Abstract
In 2013, the sugarcane aphid, Melanaphis sacchari (Zehntner) (Hemiptera: Aphididae), a new invasive pest of sorghum species in North America, was confirmed on sorghum in 4 states and 38 counties in the United States. In 2015, the aphid was reported on sorghum in 17 states and over 400 counties as well as all sorghum-producing regions in Mexico. Ability to overwinter on living annual and perennial hosts in southern sorghum-producing areas and wind-aided movement of alate aphids appear to be the main factors in its impressive geographic spread in North America. Morphological characteristics of the sugarcane aphid include dark tarsi, cornicles, and antennae, allowing easy differentiation from other aphids on the crop. Sugarcane aphid damages sorghum by removing sap and covering plants with honeydew, causing general plant decline and yield loss. Honeydew and sooty mold can disrupt harvesting. The aphid’s high reproductive rate on susceptible sorghum hybrids has resulted in reports of yield loss ranging from 10% to greater than 50%. In response, a combination of research-based data and field observations has supported development of state extension identification, scouting, and treatment guides that aid in initiating insecticide applications to prevent yield losses. Highly efficacious insecticides have been identified and when complemented by weekly scouting and use of thresholds, economic loss by sugarcane aphid can be minimized. Some commercial sorghum hybrids are partially resistant to the aphid, and plant breeders have identified other lines with sugarcane aphid resistance. A very diverse community of predators and parasitoids of sugarcane aphid has been identified, and their value to limit sugarcane aphid population growth is under investigation.
Keywords: identification, scouting, insecticide, host plant resistance, biological control
In 2013, a new aphid pest was reported damaging sorghum, Sorghum bicolor (L.) Moench, along the Texas Gulf Coast (Villanueva et al. 2014), and it was later identified as sugarcane aphid, Melanaphis sacchari (Zehntner) (Hemiptera: Aphididae). Sugarcane aphid is found worldwide on many grass genera (Poaeceae). Sugarcane, Saccharum officinarum L., and sorghum are important cultivated hosts, and sugarcane aphid is an economic pest of sorghum in Asia, Africa, Australia, and South America (Singh et al. 2004). In North America, the recent widespread occurrence of sugarcane aphid and economic impact on sorghum have led to significant research, extension, and industry response (Brewer et al. 2016, Villanueva et al. 2014).
The sugarcane aphid was first detected in sorghum along the Texas Gulf Coast and Louisiana in 2013, where abundant populations caused significant sorghum yield losses due to poor plant vigor and head emergence, and abundant honeydew affecting harvest efficiency (Villanueva et al. 2014). Later in 2013, the aphid was also detected in selected parishes and counties in Louisiana, Oklahoma, one county in Mississippi, and three northeastern states of Mexico. By the end of 2015, the aphid was reported on grain sorghum, sorghum–sudan hybrids, sweet sorghum, some millet varieties, and Johnsongrass in 17 states and over 400 counties in the United States and in all sorghum-producing regions in Mexico (Bowling et al. 2015). Sugarcane aphid has been observed on corn and cotton but does not reproduce on these crops. In response, a combination of research-based data and expert opinion has supported development of state identification, scouting, and treatment guides to aid in use of insecticides. Also, sorghum hybrid screening for host plant resistance, and identification and evaluation of the natural enemy community that preys on sugarcane aphid on sorghum have been undertaken. After three annual growing seasons following the aphid’s detection on sorghum in North America, we provide here the current status of its occurrence and damage to sorghum, on-going and prospective management approaches, and notes on potential for more integrated approaches to the aphid’s management.
Occurrence and Damage on Sorghum in the United States and Mexico
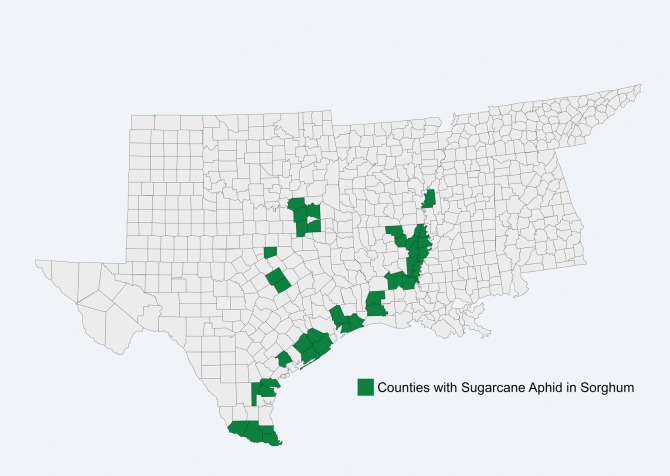
In 2013, infestations by sugarcane aphid were confirmed on sorghum in Liberty County, Texas (M.O.W., personal observation), followed closely by reports from 38 counties in four states in the United States (Fig. 1) as well as three northeastern states of Mexico. While previously known from the United States, its expansion into sorghum was an unexpected and significant event. It was first reported in North America on sugarcane in Florida in 1977 (Mead 1978) and Louisiana in 1999 (White et al. 2001). Denmark (1988) reported sugarcane aphid feeding on sorghum in Florida, but it was not considered an economic pest of this crop. The surprising occurrence of sugarcane aphid on sorghum in 2013 has led to speculation about the source of the aphid, including the possibilities of a host plant shift from sugarcane to sorghum in North America or an existing biotype that prefers sorghum entering North America through wind-aided movement or human activities. The genetic diversity is just beginning to be understood (Nibouche et al. 2015), and its capacity for long-range movement can be inferred by field observations and research on other aphids in North America (Irwin and Thresh 1988).
Fig.1.
Occurrence of sugarcane aphid on sorghum in the United States, 2013.
