Abstract
Chronic pain represents a frequent and poorly understood public health issue. Numerous studies have documented the key significance of plastic changes along the somatosensory pain pathways in chronic pain states. Our recent study demonstrated that the cGMP-dependent protein kinase I (PKG-I) specifically localized in nociceptors constitutes a key mediator of hyperexcitability of primary sensory neurons and spinal synaptic plasticity after inflammation. However, whether PKG-I in nociceptors further affects the cortical plasticity in the ascending pain pathways under pathological states has remained elusive. The immediate-early gene c-fos and phosphorylated ERK1/2 (pERK1/2) are considered reliable indicators for the neuronal activation status and it permits a comprehensive and large-scale observation of nociceptive neuronal activity along the ascending pain pathways subjected to tissue injury. In the present study, we systemically demonstrated that peripheral injury in PKG-Ifl/fl mice produced a significant upregulation of c-Fos or pERK1/2 over from the periphery to the cortex along the pain pathways, including dorsal root ganglion, spinal dorsal horn, ventral posterolateral thalamus, primary somatosensory hindlimb cortex, anterior cingulate cortex, basolateral amygdala, periaqueductal gray, and parabrachial nucleus. In contrast, very few cells in the above regions showed c-Fos or pERK1/2 induction in nociceptor-specific knockout mice lacking PKG-I (SNS-PKG-I−/− mice). Our results indicate that PKG-I expressed in nociceptors is not only a key determinant of dorsal root ganglion hyperexcitability and spinal synaptic plasticity but also an important modulator of cortical neuronal activity in pathological pain states and represent what we believe to be novel targets in the periphery for pain therapeutics.
Keywords: PKG-I, nociceptor, neuronal plasticity, pain hypersensitivity, c-Fos, phosphorylated ERK
Background
Chronic pain represents a frequent and poorly understood public health issue all over the world and is caused by nerve or tissue injury under different disease conditions. Integrative research approaches have consistently suggested that chronic pain produces long-term plastic changes along the ascending somatosensory pain pathways. Plastic changes not only take place in peripheral nociceptors (peripheral sensitization) but also in the spinal and supraspinal areas (central sensitization) that are involved in the processing of painful information.1–4 Thus, treating chronic pain requires better understanding of the mechanisms of plastic changes in the somatosensory pathways.
Numerous studies have documented the key significance of spinal NMDA receptor-NO-cGMP pathway in the synaptic plasticity and chronic pain states.5–8 NMDA receptor activation leads to the production of the diffusible gas NO via activation of diverse NO synthases (NOS isoforms), which in turn, stimulates soluble guanylyl cyclases to produce cGMP. Studies on several different biological systems have shown that cGMP regulates multiple cellular targets, including diverse cGMP-gated ion channels, such as cyclic nucleotide-gated and hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, the cGMP-dependent protein kinases, PKG-I/cGK-I and PKG-II/cGK-II, as well as diverse phosphodiesterases.9,10 However, the precise locus and downstream targets of cGMP in the spinal nociceptive system has remained elusive. Among these targets, PKG-I has been reported to be highly expressed in the primary sensory neurons in the dorsal root ganglia (DRG).11,12 Previous studies have shown that cGMP concentration and PKG-I expression in the DRG is strongly upregulated after injury or inflammation.13 Increased PKG-I activity is required to maintain long-term hyperexcitability in sensory neurons in Aplysia14 and rodents15,16 under pathological states. Because PKG-I is also expressed in Schwann cells in the DRG,17 the use of pharmacological agents above does not fully permit the dissection of PKG-I contribution at different loci in the modulation of pain in vivo. We reasoned that conditional, region-specific gene deletion at particular loci in the somatosensory pain pathway might constitute the most unambiguous approach towards clarifying the functional relevance of the cGMP-PKG-I pathway in modulation of pain. By using the Cre-loxP system, we generated transgenic mice that selectively lack PKG-I in nociceptors (SNS-PKG-I−/− mice), the peripheral arm of the somatosensory pain pathway, preserving expression in the spinal neurons, brain, and all other organs. Unlike the global PKG-I mutant mice with serious developmental deficits and early post-natal lethality,18 conditional transgenic mice lacking PKG-I specifically in nociceptors are viable and with normal development. Our recent study demonstrated by electrophysiological recordings that PKG-I localized in nociceptors constitutes a key mediator of hyperexcitability of primary sensory neurons and spinal synaptic plasticity after injury or inflammation.3,19 However, it has remained elusive how peripheral excitability and spinal potentiation affect cortical neuronal activity in different brain regions that are involved in the pain processing and whether PKG-I in nociceptors mediates this action.
