Abstract
Background:
Once considered a disease of childhood, celiac disease (CD) is now seen quite frequently in adults also, but with different and various clinical presentation. Little data is currently available about pediatric and adult CD features in Romanian patients.
Methods:
38 newly-diagnosed CD patients (17 adults and 21 children) were recruited for this study. The two groups (adult and pediatric) were compared regarding demographic, clinical, serologic and histological data.
Results:
Regarding demographic data, female gender was predominant in both groups (71% and 67% respectively). Median age was 42 (range 23-83) in the adult CD group and 4 (1-17) in the pediatric CD group. Classic presentation was more frequently seen in children than adults (62% vs. 53%). Altered liver function tests, anemia and iron deficiency were more prevalent in the pediatric group. Children with CD also had higher titers of tTG antibodies (81% over 200 U/l, compared to 29% adults) and a higher frequency of destructive histology on small bowel biopsy (95% Marsh>3a, compared to 76% adults).
Conclusion:
Significant differences in pediatric and adult CD were seen in our study cohort, regarding clinical, laboratory and histological parameters. CD manifests differently in children and adults.
Keywords:celiac disease, diagnose
INTRODUCTION
In the past, celiac disease (CD) was considered a disease of childhood. The last decades have shown that CD can develop at any age, with high prevalence in adulthood also and even in the elderly (1). Still, the prevalence of CD is considered to be higher in children than in adults (2,3). CD might develop early in life, with the introduction of gluten-containing cereals in the children’s diet. Untreated childhood CD is more frequently reported to present classic malabsorption syndrome consisting of chronic diarrhea, abdominal distension, weight loss and failure to thrive. In contrast, adulthood CD rarely displays such typical features. There are multiple data showing that adult CD is atypical, meaning either vague digestive symptoms or extra-digestive complaints, which can be challenging to recognize (4). Due to these protean manifestations, the diagnosis of adult CD is also more difficult than in children. Previous studies have shown that the CD prevalence among risk groups is similar with those reported in other countries (5,6).
Celiac disease is a frequently addressed topic in pediatric research in Romania. However, there is little data about pediatric and adult CD characteristics in Romania. In this study, we aimed to evaluate celiac disease phenotype in clinically diagnosed Romanian adults and children.
METHODS
Patients and data collection
Altogether 38 individuals newly diagnosed with CD have been prospectively included in the study. The diagnosis of CD for the 17 adults (age at diagnosis more than 18 years) and for the 21 children was established at the Gastroenterology Department at “Dr. Carol Davila” Central Military Emergency University Hospital, Bucharest and Pediatric Department at “Alfred Rusescu” Institute for Mother and Child Care, Bucharest, respectively.
Demographic data, medical history, routine laboratory parameters, CD specific serology and histology data were recorded for each patient at the time of diagnosis.
Serum autoantibody measurements
Serum CD autoantibodies were measured for both adults and children at the pediatric center. IgA anti-tissue transglutaminase 2 antibodies (tTG-2) were measured using enzymelinked immunosorbent assay (ELISA; Quanta Lite h-tTGIgA, Inova Diagnostics, CA, USA with recommended cut-off for positivity 20U), while anti-endomysial antibodies (EMA) were analyzed by an experienced examiner using indirect immunofluorescence microscopy (Nova Lite INOVA Diagnostics, CA, USA with a cutoff for positivity set at a serum dilution of 1:5).
Upper gastrointestinal endoscopies
All patients underwent upper gastrointestinal endoscopy with multiple duodenal biopsies according to current diagnosis criteria, at least 1 biopsy from the duodenal bulb and at least 4 biopsies from the distal duodenum (7,8). Histology samples were assessed by 2 experienced pathologists and graded according to Marsh- Oberhuber classification (9).
Statistical analysis
Statistical analysis was carried out using SPSS 17 (SPSS Inc., Chicago, IL) and Epi Info 7.1.5 (CDC, Atlanta, GA). Normal distribution of quantitative variables was assessed using the Kolmogorov-Smirnov test. Comparisons between groups were done using Student’s t-test for variables with normal distribution or Mann- Whitney U-test for non-parametric variables. A p value under 0.05 was considered statistically significant.