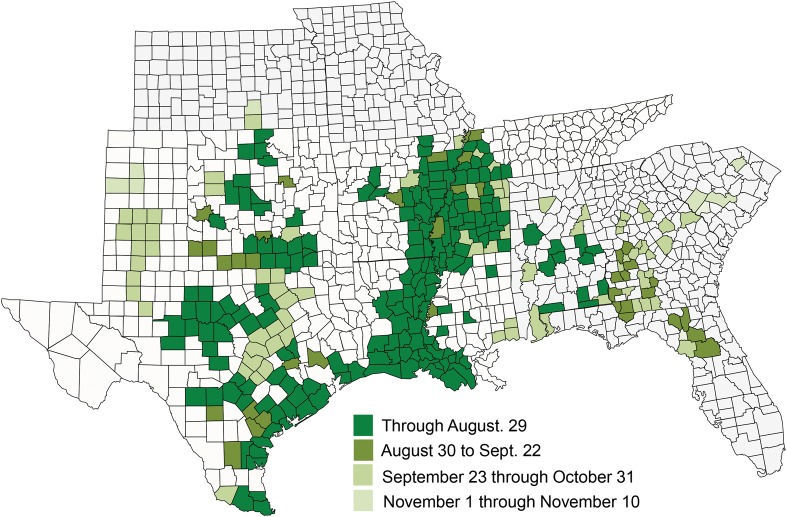
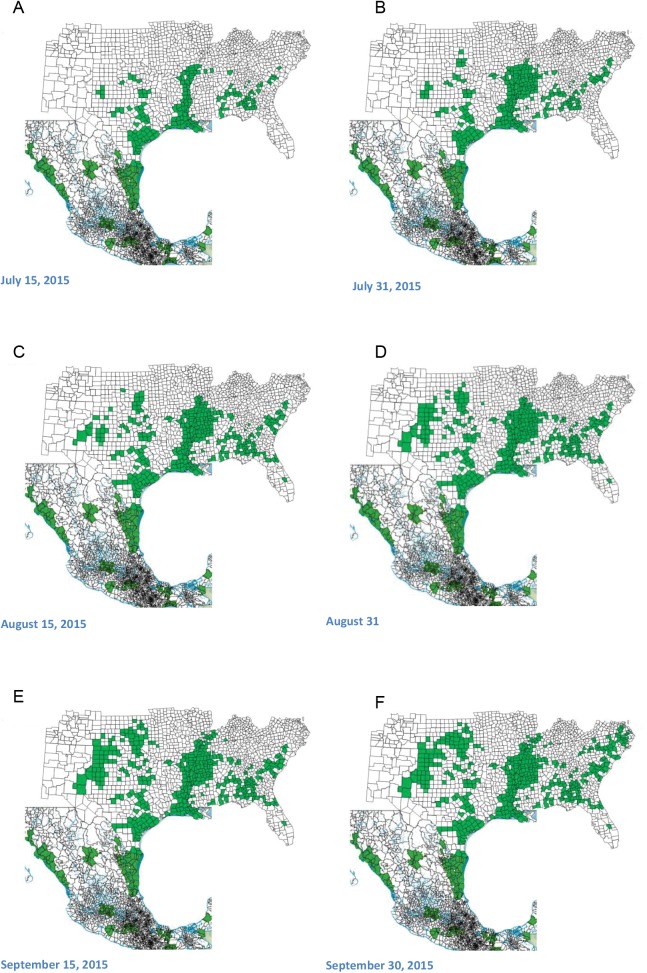
The sugarcane aphid rapidly displayed its ability to spread and put at risk greater than 90% of the sorghum-producing areas of North America. In 2014, the aphid expanded its range to 12 states in the United States and over 300 counties, with a generally later-season occurrence observed in more northern and eastern locations (Fig. 2). As of fall 2015, the sugarcane aphid has been confirmed on sorghum from 17 states in the United States and over 400 counties and all sorghum-producing regions in Mexico (Fig. 3). The most parsimonious explanation for this rapid expansion is through wind-aided movement of alates coming from maturing sorghum in southern production areas of Texas and Mexico. The aphid has been a consistent economic problem on grain sorghum in Mexico, the Texas Gulf Coast, and much of Louisiana since 2013.
Fig. 2.
Seasonal progression of sugarcane aphid on sorghum in the United States (color coded by month), 2014.
Fig. 3.
Bi-weekly occurrence of sugarcane aphid on sorghum in North America from July 15 (Panel A) through September 30 (Panel F), 2015.
Seasonal patterns of detection by the authors and from reporting by extension, research, and industry collaborators (see acknowledgments section) in 2014 and 2015, coupled with the aphid’s biology (see next section), are consistent with this explanation of overwintering survival in southern production regions and wind-aided movement. In late August of 2014, numerous confirmed reports of sugarcane aphid on sorghum from multiple states eclipsed those in 2013. In total, 191 counties from Texas, Oklahoma, Arkansas, Louisiana, Mississippi, Tennessee, and Alabama reported sugarcane aphid on sorghum. In less than a month, in late September, the sugarcane aphid had expanded its range to include Florida and Georgia. Overall in 2014, the aphid expanded its range to include records as far north as Kansas; east to South Carolina, Georgia, and Florida; and west to the Texas High Plains (Fig. 2).
In May of 2015, the sugarcane aphid was reported on sorghum in the Lower Rio Grande Valley, Coastal Bend, and Winter Garden areas of Texas. By mid-July of 2015, sugarcane aphid was reported on sorghum in the southern High Plains of Texas and much of the mid-south and southeastern United States. Confirmations of the aphid on sorghum in the southern High Plains of Texas, Missouri, and South Carolina occurred two months before 2014 reports in the same areas (Figs. 2 and 3). In late August of 2015, the aphid was confirmed at economically harmful levels on sorghum in Kentucky and central and eastern Kansas (Fig. 3). New state records for sugarcane aphid on sorghum in Illinois and Virginia were reported in September (Fig. 3). The latest report for sugarcane aphid in sorghum in 2015 showed a continued expansion north into northeastern Kansas. Overall in 2015, the 17 states in the United States with confirmed sugarcane aphid on sorghum accounted for 97% (7,405,000 acres [2,997,976 hectares]) of the sorghum acres and 98% (15,687,084 tons [14,230,426 metric tons]) of the total sorghum production in the United States (USDA NASS 2016).
