Abstract
Arterial dissections are among the most frequent causes of stroke in young adults. Usually they are associated with trauma, but as the modern imaging tools are evolving, more dissections are being diagnosed and more etiologies are being described. Vertebral artery dissections (VADs) have the distinct particularity that they can cause ischemic stroke (in the brainstem, cerebellum or even the spinal cord), but also subarachnoid hemorrhage, when the dissection occurs in the intracranial segment of the vertebral artery. We present a review of the literature, going over etiology, clinical aspects, diagnosis and treatment of VADs and we illustrate the theory with three different types of VAD from our clinical experience.
Keywords:vertebral artery dissection, ischemic stroke, subarachnoid hemorrhage, doppler ultrasound
INTRODUCTION
The vertebral artery has its origin in the subclavian artery and is divided into four segments: V0 (the origin), V1 (short segment before taking the laterocervical pathway), V2 (the passage through the transverse apophyses of the cervical spine), V3 (the retromastoid segment), and V4 (the intracranial portion, up to the point where it joins the other vertebral artery to form the basilar artery). Dissections can occur in any segment of the vertebral artery, but opinion differs among authors regarding the susceptibility to dissection of the four segments. The distal V1- and the proximal V2-segment (at the level of C6 vertebral body) were the most frequent locations of dissections (43% of cases) in a study published by Bartels which included 28 patients (1), while other authors found that the location of the dissection was more often in the pars transversaria (V2; 35%) or atlas loop (V3; 34%) than in the prevertebral (V1; 20%) or intracranial (V4; 11%) segment (2). The V4 segment has a different histological structure, compared with the other segments of the vertebral artery, as the thickness of the tunica media and the adventitia tapers when the vessel pierces the dura. Also, the vessel is subject to shearing forces associated with head motion (3). In a study performed on 983 cases of cervical artery dissections (CeAD), 149 (15.2%) presented with multiple CeADs (4).
In the majority of cases, dissection occurs at the endothelial level, with rupture of the arterial intima, and the development of a false lumen. Blood will enter between the intima and the media, will quickly coagulate, and will lead to different degrees of stenosis or even an occlusion. The hematoma is distributed around the circumference of the vessel, and has a crescent- shape, which is clearly visible on axial MRI sections (at cervical or cranial level). In the next weeks, up to 60% can recanalize, by resorbtion of the hematoma, 39% reaching complete recanalization (5). The other histological site of dissection is much more rare, consists of the cleavage of the arterial wall between adventitia and media, with leakage of the blood in the subarachnoid space, and occurs in V4 segment. Clinical presentation with subarachnoid hemorrhage (SAH) is more rare, but it is associated with worse outcome compared with non-SAH VAD, one of the causes being the high incidence of rebleeding (6).
There are several instances which favor the occurrence of a dissection: trauma, infection and inflammation, smoking, a particular genetic background, or certain diseases such as Ehlers-Danlos or Marfan disease.
A person who has a dissection of the vertebral artery can display an entire range of symptoms, from completely asymtomatic to a severe stroke in the vertebro-basilary territory. The consequences of a dissection depend on several factors: the location of the dissection, the degree of obstruction, the functionality of the collateral circulation. Vertebral artery dissection is one of the most frequent causes of stroke in people aged between 18-45 years, the estimated frequency being between 1-2.6 per 100000 (7). Strokes occur in the medulla or in the cerebellum (territory of the postero-inferior cerebellar artery, PICA), but infarction can appear also in the distal part of the vertebro-basilary system or in the spinal cord (since the anterior and posterior spinal arteries have their origin in the vertebral arteries).
Case 1. A 58-year–old male smoker, with no other vascular risk factors, presented to our Emergency Department because of the abrupt occurrence of postural imbalance and numbness of the left face and lower right limb. The patient denied any neck pain or any acute traumatic injury at the cervical level. Upon admission, the neurologic examination revealed a left sided Horner syndrome, decreased pinprick and thermal sensation in the entire territory of the left trigeminal nerve and in the right lower limb. He had a wide-based, unsteady gait. No other neurological abnormalities were noticed.