Furthermore, although electrophysiological recording technique can identify electrophysiological properties of neurons involved in PKG-I mediated changes, the spatial and temporal involvement of neuronal activity in the somatosensory pain pathways is difficult to be evaluated and the number of neurons is also limited by using this technique. To get further understanding about the populations of neurons in the somatosensory pain pathways involved in PKG-I-mediated pain hypersensitivity, it is necessary and important to assess populated neuronal activity by a functional marker. It has been well established that proto-oncogene immediate-early gene c-fos and its product c-Fos protein is a very useful marker for monitoring neuronal activity in the central pathways of sensory system temporally and spatially.20,21 c-Fos proteins and phosphorylation of ERK1/2 (extracellular receptor-activated MAP Kinases 1/2) (pERK1/2) can be stably activated by a variety of noxious stimuli such as mechanical, thermal, and chemical modalities.22,23 Such activation can be inhibited or blocked by analgesics for the treatment of chronic pain.21 It is believed that these activity-activated immediate-early genes may contribute to long-term plastic changes in the somatosensory pathways, and thus contribute to behavioral sensitization. So far as we know, whether or to what extent PKG-I in nociceptors affect the populated neuronal activity within neighboring and distant regions in the somatosensory pain pathways after injury or inflammation is still unclear. To address this question, in the present study, we studied induction of c-Fos in the somatosensory pain pathways following various irritants challenge, i.e., hindlimb formalin and capsaicin injection as well as muscular acidic saline injection in PKG-Ifl/fl and SNS-PKG-I−/− mice. We demonstrated that irritants-challenged PKG-Ifl/fl mice showed robust c-Fos or pERK1/2 induction in the DRG and spinal dorsal horn, which is significantly reduced in SNS-PKG-I−/− mice. This is consistent with our previous study showing the crucial role of PKG-I in DRG hyperexcitability and spinal synaptic plasticity. More importantly, specific deletion of PKG-I inhibited the upregulation of c-Fos in the brain regions that are involved in the sensory-discriminative and aversive components of pain, i.e., the ventral posterolateral nucleus of the thalamus (VPL), the primary somatosensory hindlimb cortex (S1HL), the anterior cingulate cortex (ACC), prefrontal cortex (PFC), basolateral amygdala, insular cortex, periaquaductal gray (PAG), and parabrachial nucleus (PBN). Behavioral surveys demonstrated that PKG-I activation in nociceptors is functionally associated with pain hypersensitivity associated with peripheral injury in vivo. Taken together, our results indicate that PKG-I expressed in nociceptors is not only a key determinant of DRG hyperexcitability and spinal synaptic plasticity but also an important modulator of cortical neuronal activity observed in pathological pain states. This study presents a strong basis for the PKG-I as a new class of targets in the periphery for pain therapeutics.
Methods
Genetically modified mice
Homozygous mice carrying the floxed allele of the mouse prkg1 gene, which encodes the cGMP-dependent kinase 1 (PKG-Ifl/fl) have been described previously in details.24 PKG-Ifl/fl mice were crossed with SNS-Cre mice,25 which express the Cre recombinase under the influence of the mouse Scn10a promoter (encoding Nav1.8) to obtain litters consisting of PKG-Ifl/fl; SNS-Cre+ mice (referred to as SNS-PKG-I−/− mice in this manuscript) and PKG-Ifl/fl mice (control littermates). Mice of all genotypes were individually backcrossed into the C57BL6 background for more than eight generations before being crossed with each other. In all experiments, littermates were strictly used to control for genetic effects of the background.
Behavioral analysis
All animal use procedures were approved by the Institutional Animal Use and Protection Committee, Fourth Military Medical University. All the testing was carried out in accordance with the approved guidelines. All behavioral measurements were done in awake, unrestrained, and age-matched adult (more than three-month-old) mice of both sexes by individuals who were blinded to the genotype of the mice being analyzed. The animals were housed in plastic boxes at 22℃–26℃ with food and water available ad libitum in the colony room. A 12:12 h light dark cycle with lights on at 08:00 was maintained and testing was done between 09:00 and 18:30. Mice were acclimatized to the laboratory and habituated to the experimental setups for at least 30 min each day for five days before testing.
Spontaneous pain observation induced by s.c. formalin injection
A transparent plexiglas test box with a transparent glass floor was placed on a supporting frame of 30 cm high above the experimental table to allow the experimenters to observe the paws of the animals without obstruction. Mice were placed in the test box for at least 30 min for acclimation before administration of the chemical agents. After the acclimation period, s.c. injection of formalin (1%, 20 µl) was made into the center of the plantar surface of one hindpaw of PKG-Ifl/fl and SNS-PKG-I−/− mice. Mice were then replaced in the test box, and spontaneous pain response was recorded for a period of 1–2 h. The spontaneous nociceptive behavior was determined by measuring the duration mice spent in flinching, lifting, and licking the injected hindpaw during 5 min intervals following injection.19,26
Examination of mechanical hypersensitivity induced by lower thigh injection of capsaicin
Mice were injected with 10 µl of capsaicin (0.06 %) into the lower thigh of one leg. Capsaicin-induced flare reached up to the ankle, but not the plantar hindpaw surface. Mechanical hyperalgesia and allodynia were tested with manual application of Von Frey hairs with bending force ranging from 0.04 to 4.0 g to the plantar surface of the hindpaw at various time points after capsaicin injection. Mice were placed on a metal mesh floor covered with a plexiglas chamber and von Frey filaments were applied from underneath the metal mesh floor to the testing site of the hindpaw. A “response” to the von Frey stimuli was defined as an abrupt foot lift upon application of the von Frey filament. Each filament was applied 10 times and the paw withdrawal response frequency (the percentage of positive responses to the stimulus) was recorded. The force of a particular filament required to elicit 50% frequency of paw withdrawal was expressed as the mechanical threshold.
Induction of muscle pain and examination of mechanical hypersensitivity
Mice were injected twice with 20 µl of acidic saline, pH 4, into one gastrocnemius muscle two times at an interval of three days. Plantar application of von Frey hairs to the plantar surface of bilateral hindpaws was performed to test mechanical hyperalgesia and allodynia at 24 h through three weeks after the second injection.27 The response frequency and threshold to mechanical stimuli at each point were averaged from 10 mechanical stimuli applied.
Measurement of c-Fos expression and ERK1/2 phosphorylation in the DRG and spinal cord and brain in vivo
Mice in various treatment groups were subjected to hindlimb injection with formalin (1%, 20 µl), capsaicin (0.06%, 20 µl), and muscular injection of acidic saline (pH 4.0, 20 µl), killed and perfused transcardially with 4% paraformaldehyde at 1 h after formalin and capsaicin, and 24 h after second acidic saline injection. The DRG, spinal cord, and brain were removed, trimmed into several blocks, and postfixed in the same fixative for 48 h, and then cryoprotected in 0.1 M PB containing 30% sucrose until the tissue block sank onto the bottom of the container. Vibratome sections (50 µm) of the spinal cord and brain or Cyrostat-sections (16 µm) of the L4/L5 DRG were immunostained with anti-Fos antibody (Chemicon, 1:8000) or anti-phospho-ERK1/2 antibody (Cell signaling, 1:200). Immunoreactive cells in laminae I and II of the spinal dorsal horn and different brain regions were microscopically counted in five to six sections per mouse from four mice per treatment group. Similarly, the number of immunoreactive neurons per DRG section was counted and numbers were averaged over 10 sections per mouse and four mice per treatment group.