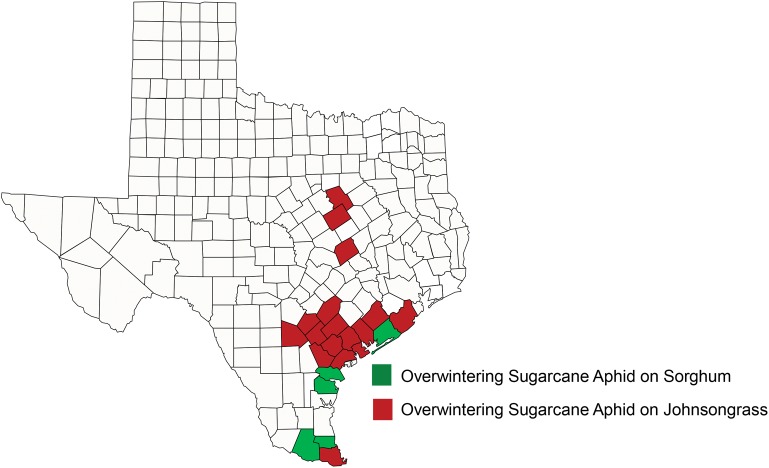
There has been speculation regarding the rapid and widespread geographic expansion of this aphid on sorghum. Is the overwintering capability of the aphid broader than assumed based on its predominant worldwide distribution on sorghum and sugarcane in semi-tropical regions (Singh et al. 2004) or have yearly wind-aided movement and favorable weather been sufficient to support aphid population development resulting in economic damage of sorghum grown in more northern locations of North America? Field observations to date indicate only asexual reproduction of sugarcane aphid in the United States, and overwintering dependent upon nymphs and adults surviving on remnant and ratoon sorghum and perennial grass hosts. Populations during the winter are readily found on remnant sorghum and Johnsongrass, Sorghum halepense (L.) Pers., along the Texas Gulf Coast and southern Louisiana, and ratoon sorghum grown for production in the Rio Grande Valley of Texas and into Mexico. In more northern locations, overwintering populations of sugarcane aphid were observed on Johnsongrass in northcentral Texas in January and February of 2015, despite deterioration of much of the visible vegetation by periodic freezing temperatures (Fig. 4). No living remnant sorghum was observed in these areas in January and February. The aphid as a permanent resident in Mexico through the Texas Gulf Coast appears assured due to typical mild winters, while survival in temperate northern sorghum-producing regions in the United States may be possible depending on the growth of winter host plants. The occurrence of sugarcane aphid in Florida and Puerto Rico likely adds to known overwintering sites in Texas, Louisiana, and Mexico.
Fig. 4.
Texas counties with overwintering sugarcane aphid on sorghum and Johnsongrass, 2014.
Identification and Biology
The sugarcane aphid body color ranges from gray to tan to light yellow. Morphological characteristics unique to sugarcane aphid include dark cornicles, tarsi, and antennae (Villanueva et al. 2014; Fig. 5). The gray cast body color is typically observed during cool conditions common in the winter and spring. Alate (winged) sugarcane aphids look like large apterous (non-winged) aphids, except they frequently possess black markings along the dorsal sclerites (Fig. 5; Eastop 1955, Blackman and Eastop 1984) and always possess black hardened structures at the base of the wings. Other common aphid species on sorghum in the United States include the corn leaf aphid, Rhopalosiphum maidis (Fitch); greenbug, Schizaphis graminum (Rhondani); and yellow sugarcane aphid, Sipha flava (Forbes). These aphids are readily distinguished from sugarcane aphid as nymphs and adults, whether alate or apterous. The aid of 10 power magnification may be needed to distinguish small nymphs. The corn leaf aphid (Fig. 6A) exhibits a bluish-green coloration, black legs, antennae, and cornicles. The greenbug is a light-green aphid with a dark-green stripe down its back, dark tarsi, light-colored cornicles with black tips, and dark antennae (Royer et al. 2015; Fig. 6B). The yellow sugarcane aphid is bright yellow with numerous hairs covering its body and light-colored antennae, and legs, and cornicles are very short and not detectable with the naked eye (Fig. 6C).
Fig. 5.
Sugarcane aphid on sorghum. Morphological characteristics of sugarcane aphid include dark tarsi (feet), cornicles (tailpipes), and antennae. Apterous sugarcane aphid color may vary from tan to light yellow. A mix of sugarcane aphid color morphs are most common in overwintering populations (A). Summer populations are typically lighter morphs. Winged aphids can have black markings on the dorsal sclerites (B).
Fig. 6.
Other aphids occurring on sorghum include: the corn leaf aphid, Rhopalosiphum maidis (Fitch), with bluish-green coloration, black legs, antennae, and cornicles (A); greenbug, Schizaphis graminum (Rhondani), with a commonly visible dark green stripe on the dorsum of the abdomen (B); and yellow sugarcane aphid, Sipha flava (Forbes), with uniform body coloration, very short cornicles, and many fine hairs covering its body (C). Ten power magnification is needed to observe these features.
The sugarcane aphid is an anholocyclic, parthenogenic, viviparous species, which means that it feeds on its annual hosts (sorghum species) only in the spring and summer, and the same hosts that persist through the fall and winter months (Johnsongrass but also remnant sorghum). All aphids are female and produce live young asexually in North America, with the exception of one report of egg production from female aphids collected from three Mexican states (Peña-Martinez et al. 2016). Sexual forms have previously been reported on sorghum in China, India, and Japan (Wang 1961, Yadava 1966, Setokuchi 1975, David and Sandhu 1976). The aphid has four nymphal stadia (non-winged nymphs). It takes about 4–12 d for development from birth to adult, depending on temperature (Chang et al. 1982). Adult longevity ranges from 10–37 d (Chang et al. 1982, Singh et al. 2004), may be apterous or alate, with a reproductive potential ranging from 34 to 96 nymphs per female depending on temperature and nutrition (Chang et al. 1982, Singh et al. 2004).
Population increase is influenced by temperature and rainfall pattern (Chang et al. 1982). Sugarcane aphid population growth increases rapidly during warm, dry climatic conditions (Singh et al. 2004), which are common in much of the sorghum-producing regions of North America. Local and long-distance dispersal of alate sugarcane aphids is likely greatly influenced by wind-aided movement. This wind-aided movement distributes aphids locally and over long distances, as inferred by field observations of large concentrated areas of alates (R.B. and M.J.B., personal observation) and as supported by known aphid movement by other species (Irwin and Thresh 1988). Wind-aided movement is especially relevant in North America, where the aphid’s hosts are not available during the winter months. Alates dispersing by wind are typically found on the underside of upper canopy leaves when they first infest a sorghum field (Wallin and Loonan 1971, Irwin and Thresh 1988, M.J.B. and R.B. personal observation). Once established, a colony can undergo exponential growth during sorghum plant development, with populations reaching as many as 30,000 aphids on a single plant (Singh et al. 2004). In south Texas, populations exceeding 10,000 aphids per plant have been observed in commercial grain sorghum-producing fields (M.J.B., personal observation). At such high densities, host plant quality rapidly declines and natural enemies may become very common (R.B. and M.J.B., personal observation). Poor plant quality associated with high aphid populations and dry windy weather can induce significant alate production and wind-aided dispersal (Singh et al. 2004).
