramoto H, Murakami T, Ise T, Yoshida Y, Tanabe M, Nomoto M, Itoh H, et al. Enhanced expression of the human vacuolar H+-ATPase c subunit gene (ATP6L) in response to anticancer agents. J Biol Chem. 2002;277:36534–36543. doi: 10.1074/jbc.M202605200. [DOI] [PubMed] [Google Scholar]
- 16.Marquardt D, Center MS. Involvement of vacuolar H(+)-adenosine triphosphatase activity in multidrug resistance in HL60 cells. J Natl Cancer Inst. 1991;83:1098–1102. doi: 10.1093/jnci/83.15.1098. [DOI] [PubMed] [Google Scholar]
- 17.Mattsson JP, Väänänen K, Wallmark B, Lorentzon P. Omeprazole and bafilomycin, two proton pump inhibitors: differentiation of their effects on gastric, kidney and bone H(+)-translocating ATPases. Biochim Biophys Acta. 1991;1065:261–268. doi: 10.1016/0005-2736(91)90238-4. [DOI] [PubMed] [Google Scholar]
- 18.Moriyama Y, Patel V, Ueda I, Futai M. Evidence for a common binding site for omeprazole and N-ethylmaleimide in subunit A of chromaffin granule vacuolar-type H(+)-ATPase. Biochem Biophys Res Commun. 1993;196:699–706. doi: 10.1006/bbrc.1993.2306. [DOI] [PubMed] [Google Scholar]
- 19.Mizunashi K, Furukawa Y, Katano K, Abe K. Effect of omeprazole, an inhibitor of H+,K(+)-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53:21–25. doi: 10.1007/BF01352010. [DOI] [PubMed] [Google Scholar]
- 20.Sabolić I, Brown D, Verbavatz JM, Kleinman J. H(+)-ATPases of renal cortical and medullary endosomes are differentially sensitive to Sch-28080 and omeprazole. Am J Physiol. 1994;266:F868–F877. doi: 10.1152/ajprenal.1994.266.6.F868. [DOI] [PubMed] [Google Scholar]
- 21.Yeo M, Kim DK, Kim YB, Oh TY, Lee JE, Cho SW, Kim HC, Hahm KB. Selective induction of apoptosis with proton pump inhibitor in gastric cancer cells. Clin Cancer Res. 2004;10:8687–8696. doi: 10.1158/1078-0432.CCR-04-1065. [DOI] [PubMed] [Google Scholar]
- 22.De Milito A, Iessi E, Logozzi M, Lozupone F, Spada M, Marino ML, Federici C, Perdicchio M, Matarrese P, Lugini L, et al. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 2007;67:5408–5417. doi: 10.1158/0008-5472.CAN-06-4095. [DOI] [PubMed] [Google Scholar]
- 23.Morimura T, Fujita K, Akita M, Nagashima M, Satomi A. The proton pump inhibitor inhibits cell growth and induces apoptosis in human hepatoblastoma. Pediatr Surg Int. 2008;24:1087–1094. doi: 10.1007/s00383-008-2229-2. [DOI] [PubMed] [Google Scholar]
- 24.Marino ML, Fais S, Djavaheri-Mergny M, Villa A, Meschini S, Lozupone F, Venturi G, Della Mina P, Pattingre S, Rivoltini L, et al. Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis. 2010;1:e87. doi: 10.1038/cddis.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Udelnow A, Kreyes A, Ellinger S, Landfester K, Walther P, Klapperstueck T, Wohlrab J, Henne-Bruns D, Knippschild U, Würl P. Omeprazole inhibits proliferation and modulates autophagy in pancreatic cancer cells. PLoS One. 2011;6:e20143. doi: 10.1371/journal.pone.0020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luciani F, Spada M, De Milito A, Molinari A, Rivoltini L, Montinaro A, Marra M, Lugini L, Logozzi M, Lozupone F, et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J Natl Cancer Inst. 2004;96:1702–1713. doi: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]
- 27.Bellone M, Calcinotto A, Filipazzi P, De Milito A, Fais S, Rivoltini L. The acidity of the tumor microenvironment is a mechanism of immune escape that can be overcome by proton pump inhibitors. Oncoimmunology. 2013;2:e22058. doi: 10.4161/onci.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calcinotto A, Filipazzi P, Grioni M, Iero M, De Milito A, Ricupito A, Cova A, Canese R, Jachetti E, Rossetti M. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012;72:2746–2756. doi: 10.1158/0008-5472.CAN-11-1272. [DOI] [PubMed] [Google Scholar]
- 29.Jansen JB, Lundborg P, Baak LC, Greve J, Ohman M, Stöver C, Röhss K, Lamers CB. Effect of single and repeated intravenous doses of omeprazole on pentagastrin stimulated gastric acid secretion and pharmacokinetics in man. Gut. 1988;29:75–80. doi: 10.1136/gut.29.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cederberg C, Andersson T, Skånberg I. Omeprazole: pharmacokinetics and metabolism in man. Scand J Gastroenterol Suppl. 1989;166:33–40; discussion 41-2. doi: 10.3109/00365528909091241. [DOI] [PubMed] [Google Scholar]
