Abstract
Background
Psoriasis is a debilitating chronic inflammatory autoimmune disease affecting approximately 7.4 million adults in the United States. Plaque psoriasis is the most common form, affecting 80% to 90% of patients.
Objectives
To describe the impact and challenges that psoriasis presents for various stakeholders, and to provide nondermatologist healthcare decision makers with information to enhance their contributions to drug and pharmacy benefit design discussions.
Discussion
Psoriasis carries an increased risk for early mortality and an increased prevalence of comorbidities, including psoriatic arthritis, cardiovascular disease, and diabetes. It is also associated with anxiety, depression, and social isolation, and can negatively impact patients' relationships, productivity, and careers. The physical, psychologic, social, and economic impact of psoriasis, plus the associated stigma, result in cumulative impairment over a patient's lifetime. The current treatments for moderate-to-severe psoriasis include topical therapy, phototherapy, and systemic drugs (nonbiologic and biologic); however, patient satisfaction remains low, combination therapy and treatment switching are common, and many patients remain untreated or undertreated. Clinicians should consider the patient holistically, and should select treatment based on a range of factors, including disease severity (with physical and psychosocial manifestations), susceptibility to cumulative life-course impairment (considering personality, behavior, and cognition), comorbidities, concomitant medication, and patient preference. It is estimated that the total annual direct cost of treating psoriasis in the United States in 2015 exceeded $12.2 billion.
Conclusion
Psoriasis is a complex disease, and appropriate management is correspondingly complex. Newer psoriasis treatments provide improved efficacy and safety versus traditional treatments, but challenges remain in ensuring patients access to these medications. An improved understanding of the barriers to appropriate treatment is needed, as well as clear and accessible information for payers and clinicians on current treatment options, to ensure that decision makers can control costs while providing patients with optimal care.
Keywords: chronic disease, cost, disease burden, disease management, healthcare decision makers, plaque psoriasis, psoriasis, treatment, unmet medical needs
The increasing prevalence of noncommunicable chronic diseases is widely recognized as a significant challenge to US and global healthcare. Despite such recognition focusing primarily on life-threatening conditions (eg, cardiovascular disease, respiratory disease, diabetes, and cancer), there is also a recognition of the growing burden imposed by chronic diseases that result in significant morbidity rather than mortality.1 In 2014, the World Health Organization adopted a resolution characterizing psoriasis as “a chronic, non-communicable, painful, disfiguring and disabling disease for which there is no cure.”1
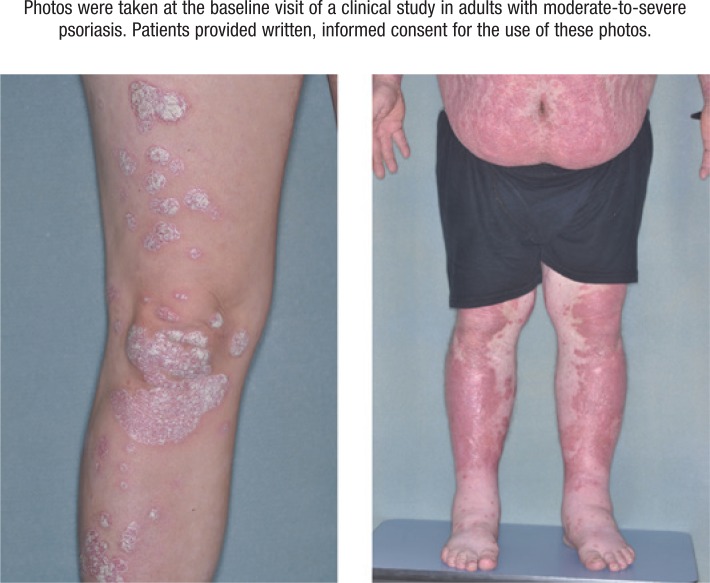
Psoriasis is a chronic systemic inflammatory autoimmune disease characterized by skin lesions, with the most common subtype being plaque psoriasis, which accounts for 80% to 90% of all cases.2 Plaque psoriasis manifests as red, raised lesions covered in dead skin cells with a silvery appearance (Figure).2 Plaques vary in their extent and location, but they often have a bilateral symmetrical distribution and most frequently occur on the elbows, knees, genitals, scalp, lower back, and buttocks.2
Figure. Patients with Moderate-to-Severe Psoriasis.
Psoriasis is also associated with an increased incidence of chronic comorbid conditions related to the systemic inflammatory nature of the disease.3 For example, the risk for a myocardial infarction in a 30-year-old patient with severe psoriasis is 3-fold higher than in the general population.4 Patients with psoriasis also have an increased risk for metabolic syndrome, including obesity, diabetes, and hyperlipidemia,5 with an increased overall mortality risk in patients with severe psoriasis,6,7 or with varying severity of disease.8 Cardiovascular disease is the main driver of excess mortality in absolute terms, but one study suggested that the relative risk for premature death from renal or hepatic causes is also elevated in patients with psoriasis compared with matched controls.8 The relative risk for cardiovascular death associated with severe psoriasis is higher in younger patients.6 In addition, up to 30% of patients with psoriasis also have psoriatic arthritis, which manifests as generalized fatigue, pain, stiffness, and swelling in and around the joints, potentially resulting in joint destruction and further disability.9
Psoriasis is primarily diagnosed in young people; 75% of individuals are aged <40 years at the time of diagnosis.10 For patients diagnosed before age 40 years, peak onset occurs between the ages of 16 and 22 years; diagnosis after age 40 years has a peak age of onset when patients are in their mid-to-late 50s.10 Estimates of psoriasis prevalence in the United States range between 1% and 5%11,12; in 2013, approximately 7.4 million US adults had psoriasis.11 In the United States, approximately 150,000 cases of psoriasis are diagnosed annually, and 3 million visits to doctors' offices or hospitals are a result of psoriasis.2
The proportion of US patients with plaque psoriasis who have moderate-to-severe disease is estimated to be approximately 20%.13 However, of the 5604 US patients who responded to the National Psoriasis Foundation's biannual survey between 2003 and 2011, 36% considered themselves to have moderate disease and 34% severe disease,14 and in a large international survey, responding dermatologists considered approximately 20% of their patients to have severe psoriasis.15 Patients with more severe disease are more likely to consult a clinician, and a substantial number of patients with mild psoriasis do not seek treatment from a clinician.14
KEY POINTS
-
▸
Psoriasis is a chronic inflammatory autoimmune disease affecting >7 million US adults.