The sections were immunostained for c-Fos protein with the avidin–biotin–peroxidase complex (ABC) methods. The sections of DRG, spinal cord, and brain were rinsed twice in 0.01 M PBS and then incubated with a solution containing 2.5% Triton X-100 and 3% bovine serum albumin for 30 min at room temperature (22℃–25℃). The sections were further incubated with a polyclonal antibody raised in rabbit against c-fos and phospho-ERK1/2 for 24 h at 4℃ and then incubated with biotinylated goat anti-rabbit IgG (Vector, 1:200) and ABC complex (Vector, 1:200). The reaction product was visualized with 0.01% hydrogen peroxide and 0.05% diaminobenzidine in 0.05 M Tris–HCl buffer (pH 7.6) or AEC. Between incubations, the sections were rinsed three times in 0.01 M PBS, each for 10 min. There was no positive staining when PBS or normal rabbit serum was used instead of the antibody. The sections were then mounted on slides, dried, dehydrated, cleared, and coverslipped.
Western blotting
DRGs, spinal cords, and brains were collected and homogenised in ice-cold lysis buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.5% Sodium deoxycholate, 0.1% SDS, and standard protease inhibitors. Insoluble material was removed by centrifugation (13,000 r/min × 10 min) and supernatant were collected. Protein concentration for each sample was determined by the bicinchoninic acid method using the MICRO bicinchoninic acid protein assay kit (Pierce). Proteins were loaded on a polyacrylamide gel and separated by electrophoresis. The membrane blots were blocked with 10% non-fat dry milk for 12 h and incubated with primary antibodies: anti-PKG-I (1:100) overnight at 4℃. The membranes were then incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:10000, Amersham Biosciences) for 2 h at room temperature. To normalize the loaded samples, mouse monoclonal anti-tubulin antibody (1:5000; GE Healthcare) was used, followed by incubation with HRP-conjugated goat anti-mouse IgG (1:5000; Pierce). Membranes were incubated with enhanced chemiluminescence reagents (Pierce), and images of the membrane were acquired with the CHEMIL-MAGER chemiluminescence imaging system and analyzed with Image J software. The density of the band of interest was measured and normalized to the density of the band of tubulin.
Statistical analysis
All results are presented as mean ± SEM. Analysis of variance for random measures was carried out, followed by post hoc Fisher’s test or Dunnett’s test to determine statistically significant differences for all data. P < 0.05 was considered to be significant.
Results
Conditional, nociceptor-specific deletion of PKG-I in mice
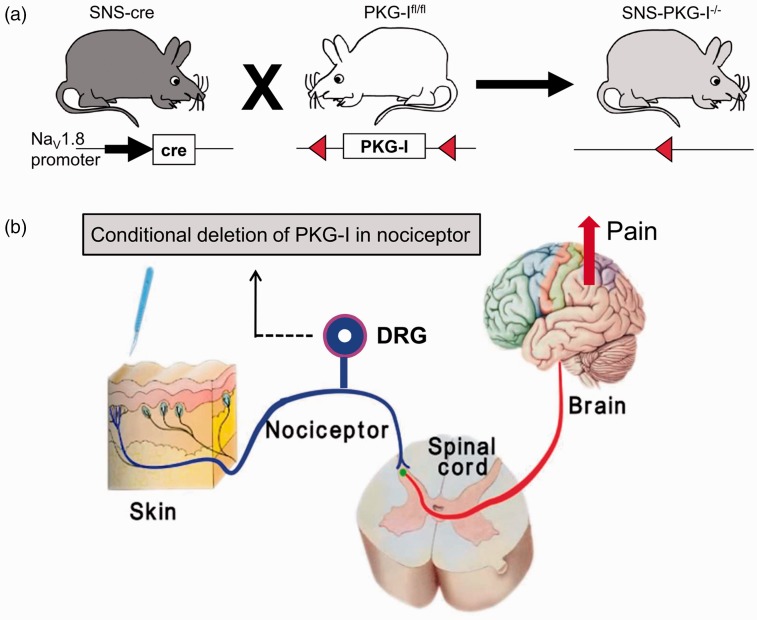
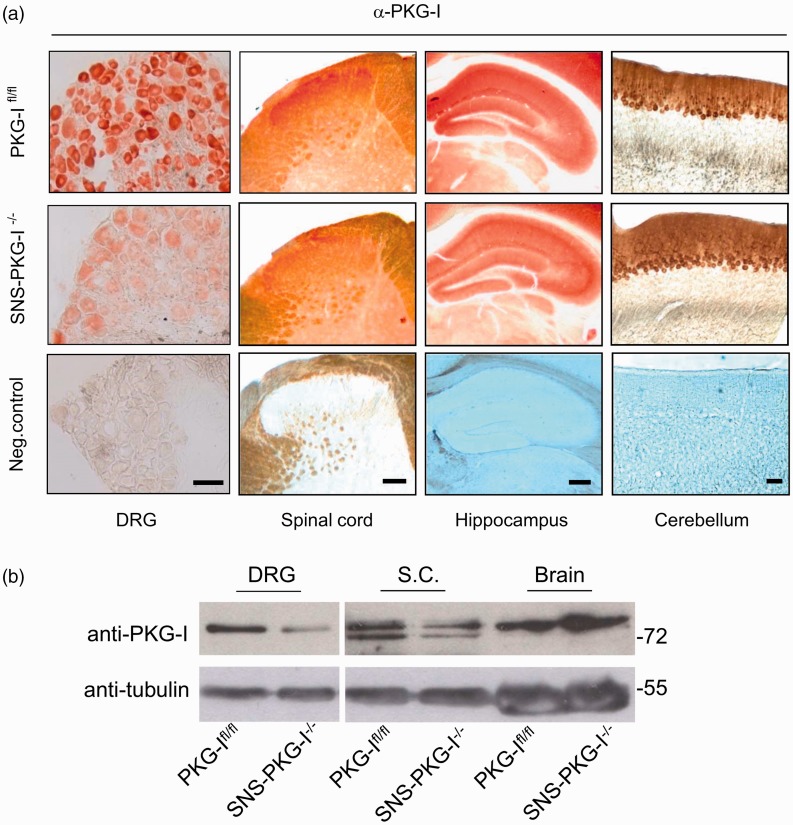