Ethical considerations
The study was approved by Ethical Committees of “Alfred Rusescu” Institute of Mother and Child Care and “Dr Carol Davila” Central Military Emergency University Hospital.
Funding
Romanian Authority for Scientific Research CNDI-UEFISCDI project number 111/2012.
RESULTS
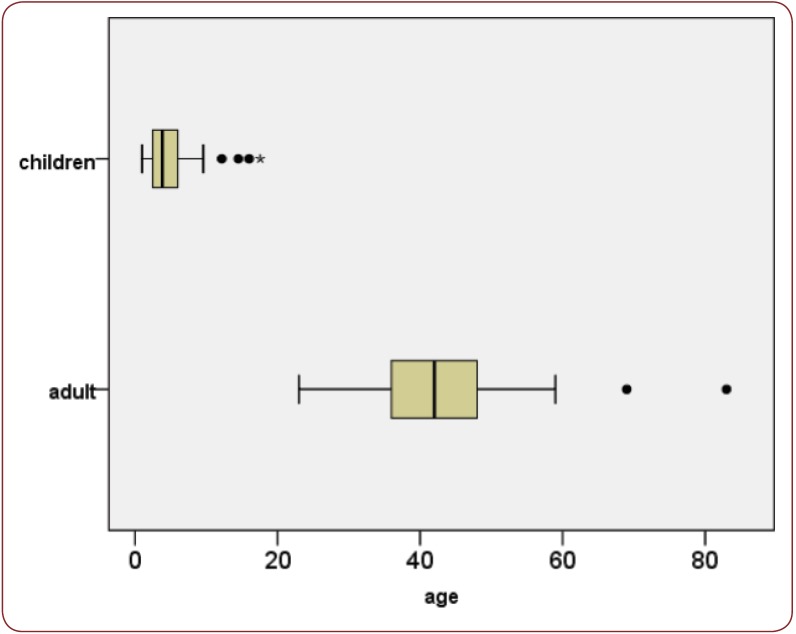
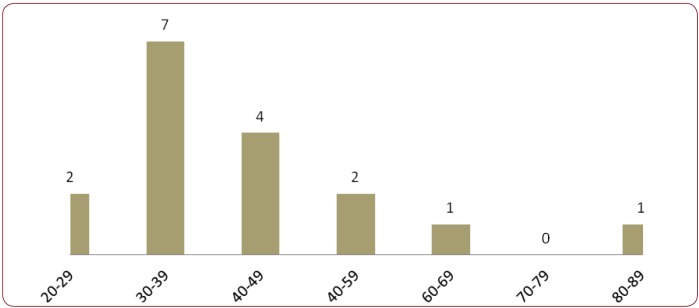
Among the 38 CD patients, there was a female predominance in both groups (12/17 representing 70.59% in the adult group and 14/21 representing 66.67% in the pediatric group). Median age was 42 years in adults (range 23-83) and 4 years in children (range 1-17) – Figure 1. Regarding age distribution in the adult CD group, there was a clear predominance for the 4th and 5th decade – Figure 2.
With respect to nutritional status evaluated by body mass index (BMI), most adults were of normal weight (82.35%), with 11.76% underweight and 5.88% overweight (none obese), while children over 2 years were 35% underweight, 50% normal weight and 15% overweight or obese according to CDC growth charts (10); a child aged 1 diagnosed with CD had low weight and low height for his age.