-
▸
Plaque psoriasis is the most common form, affecting 80% to 90% of patients.
-
▸
Psoriasis has a physical, emotional, social, and economic impact on patients, and is associated with reduced health and productivity, lost work days, and an increased incidence of comorbidities.
-
▸
Current treatment options include topical therapies, phototherapies, oral systemic therapies, and a wide range of biologics.
-
▸
New therapies are needed to help address long-term efficacy and safety concerns, low patient satisfaction, and poor adherence.
-
▸
Payers face challenges in deciding which treatments for moderate-to-severe psoriasis to include on their formularies because of increasing options and costs.
In light of the increasing prevalence and burden of psoriasis and the rapidly changing treatment landscape of the disease, the purpose of this article is to provide an overview of the key aspects of moderate-to-severe psoriasis, including the methods by which it is assessed, current treatment options, and unmet treatment needs. The impact of psoriasis on patients, clinicians, payers, and employers will also be discussed.
Disease Assessment
Several measures are used to assess the severity of psoriasis as a measure of treatment response in clinical trials and in day-to-day clinical practice. According to the American Academy of Dermatology guidelines, the Psoriasis Area and Severity Index (PASI) is often used to assess the overall severity of psoriasis in clinical trials; a 75% improvement in the PASI score (PASI75) is considered to represent a clinically relevant treatment response.13 Other frequently used assessment tools include the Physician's Global Assessment, and an assessment of the percent of body surface area affected.13 The Simple-Measure for Assessing Psoriasis Activity, which is calculated by multiplying the Physician's Global Assessment by the body surface area, is gaining favor as a way to assess psoriasis severity and response to treatment.16 To determine psoriasis severity in clinical practice, current guidelines recommend that clinicians consider objective evaluations, including body surface area involvement, the location and thickness of lesions, symptoms, and the presence or absence of psoriatic arthritis, with a subjective assessment of the physical, emotional, and financial impact on the patient.13
Several questionnaires have been used in the research setting to assess the impact of psoriasis on health-related quality of life (QOL), including generic (eg, EuroQoL 5 dimensions [EQ-5D], Sickness Impact Profile, Medical Outcomes Study 36-Item Short-Form Health Survey [SF-36]), dermatology-specific (eg, Dermatology Life Quality Index [DLQI], Skindex), and psoriasis-specific (eg, Psoriasis Quality of Life, Psoriasis Disability Index) instruments.3 The Koo-Menter Psoriasis Instrument is a diagnostic tool that can assist clinicians in identifying patients with a significantly reduced health-related QOL warranting systemic treatment, and help to document the rationale for treatment decisions for healthcare payers.17 However, a recent systematic review concluded that a valid, sensitive, specific, and acceptable measure is still needed that assesses the full impact of psoriasis on patients, and can assist in the clinical management of patients with psoriasis.18
In the clinical practice setting, formal measures are often not used, relying instead on subjective assessments, particularly patient satisfaction, to drive treatment decision-making. The paucity of objective clinical outcomes, and the major limitation of risk adjustment, are significant hurdles to assessing the quality of the management of patients with psoriasis in the clinical setting.
Current Treatment Options
Various options are available for the treatment of psoriasis, including topical agents, phototherapies and photochemotherapy, and systemic nonbiologic drugs, such as methotrexate (MTX) and cyclosporine (both immunosuppressive drugs), acitretin (a second-generation retinoid), and apremilast (an oral small-molecule inhibitor of phosphodiesterase-4), as noted in Table 1.
Table 1.
FDA-Approved Treatments for Plaque Psoriasis
| Therapy | Therapy line |
|---|---|
| Topical agents | |
| Anthralin | First line in mild disease; second line in combination with other treatments in mild, moderate, or severe disease |
| Corticosteroids | First line in mild disease; second line in combination with other treatments in mild, moderate, or severe disease |
| Coal tar | First line in mild disease; second line in combination with other treatments in mild, moderate, or severe disease |
| Salicylic acid | First line in mild disease; second line in combination with other treatments in mild, moderate, or severe disease |
| Vitamin D analogs | First line in mild disease; second line in combination with other treatments in mild, moderate, or severe disease |
| Vitamin A analogs | First line in mild disease; second line in combination with other treatments in mild, moderate, or severe disease |
| Phototherapy | |
| Narrowband UVB | First line in moderate-to-severe disease or mild disease in vulnerable areas; second line (in combination with other treatments) in mild, moderate, or severe disease |
| Excimer 308-nm laser narrowband UVB | First line in moderate-to-severe disease or mild disease in vulnerable areas; second line (in combination with other treatments) in mild, moderate, or severe disease |
| PUVA | First line in moderate-to-severe disease or mild disease in vulnerable areas; second line (in combination with other treatments) in mild, moderate, or severe disease |
| Nonbiologic systemic therapies | |
| Methotrexate | Second line in severe, recalcitrant, disabling disease that is not adequately responsive to other forms of therapy |
| Cyclosporine | First line in severe, recalcitrant disease in patients for whom other systemic therapies are contraindicated; second line in severe, recalcitrant disease in patients who have failed to respond to at least 1 systemic therapy or cannot tolerate other systemic therapies |
| Acitretin | First line in severe disease |
| Apremilast (Otezla) | First line in moderate-to-severe disease |
| Biologic systemic therapies | |
| Adalimumab (Humira) | First line in moderate-to-severe disease |
| Etanercept (Enbrel) | First line in moderate-to-severe disease |
| Infiiximab (Remicade) | First line in severe disease |
| Ixekizumab (Taltz) | First line in moderate-to-severe disease |
| Secukinumab (Cosentyx) | First line in moderate-to-severe disease |
| Ustekinumab (Stelara) | First line in moderate-to-severe disease |
FDA indicates US Food and Drug Administration; PUVA, psoralen combined with ultraviolet A; UVB, ultraviolet light B.
In addition, several systemic biologic therapies are currently approved (as of March 2016) by the US Food and Drug Administration (FDA) for the treatment of chronic, moderate-to-severe plaque psoriasis, including infliximab, etanercept, adalimumab, ustekinumab, secukinumab, and ixekizumab (Table 1).