Damage to Sorghum
Damage by sugarcane aphid on sorghum produced for grain and forage occurs in several ways, depending on the stage of plant development when aphids infest the crop, aphid population increase, and use of the crop. Sugarcane aphids remove plant sap when feeding on the underside of sorghum leaves and along the stalk. To date, there has been no indication of plant pathogen transmission. Based on field observations and the literature, plant damage from sugarcane aphid results from a combination of direct loss of plant nutrients and sugars during feeding, which can be exacerbated by plant water stress, and reduction in photosynthetic efficiency due to sooty mold buildup from honeydew excreted by aphids (Singh et al. 2004, R.B. and M.J.B. personal observation). Symptoms of aphid damage include purpling of young plants, which can lead to stunting, chlorosis, and necrosis of maturing leaves (Singh et al. 2004). Often during initial field infestation and populations increase, the foliage remains green despite readily detectable aphids on the underside of leaves. As aphid population increase and feeding injury intensifies, leaves change color to yellow, purple, and, finally, brown as leaf health declines (Fig. 7).
Fig. 7.
Sorghum damaged by sugarcane aphid feeding (foreground). Plant damage symptoms associated with sugarcane aphid feeing include leaf discoloration (yellow, purple, and, eventually, necrosis), reduced panicle formation, and delayed development (compare sugarcane aphid damaged sorghum in foreground with aphid free sorghum in the background). Severe plant damage by sugarcane aphid resulted in poor head emergence and no pollination (image on right).
Excessive aphid infestations on pre-flowering sorghum and infestations present during grain development can reduce yield through reduction in number of heads, reduced seed weight, delayed development and maturity, and plant death (Fig. 7). Additional losses in grain sorghum are possible at harvest as a consequence of reduction in harvest efficiency (Kerns et al. 2015). Such yield loss can be attributed to “sticky” upper leaves and heads resulting from excess honeydew as they pass through the combine. Consequently, material may build up in the separator, leading to as much as a 50% reduction in the recovery of the grain during harvest and delay in harvest. In sorghum grown for forage or hay, the buildup of honeydew can lead to mechanical issues during cutting and baling, as well as decreased quality due to mold, and additional time required for proper drying down and curing of the cut foliage.
Field experiments (2014 and 2015) manipulating aphid densities between 50 and 500 per leaf with an insecticide on pre-flowering sorghum, as well as estimates taken from commercial fields infested with sugarcane aphid, indicated that yield losses in grain sorghum ranged from 100 to 400 pounds per acre (45 to 181 kg per hectare). The range of yield loss appeared dependent on environment and production approach (e.g., warm and dry weather) is conducive to high population increase and dryland systems are more susceptible to stress factors (Singh et al. 2004), and hybrids grown (e.g., current commercial hybrids vary in aphid susceptibility [see section below]). At current U.S. grain prices, a monetary loss of between $25 and $175 per acre ($62 and $432 per hectare) may occur as a result of large aphid infestations exceeding 250 aphids per leaf in warm dry growing conditions (Fig. 8B). Smaller populations of aphids, such as those at average densities below 50 aphids per leaf (Fig. 8A), do not appear to justify insecticide use at current grain value, insecticide costs, and reduction of grain resulting from the infestation. However, the potential for rapid population increase especially in warm and dry conditions warrants regular scouting (see next section).
Fig. 8.
Grain sorghum injured by sugarcane aphid. Grain sorghum infested with an average of 50 (A), 250 (B), and 500 (C) aphids per leaf. Panel B illustrates 250 sugarcane aphids per leaf with visible signs of plant damage and honeydew buildup caused by its feeding activity. Plant injury, yield reduction, and head deformation are visible when sugarcane aphid populations build at or above 500 per leaf (Panel C).
Populations of aphids increasing after flowering are less likely to directly affect grain set, but grain quality and harvest efficiency may be substantially affected. Inspection for aphids in the heads and associated honeydew is also critical when green leaf tissue is drying down through natural chemical means prompting movement by aphids to the head. Sugarcane aphid has also been a pest in sorghums used for fresh forage and hay, but formal considerations for controlling aphids on forages have been challenging because forage sorghum has a dense canopy and tall plant stature that makes penetration of insecticides difficult (Knutson et al. 2016).
Sampling and Management With Insecticides
With the potential for losses caused by sugarcane aphid, management with insecticide is justified to prevent economic loss, as experienced in past aphid invasions affecting sorghum in North America. Insecticidal seed treatments are very useful as a first line of defense and may delay or negate a foliar spray especially in double-crop or late-planted sorghum. Insecticide-treated seed available for grain sorghum may provide about three weeks to a month of insect protection (Jones et al. 2015), but this approach has limitations. Insecticide-treated seed is not in common practice for forage sorghums, and the period of concern for aphid infestations and plant damage of grain sorghums extends considerably past this window of protection. Sugarcane aphid infestations can occur quickly in both grain and forage sorghums, from locally overwintering aphids and winged aphids blown by the wind landing on the crop. The first steps to management are proper identification (see above) and scouting. Balancing needs to inspect many fields for first arrival of aphids and evaluating economic risk in fields with established infestations, sampling protocols can be separated into a more rapid first detection procedure (early detection of an infested field) and a more thorough evaluation of plant damage risk (scouting infested fields) once an infestation is detected.