Doppler ultrasound of the cervical arteries showed a high-grade left vertebral artery stenosis in the V1/V2 segment; in the V3-V4 segments the flow velocities were lower, with a poststenotic shape. The presence of a stenosis associated with the absence of other atherosclerotic lesions raised the suspicion of a dissection in the V1 segment of the left vertebral artery. A brain MRI was performed on the day of admission. Diffusion-weighted (DWI) MRI showed a small lesion in the dorsolateral portion of the left lower medulla, behind the retro-olivary sulcus, not visible on T2 sequences (Figure 1). On the contrast enhanced T1-weighted images of the neck, the lumen of the left vertebral artery was thinned out throughout the entire course. A thickening of the wall with nonspecific signal transduction was observed in the distal V2 segment. MRI findings were consistent with a dissection of the left V2 and V3 vertebral artery, in the subacute stage.
Resting 12 lead ECG revealed a left bundle branch block. Transthoracic echocardiography showed left ventricle concentric hypertrophy and grade I diastolic dysfunction. Routine blood analysis highlighted a slight disturbance of the lipid profile (total serum cholesterol was 239 mg/dl and HDL cholesterol was 40 mg/dl).
The patient was given unfractionated heparin for 5 days and then switched to dual antiplatelet therapy (aspirin 75 mg daily and clopidogrel 75 mg daily) and statin (rosuvastatin 20 mg daily). His clinical condition improved within a few days. Upon discharge, 9 days after admission, he no longer had numbness of the left face or postural imbalance, and he was able to walk unassisted. At discharge: left Horner syndrome and a mild impairment of temperature and pinprick sensation in the right lower limb. At the 30 days follow-up, the Doppler ultrasound showed partial recanalization of the left vertebral artery, and a minimal hypoesthesia in the right lower limb.
Case 2. A 61-year old male, with a history of alcohol abuse disorder, presented to the Emergency Department for the sudden onset of slurred speech, dysphagia and dysphonia, followed by drooping of the left eyelid, numbness on the left side of his face, unsteady gait, difficulty when using his left limbs and vertigo. The symptoms began several hours before arriving at the hospital; he mentions he felt slight pain on the left side of his neck prior to symptom onset.
On clinical examination he had moderate dysarthria and dysphonia, inward deviation of the left eye, limitation of conjugated eye movements towards the left, peripheral facial palsy, rotational nystagmus on looking towards the right and upwards, and difficulty swallowing, with an impaired pharyngeal reflex on the left side. He also had ataxia of all limbs, more severe on the left side, left inferior limb paresis, brisk reflexes and clonus of the foot bilaterally, a wide-based gait, could not stand upright unaided, and thermal and pain sensation were decreased on the right side of his body. Given the sudden onset and the presenting symptoms, a posterior circulation stroke was the most likely cause, and given the associated left cervical pain we suspected a vertebral artery dissection. Initial cerebral CT scan was normal.
Doppler ultrasound examination of the cervical and cerebral arteries revealed a systolic flow speed of 180 cm/sec in the left vertebral artery (V2 segment) and absence of the flow in V4 segment, supportive of our clinical suspicion.
Brain MRI (performed 9 days from onset) showed a subacute ischemic stroke in the territory of the postero-inferior cerebellar artery. The left vertebral artery had a thick wall, isointense on T1 and T2 weighted sequences, and, on MRA, a discontinuous flow in the second and third segment and slow flow in the fourth segment, consistent with a left vertebral artery dissection in the second and third segment.
The patient received unfractionated heparin and was afterwards switched to aspirin, clopidogrel and a statin. The patient had a satisfactory evolution during his stay in our clinic, with improvement of the eye movement, ataxia and dysarthria. The swallowing difficulty persisted on discharge, albeit slightly improved, and we decided to keep the nasogastric tube we inserted. The patient returned for follow-up one month later, with significant improvement in gait and coordination, dysphonia and nystagmus, but also being able to swallow food of moderate consistency and not requiring the nasogastric tube. Ultrasound examination showed the same appearance.
Case 3.A 68 year-old female, was admitted to our clinic for dizziness, a balance disorder, vertigo, vomiting and transient neck pain which had begun 4 days prior to her admittance. Her medical history included diabetes mellitus and arterial hypertension. On neurologic examination, the patient had a positive Romberg sign with a tendency to retropulsion and no other neurological signs. Her CT scan showed no recent intracranial vascular lesions, but revealed a spontaneously hyperdense left vertebral artery with a larger diameter; ultrasound examination showed high velocities on the vertebral arteries, suggesting a 50% left vertebral artery stenosis and a 60-70% right vertebral artery stenosis. A few days after her admittance to our department she developed intense headache, photophobia, phonophobia, vomit and neck stiffness. An emergency CT scan excluded new lesions. Spinal tap revealed a hemorrhagic cerebrospinal fluid (traumatic spinal tap was excluded). A subarachnoid hemorrhage was diagnosed, due to a vertebral artery dissection. MRI confirmed the vertebral artery dissection, with occlusion of the left vertebral artery, and a stenosis >70% of the right vertebral artery, without any image of ischemic stroke (Figure 3).