In a survey conducted in the United States, Canada, France, Germany, Italy, Spain, and the United Kingdom, dermatologists reported that among patients with moderate-to-severe psoriasis, approximately 75%, 20%, and 20% of patients were receiving topical therapy, conventional oral therapy, and biologics, respectively.15 The American Academy of Dermatology has developed algorithms identifying recommended treatment options for psoriasis,19 and several drugs are currently under investigation for the treatment of psoriasis.
The efficacy of conventional systemic agents (ie, MTX and cyclosporine) and of biologic agents has been widely documented.13,20 A systematic review and meta-analysis of 48 randomized controlled trials of therapies approved for the treatment of moderate-to-severe psoriasis reported biologics to have higher efficacy than conventional systemic agents, with infliximab having the highest efficacy, followed by adalimumab and ustekinumab.20 Newer agents (ie, secukinumab, ixekizumab, and apremilast) were not included in this systematic review, because it was conducted before their availability.
Patients' response to biologic agents can also decrease over time as a result of immunogenicity and antidrug antibodies.13 Furthermore, there is evidence of safety issues related to nonbiologic and biologic therapies, including end-organ toxicity, that preclude the long-term use of conventional systemic agents.3 Biologics also have rare safety concerns, including possible increased risk for serious infections, nonmelanoma skin cancer, and malignancies.21
At the time of this writing, the efficacy and safety of 35 drugs were being evaluated in phase 2 or 3 clinical trials for the treatment of psoriasis.22
Unmet Medical Needs
National surveys by the National Psoriasis Foundation between 2003 and 2011 reported that approximately 37% to 49%, 24% to 36%, and 9% to 30% of patients with mild, moderate, and severe psoriasis, respectively, were not receiving treatment.14 An analysis of claims data from a US healthcare plan estimated that approximately 60% of 1.7 million eligible patients with moderate-to-severe psoriasis (based on self-reported body surface area involvement ≥3%) had not received therapy in the 12 months before September 2012, and approximately 33% had not received treatment within the previous 5 years.23 In that analysis, 50.2% of the patients who received treatment lapsed therapy within 12 months.23
Delays in initiating systemic treatment, switching treatment, discontinuation and restarting, and dose escalation and reduction are common events in the treatment of patients with moderate-to-severe psoriasis,23,24 as is the use of a combination of ≥2 therapies with differing mechanisms of action.25 Dermatologists in the United States, Canada, France, Germany, Italy, Spain, and the United Kingdom acknowledge that psoriasis is undertreated and recognize that there is an unmet treatment need for patients with psoriasis.15 The main reasons for physicians not initiating or maintaining treatment reported in this survey were related to concerns about the long-term safety or tolerability and efficacy of currently available therapies.15
Furthermore, studies demonstrate that up to 40% of individuals with psoriasis do not use their medication as recommended by their clinician.26 Moreover, data from National Psoriasis Foundation surveys show that satisfaction with psoriasis treatments is low: more than 50% of US patients are dissatisfied with their treatment.14 In US clinical practice, patients with moderate-to-severe psoriasis who were receiving biologic monotherapy, adalimumab in combination with MTX, or phototherapy had higher overall satisfaction scores, whereas those receiving topical therapy alone had significantly lower overall satisfaction scores compared with patients receiving MTX monotherapy.27
Other studies also showed that patient satisfaction with systemic therapy was higher compared with topical treatment.28 Topical therapy requires a cream or gel to be spread over the affected area of skin, often more than once daily, which is time-consuming and messy; this, and the poor perceived efficacy of topical treatment in moderate-to-severe psoriasis, may account for the low level of patient satisfaction with topical therapy.27,28 Satisfaction with phototherapy is broadly similar to,28 or less than,29 satisfaction with systemic treatment. Most forms of phototherapy are administered in a clinical setting, frequently requiring multiple sessions weekly over a prolonged period, thus presenting a substantial time commitment burden on patients.30 In addition, patients may have a copayment for each phototherapy treatment; this, and the time commitment, may be barriers for patients. Patients with psoriasis who receive biologic therapies have reported higher treatment satisfaction than biologic-naïve patients.31
Treatment time was the strongest of 12 predictors of health-related QOL in a cross-sectional survey of patients with psoriasis.32 Overall, patients express greater satisfaction with biologics and oral systemic treatments than more time-consuming forms of therapy (including topical and phototherapy).28,29 Although many patients react with anxiety or fear to the idea of self-administration of subcutaneous treatment,33 which may present a barrier to biologic treatment for some patients, treatment satisfaction is often high with injectable biologic agents because of their efficacy.31
Patients with psoriasis place considerable importance on treatment attributes that are compatible with their personal and professional lives, such as treatment location (clinic or home), safety, cost, monitoring requirements, and administration route.34 Of the 35 drugs that are currently in clinical development for psoriasis, approximately 29% require administration by injection, 31% are oral therapies, and 40% are topical therapies.22
Taken together, the potential for low satisfaction with psoriasis treatment, the rates of untreated and undertreated patients, frequent treatment switching, and the widespread use of combination therapy in the attempt to try to achieve a satisfactory response represent unmet treatment needs in patients with psoriasis of all severities.