We generated mice lacking PKG-I specifically in a primary nociceptor-specific manner (SNS-PKG-I−/−) via Cre/loxP-mediated recombination by mating mice carrying the floxed prkg1 allele (PKG-Ifl/fl) with a mouse line expressing Cre recombinase under control of the Nav1.8 promoter (SNS-Cre)24,25 (Figure 1(a)). Previous studies by Agarwal et al.25,28 have demonstrated that SNS-Cre mice enable gene recombination commencing at birth selectively in nociceptive (Nav1.8-expressing) sensory neurons, without affecting gene expression in the spinal cord, brain, or any other organs in the body. Thus, SNS-PKG-I−/− mice would show a selective deletion of PKG-I in nociceptive DRG neurons in ascending pain pathways without any change of PKG-I in other regions (Figure 1(b)). As shown in Figure 2(a), an anti-PKG-I antibody yielded specific and intense staining in the peripheral and central nervous system including DRG, spinal cord, hippocampus, and cerebellum in PKG-Ifl/fl mice (Figure 2(a)). Immunohistochemical staining revealed that a majority of DRG neurons expressing PKG-I in PKG-Ifl/fl mice are small to medium-diameter neurons, which was completely devoid of PKG-I expression in SNS-PKG-I−/− mice (Figure 2(a), panels in the left column). In the spinal cord, anti-PKG-I immunoreactivity was strongly seen in the superficial dorsal laminae of PKG-Ifl/fl mice, which represent the major termination zones of the nociceptive primary afferents. In contrast, the intensity of PKG-I immunoreactivity was markedly decreased in the superficial laminae of spinal cord in SNS-PKG-I−/− mice. PKG-I expression in spinal cord neurons leaved intact and appeared particularly conspicuous due to the loss of PKG-I labeling in afferent terminals in SNS-PKG-I−/− mice (Figure 2(a), panels in the second column). Anti-PKG-I immunoreactivity is entirely unaltered in the brains of SNS-PKG-I−/− mice, with examples of expression in the hippocampus and cerebellum shown in the right two panels in Figure 2(a). Furthermore, Western blot analysis with anti-PKG-I antibody confirmed that SNS-PKG-I−/− mice show a DRG-specific loss of PKG-I while retaining expression in the brain (Figure 2(b)). Taken together, SNS-PKG-I−/− mice exhibit a nociceptor-specific loss of PKG-I while retaining expression in neurons of the central nervous system.
Figure 1.
(a) Generation of nociceptor specific knockout mice lacking PKG-I (SNS-PKG-I−/−) via Cre/loxP-mediated recombination by mating mice carrying the floxed prkg1 allele (PKG-Ifl/fl) with a mouse line expressing Cre recombinase under control of the Nav1.8 promoter (SNS-Cre). (b) Schematic diagram showing SNS-PKG-I−/− mice with a selective deletion of PKG-I in nociceptive DRG neurons in the ascending pain pathways, but without any change of PKG-I expression in the brain regions.
Figure 2.
Identification of specific deletion of PKG-I in nociceptors in SNS-PKG- I−/− mice. (a) Immunohistochemical staining showing that PKG-I is specifically deleted in small- to medium-diameter DRG neurons and superficial lamina of spinal dorsal horn where nociceptive primary afferents mainly terminates, but without alterations in the brain regions, such as hippocampus and cerebellum. (b) Western blot analysis with anti-PKG-I antibody confirmed that SNS-PKG-I−/− mice show a DRG-specific loss of PKG-I while retaining expression in the brain. Scale bars represent 50 µm for DRG, 100 µm for spinal cord, hippocampus, and cerebellum.