The three cases illustrate the clinical versatility of the vertebral artery dissection (with two different location of ischemic stroke for the same site of the VAD, and one subarachnoid hemorrhage), and the importance of chosing the right method for diagnosing a stroke (inccluding the etiological diagnosis).
Ultrasound examination can be a very useful tool for diagnosing blood flow disturbances in cervical and cerebral arteries Typical configurations for dissection include narrow, bi-directional complexes (with positive and negative flow), caused by an increased resistance to the blod flow, when dissection is in the upper segment of the vertebral arteries and double lumen or an intimal arterial flap in the lumen, but this is rarely seen; stenosis or occlusion can be frequently observed, but ultrasound cannot point out exactly the site of the stenosis, and cannot differentiate between stenosis due to atherosclerosis or dissection. When the patient has no other atherosclerotic lesions, and is young, the scales can easily sway towards dissection, but in older people, other methods must be used to prove it. Ultrasound criteria for diagnosing a vertebral artery stenosis are less precise compared to those for internal carotid artery.
Other options to show a vertebral artery stenosis or occlusion are CTA, MRA or DSA, but the hallmark of the dissection is the presence of the arterial wall hematoma, identifiable on axial MRI slices. For occluded arteries the sensitivity of duplex without colour, duplex with colour, time of flight (TOF) MRA and contrast enhanced (CE)-MRA was over 98%, with a specificity ranging from 90.8% (95% CI 89.4 to 100) for duplex to 100% (95% CI 97.5% to 100) for both non contrast and CE- MRA (8).
MRA (magnetic resonance angiography) has a tendency to overrate the degree of stenosis, and data regarding the sensitivity and sensibility of the method given by different studies ranges between 53.8% to 100%, for both noncontrast and contrast enhanced MRA (9-12).
CTA (computed tomography angiography) has few studies but the results suggest that the method can be superior to TOF MRA, when slow flow is present, or for the detection of intracranial vertebral artery stenosis or occlusion (13). DSA (digital substraction angiography) remains the gold standard for exploring the residual aterial lumen. Exploring the internal carotid artery, the flame shape of the occlusion can be a hint for dissection, but in vertebral arteries this is less obvious. Other changes (thrombembolism or atherosclerosis) can produce a stenosis or occlusion, so catheter angiographic findings are nonspecific. For patients who presented with SAH, the most frequently reported angiographic findings were fusiform aneurysms (70.8%), and pearl-and-string lesions (24.5%), meaning a succesion of aneurysmal dilatations and vessel constrictions (6).
MRI is the only reliable method for exploring the arterial wall. A hyperintense, crescentshaped intramural hematoma is specific for arterial dissection, on axial cuts at cervical or cranial level (depending on the site of the dissection). If there is a residual patent lumen this will appear as an eccentric flow void. The aspect of the intramural hematoma is time dependent. In the first 3 days it has a high signal intensity on T2-weighted images with intermediate signal intensity on T1-weighted images followed in the next days in most cases by a slightly or definitively increased signal intensity on T1- and T2-weighted images. The increased signal will remain high for approximately 2 months (14).
Appart from T1- and T2- weighted images, other MRI techniques were studied in order to improve the accuracy of the diagnosis. The intramural hematoma sign is considered positive if the patient has an eccentric or concentric hypointense signal lesion in the vertebral artery on susceptibility weighted imaging (SWI), a corresponding hyperintense signal on phase map and no evidence of calcification on the brain CT (15). Another technique, black blood T1-weighted imaging (3D-BB-T1WI) revealed the characteristic crescent shape of the intramural haematoma in 14 cases (87.5%), being considered a promising technique (16). Two other techniques are mentioned for detection of VAD. One is BPAS (BasiParallel Anatomic Scanning) which was designed to visualize the surface appearance of the vertebrobasilar artery within the cistern and the other is VISTA (Volumetric isotropic TSE acquisition) based on black blood imaging method, designed to evaluate the arterial wall and lumen. Although the sensitivity is still low, these are considered promising techniques for diagnosing VAD. A recent study published by Natori (17) highlights the advantages of 3D-T1-weighted imaging, upon BPAS + MRA, MRA alone or MRI alone, the technique being able to identify luminal stenosis, aneurysmal dilatation, intramural hematoma or intimal flap in 100% of cases.