Patient Perspective
Psoriasis is associated with physical symptoms, with the most common patient-reported symptoms being itch and pain.33 However, the impact of psoriasis on the patient extends far beyond these physical manifestations and can have a profound psychosocial effect that does not always correlate with the severity of skin lesions.33 The appearance of psoriatic plaques can lead to negative reactions from other people, including repulsion and fear. Psoriasis is therefore associated with social stigma, causing anxiety, depression, and social isolation that can affect a patient's interpersonal relationships and intimacy.35,36
The prevalence of depression is higher in patients with psoriasis compared with the general population,35,37 and patients with psoriasis are more likely to have suicidal thoughts or to attempt suicide than healthy individuals.37 Moreover, among patients with moderate-to-severe psoriasis, patients' subjective ratings of disease severity have been reported to be higher than physicians' objective ratings, which may be caused by the psychosocial aspects of the disease.38
The impact of psoriasis on a patient's health-related QOL has been shown in various studies using different instruments, including the DLQI, the Psoriasis Disability Index, the EQ-5D, the Sickness Impact Profile, and the SF-36.33,39–41 An increase in DLQI score, indicating reduced health-related QOL, is correlated with the severity of disease, but even in patients with ≤3% body surface area (ie, mild disease), almost 25% report a substantial impact on health-related QOL.33 A review of 10 years of DLQI use identified studies that included 2468 patients with psoriasis and demonstrated reduced health-related QOL compared with the general population.42
Further evidence of the impact of psoriasis on health-related QOL comes from a systematic review, showing that the impact of psoriasis on patients' health status is similar to that experienced by patients with other chronic diseases, with the mean EQ-5D scores reported to be 0.52 to 0.90 for psoriasis, 0.24 to 0.90 for cardiovascular disease, 0.20 to 0.88 for type 2 diabetes, 0.44 to 0.86 for end-stage renal disease, 0.66 to 0.79 for liver disease, 0.33 to 0.93 for cancer, and 0.64 to 0.89 for visual disorders.39
Psoriasis has also been shown to result in a reduction in patient-reported physical and mental functioning comparable with other diseases, with mean SF-36 physical component summary and mental component summary scores of 41.2 and 45.7, respectively, in psoriasis compared with 45.1 and 48.8, respectively, in cancer; 43.2 and 48.8, respectively, in arthritis; 44.3 and 52.2, respectively, in hypertension; 41.5 and 51.9, respectively, in type 2 diabetes; and 45.0 and 34.8, respectively, in depression.41 Some forms of psoriasis have a particularly high impact on patients' QOL, including genital psoriasis, palmoplantar psoriasis, and the comorbidity psoriatic arthritis.33,43,44
The combination of the physical and psychosocial impact, and the resultant reduction in health-related QOL associated with psoriasis has a substantial, often unrecognized, effect on individuals with this disease. A large, population-based survey in the United States reported that approximately 28% of patients with psoriasis considered it a “problem” in their everyday lives, and another 12% rated their psoriasis as a “large problem.”45 In a multinational survey, approximately 92% of responding dermatologists agreed that the clinical disease burden of psoriasis was frequently underestimated: approximately 79% thought psoriasis had a social and/or emotional impact, and approximately 63% considered it to affect patients' daily activities and/or work.15
Moderate-to-severe psoriasis adversely affects employment opportunities, career prospects, and earning potential.46 Indeed, the level of income in patients with psoriasis correlates inversely with disease severity.47 In a recent study of 201 patients with psoriasis, of whom 50% had moderate-to-severe disease, the mean productivity loss was 7.6%, resulting from presenteeism, with an additional mean 6.6% loss of working time (ie, absenteeism) over a 4-week period, as assessed via the Work Limitations Questionnaire.48 These results are consistent with those of a study of 200 patients with moderate-to-severe plaque psoriasis, which showed a 14% overall work impairment, with the majority of impairment resulting from presenteeism rather than absenteeism.49
Kimball and colleagues suggested that the negative psychological, social, and economic impact of psoriasis on a patient accumulates over time; they proposed the concept of Cumulative Life Course Impairment (CLCI) to assess the additive burden of psoriasis, its associated comorbidities, and stigma over a patient's lifetime.50 Assessment of health-related QOL is generally cross-sectional, and the findings are representative of only a single point in time for a patient; however, CLCI represents the impact of psoriasis on the patient's physical and psychological well-being, social and emotional relationships, and vocational and employment decisions over time. CLCI may vary depending on factors such as stigmatization, comorbid conditions (physical and psychological), coping mechanisms, external factors, and the patient's personality.50,51 Many patients consider psoriasis to have altered the course of their lives, such that their abilities to pursue their chosen career, develop relationships, and fully enjoy family life are substantially compromised.51
Clinician Perspective
Although patients with psoriasis may be treated by a general physician, they are usually managed by dermatologists (who may refer patients to an infusion center, a rheumatologist, or a gastroenterologist for infusions, if required).2 In developing treatment plans, clinicians select a treatment from different topical therapies, systemic therapies, and phototherapies, and should consider disease severity, relevant comorbidities, and patient preference, as well as the patient's insurance coverage and treatment availability.52 It is important for physicians to ensure that patients understand the efficacy, safety, convenience, and insurance coverage of appropriate treatment options, so that they can be involved in the treatment decision and potentially maximize adherence.53
Treatment should reflect the severity of disease, as is recommended by current treatment guidelines19 rather than being initiated using a stepwise approach. Treatment decisions are often difficult for physicians, because many medical management policies require a step-edit approach as a condition for reimbursement, requiring that the patient's disease fail to respond adequately to topical and/or conventional systemic therapies before being able to prescribe biologics.54 This may limit clinicians' ability to aggressively treat patients with moderate-to-severe disease, despite the recommended treatment guidelines.
In addition, clinicians managing patients with psoriasis should consider a holistic approach that considers the psychosocial and the physical aspects of the disease.55 However, understanding the critical factors, including behavioral and cognitive patterns and personality, that affect the cumulative effects of the disease are essential to inform treatment decisions earlier in the course of the disease.51
Current biologic therapies are administered via subcutaneous injection or intravenous infusion and are effective in moderate-to-severe psoriasis.3 However, their use is often restricted by payers, requiring completion of prior authorization forms or other processes to justify use,54 which may contribute to the undertreatment of moderate-to-severe psoriasis, despite evidence that such requirements for other types of therapies do not lead to reduced costs.56
The ever-growing challenges and opportunities associated with the increasing number of treatment options available for psoriasis must be considered against a backdrop of changes that add to the pressures on the clinician. Physician performance measurements attempt to assess the quality and efficiency of clinicians' performance.57 Linked to this, tiered-provider networks have been introduced with clinician tiering based on performance ratings (including perceived quality of care and cost-effectiveness) that may not take into account the case mix of patients or the treatment efficacy (which may not be measurable from billing information), and with patients being offered lower copayments for consulting with clinicians who have better ratings.57
As a consequence of the links between the cost of care, patient case mix, and the severity of disease, the inclusion of financial considerations in clinician performance ratings raises concerns among dermatologists that a dermatologist who manages patients with severe psoriasis could receive a lower rating than another clinician who only manages patients with mild psoriasis or who undertreats patients with moderate-to-severe psoriasis.