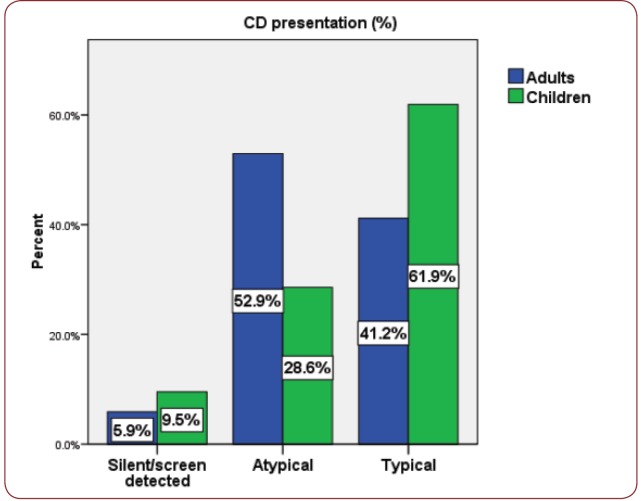
Although classic presentation of CD was more frequently seen in children (62%) and atypical forms predominated among adults (53%), the difference did not achieve statistical significance (p>0.05) – Figure 3. Dermatitis herpetiformis was seen in 3 adults and 3 children.
Two patients in each group had a first-degree relative diagnosed with CD, yielding values of 11.76% and 9.52% respectively for positive CD family history.
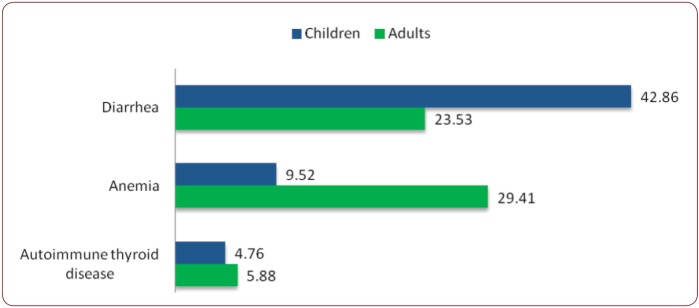
There were similar frequencies of associated diseases in the two groups, such as autoimmune thyroiditis, but differences were noted regarding the personal history of anemia or chronic diarrhea, with the latter being clearly more common in the pediatric CD (42.86% vs. 23.53%, p=NS) - Figure 4. Altered liver function tests were also seen more frequently in children than adults, about one in three having elevated transaminases (30.77% vs. 18.75%, p=NS).
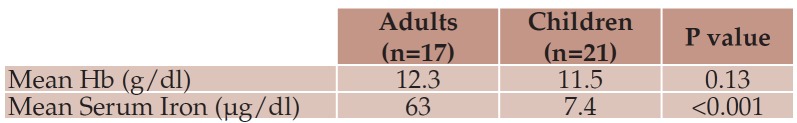
At diagnosis, anemia was slightly more prevalent in children (57.14% vs. 42.86%), with a mean hemoglobin value of 11.52 ± 1.65 g/dl, versus 12.35 ± 1.68 g/dl in adults (p=0.13). Children were also more likely to be iron deficient with average serum iron of 7.44 compared to 63.03 ìg/dL in the adult CD group (p<0.001) – Table 1.
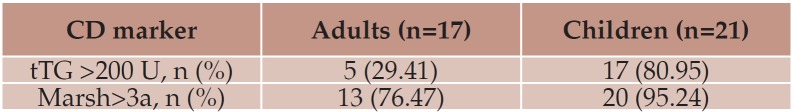
All CD patients showed positive EMA. There was also a pronounced difference regarding the titers of tTG antibodies (80.95% of children with values over 200 U, compared to 29.41% adults).
All patients showed mucosal injury in both bulb and duodenum. The frequency of severe lesions on histology was higher in children (Marsh>3a, 95% vs. 76%) – Table 2.
Figure 1.
Figure 1. Boxplot for age distribution in adults and children with CD.
Figure 2.
Figure 2. Age distribution by decades in adult CD patients.
Table 1.
Table 1. Laboratory parameters in the two CD groups.
Table 2.
Table 2. Immunologic and histologic activity in the two CD groups.
Figure 3.
Figure 3. CD presentation in adults and children.
Figure 4.
Figure 4. Medical history (% positive) in two groups.