- 31.Tolman KG, Sanders SW, Buchi KN, Karol MD, Jennings DE, Ringham GL. The effects of oral doses of lansoprazole and omeprazole on gastric pH. J Clin Gastroenterol. 1997;24:65–70. doi: 10.1097/00004836-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Andersson T, Andrén K, Cederberg C, Lagerström PO, Lundborg P, Skånberg I. Pharmacokinetics and bioavailability of omeprazole after single and repeated oral administration in healthy subjects. Br J Clin Pharmacol. 1990;29:557–563. doi: 10.1111/j.1365-2125.1990.tb03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McTavish D, Buckley MM, Heel RC. Omeprazole. An updated review of its pharmacology and therapeutic use in acid-related disorders. Drugs. 1991;42:138–170. doi: 10.2165/00003495-199142010-00008. [DOI] [PubMed] [Google Scholar]
- 34.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 35.De Milito A, Canese R, Marino ML, Borghi M, Iero M, Villa A, Venturi G, Lozupone F, Iessi E, Logozzi M, et al. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int J Cancer. 2010;127:207–219. doi: 10.1002/ijc.25009. [DOI] [PubMed] [Google Scholar]
- 36.Lambert AA, Lam JO, Paik JJ, Ugarte-Gil C, Drummond MB, Crowell TA. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy: a systematic review and meta-analysis. PLoS One. 2015;10:e0128004. doi: 10.1371/journal.pone.0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 38.Fais S. Proton pump inhibitor-induced tumour cell death by inhibition of a detoxification mechanism. J Intern Med. 2010;267:515–525. doi: 10.1111/j.1365-2796.2010.02225.x. [DOI] [PubMed] [Google Scholar]
- 39.Fais S, De Milito A, You H, Qin W. Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Res. 2007;67:10627–10630. doi: 10.1158/0008-5472.CAN-07-1805. [DOI] [PubMed] [Google Scholar]
- 40.De Milito A, Fais S. Proton pump inhibitors may reduce tumour resistance. Expert Opin Pharmacother. 2005;6:1049–1054. doi: 10.1517/14656566.6.7.1049. [DOI] [PubMed] [Google Scholar]
- 41.De Milito A, Marino ML, Fais S. A rationale for the use of proton pump inhibitors as antineoplastic agents. Curr Pharm Des. 2012;18:1395–1406. doi: 10.2174/138161212799504911. [DOI] [PubMed] [Google Scholar]
- 42.Spugnini EP, Citro G, Fais S. Proton pump inhibitors as anti vacuolar-ATPases drugs: a novel anticancer strategy. J Exp Clin Cancer Res. 2010;29:44. doi: 10.1186/1756-9966-29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martínez-Zaguilán R, Seftor EA, Seftor RE, Chu YW, Gillies RJ, Hendrix MJ. Acidic pH enhances the invasive behavior of human melanoma cells. Clin Exp Metastasis. 1996;14:176–186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 44.Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006;66:6699–6707. doi: 10.1158/0008-5472.CAN-06-0983. [DOI] [PubMed] [Google Scholar]
- 45.Moellering RE, Black KC, Krishnamurty C, Baggett BK, Stafford P, Rain M, Gatenby RA, Gillies RJ. Acid treatment of melanoma cells selects for invasive phenotypes. Clin Exp Metastasis. 2008;25:411–425. doi: 10.1007/s10585-008-9145-7. [DOI] [PubMed] [Google Scholar]
- 46.Smallbone K, Gavaghan DJ, Gatenby RA, Maini PK. The role of acidity in solid tumour growth and invasion. J Theor Biol. 2005;235:476–484. doi: 10.1016/j.jtbi.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005;5:786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- 48.Katagiri F, Inoue S, Itoh H, Takeyama M. Omeprazole raises somatostatin and motilin in human plasma. Biol Pharm Bull. 2005;28:370–373. doi: 10.1248/bpb.28.370. [DOI] [PubMed] [Google Scholar]
- 49.Ishiguro T, Ishiguro R, Ishiguro M, Iwai S. Co-treatment of dichloroacetate, omeprazole and tamoxifen exhibited synergistically antiproliferative effect on malignant tumors: in vivo experiments and a case report. Hepatogastroenterology. 2012;59:994–996. doi: 10.5754/hge10507. [DOI] [PubMed] [Google Scholar]
- 50.Ishiguro T, Ishiguro M, Ishiguro R, Iwai S. Cotreatment with dichloroacetate and omeprazole exhibits a synergistic antiproliferative effect on malignant tumors. Oncol Lett. 2012;3:726–728. doi: 10.3892/ol.2012.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balakrishnan VS. Low BMI linked to worse colorectal cancer outcomes. Lancet Oncol. 2015;16:e593. doi: 10.1016/S1470-2045(15)00475-1. [DOI] [PubMed] [Google Scholar]