Although the objective of tiered networks is to improve the quality of care that clinicians provide and to encourage patients to identify higher quality and more cost-effective care, there is a potential that such incentive structures would discourage dermatologists from adequately treating (or even seeing) patients with severe psoriasis.57 The introduction of tight networks, whereby an insurance company includes a limited number of specialists (eg, dermatologists) in their network, can limit patients' access to required expertise, and may potentially present even bigger challenges than tiered networks to patients accessing optimal treatment.58
Economic Implications and Payer Perspective
The annual costs, based on wholesale acquisition cost (WAC), for select systemic therapies currently approved by the FDA for the treatment of chronic, moderate-to-severe plaque psoriasis are considerable (Table 2). In addition, the recent approval of extremely effective but costly treatments for patients with hepatitis C has resulted in extensive debate regarding the use of such treatments.59
Table 2.
Annual Maintenance Costs in 2015 of Select Systemic Therapies Approved by the FDA for Moderate-to-Severe Plaque Psoriasis, Based on Approved Dosage Schedulesa
| Drug | Mechanism of action | Route of administration | Dosing schedule | Annual cost, $ (WAC)b |
|---|---|---|---|---|
| Secukinumab (Cosentyx) | IL-17A antagonist | Subcutaneous injection | 150 mg on wks 0, 1, 2, 3, and 4, then 150 mg every 4 wks or 300 mg on wks 0, 1, 2, 3, and 4, then 300 mg every 4 wks | 33,220 66,440 |
| Etanercept (Enbrel) | TNF-α inhibitor | Subcutaneous injection | 50 mg/wk | 48,472 |
| Adalimumab (Humira) | TNF-α inhibitor | Subcutaneous injection | 40 mg every other wk after an initial single 80-mg dose | 51,260 |
| Ustekinumab (Stelara) | IL-12 and IL-23 antagonist | Subcutaneous injection | 45 mg on wks 0 and 4 then every 12 wks (patients ≤100 kg) or 90 mg on wks 0 and 4 then every 12 wks (patients >100 kg) | 44,201 88,402 |
| Infliximab (Remicade) | TNF-α inhibitor | Intravenous infusion | 5 mg/kg on wks 0, 2, and 6 then every 6–8 wks (80-kg patient) or 5 mg/kg on wks 0, 2, and 6 then every 6–8 wks (100-kg patient) | 30,001 37,502 |
| Ixekizumab (Taltz) | IL-17A antagonist | Subcutaneous injection | 80 mg × 2 then 80 mg every 2 wks for 12 wks, then 80 mg every mo | 69,762 |
| Apremilast (Otezla) | PDE-4 inhibitor | Oral | 30 mg twice daily | 31,035 |
Costs shown are based on approved dosage schedules; these may be adapted in clinical practice and may be discounted depending on contracts between purchasers and manufacturers.
AnalySource. 2015. www.analysource.com/. Accessed August 24, 2015.
FDA indicates US Food and Drug Administration; IL, interleukin; PDE-4, phosphodiesterase-4; TNF, tumor necrosis factor; WAC, wholesale acquisition cost.
Systematic reviews of the economic burden of psoriasis in the United States have reported high direct costs of treating psoriasis, although there is no clear consensus on the actual figure, with estimates of the total annual direct cost in 2013 ranging from $12.2 billion60 to $63.2 billion.61 This wide variation in cost estimates may result in part from the publication date of articles included in the systematic reviews, because biologic therapies with higher costs have become available in more recent years. The annual WAC for newer systemic and biologic therapies range from $30,001 to $88,402 (Table 2). A recent US observational study reported the mean 6-month direct costs for patients with moderate-to-severe psoriasis to be an annual direct cost of $22,582 per patient; in this study, 60% of patients received biologic therapy, with 36% using a self-administered biologic.49
In a retrospective analysis of a large US healthcare claims database, the mean annualized total healthcare cost for patients with moderate-to-severe psoriasis receiving biologic therapy between January 2007 and March 2012 was $30,568.24 This cost was broadly consistent with the findings of another retrospective analysis of a US commercial claims database, in which the mean annual direct cost (including the cost of the index biologic and any biologic used after discontinuation of the index drug) per patient with psoriasis and a claim for a biologic therapy ranged from $22,474 for patients continuing etanercept therapy to $47,701 for patients who were newly prescribed infliximab.62 The acquisition costs for some therapies have changed since this analysis was reported (Table 2). The annual economic cost in the United States resulting from reduced health-related QOL associated with psoriasis has been estimated to be $11.8 billion.60
In an analysis of the monthly direct cost to achieve PASI75, traditional systemic therapies (ie, MTX and cyclosporine) had the best cost-efficacy of the treatments analyzed, whereas infliximab 100 mg and ustekinumab 90 mg were the most expensive.63 However, the results of relatively few clinical trials of MTX or cyclosporine have been published (3 for MTX and 1 for cyclosporine were included in this review), resulting in uncertainty in the number needed to treat to achieve PASI75 response. Other biologic therapies (adalimumab 40 mg, ustekinumab 45 mg, and etanercept 25 mg and 50 mg) had intermediate cost-efficacy.63 However, this analysis did not take into account the cost of managing treatment side effects63; MTX and cyclosporine have significant safety concerns that may lead to increased total cost of care, with hepatotoxicity and bone marrow suppression reported for MTX and hypertension and kidney failure for cyclosporine.3
Estimates of annual US indirect costs of productivity loss related to psoriasis vary, with reported costs of up to $35.4 billion.60,61 However, recent research has suggested that the productivity loss related to psoriasis might have been overestimated in several studies that did not exclude absenteeism or presenteeism in patients with psoriasis resulting from other causes.64
Payers face substantial challenges when considering which psoriasis treatments to include in their plan for reimbursement, particularly in the setting of moderate-to-severe psoriasis for which biologic or oral therapies may be indicated. Payers have to balance the potential benefit of treatments with any risk of side effects, as well as take into account the cost of the therapy (including the cost of monitoring for side effects and the likely cost of managing disease sequelae in the absence of treatment). As a consequence, payers often support a traditional stepwise approach to treatments for psoriasis.54 Traditional nonbiologic therapies with much lower acquisition costs may be required as first-line treatments even for patients with moderate-to-severe psoriasis,54 for whom some dermatologists think a biologic or a recently approved nonbiologic drug with higher acquisition costs are warranted for initial therapy.65
Most biologic therapies, and some of the newer nonbiologic agents, are effective in multiple autoimmune (and nonautoimmune) diseases, but have different dosage regimens across indications, which can present a challenge to payers.66 Payer formulary strategy and utilization management for FDA-approved specialty drugs are frequently based on a collective therapeutic review for all indicated autoimmune diseases. Such reviews are often based on the autoimmune disease with the largest patient population, which is rheumatoid arthritis.66 This strategy may result in a formulary that may limit or even preclude access to some treatment options for patients with other conditions, such as moderate-to-severe psoriasis.