DISCUSSION
Demographic data
In our study population, CD was more frequent in females, both in adults and children; significant female predominance has been described in the literature (11), although recent data suggests that this gender difference isn’t very high (12). With respect to positive family history of CD, a similar proportion was seen in the two study groups.
Remarkably, the median age of diagnosis in the pediatric group was quite low (4 years), which is probably explained by the higher frequency in diarrhea-predominant presentation. This is a strong indicator of severe underdiagnoses in pediatrics due to unrecognized atypical manifestation of CD; children with vague manifestations are not referred for celiac disease investigations, their complaints are overlapping with functional gastrointestinal disorders.
Synthetic nutritional evaluation using BMI revealed that most adults were normal weight, while in the children’s group over one third was underweight and half normal weight. This is discordant to data from other studies, which have shown that a significant proportion of both children and adult CD are overweight or even obese at presentation (13).
Clinical presentation
As shown in previous studies, typical CD was more frequent in children than in adults in our cohort also – 61.9% vs. 41.2% (13). The reason for the higher proportion of classical CD in children could be related to the higher prevalence of destructive lesions on small bowel histology in children (14), which leads to overt malabsorption. The early and more severe presentation of CD in children is theorized to be associated with the age of gluten-introduction, the influence of breastfeeding, infections such as rotavirus and interestingly with a gene dose effect seen in several studies (double-dose DQB1*02 allele being associated with classical presentation) (15-17). Regarding the adult CD group, most cases were probably adult-onset CD, as only 2/17 (11.76%) reported chronic digestive symptoms since childhood and only 4/17(23.52%) had short stature (18), which could reflect poor growth during childhood.
However, this trend of typical presentation in early-onset CD and atypical in late-onset CD seems to be changing over time, as in a large Italian study the proportion of atypical CD in children went from 16% in 1990 to 30% in 1994 (19); this has been also seen in the experience of Roma et al. (20), who reported a 50% reduction in classical presentation of children diagnosed with CD between 1978 and 2007, with a concomitant increase in the mean age of diagnosis (from 25 months in 1978-1987 to 73 months in 1998–2007).
This significant difference in adult and pediatric CD presentation is a counter-argument for the use of a non-biopsy strategy in adults: one of the triple ESPGHAN criteria (7) is that of characteristic CD symptoms, and this cannot be checked in a large proportion of adult CD patients, who have atypical or subclinical presentations.
Associated diseases
The prototype of CD associated disease is type 1 diabetes mellitus (DM), the two having shared genetic susceptibility (21). Literature data state there are also differences regarding the associated diseases among children and adults with CD. The most common associated diseases in childhood CD are type 1 DM and autoimmune thyroid diseases, while rheumatic disorders and infertility seem to be the most frequent associations in adulthood CD (14).
We did not see these differences in our population but this could be due to the low number of patients included, which is a limitation of our study.
Laboratory work-up and histology
Anemia was prevalent in both groups at diagnosis, almost half of the study population having this condition. This builds up to the current evidence that anemia is often recorded in CD, that it can be the presenting clinical manifestation and sometimes the only pathologic laboratory finding (22).
Interestingly, almost one third of children with CD had elevated liver function tests. While some studies reported this abnormality more frequently in adult CD (23,24), recent data has shown that cryptogenic hypertransaminasemia is quite frequent in the CD pediatric population also (25). The so-called “celiac hepatitis” resolves on the gluten-free diet (26).
Regarding the immunologic activity of the disease, tTG values over 200 U were more frequently encountered in children than adults (81% vs 29%). We also saw a higher frequency of destructive histology on small bowel biopsy specimens of children than that of adults. This is similar to other studies in the literature (23) and supports the theory that tTG antibody titers and histologic lesions inversely correlate with age – meaning that with increasing age there is less marked architectural damage and lower titers of tTG (14). It is not rare to find only inflammation in the duodenum of adult CD (intraepithelial lymphocytosis), maybe with crypt hyperplasia but without villous atrophy (14).