Treatment of the VAD is still a subject of debate. Should we use anticoagulants or antiplatelets? Is it safe to administer fibrinolitic treatment in the setting of an ischemic stroke due to VAD? When should we use endovascular treatment? These are just several questions found in currently daily practice, answers being based more on the personal experience or local habbits, than on evidence based data.
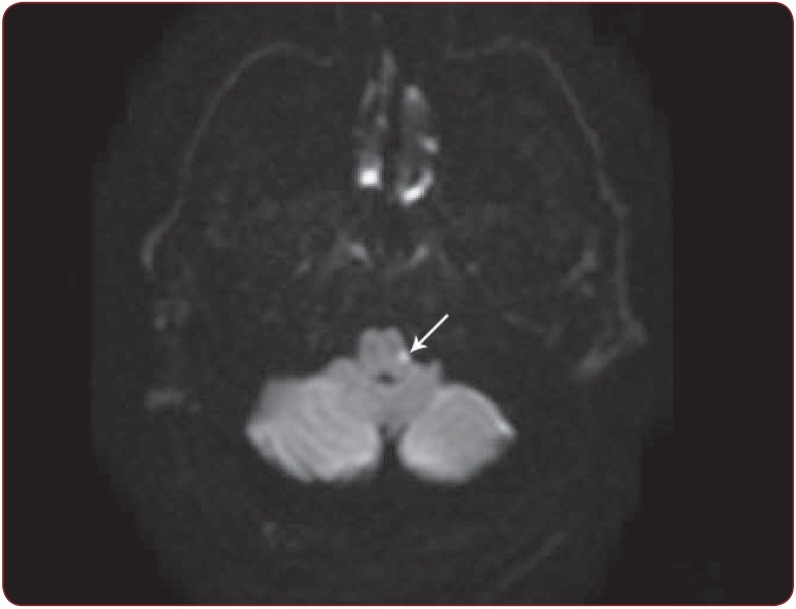
Figure 1.
Figure 1. Case 1. DWI-MRI shows an acute ischemic stroke (the white arrow: hyperintense signal) in the left part of the lower medulla, behind the retro-olivary sulcus.
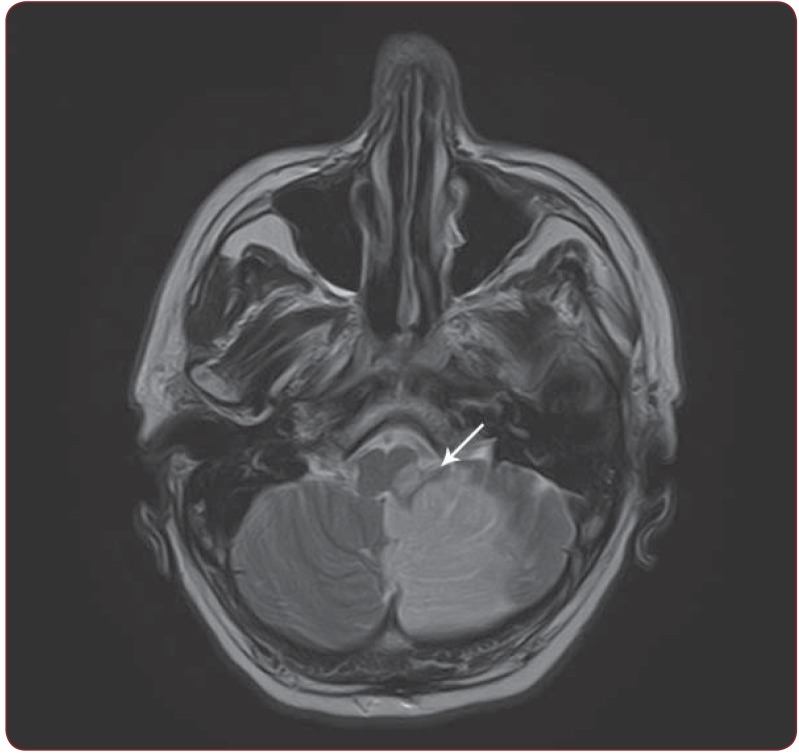
Figure 2.