Given the prevalence of psoriasis and the rising costs associated with effective treatment, cost management has become a major concern for payers. Specialty medications accounted for more than 30% of the total drug spending billed through pharmacy benefit managers in 2014.67 Furthermore, medications for inflammatory conditions (including psoriasis) accounted for the highest spending within specialty medications.67 Drug spending in 2014 increased by approximately 13%, with specialty drugs responsible for approximately 30% to 50% of that increase.67,68
Conclusion
Decision makers have to face difficult choices in sharing limited resources not just between competing treatments within a disease but between different disease states, and it is critical that all stakeholders have an informed understanding of this.
The high incidence of undertreatment of moderate-to-severe psoriasis and the variability in patient response highlight that despite a large range of available treatments, there remains an unmet need for new and effective treatments, as well as improved access to existing treatments. Continued research is needed to develop effective treatments for psoriasis with acceptable safety profiles and convenient administration routes, as well as regimens that will address issues of low treatment satisfaction and poor adherence among patients. Action is also needed to ensure that US treatment guidelines allow decision makers to control costs while providing patients with optimal care, and that such guidelines are followed by providers. Recent advances have led to the discovery of new agents for the treatment of psoriasis, providing broader treatment choices than those available only a few years ago.
Improved education of clinicians and payers in terms of the continually evolving nature of available treatments and their different mechanisms of action, efficacy, potential side effects, and position in treatment algorithms is needed to help inform treatment choices. Treatment plans should be developed in discussion with the patient to enhance adherence to their treatment regimens and to optimize patient outcomes.
Acknowledgments
Medical writing support, under direction from the authors, was provided by Carole Evans, PhD, of Complete Medical Communications.
Contributor Information
Steven R. Feldman, Professor of Dermatology, Department of Dermatology, Wake Forest University School of Medicine, Winston-Salem, NC.
Bernard Goffe, Dermatologist, Dermatology Associates, and Division of Dermatology, University of Washington, Seattle.
Gary Rice, Senior Vice President, Diplomat Specialty Pharmacy, Flint, MI.
Matthew Mitchell, Director, Pharmacy Services, SelectHealth, Murray, UT.
Mandeep Kaur, Senior Medical Director, Pfizer, Collegeville, PA.
Debbie Robertson, Senior Director of Medical Affairs, Pfizer.
Debra Sierka, Director of Medical Affairs, Pfizer.
Jeffrey A. Bourret, Senior Director, North America Medical Affairs, and Medical Lead, Specialty Payer & Channel Customer Strategy, Pfizer.
Tamara S. Evans, Field Medical Director, Pfizer, Indianapolis, IN.
Alice Gottlieb, Professor of Dermatology, Department of Dermatology, New York Medical College, Valhalla, NY..
Source of Funding
The writing and preparation of this article were funded by Pfizer, Inc.
Author Disclosure Statement
Available online at www.AHDBonline.com.
Dr Feldman has received research, speaking and/or consulting support from a variety of companies including AbbVie, Advance Medical, Suncare Research, Anacor, Astellas, Baxter, Boehringer Ingelheim, Janssen, Lilly, Merck, Merz, Mylan, Caremark, Celgene, Cosmederm, Galderma, GSK/Stiefel, Informa, Leo Pharma, National Biological Corporation, National Psoriasis Foundation, Novartis, Pfizer Inc, Qurient, UpToDate, and Valeant. He is founder and majority owner of www.DrScore.com, and founder and part-owner of Causa Research, a company dedicated to enhancing patients' adherence to treatment. Dr Feldman has participated in advisory boards for AbbVie, Boehringer Ingelheim, Merck, Novartis, and Pfizer Inc; has served as a consultant for AbbVie, Amgen, Celgene, Galderma Laboratories, Lilly, Mylan, Novartis, and Pfizer Inc; and is a member of speakers' bureau for Celgene, Galderma, and Janssen. Dr Feldman has received research grant support from Galderma Laboratories, Celgene, Novartis, and National Biological Corporation.
Dr Gottlieb has current consulting or advisory board agreements with Abbott Labs (AbbVie), Actelion, Amgen Inc, Astellas, Akros, Baxalta, Beiersdorf, Inc, Bristol-Myers Squibb Co, Canfite, Catabasis, Celgene Corp, Centocor (Janssen), Coronado, CSL Behring Biotherapies for Life, Dermipsor Ltd, Glaxo Smith Kline, Genentech, Incyte, Karyopharm, Kineta One, KPI Therapeutics, Lilly, Novartis, Meiji Seika Pharma Co, Ltd, Mitsubishi Tanabe Pharma Development America, Inc, Novo Nordisk, Pfizer Inc, Takeda, TEVA, UCB, Vertex, and Xenoport; and has received research/educational grants (paid to Tufts Medical Center) from Abbott (AbbVie), Amgen, Baxalta, Celgene, Centocor (Janssen), Dermira, Levia, Lilly, Merck, Novartis, Pfizer Inc, and Xenoport.
Dr Goffe has research, speaking, and/or consulting support from AbbVie, Amgen, Astellas, Celgene, Centocor, Lilly, Novartis, and Pfizer Inc.
Mr Rice has participated in advisory boards for AbbVie, Biogen Idec, Janssen, Novartis, and Pfizer Inc; has served as a consultant for Gilead and is a member of speakers' bureau for Novartis.
Dr Mitchell reported no conflicts of interest.
Dr Kaur, Ms Robertson, Dr Sierka, Dr Bourret, and Dr Evans are employees and shareholders of Pfizer Inc.
References
- 1. World Health Organization. Psoriasis. Agenda item 13.5. World Health Assembly; Geneva, Switzerland; May 24, 2014. http://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_R9-en.pdf. Accessed August 24, 2016. [Google Scholar]
- 2. Lo Sicco K, Camisa C, Grandinetti L. Psoriasis. September 2013. www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/dermatology/psoriasis-papulosquamous-skin-disease/#cesec2. Accessed August 24, 2016.