Reduced spontaneous pain behaviors induced by formalin after deletion of PKG-I in nociceptors
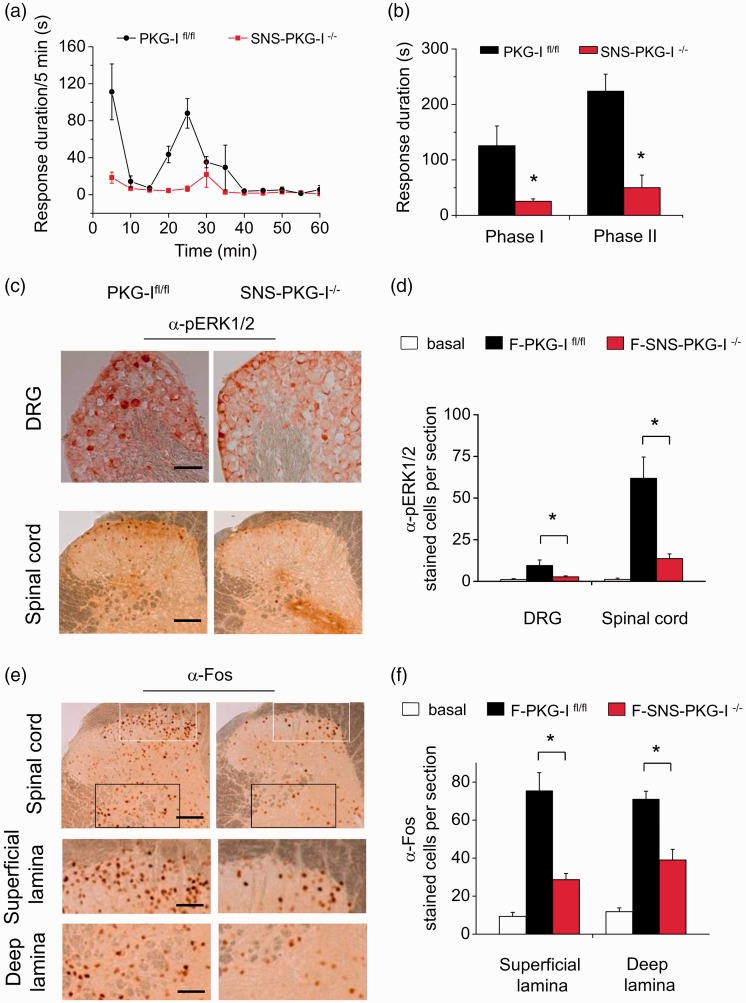
Intraplantar injection of formalin induced a biphasic spontaneous nocifensive response in PKG-Ifl/fl mice, which includes an initial robust phase in which paw lifting, licking, and flinching are scored during the first 10 min, followed by a transient decline in these behaviors and a subsequent second phase of behavior lasting 30–60 min (Figure 3(a)). Analysis of the duration of the mice spent on flinching, lifting, and licking behavior revealed that SNS-PKG-I−/− mice showed a significant reduction of formalin-induced spontaneous pain responses in both phases as compared to PKG-Ifl/fl mice (Figure 3(a) and (b), P < 0.05, n = 10–12 mice). This suggests a pivotal role of PKG-I in nociceptors involved in the spontaneous nociception produced by noxious irritation.
Figure 3.
Time course (a) and quantification analysis (b) showing that SNS-PKG-I−/− mice displayed a significant reduction of formalin-induced spontaneous pain responses in both phases as compared to PKG-Ifl/fl mice. (c) Typical examples showing that phosphorylated ERK1/2 (pERK1/2) immunoreactivity was induced in the DRG (upper panels) and spinal cord (lower panels) at 1 h following intraplantar injection of formalin in PKG-Ifl/fl mice and SNS-PKG-I−/− mice. (d) Quantitative summary of the number of pERK1/2-positive cells in the DRG and spinal cord derived from control and formalin-treated mice. (e) Typical examples showing that c-Fos immunoreactivity was induced in both the superficial and deep lamina of the spinal cord at 1 h following intraplantar injection of formalin in PKG-Ifl/fl mice and SNS-PKG-I−/− mice (upper panels). Magnification of superficial (middle panels) and deep lamina (lower panels) are shown. (f) Quantitative summary of the number of c-Fos-positive cells in both the superficial and deep lamina of spinal cord derived from control and formalin-treated mice. * indicates statistically significant differences (P < 0.05) between PKG-Ifl/fl mice and SNS-PKG-I−/− mice (ANOVA, post hoc Fisher’s test, n = 4 mice per genotype). Scale bars represent 100 µm in (c) and upper panels in (e), 50 µm in middle and lower panels in (e).
Reduced c-Fos and pERK1/2 induction in the DRG and spinal cord after formalin injection in SNS-PKG-I−/− mice
Previous studies have proposed that phase I of formalin response results from direct activation of primary afferent sensory neurons, whereas phase II reflects the combined effects of afferent input and central sensitization in the spinal dorsal horn.29–31 We further addressed whether PKG-I in nociceptors affects excitability of DRG neurons and its neighboring spinal dorsal horn neurons. As c-Fos is not well expressed in the DRG,32 we studied induction of pERK1/2, an alternate neuronal activity marker,23 following formalin injection in PKG-Ifl/fl and SNS-PKG-I−/− mice. As shown in Figure 3(c), formalin-injected PKG-Ifl/fl mice showed marked pERK1/2 induction in the DRG. In contrast, the number of neurons expressing pERK1/2 was markedly reduced in the DRG of SNS-PKG-I−/− mice (see Figure 3(c) for typical examples and Figure 3(d) for quantitative summary, P < 0.05, n = 4 mice). In the spinal cord, intense pERK1/2 induction was seen in the superficial lamina of the dorsal horn where the nociceptive primary afferents mainly terminates following formalin injection in PKG-Ifl/fl mice (Figure 3(c), lower panels). In striking contrast, SNS-PKG-I−/− mice showed a much less pERK1/2 expression in the superficial layers of spinal dorsal horn (see Figure 3(c) for typical examples and Figure 3(d) for quantitative summary, P < 0.05, n = 4 mice). Similarly, upon formalin injection, strong Fos-immunoreactivity was seen in the superficial and deep lamina of the dorsal horn following formalin injection in PKG-Ifl/fl mice (Figure 3(e)). In striking contrast, SNS-PKG-I−/− mice showed a much less Fos-positive cells in both the superficial and deep layers of spinal dorsal horn (see Figure 3(e) for typical examples and Figure 3(f) for quantitative summary, P < 0.05, n = 4 mice).