These lower values of tTG and milder histological lesions in adults add up to the diagnostic challenge of adult CD compared to the pediatric population.
The differences highlighted above can sometimes create a gap between pediatric and adult gastroenterologists. A strong collaboration between the two is critical to avoid discontinuity in transition of CD patients from childhood to adulthood, which unfortunately is currently unmet (27).
Besides the reduced sample size, another limitation of our study is lack of data regarding long-term follow-up. Current evidence shows that the response to gluten free diet is similar between the two groups; malignant CD-related complications are almost exclusively seen in adults (13).
CONCLUSION
Despite some similarities, the differences regarding clinical presentation, serological activity and histological changes support the conclusion that although it is the same disorder, CD manifests differently in children and adults. CD is a good example to support the saying that “children are not little adults”.
Conflict of interests: none declared.
Financial support: The authors declare that this work was supported by Romanian Authority for Scientific Research CNDI-UEFISCDI [project number 111/2012].
Contributor Information
Vasile Balaban, ”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Vasile Balaban, ”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania.
Alina Popp, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; “Alfred Rusescu” Institute for Mother and Child Care, Bucharest, Romania.
Florina Vasilescu, ”Dr Carol Davila” Central University Emergency Military Hospital, Bucharest, Romania.
Adina Ene, “Alfred Rusescu” Institute for Mother and Child Care, Bucharest, Romania.
Mariana Jinga, ”Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; ”Dr Carol Davila” Central University Emergency Military Hospital, Bucharest, Romania.
REFERENCES
- Vilppula A, Kaukinen K, Luostarinen L, et al. - Increasing prevalence and high incidence of celiac disease in elderly people: a population based study. BMC Gastroenterol. 2009;9:49. doi: 10.1186/1471-230X-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marine M, Farre C, Alsina M, et al. - The prevalence of coeliac disease is significantly higher in children compared with adults. Aliment Pharmacol Ther. 2011;33:477–86. doi: 10.1111/j.1365-2036.2010.04543.x. [DOI] [PubMed] [Google Scholar]
- Makharia GK, Verma AK, Amarchand R, et al. - Prevalence of celiac disease in the northern part of India: a community based study. J Gastroenterol Hepatol. 2011;26:894–900. doi: 10.1111/j.1440-1746.2010.06606.x. [DOI] [PubMed] [Google Scholar]
- Fernández A, González L, de la Fuente J - Coeliac disease: clinical features in adult population. Rev Esp Enferm Dig. 2010;102:466–71. doi: 10.4321/s1130-01082010000800002. [DOI] [PubMed] [Google Scholar]
- Popp A, Mihu M, Munteanu M, et al. - Prospective antibody case finding of coeliac disease in type-1 diabetes children: need of biopsy revisited. Acta Paediatr. 2013;102:e102–6. doi: 10.1111/apa.12117. [DOI] [PubMed] [Google Scholar]
- Popp A, Jinga M, Jurcut C, et al. - Fingertip rapid point-of-care test in adult case-finding in coeliac disease. BMC Gastroenterol. 2013;13:115. doi: 10.1186/1471-230X-13-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby S, Koletzko S, Korponay-Szabó IR, et al. - PrognosticESPGHAN Working Group on Coeliac Disease Diagnosis, ESPGHAN Gastroenterology Committee, European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH, Murray JA - American College of Gastroenterology.ACG clinical guidelines: diagnosis and management of celic disease. Am J Gastroenterol. 2013;108:656–76. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhüber G, Granditsch G, Vogelsang H - The histopathology of coeliac disease. Time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185–94. doi: 10.1097/00042737-199910000-00019. [DOI] [PubMed] [Google Scholar]