- 3. Pathirana D, Ormerod AD, Saiag P, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009; 23 (suppl 2): 1–70. Erratum in: J Eur Acad Dermatol Venereol. 2010; 24:117–118. [DOI] [PubMed] [Google Scholar]
- 4. Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006; 296: 1735–1741. [DOI] [PubMed] [Google Scholar]
- 5. Cohen AD, Sherf M, Vidavsky L, et al. Association between psoriasis and the metabolic syndrome: a cross-sectional study. Dermatology. 2008; 216: 152–155. [DOI] [PubMed] [Google Scholar]
- 6. Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010; 31: 1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007; 143: 1493–1499. [DOI] [PubMed] [Google Scholar]
- 8. Svedbom A, Dalén J, Mamolo C, et al. Increased cause-specific mortality in patients with mild and severe psoriasis: a population-based Swedish register study. Acta Derm Venereol. 2015; 95: 809–815. [DOI] [PubMed] [Google Scholar]
- 9. National Psoriasis Foundation. Psoriatic arthritis. www.psoriasis.org/psoriatic-arthritis. Accessed August 24, 2016.
- 10. Queiro R, Tejón P, Alonso S, Coto P. Age at disease onset: a key factor for understanding psoriatic disease. Rheumatology (Oxford). 2014; 53: 1178–1185. [DOI] [PubMed] [Google Scholar]
- 11. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014; 70: 512–516. [DOI] [PubMed] [Google Scholar]
- 12. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; for the Identification and Management of Psoriasis and Associated Comorbidity (IMPACT) Project Team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013; 133: 377–385. [DOI] [PubMed] [Google Scholar]
- 13. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008; 58: 826–850. [DOI] [PubMed] [Google Scholar]
- 14. Armstrong AW, Robertson AD, Wu J, et al. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013; 149: 1180–1185. Errata in: JAMA Dermatol 2014; 150: 103,; JAMA Dermatol. 2014; 150: 337. [DOI] [PubMed] [Google Scholar]
- 15. van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis survey. J Eur Acad Dermatol Venereol. 2015; 29: 2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garber C, Plotnikova N, Au SC, et al. Biologic and conventional systemic therapies show similar safety and efficacy in elderly and adult patients with moderate to severe psoriasis. J Drugs Dermatol. 2015; 14: 846–852. [PubMed] [Google Scholar]
- 17. Feldman SR, Koo JY, Menter A, Bagel J. Decision points for the initiation of systemic treatment for psoriasis. J Am Acad Dermatol. 2005; 53: 101–107. [DOI] [PubMed] [Google Scholar]
- 18. Kitchen H, Cordingley L, Young H, et al. Patient-reported outcome measures in psoriasis: the good, the bad and the missing! Br J Dermat l. 2015; 172: 1210–1221. [DOI] [PubMed] [Google Scholar]
- 19. Menter A, Korman NJ, Elmets CA, et al; for the American Academy of Dermatology Work Group. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011; 65: 137–174. [DOI] [PubMed] [Google Scholar]
- 20. Schmitt J, Rosumeck S, Thomaschewski G, et al. Efficacy and safety of systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials. Br J Dermatol. 2014; 170: 274–303. [DOI] [PubMed] [Google Scholar]
- 21. Mansouri Y, Goldenberg G. Biologic safety in psoriasis: review of long-term safety data. J Clin Aesthet Dermatol. 2015; 8: 30–42. [PMC free article] [PubMed] [Google Scholar]
- 22. National Psoriasis Foundation. Drug pipeline. http://services.psoriasis.org/drug-pipeline/index.php. Accessed August 24, 2016.
- 23. Armstrong AW, Koning JW, Rowse S, et al. Under-treatment of patients with moderate to severe psoriasis in the United States: a study of medication usage with health-plan data. Poster presented at the National Psoriasis Foundation Research Symposium and National Volunteer Conference; July 24–26, 2015; San Francisco, CA.
- 24. Feldman SR, Zhao Y, Navaratnam P, et al. Patterns of medication utilization and costs associated with the use of etanercept, adalimumab, and ustekinumab in the management of moderate-to-severe psoriasis. J Manag Care Spec Pharm. 2015; 21: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cather JC, Crowley JJ. Use of biologic agents in combination with other therapies for the treatment of psoriasis. Am J Clin Dermatol. 2014; 15: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richards HL, Fortune DG, Griffiths CE. Adherence to treatment in patients with psoriasis. J Eur Acad Dermatol Venereol. 2006; 20: 370–379. [DOI] [PubMed] [Google Scholar]
- 27. Callis Duffin K, Yeung H, Takeshita J, et al. Patient satisfaction with treatments for moderate-to-severe plaque psoriasis in clinical practice. Br J Dermatol. 2014; 170: 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Finch T, Shim TN, Roberts L, Johnson O. Treatment satisfaction among patients with moderate-to-severe psoriasis. J Clin Aesthet Dermatol. 2015; 8: 26–30. [PMC free article] [PubMed] [Google Scholar]
- 29. Schaarschmidt ML, Kromer C, Herr R, et al. Treatment satisfaction of patients with psoriasis. Acta Derm Venereol. 2015; 95: 572–578. [DOI] [PubMed] [Google Scholar]
- 30. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010; 62: 114–135. [DOI] [PubMed] [Google Scholar]
- 31. Zhang M, Brenneman SK, Carter CT, et al. Patient-reported treatment satisfaction and choice of dosing frequency with biologic treatment for moderate to severe plaque psoriasis. Patient Prefer Adherence. 2015; 9: 777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blome C, Simianer S, Purwins S, et al. Time needed for treatment is the major predictor of quality of life in psoriasis. Dermatology. 2010; 221: 154–159. [DOI] [PubMed] [Google Scholar]
- 33. Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014; 70: 871–881.e30. [DOI] [PubMed] [Google Scholar]
- 34. Schaarschmidt ML, Schmieder A, Umar N, et al. Patient preferences for psoriasis treatments: process characteristics can outweigh outcome attributes. Arch Dermatol. 2011; 147: 1285–1294. [DOI] [PubMed] [Google Scholar]
- 35. Dowlatshahi EA, Wakkee M, Arends LR, Nijsten T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014; 134: 1542–1551. [DOI] [PubMed] [Google Scholar]