Early Detection of an Infested Field
Initial sampling protocols (Bowling et al. 2016) have been developed based on field observations and early sampling experiments conducted in south Texas, Arkansas, Oklahoma, Louisiana, and Kansas. To first detect aphids, check foliage (and heads if present) along field edges up to 25 feet (7.6 m) into the field, and continue inspections until an infestation is detected or the seed head begins to harden. At each sampling site, examine 15–20 plants as you walk down 50 feet (15 m) of row. Inspect undersides of leaves from both the upper and lower canopy, particularly to areas that appear to have honeydew (the shiny sugary aphid excretion). Honeydew detection should be used as a supplement only to checking the leaves for aphid presence, because lack of honeydew does not guarantee absence of aphids, especially aphids increasing in the lower canopy and newly arrived winged aphids. Focus on field edges where wind direction is toward the field and where unmanaged lands are adjacent to the field because of overwintering potential and wind-aided movement of aphids. Simply note at the site if any aphids were detected and whether they were newly arrived winged aphids or established colonies. If no aphids are detected, repeat the procedure at other field locations, depending on field size and variability. If aphids are detected, declare the field infested and begin a more detailed evaluation to determine if an insecticide is needed. The authors here have found that this relatively quick detection protocol can be completed in less than 5 min at each sampling site. The importance of efficient first aphid detection should be emphasized especially when many fields need to be inspected during the period of possible aphid movement into the fields.
Scouting Infested Fields
Once a field is classified as infested, evaluate aphid risk twice weekly to determine if and when an insecticide is needed. Under optimal conditions, sugarcane aphid infestations can increase very quickly, going from as few as 50 per leaf to 500–600 per leaf over a 7-d period. Research has demonstrated that delaying an insecticide application for 5 d targeting a sugarcane aphid infestation averaging 800 sugarcane aphids per leaf can result in a 75% reduction in yield (Kerns unpublished data). Thus, timeliness of insecticide applications targeting high aphid population is critical. Economic thresholds based on experimental data and field observations have been proposed in extension materials in North America, currently taking the form of either an estimate of aphids per leaf or percent of plants infested with established colonies (Brown et al. 2015, Catchot et al. 2015, Seiter et al. 2015, Knutson et al. 2016). One protocol to obtain a quick estimate of aphid populations is based on an average of visual observations of the upper leaf (leaf below flag leaf) and lowest green leaf taken from experiments conducted in south Texas, Louisiana, Arkansas, Oklahoma, and Georgia. In the research from south Texas, visually categorizing aphid populations has been sufficient and greatly reduces the time spent sampling (Fig. 8, Bowling et al. 2016). Further reductions in sampling time while maintaining good estimation may be possible, based on past efforts in sampling for other aphids infesting North American grains (Elliott et al. 2004).
Insecticide Control
Insecticides labeled against Hemiptera on sorghum prior to the sugarcane aphid invasion were inconsistent in their performance against the sugarcane aphid. More hemipteran-specific insecticides were tested for their efficacy against sugarcane aphid on sorghum within one year of its invasion in North America. Based on this and other work, two products have become available for use to control sugarcane aphid in the United States. Much of the supporting data have been acquired from 2013 to 2015 and are available from various Web sources (Gordy et al. 2015, Buntin and Roberts 2016, Seiter 2016).
Transform (Dow AgroSciences, Indianapolis, IN, 50% sulfoxaflor), an Insecticide Resistance Action Committee (IRAC) sub-group 4C insecticide (sulfoximines), received a Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) Section 18 Emergency Exemption in most southern states specifically for control of sugarcane aphid on sorghum in 2014, 2015, and 2016. In 2015, Sivanto Prime (Bayer CropScience, Leverkusen, Germany, 17.09% flupyradifurone), a IRAC sub-group 4D insecticide (butenolides), received U.S. Environmental Protection Agency approval for a FIFRA Section 3 federal registration. Both these insecticides provide very high mortality of sugarcane aphid (>98%), with minimum activity of 7–10 d, and absence of economic populations of sugarcane aphid up to 21 d after application. Insecticides that have specificity on target pests and with minimal impact on beneficial arthropods can be especially advantageous in suppressing outbreaks of secondary pests or preventing resurgence of the target pest. Sulfoxaflor and flupyradifurone have low toxicity to aphid-specific natural enemies, making these products a good fit for sugarcane aphid management (Michaud et al 2016). Flupyradifurone has the added benefit of low toxicity to pollinators such as honeybees, although sorghum is not pollinated by insects. In Mexico, foliar-applied imidacloprid, an IRAC sub-group 4A insecticide (neonicotinoids), is available for use in grain sorghum and has demonstrated effectiveness in management of sugarcane aphid. Other products have selective places for use on sorghum in the United States, particularly when other pests co-occur with sugarcane aphid.
Biological Control
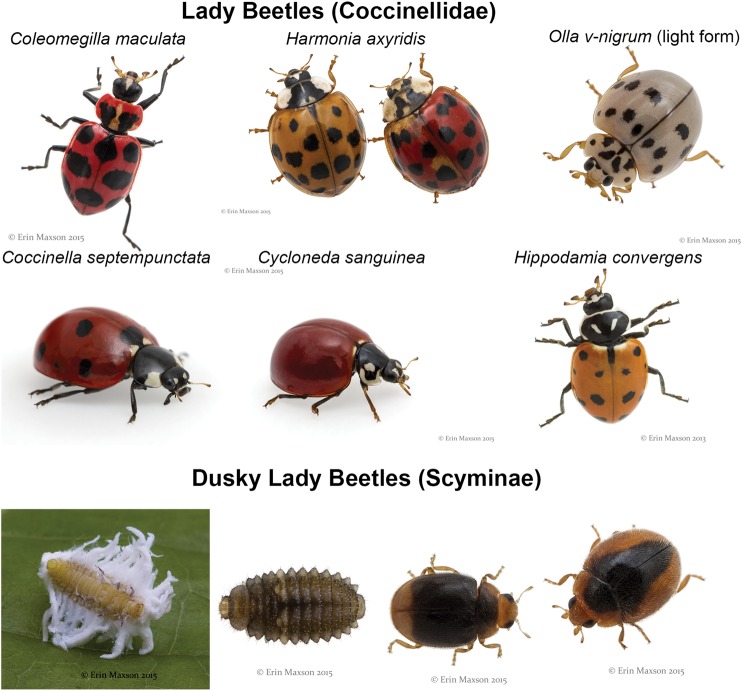
Natural enemies may be detected during sampling for sugarcane aphid or soon after the aphid’s first detection on sorghum. Compared with field observations in 2014, the natural enemy complex was more abundant and more diverse in 2015 (E.M. and M.J.B., personal observation). A natural enemy species census was conducted in sorghum where sugarcane aphid was detected in south (Nueces County) and central (Burleson County) Texas in 2015. The natural enemy communities in both areas had nearly identical species composition. Nine species of lady beetle (Coleoptera, Coccinellidae) were observed: Coccinella septempunctata L., Coleomegilla maculata (Degeer), Cycloneda sanguinea Casey, Harmonia axyridis Pallas, Hippodamia convergens Guérin-Méneville, Olla v-nigrum (Mulsant), and three morphospecies of dusky lady beetles (Coccinellidae: Scymninae) (Fig. 9). Brown lacewings (Neuroptera: Hemerobiidae) of the genus Hemerobius were present, as were the green lacewings (Neuroptera: Chrysopidae) Ceraeochrysa valida (Banks), Chrysopa quadripunctata Burmeister, Chrysoperla externa (Hagen), Chrysoperla rufilabris Burmeister, and Chrysoperla plorabunda (Fitch) (Fig. 10A). Hoverflies (Diptera: Syrphidae) detected included Allograpta obliqua (Say), Pseudodoros clavatus (Fabricius), and Eupeodes americanus (Wiedemann) (Syrphidae: Syrphini) (Fig. 10B). The minute pirate bug, Orius insidiosus (Say) (Hemiptera: Anthocoridae), was observed in trace numbers.