Figure 2. Case 2. Axial T2 sections, hyperintense signal in the left medulla and left cerebellar hemisphere corresponding to the subacute ischemic stroke (white arrow).
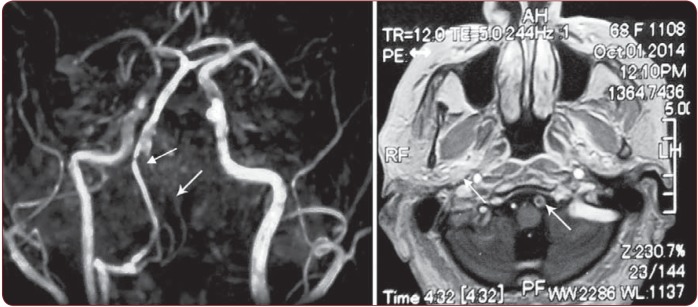
Figure 3.
Figure 3. MRA (left panel, white arrow): occlusion of the left vertebral artery; MRI (Right panel, white arrow): Enlargement of the diameter of the VA with thrombus in the left vertebral artery.
MEDICAL TREATMENT
Current guidelines for the treatment of acute ischemic stroke (18) do not list arterial dissection among the contraindications of thrombolysis. Thrombolysis was independently associated with neither an unfavourable outcome, nor with an excess of symptomatic bleedings in an analysis performed on 616 cervical artery dissections from a multicentric stroke data base – CADISP (19). However, the lack of any trend towards a benefit of thrombolysis may sustain the search for more efficient treatment options including mechanical revascularization strategies.
The choice between anticoagulant and antiplatelet therapy has not been settled yet. There are no reliable data from randomised trials to decide whether anticoagulants or antiplatelet agents are better to prevent further thromboembolic events after cervical arterial dissection.
Most neurologist favor anticoagulants, especialy in the VAD, but this is sustained more by personal experience and not evidence-based data. A meta-analysis (20) performed on 34 non- randomised studies, which encompassed 762 patients, showed no significant difference with regard to the risk of death (antiplatelet 5/268 (1.8%), anticoagulation 9/494 (1.8%), p = 0.88); stroke (antiplatelet 5/268 (1.9%), anticoagulant 10/494 (2.0%), p = 0.66), or stroke and death.
The endovascular approach is an attractive alternative for the treatment of vertebral artery dissection in the acute setting. However, while various techniques have been used (although a large part of the published data refers to the treatment of local pseudoaneurysms following the dissection), these haven’t been studied in a manner which allows for the issuing of recommendations and therefore, at least for the time being, angioplasty and stenting of the vertebral arteries in the setting of an acute stroke due to VAD remain an option in selected cases.
CONCLUSION
Arterial dissections are more frequently diagnosed during the last years, as the access to performant imagistic tools has increased. VAD has the particularity to cause both ischemic stroke and subarachnoid hemorrhage and should be taken into account as a possible etiology for these diseases, especially in young adults. Medical treatment (either antiplatelet or anticoagulant) is the first option for secondary prevention of stroke, but endovascular treatment with stenting is emerging.
Conflict of interests: none declared.
Financial support: none declared.
Contributor Information
Cristina Tiu, Department of Neurology, Emergency University Hospital, Bucharest, Romania.
Elena Terecoasa, Department of Neurology, Emergency University Hospital, Bucharest, Romania.
Nicolae Grecu, Department of Neurology, Emergency University Hospital, Bucharest, Romania.
Raluca Nistor, Department of Neurology, Emergency University Hospital, Bucharest, Romania.
Smaranda Frangu, Department of Neurology, Emergency University Hospital, Bucharest, Romania.
Florina Antochi, Department of Neurology, Emergency University Hospital, Bucharest, Romania.