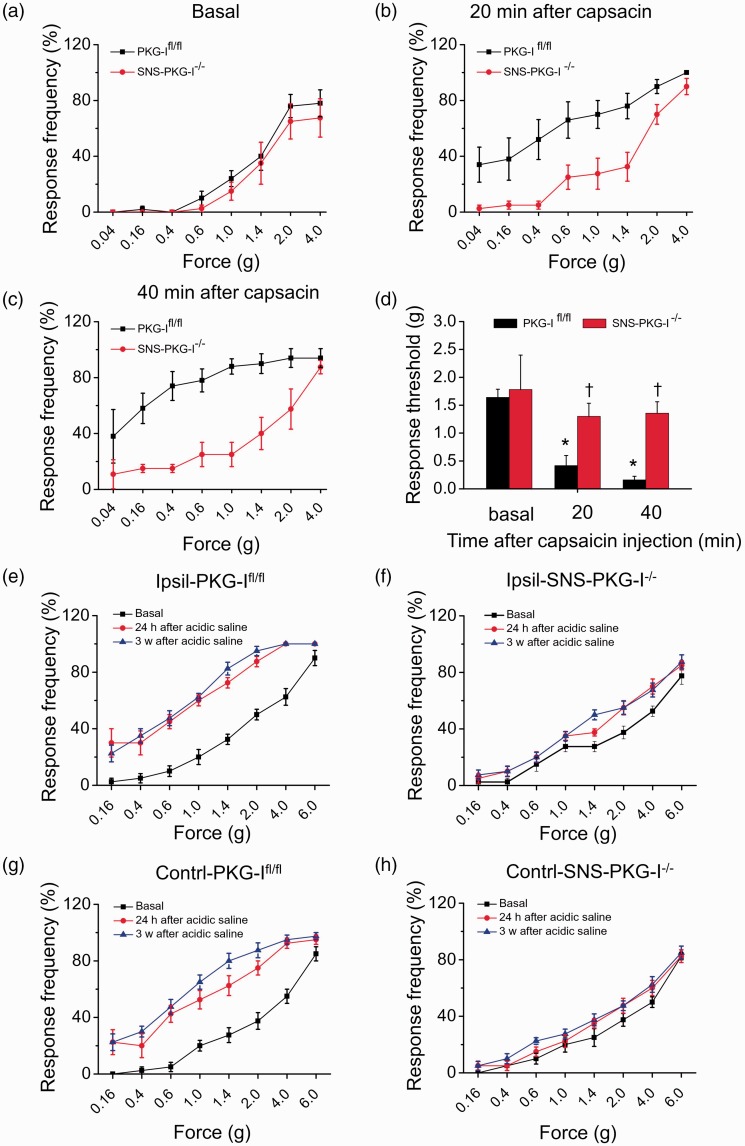
Reduced hyperalgesia and allodynia induced by lower thigh injection of capsaicin and muscular acidic saline injection in SNS-PKG-I−/− mice
We next examined whether peripherally localized PKG-I plays a key role in the secondary hyperalgesia and allodynia under pathological states, which reflects central amplification processes. To address this question, we take two pain models induced by subcutaneous (s.c.) lower thigh injection of capsaicin and muscular injection of acidic saline. Development of capsaicin-induced secondary hyperalgesia and allodynia was assessed in the hindpaw plantar surface at 20–40 min following unilateral injection of capsaicin in the skin of the lower thigh. The plantar surface was outside the area of capsaicin-induced flare. Upon capsaicin injection, PKG-Ifl/fl mice demonstrated the characteristic leftward and upward shift in the stimulus-response curve over basal curves, reflecting allodynia (measured here as responses to 0.04–0.6 g force, which are non-noxious in wild-type mice) as well as mechanical hyperalgesia (measured here as responses to 1.0–4.0 g force, which elicit withdrawal behavior in wild-type mice) (black squares in Figure 4(a) to (c)). In striking contrast to PKG-Ifl/fl mice, SNS-PKG-I−/− mice demonstrated very little deviation from baseline behavior upon capsaicin injection (red circles in Figure 4(a) to (c)). Furthermore, the relative drop in response thresholds to von Frey hairs (defined here as minimum force required to elicit 50% response frequency) in the inflamed state over basal (pre-capsaicin) state was markedly attenuated in SNS-PKG-I−/− mice as compared to PKG-Ifl/fl mice (Figure 4(d), n = 10–12 mice, P < 0.05).
Figure 4.
(a–c) Time course showing secondary mechanical hyperalgesia and allodynia induced by lower thigh injection of capsacin in PKG-Ifl/fl mice and SNS-PKG-I−/− mice. (d) Quantitative summary of response threshold to Von Frey filament applied to hindpaw plantar surface showing that capsaicin-induced secondary mechanical hyperalgesia and allodynia was significantly reduced in SNS-PKG-I−/− mice as compared to PKG-Ifl/fl mice. (e–h) Bilateral mechanical hyperalgesia and allodynia in a model of chronic muscle pain is reduced upon a specific loss of PKG-I in nociceptors. Intramuscular injection of acidic saline in PKG-Ifl/fl mice produced a dramatic, long-lasting mechanical hyperalgesia and allodynia in ipsilateral (e) and contralateral (g) hindpaws, displaying as a leftward and upward shift of stimulus-response curves over basal curves at 24 h and 3 weeks after second muscle acidic saline injection. Both ipsilateral (f) and contralateral (h) hypersensitivity was almost abolished by the deletion of PKG-I in nociceptors, displaying as very little deviation of stimulus-response curves from basal curves upon muscular acidic saline injection. *indicates statistically significant differences (P < 0.05) between basal and capsaicin treatment in PKG-Ifl/fl mice. †indicates statistically significant differences (P < 0.05) between PKG-Ifl/fl mice and SNS-PKG-I−/− mice upon capsaicin treatment (ANOVA, post hoc Fisher’s test, n = 8–10 mice per genotype).
Similarly, following two intramuscular injections of acidic saline three days apart, PKG-Ifl/fl mice produced a dramatic, long-lasting mechanical hyperalgesia and allodynia in bi-lateral hindpaws, displaying as a leftward and upward shift of stimulus-response curves over basal curves (Figure 4(e) and (g), n = 10–12 mice). The response thresholds to von Frey hairs in bilateral hindpaws were significantly lowered after acidic saline injection in PKG-Ifl/fl mice (Figure 4(e) and (g)). In contrast, this pain hypersensitivity was almost abolished by the deletion of PKG-I in nociceptors, displaying as very little deviation of stimulus-response curves from basal curves upon muscular acidic saline injection (Figure 4(f) and (h), n = 10–12 mice). The response thresholds to mechanical stimulation were not significantly altered after acidic saline injection in SNS-PKG-I−/− mice (Figure 4(f) and (h)).