Fig. 9.
At least nine species of lady beetle (Coleoptera: Coccinellidae) were observed feeding on sugarcane aphid in south and central Texas, 2015.
Fig 10.
Other predators of sugarcane aphid observed in south and central Texas in 2015 include brown lacewings (Neuroptera: Hemerobiidae), green lacewings (Neuroptera: Chrysopidae) (A), and hoverflies (Diptera: Syrphidae) (B).
The predominant primary parasitoid reared from sugarcane aphid mummies was Aphelinus sp. varipes group (Hymenoptera: Aphelinidae) (Fig. 11). The mummy is elongate and streamlined, shiny to dull black with a bluish-hue in coloration. It is readily distinguishable from live aphids (Fig. 11). A hyperparasitoid of the aphelinid, Syrphophagus aphidivorus (Mayr) (Hymenoptera: Encyrtidae), was also detected (Fig. 11). Lysiphlebus testaceipes (Cresson) (Hymenoptera: Braconidae), reared from a few sugarcane aphid mummies that were tan in color and bulbous in shape, was rarely encountered.
Fig. 11.
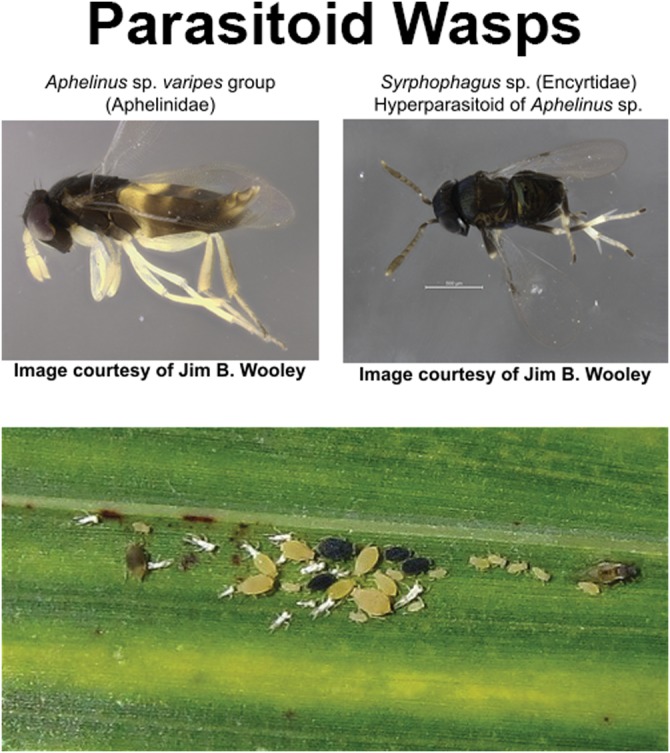
The predominant primary parasitoid reared from aphid mummies in south and central Texas in 2015 was Aphelinus sp. varipes group (Hymenoptera: Aphelinidae). A hyperparasitoid of the aphelinid, Syrphophagus aphidivorus (Hymenoptera: Encyrtidae), was also detected. The Aphelinus parasitoid mummies are shiny to dull black with a bluish-hue and elongate, making them readily distinguished from live aphids (bottom image).
All aphidophagous predator species were present on aphid-infested sorghum in both juvenile and adult life stages, suggesting that these natural enemies are successfully utilizing sugarcane aphid as a prey item and reproducing representative immatures of most species encountered. In experimental work by Colares et al. (2015), four of these species detected in the south and central Texas census (Coleomegilla maculata, Hippodamia convergens, Chrysoperla carnea (Stephens), and Orius insidiosus) were reared successfully on sugarcane aphid and on the well-established greenbug (Schizaphis graminum). Colleagues in other states also report a similar range of aphidophagous taxa in sugarcane aphid-infested fields (D.B., D.L.K., and N.S., personal observation), again suggesting adaptation of established aphidophagous species to sugarcane aphid invading commercial sorghum production in North America.
Despite the diversity of natural enemies encountered, field observations have shown that aphids are capable of regularly increasing above economically damaging populations even in the presence of natural enemies occurring in sorghum. They may become a more significant mortality factor for the aphid as they adapt to the new prey item (Colares et al. 2015), and more sorghum hybrids with at least partial aphid resistance are grown.
Host Plant Resistance
Genetic resistance in field crops to insect pests is an attractive aphid pest management tactic because of its ease of use and potential affordability and compatibility with natural enemies (Brewer and Elliott 2004). There has been substantial use of sorghum hybrids with traits resistant to greenbug in North America (Michels and Burd 2007). Research has begun to evaluate parental lines and commercially available hybrids for resistance to sugarcane aphid in North America, adding to existing international efforts (Singh et al. 2004). Most recently, sorghum parental types SC110 and SC170, Tx2783, and Texas A&M sorghum lines and hybrids Tx2783, Tx3408, Tx3409, B11070, B11070, AB11055-WF1-CS1/RTx436, and AB11055-WF1-CS1/RTx437 have shown high levels of resistance to sugarcane aphid in greenhouse and field tests (Armstrong et al. 2015, Mbulwe et al. 2015). The parental lines may provide traits necessary to breed widely adapted commercial sorghum hybrids with resistance to sugarcane aphid. Also, field observations in 2015 have shown abundance of natural enemies adjusted by aphid density (i.e., natural enemies per 100 aphids) to be similar on hybrids resistant and susceptible to sugarcane aphid (E.M. and M.J.B., personal observation). We anticipate that current commercial hybrids with partial resistance to sugarcane aphid will provide a bridge to improved hybrids, and information on commercial hybrid resistant to sugarcane aphid has been distributed (Anonymous 2016, Brown and Kerns 2016).