- 36. Bewley A, Burrage DM, Ersser SJ, et al. Identifying individual psychosocial and adherence support needs in patients with psoriasis: a multinational two-stage qualitative and quantitative study. J Eur Acad Dermatol Venereol. 2014; 28: 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010; 146: 891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paul C, Bushmakin AG, Cappelleri JC, et al. Do patients and physicians agree in their assessment of the severity of psoriasis? Insights from tofacitinib phase 3 clinical trials. J Dermatol Clin Res. 2015; 3: 1048. [Google Scholar]
- 39. Møller AH, Erntoft S, Vinding GR, Jemec GB. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ-5D-derived utility values. Patient Relat Outcome Meas. 2015; 6: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Korte J, Sprangers MA, Mombers FM, Bos JD. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc. 2004; 9: 140–147. [DOI] [PubMed] [Google Scholar]
- 41. Rapp SR, Feldman SR, Exum ML, et al. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999; 41 (3 pt 1):401–407. [DOI] [PubMed] [Google Scholar]
- 42. Lewis V, Finlay AY. 10 years experience of the Dermatology Life Quality Index (DLQI). J Investig Dermatol Symp Proc. 2004; 9: 169–180. [DOI] [PubMed] [Google Scholar]
- 43. Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015; 72: 978–983. [DOI] [PubMed] [Google Scholar]
- 44. Chung J, Callis Duffin K, Takeshita J, et al. Palmoplantar psoriasis is associated with greater impairment of health-related quality of life compared with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2014; 71: 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004; 9: 136–139. [DOI] [PubMed] [Google Scholar]
- 46. Ayala F, Sampogna F, Romano GV, et al; for the Daniele Study Group. The impact of psoriasis on work-related problems: a multicenter cross-sectional survey. J Eur Acad Dermatol Venereol. 2014; 28: 1623–1632. [DOI] [PubMed] [Google Scholar]
- 47. Horn EJ, Fox KM, Patel V, et al. Association of patient-reported psoriasis severity with income and employment. J Am Acad Dermatol. 2007; 57: 963–971. [DOI] [PubMed] [Google Scholar]
- 48. Schmitt J, Küster D. Correlation between Dermatology Life Quality Index (DLQI) scores and Work Limitations Questionnaire (WLQ) allows the calculation of percent work productivity loss in patients with psoriasis. Arch Dermatol Res. 2015; 307: 451–453. [DOI] [PubMed] [Google Scholar]
- 49. Schaefer CP, Cappelleri JC, Cheng R, et al. Health care resource use, productivity, and costs among patients with moderate to severe plaque psoriasis in the United States. J Am Acad Dermatol. 2015; 73: 585–593.e3. [DOI] [PubMed] [Google Scholar]
- 50. Kimball AB, Gieler U, Linder D, et al. Psoriasis: is the impairment to a patient's life cumulative? J Eur Acad Dermatol Venereol. 2010; 24: 989–1004. [DOI] [PubMed] [Google Scholar]
- 51. Warren RB, Kleyn CE, Gulliver WP. Cumulative life course impairment in psoriasis: patient perception of disease-related impairment throughout the life course. Br J Dermatol. 2011; 164 (suppl 1): 1–14. [DOI] [PubMed] [Google Scholar]
- 52. Feldman SR. Treatment of psoriasis. UpToDate. www.uptodate.com/contents/treatment-of-psoriasis#H2. Accessed August 24, 2016.
- 53. Wilson SR, Strub P, Buist AS, et al; for the Better Outcomes of Asthma Treatment (BOAT) Study Group. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010; 181: 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stern D. Benefit design innovations to manage specialty pharmaceuticals. J Manag Care Pharm. 2008; 14 (4 suppl A): S12–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Menter A, Griffiths CE. Current and future management of psoriasis. Lancet. 2007; 370: 272–284. [DOI] [PubMed] [Google Scholar]
- 56. Balkrishnan R, Bhosle MJ, Fleischer AB, Jr, Feldman SR. Prior authorization for topical psoriasis treatments: is it cost-beneficial for managed care? J Dermatolog Treat. 2010; 21: 178–184. [DOI] [PubMed] [Google Scholar]
- 57. Freedman JD, Gottlieb AB, Lizzul PF. Physician performance measurement: tiered networks and dermatology (an opportunity and a challenge). J Am Acad Dermatol. 2011; 64: 1164–1169. [DOI] [PubMed] [Google Scholar]
- 58. American Academy of Dermatology. President-Elect calls on members to come together. Dermatology World Meeting News. March 21, 2014. www.aadmeetingnews.org/2014-annual-meeting-daily/president-elect-calls-on-members-to-come-together/. Accessed August 24, 2016.
- 59. Owens GM. Revolutionizing treatment outcomes in hepatitis C: managed care implications and considerations—the new and evolving standards of care. Am J Manag Care. 2015; 21 (5 suppl): S97–S105. [PubMed] [Google Scholar]
- 60. Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015; 72: 961–967.e5. [DOI] [PubMed] [Google Scholar]
- 61. Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015; 151: 651–658. [DOI] [PubMed] [Google Scholar]
- 62. Howe A, Eyck LT, Dufour R, et al. Treatment patterns and annual drug costs of biologic therapies across indications from the Humana commercial database. J Manag Care Spec Pharm. 2014; 20: 1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. D'Souza LS, Payette MJ. Estimated cost efficacy of systemic treatments that are approved by the US Food and Drug Administration for the treatment of moderate to severe psoriasis. J Am Acad Dermatol. 2015; 72: 589–598. [DOI] [PubMed] [Google Scholar]
- 64. Mustonen A, Mattila K, Leino M, et al. How much of the productivity losses among psoriasis patients are due to psoriasis. BMC Health Serv Res. 2015; 15: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hagland M. Step therapy and biologics: no easy answers. Biotechnol Healthc. 2006; 3: 32–40. [PMC free article] [PubMed] [Google Scholar]
- 66. Cohen M, Morrow T, Penna P. Managing the expanded use of biologics across therapeutic areas: an example from B-cell targeted therapies. Am J Manag Care. 2006; 12 (2 suppl): S24–S37, quiz S38. [PubMed] [Google Scholar]
- 67. Express Scripts. Express Scripts 2015 Drug Trend Report. March 2016. http://lab.express-scripts.com/lab/drug-trend-report. Accessed August 24, 2016.
- 68. CVS Health. Taking aim at trend. Insights. Spring 2015. www.cvshealth.com/sites/default/files/INSIGHTS_Trend_2015.pdf. Accessed August 24, 2016.