Reduced c-Fos and pERK1/2 induction in the DRG and spinal cord after capsaicin and acidic saline injection in SNS-PKG-I−/− mice
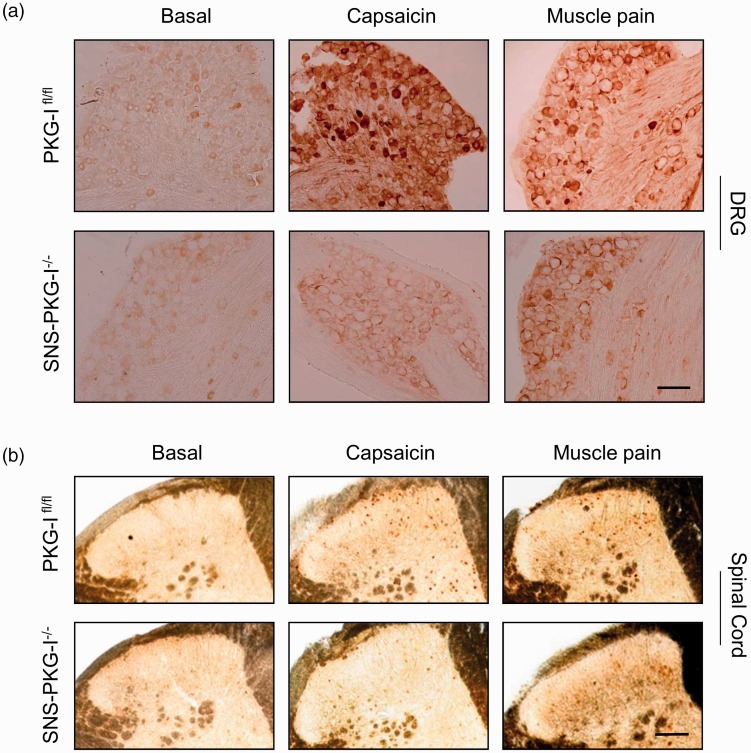
DRG
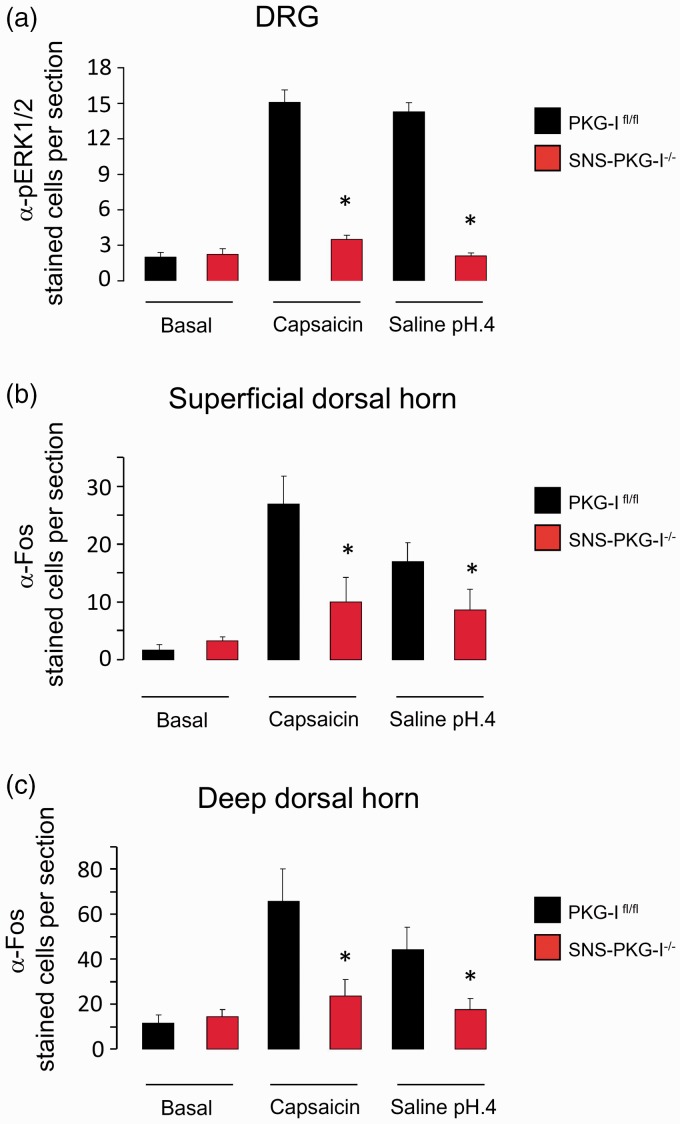
We further addressed whether PKG-I in nociceptors affects excitability of DRG neurons and its neighboring spinal dorsal horn neurons in hindlimb capsaicin and muscle pain models. As shown in Figure 5(a), capsaicin-treated PKG-Ifl/fl mice displayed pronounced pERK1/2 induction in the DRG. In striking contrast, very less pERK1/2 was induced in the DRG of mice lacking PKG-I in nociceptors (see Figure 5(a) for typical examples and Figure 6(a) for quantitative summary, P < 0.05, n = 4 mice). It also holds true for muscle pain model. Evaluation of pERK1/2 expression in the DRG induced by intramuscular acidic saline injection revealed a significant difference between PKG-Ifl/fl and SNS-PKG-I−/− mice (see Figure 5(a) for typical examples and Figure 6(a) for quantitative summary, P < 0.05, n = 4 mice).
Figure 5.
(a) Typical examples showing that induction of pERK1/2 was observed in the DRG at 1 h following hindlimb injection of capsaicin or acidic saline in PKG-Ifl/fl mice and SNS-PKG-I−/− mice. (b) Typical examples showing that c-Fos immunoreactivity was induced in both the superficial and deep lamina of the spinal cord at 1 h following hindlimb injection of capsaicin or acidic saline in PKG-Ifl/fl mice and SNS-PKG-I−/− mice. Scale bars represent 50 µm for DRG and 100 µm for spinal cord.