Further breeding work will add resistance sources to those identified during the early phase of sugarcane aphid invasion in North America. Work on characterization of resistance mechanisms will provide base data to anticipate trait durability, compatibility with other control strategies, best means to deploy resistance, and adjusting economic thresholds.
In summary, the sugarcane aphid is a new invasive pest of sorghum in the United States. In 2013, it was isolated in a small number of counties, primarily in Texas and Louisiana. In 2015, the aphid expanded its range, threatening over 90% of sorghum production in North America. Key morphological characteristics of the sugarcane aphid include dark cornicles (tailpipes), dark feet (tarsi), and dark antennae. These characteristics may be used to distinguish sugarcane aphid from other aphids occurring on sorghum. Sugarcane aphid damages sorghum by removing sap from plants, which directly reduces yield, and the accumulation of honeydew on leaves and in the panicle interferes with harvest operations, which can further reduce yield.
Revising sorghum pest management to include the sugarcane aphid is underway in order to maintain a sustainable state of sorghum production in North America. A combination of expert opinion and replicated research conducted within two years of its invasion on sorghum has led to field identification and sampling protocols, information on insecticide efficacy and guidance for insecticidal use, and initial information on host plant resistance and natural enemies.
Acknowledgments
Photos appearing in this publication were provided by Gary Odvody and Jason Thomas (Fig. 5), Jason Thomas (Fig. 6), Robert Bowling (Fig. 7), John Gordy (Fig. 8), and Erin Maxson (Figs. 9–11). We thank members of the Sugarcane Aphid Task Force who posted seasonal detections of sugarcane aphid on sorghum in 2014 and 2015. We thank the Texas Grain Sorghum Board, United Sorghum Checkoff Program, and the USDA-National Institute of Food and Agriculture (Southern Region IPM Program and Crop Protection and Pest Management Program) for funding research and extension activities discussed in this paper.
References Cited
- Anonymous. 2016. Defense against the sugarcane aphid. United Sorghum Checkoff Program (http://www.sorghumcheckoff.com/newsroom/2016/03/28/sugarcane-aphid/).
- Armstrong J. S., Rooney W. L., Peterson G. C., Villanueva R. T., Brewer M. J., Sekula-Ortiz D. 2015. Sugarcane aphid (Hemiptera: Aphididae): host range and sorghum resistance including cross-resistance from greenbug sources. Journal of Economic Entomolology 108: 576–582. [DOI] [PubMed] [Google Scholar]
- Blackman R. L., Eastop V. F. 1984. Aphids on the world’s crops: an identification and information guide. Wiley, Hoboken, NJ. [Google Scholar]
- Bowling R., Brewer M. J., Biles S., Gordy J. 2015. 2015 occurrence of sugarcane aphid in the United States and Mexico with reference to occurrence in 2013 and 2014. Texas Plant Protection Conference, Bryan, TX. (http://ccag.tamu.edu/sorghum-insect-pests/).
- Bowling R., Brewer M. J., Knutson A., Biles S., Way M. O., Sekula-Ortiz D. 2016. Scouting sugarcane aphids in South, Central, and West Texas. Texas A&M Agrilife Extension. NTO-043, College Station, TX, 2 p. (http://ccag.tamu.edu/sorghum-insect-pests/). [Google Scholar]
- Brewer M. J., Elliott N. C. 2004. Biological control of cereal aphids and mediating effects of host plant and habitat manipulations. Annual Review of Entomology 49: 219–242. [DOI] [PubMed] [Google Scholar]
- Brewer M. J., Bowling R., Michaud J. P., Jacobson A. L. 2016. Sugarcane aphid: a new sorghum pest in North America, 2 pp. ENTO-056. Texas A&M Agrilife Extension Service, College Station, TX.
- Brown S., Kerns D. L., Beuzelin J. 2015. Sugarcane aphids: an emerging pest of grain sorghum, Pub. 3369. Louisiana State University Agricultural Center, Baton Rouge, LA: (http://www.lsuagcenter.com/topics/crops/sorghum/insects/sugarcane-aphids. [Google Scholar]
- Brown S., Kerns D. L. 2016. Sorghum hybrids that offer some protection from sugarcane aphid with expected availability in 2016, Pub. 3523. Louisiana State University Agricultural Center, Baton Rouge, LA: (http://www.lsuagcenter.com/∼/media/system/e/9/6/5/e9654cbfc316a1eccb7d7181fcae0a94/pub%203523%20sorghum%20hybrids_final.pdf.) [Google Scholar]
- Buntin G. D., Roberts P. M. 2016. Insecticide control of sugarcane aphid on grain sorghum, 2014. Arthropod Management Tests 41: tsw003. doi: http://dx.doi.org/10.1093/amt/tsw003. [Google Scholar]
- Catchot A., Gore J., Cook D. 2015. Management guidelines for sugarcane aphids in MS grain sorghum. Mississippi State University Extension, Starkville, MS: (http://www.mississippi-crops.com/category/by-crop/grain-sorghum-crops/page/5/). [Google Scholar]
- Chang C. P., Fang M. N., Tseng H. Y. 1982. Studies on the life history and varietal resistance in grain sorghum aphid, Melanaphis sacchari Zehntner in central Taiwan. Chinese Journal of Entomology. 2: 70–81. [Google Scholar]
- Colares F., Michaud J. P., Bain C. L., Torres J. B. 2015. Indigenous aphid predators show high levels of preadaptation to a novel prey, Melanaphis sacchari (Hemiptera: Aphididae). Journal of Economic Entomology 108: 2546–2555. doi: 10.1093/jee/tov235. [DOI] [PubMed] [Google Scholar]
- David S. K., Sandhu G. S. 1976. New oviparous morph of Melanaphis sacchari (Zehntner) on sorghum. Entomologist's Record 88: 28–29. [Google Scholar]
- Denmark H. A. 1988. Sugarcane aphids in Florida. Florida Department of Agriculture and Consumer Services, Division of Plant Industry. Entomology Circular 302.