- http://www.cdc.gov/growthcharts/cdc_charts.htm. Centers for Disease Control and Prevention, National Center for Health Statistics. 2016 [Google Scholar]
- Kocsis D, Miheller P, Lürinczy K, et al. - Coeliac disease in a 15-year period of observation (1997 and 2011) in a Hungarian referral centre. Eur J Intern Med. 2013;24:461–7. doi: 10.1016/j.ejim.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Singh P, Arora S, Singh A, Strand TA, Makharia GK - Prevalence of Celiac disease in Asia: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31:1095–101. doi: 10.1111/jgh.13270. [DOI] [PubMed] [Google Scholar]
- Poddar U - Pediatric and adult celiac disease: similarities and differences. Indian J Gastroenterol. 2013;32:283–8. doi: 10.1007/s12664-013-0339-9. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Kruzliak P, Cangemi GC, et al. - The Spectrum of Differences between Childhood and Adulthood Celiac Disease. Nutrients. 2015;7:8733–8751. doi: 10.3390/nu7105426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congia M, Cucca F, Lampis R, et al. - A gene dosage effect of the DQA1*0501/ DQB1*0201 allelic combination influences the clinical heterogeneity of celiac disease. Hum Immunol. 1994;40:138–42. doi: 10.1016/0198-8859(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Zubillaga P, Vidales MC, Zubillaga I, et al. – HLA-DQA1 and HLA-DQB1 genetic markers and clinical presentation in celiac disease. J Pediatr Gastroenterol Nutr. 2002;34:548–54. doi: 10.1097/00005176-200205000-00014. [DOI] [PubMed] [Google Scholar]
- Karinen H, Karkkainen P, Pihlajamaki J, et al. - Gene dose effect of the DQB1*0201 allele contributes to the severity of coeliac disease. Scand J Gastroenterol. 2006;41:191–9. doi: 10.1080/00365520500206277. [DOI] [PubMed] [Google Scholar]
- - Anthropometric Reference Data for Children and Adults. Centers for Disease Control and Prevention, National Center for Health Statistics. 2016 [Google Scholar]
- Bottaro G, Cataldo F, Rotolo N, Spina M, Corazza GR - The clinical pattern of subclinical/silent celiac disease: an analysis on 1026 consecutive cases. Am J Gastroenterol. 1999;94:691–6. doi: 10.1111/j.1572-0241.1999.00938.x. [DOI] [PubMed] [Google Scholar]
- Roma E, Panayiotou J, Karantana H, et al. - Changing pattern in the clinical presentation of pediatric celiac disease: a 30-year study. Digestion. 2009;80:185–91. doi: 10.1159/000227275. [DOI] [PubMed] [Google Scholar]
- Smyth DJ, et al. - Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008;359:2767–77. doi: 10.1056/NEJMoa0807917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman HJ - Iron deficiency anemia in celiac disease. World J Gastroenterol. 2015;21:9233–9238. doi: 10.3748/wjg.v21.i31.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo-Saez L, Fuentes-Alvarez D, Perez-Martinez I, et al. - Differences between pediatric and adult celiac disease. Rev Esp Enferm Dig. 2011;103:238–244. [PubMed] [Google Scholar]
- Castillo N, Vanga RR, Theethira TG, et al. - Prevalence of abnormal liver function tests in celiac disease and the effect of the gluten free diet in the US population. Am. J. Gastroenterol. 2015;110:1216–22. doi: 10.1038/ajg.2015.192. [DOI] [PubMed] [Google Scholar]
- Anania C, De Luca E, De Castro G, et al. - Liver involvement in pediatric celiac disease. World J Gastroenterol. 2015;21:5813–22. doi: 10.3748/wjg.v21.i19.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury A, Sanders DS, Ford AC - Meta-aalysis: Coeliac disease and hypertransaminasemia. Aliment Pharmacol Ther. 2011;34:33–40. doi: 10.1111/j.1365-2036.2011.04685.x. [DOI] [PubMed] [Google Scholar]
- O’Leary C, Wieneke P, Healy M, et al. - Celiac disease and the transition from childhood to adulthood: a 28-year follow-up. Am J Gastroenterol. 2004;99:2437–41. doi: 10.1111/j.1572-0241.2004.40182.x. [DOI] [PubMed] [Google Scholar]