REFERENCES
- Bartels E - Dissection of the extracranial vertebral artery: clinical findings and early noninvasive diagnosis in 24 patients. . J Neuroimaging. 2006;16:24–33. doi: 10.1177/1051228405280646. [DOI] [PubMed] [Google Scholar]
- Arnold M, Bousser MG, Fahrni G et al. - Vertebral artery dissection: presenting findings and predictors of outcome. Stroke. 2006;37:2499–503. doi: 10.1161/01.STR.0000240493.88473.39. [DOI] [PubMed] [Google Scholar]
- Sasaki O, Ogawa H, Koike T et al. - A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 1991;75:874–82. doi: 10.3171/jns.1991.75.6.0874. [DOI] [PubMed] [Google Scholar]
- Bejot Y, Aboa-Eboule C, Debette S, et al. - Characteristics and outcomes of patients with multiple cervical artery dissection. Stroke. 2014;45:37, 41. doi: 10.1161/STROKEAHA.113.001654. [DOI] [PubMed] [Google Scholar]
- Sengelhoff C, Nebelsieck J, Nassenstein et al. - Neurosonographical follow-up in patients with spontaneous cervical artery dissection. Neurol Res. 2008;30:687–9. doi: 10.1179/174313208X319080. [DOI] [PubMed] [Google Scholar]
- Kocaeli H, Chaalala C, Andaluz N, et al. - Spontaneous intradural vertebral artery dissection: a single-center experience and review of the literature. Skull Base. 2009;19:209–18. doi: 10.1055/s-0028-1114296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debette S, Kamatani Y, Metso TM, et al. - Common variation in PHACTR1 is associated with susceptibility to cervical artery dissection. Nat Genet. 2015;47:78–83. doi: 10.1038/ng.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S CG, Kerry S, Markus HS - Imaging of vertebral artery stenosis: a systematic review. J Neurol Neurosurg Psychiatry. 2007;78:1218–25. doi: 10.1136/jnnp.2006.111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CW, Carr JC, Futterer SF, et al. - Contrast-enhanced MR angiography of the carotid and vertebrobasilar circulations. AJNR Am J Neuroradiol. 2005;26:2095–101. [PMC free article] [PubMed] [Google Scholar]
- Leclerc X, Martinat P, Godefroy O, et al. - Contrast-enhanced three-dimensional fast imaging with steady-state precession (FISP) MR angiography of supraaortic vessels: preliminary results. AJNR Am J Neuroradiol. 1998;19:1405–13. [PMC free article] [PubMed] [Google Scholar]
- Randoux B, Marro B, Koskas F, et al. - Proximal great vessels of aortic arch: comparison of three-dimensional gadolinium-enhanced MR angiography and digital subtraction angiography. Radiology. 2003;229:697–702. doi: 10.1148/radiol.2292011648. [DOI] [PubMed] [Google Scholar]
- Wentz KU, Rother J, Schwartz A, et al. - Intracranial vertebrobasilar system: MR angiography. Radiology. 1994;190:105–10. doi: 10.1148/radiology.190.1.8259384. [DOI] [PubMed] [Google Scholar]
- Bash S, Villablanca JP, Jahan R, et al. - Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol. 2005;26:1012–21. [PMC free article] [PubMed] [Google Scholar]
- Paciaroni M, Caso V, Agnelli G - Magnetic resonance imaging, magnetic resonance and catheter angiography for diagnosis of cervical artery dissection. Front Neurol Neurosci. 2005;20:102–18. doi: 10.1159/000088155. [DOI] [PubMed] [Google Scholar]
- Kim TW, Choi HS, Koo J, et al. - Intramural hematoma detection by susceptibility-weighted imaging in intracranial vertebral artery dissection. Cerebrovasc Dis. 2013;36:292–8. doi: 10.1159/000354811. [DOI] [PubMed] [Google Scholar]
- Takano K, Yamashita S, Takemoto K, et al. - MRI of intracranial vertebral artery dissection: evaluation of intramural haematoma using a black blood, variable-flip-angle 3D turbo spin-echo sequence. Neuroradiology. 2013;55:845–51. doi: 10.1007/s00234-013-1183-4. [DOI] [PubMed] [Google Scholar]
- Natori T, Sasaki M, Miyoshi M, et al. - Detection of vessel wall lesions in spontaneous symptomatic vertebrobasilar artery dissection using T1-weighted 3-dimensional imaging. J Stroke Cerebrovasc Dis. 2014;23:2419–24. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Saver JL, Adams HP, et al. - Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- Engelter ST, Dallongeville J, Kloss M, et al. - Thrombolysis in cervical artery dissection--data from the Cervical Artery Dissection and Ischaemic Stroke Patients (CADISP) database. Eur J Neurol. 2012;19:1199–206. doi: 10.1111/j.1468-1331.2012.03704.x. [DOI] [PubMed] [Google Scholar]
- Menon R, Kerry S, Norris JW, et al. - Treatment of cervical artery dissection: a systematic review and metaanalysis. J Neurol Neurosurg Psychiatry. 2008;79:1122–7. doi: 10.1136/jnnp.2007.138800. [DOI] [PubMed] [Google Scholar]