- Eastop V. F. 1955. Notes on East African aphids. VI. Cereal and grass root feeding species. East African Agricultural and Forestry Journal 20: 209–212. [Google Scholar]
- Elliott N. C., Royer T. A., Giles K. L., Kindler S. D., Porter D. R., Elliott D. T., Waits D. A. 2004. A web-based decision support system for managing greenbugs in wheat. Crop Management 3. doi:10.1094/CM-2004-1006-01-MG. [Google Scholar]
- Gordy J., Biles S., Bowling R., Brewer M. J., Way M. O. 2015. Sugarcane aphid plant injury, residual, and yield response following insecticide applications in three Texas studies. Texas Plant Protection Conference, Bryan, TX. (http://ccag.tamu.edu/sorghum-insect-pests/).
- Irwin M. E., Thresh J. M. 1988. Long-range aerial dispersal of cereal aphids as virus vectors in North America. Philosophical Transactions of the Royal Society of London B: Biological Sciences 321: 421–446. [Google Scholar]
- Jones N., Brown S., Williams S., Emfinger K., Kerns D. 2015. Efficacy of neonicotinoid seed treatments against sugarcane aphid in grain sorghum, 2014. Arthropod Management Tests 40. doi:http://dx.doi.org/10.1093/amt/tsv139. [Google Scholar]
- Kerns D. L., Brown S., Beuzelin J., Guidry K. M. 2015. Sugarcane aphid: a new invasive pest of sorghum. Louisiana Agriculture 58: 12–14. [Google Scholar]
- Knutson A., Bowling R., Brewer M. J., Bynum E., Porter P. 2016. The sugarcane aphid: management guidelines for grain and forage sorghum in Texas. Texas A&M AgriLife Extension and Research, Texas A&M University: (http://ccag.tamu.edu/sorghum-insect-pests/). [Google Scholar]
- Mbulwe L., Peterson G. C., Armstrong J. S., Rooney W. L. 2015. Registration of sorghum germplasm Tx3408 and Tx3409 with tolerance to sugarcane aphid [Melanaphis sacchari (Zehntner)]. Journal of Plant Registrations 10: 51–56. doi:10.3198/jpr2015.04.0025crg. [Google Scholar]
- Mead F. W. 1978. Sugarcane aphid, Melanaphis sacchari (Zehntner)—Florida—new continental United States record. Cooperative Plant Pest Report 3: 475. [Google Scholar]
- Michaud J. P., Whitworth R. J., Schwarting H., McCornack B., Zukoff S. 2016. Sorghum insect management. Kansas State University Research and Extension. MF742, Manhattan, KS, 12 p. (http://entomology.k-state.edu/extension/publications/). [Google Scholar]
- Michels G. J., Jr, Burd J. D. 2007. IPM Case Studies: Sorghum 627-38. In van Emden H. F., Harrington R. (eds.), Aphids as crop pests. CAB International, Wallingford, Oxfordshire, United Kingdom. [Google Scholar]
- Nibouche S., Mississippi S., Fartek B., Delatte H., Reynaud B., Costet L. 2015. Host plant specialization in the sugarcane aphid Melanaphis sacchari. PLoS ONE 10: e0143704. doi: 10.1371/journal.pone.0143704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Martinez R., Muñoz-Viveros A. L., Bujanos-Muñiz R., Luevano-Borroel J., Tamayo-Mejia F., Cotez-Mondaca E. 2016. Sexual forms of sorghum aphid complex Melanaphis sacchari/sorghi in Mexico. Southwestern Entomologist 41: 127–131. [Google Scholar]
- Royer T. A., Pendleton B. B., Elliott N. C., Giles K. L. 2015. Greenbug (Hemiptera: Aphididae) biology, ecology, and management in wheat and sorghum. Journal of Integrated Pest Management 6: 19. doi:http://dx.doi.org/10.1093/jipm/pmv018. [Google Scholar]
- Seiter N. 2016. Sugarcane aphid in 2016: make your game plan now. Arkansas Row Crops; (http://www.arkansas-crops.com/2016/05/12/sugarcane-aphid-game/). [Google Scholar]
- Seiter N., Lorenz G., Studebaker G., Kelley J. 2015. Sugarcane aphid, a new pest of grain sorghum in Arkansas. University of Arkansas Cooperative Extension, Fayetteville, AR: (https://www.uaex.edu/publications/FSA-7087.pdf). [Google Scholar]
- Setokuchi O. 1975. The hibernation of Longiunguis sacchari (Zehentner) on sorghums. Japanese Society of Applied Entomology and Zoology 19: 296–297. [Google Scholar]
- Singh B. U., Padmaja P. G., Seetharama N. 2004. Biology and management of the sugarcane aphid, Melanaphis sacchari (Zehntner) (Homoptera: Aphididae), in sorghum: a review. Crop Protection 23: 739–755. [Google Scholar]
- United States Department of Agriculture, National Agricultural Statistics Service (USDA NASS). 2016. Crop production, 2015 summary, Cr Pr 2-1, 99 p. (http://www.usda.gov/nass/PUBS/TODAYRPT/cropan16.pdf).
- Villanueva R. T., Brewer M. J., Way M. O., Biles S., Sekula D., Bynum E., Swart J., Crumley C., Knutson A., Porter P., et al. 2014. Sugarcane aphid: a new pest of sorghum. Texas A&M Agrilife Extension. Ento-035, College Station, TX: 4 p. (http://ccag.tamu.edu/sorghum-insect-pests/). [Google Scholar]
- Wallin J. R., Loonan D. V. 1971. Low-level jet winds, aphid vectors, local weather, and barley yellow dwarf virus outbreaks. Phytopathology 61: 1068–1070. [Google Scholar]
- Wang Y. S. 1961. Studies on the sorghum aphid, Aphis sacchari Zehntner. Acta Entomologica Sinica 10: 363–380. [Google Scholar]
- White W. H., Regan T. E., Hall D. G. 2001. Melanaphis sachari (Homoptera: Aphididae), A sugarcane pest new to Louisiana. Florida Entomologist 84: 435–436. [Google Scholar]
- Yadava R. L. 1966. Oviparity in sugarcane aphid Longiunguis sacchari Zehnt (Aphididae: Homoptera). Current Science 1: 18. [Google Scholar]