Figure 6.
(a) Quantitative summary showing a dramatic reduction of pERK1/2 expression in both capsaicin and muscle pain models in mice lacking PKG-I in nociceptors, as compared to their PKG-Ifl/fl littermates. (b, c) Quantification of the number of Fos positive cells revealed that Fos immunoreactivity in both capsaicin and muscle pain models was greatly reduced in the superficial (b) and deep spinal dorsal horn (c) in SNS-PKG-I−/− mice, as compared to PKG-Ifl/fl mice. *indicates statistically significant differences (P < 0.05) between PKG-Ifl/fl mice and SNS-PKG-I−/− mice (ANOVA, post hoc Fisher’s test, n = 4 mice per genotype).
Spinal cord
In PKG-Ifl/fl mice, dense Fos-immunoreactive neurons were seen in medial half of superficial (laminae I–II) and deep (laminae V–VI) layers of ipsilateral spinal dorsal horn to the capsaicin injection side (typical examples are shown in Figure 5(b) and cell counts are shown in Figure 6(b) and (c)). In contrast, very few cells in the above regions showed c-Fos induction in capsaicin-injected SNS-PKG-I−/− mice (Figure 5(b)). Analysis of the number of c-Fos positive cells in both superficial and deep lamina revealed that there was significant difference between two genotypes (Figure 6(b) and (c), P < 0.05, n = 4 mice per group). Similar results were obtained upon studying c-Fos induction in PKG-Ifl/fl and SNS-PKG-I−/− mice 24 h after muscle injury caused by acidic saline (Figures 5(b) and 6(b), (c), P < 0.05 between two genotypes).
Reduced c-Fos induction in the supraspinal regions along ascending pain pathways after capsaicin and acidic saline injection in SNS-PKG-I−/− mice
It is well accepted that the development and maintenance of the secondary hyperalgesia and allodynia in the hindpaws induced by s.c. lower thigh capsaicin injection and intramuscular acidic saline injection is associated with the changes in the central nervous system (CNS).27,33 We were therefore interested to know whether neuronal activity in the central regions in the somatosensory pain pathways mirrored the capsaicin and acid-induced secondary hyperalgesia and allodynia and whether PKG-I in nociceptors affects the neuronal activity in the ascending pain pathways. For this purpose, we studied induction of c-Fos in the central regions following capsaicin injection and acidic saline injection in PKG-Ifl/fl and SNS-PKG-I−/− mice.
Reduced c-Fos expression in the regions responsible for sensory-discriminative components of pain in SNS-PKG-I−/− mice
The superficial and deep dorsal horn of the spinal cord receives a major input from nociceptive primary afferents. The spinothalamic tract is an important ascending pathway, providing direct spinal input to the thalamus, from which information is transmitted to primary somatosensory cortical areas that underlie the sensory-discriminative components of pain perception. We then examined the profile of c-Fos expression induced by capsaicin injection and acidic saline injection in the thalamus, primary and secondary somatosensory cortex, and whether PKG-I in nociceptors exerts any effect on the above c-Fos expression.
Thalamus
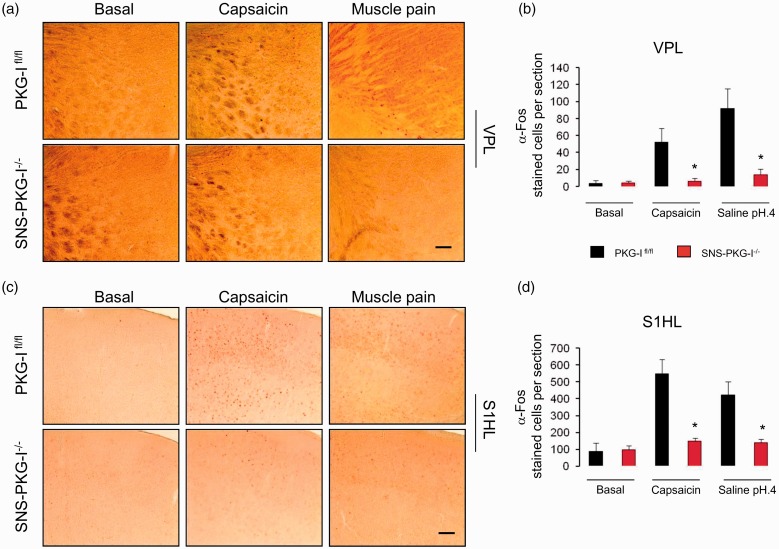
Following either capsaicin or acidic saline injection, the VPL of the thalamus showed an increased level of c-Fos expression in PKG-Ifl/fl mice as compared to control group (Figure 7(a), upper panels). In striking contrast, c-Fos expression was absent in SNS-PKG-I−/− mice by the same noxious stimulation (Figure 7(a), lower panels). Quantitative analysis of c-Fos expression in the VPL was shown in Figure 7(b). In addition, in the midline thalamus, paraventricular nucleus also showed massive c-Fos induction in PKG-Ifl/fl mice, which was profoundly reduced in SNS-PKG-I−/− mice (Supplementary Figure 1).
Figure 7.
Induction of c-Fos in the regions responsible for sensory-discriminative components of pain in the capsaicin and muscle pain model in PKG-Ifl/fl mice and SNS-PKG-I−/− mice. Fos immunoreactivity was induced in the ventral posterolateral nucleus (VPL) of the thalamus (a) and topographic hindlimb regions of primary somatosensory cortex (S1HL) (c) following capsaicin and acidic saline injection, respectively, in PKG-Ifl/fl mice and SNS-PKG-I−/− mice. (b, d) Quantification of the number of Fos positive cells revealed that Fos immunoreactivity in both capsaicin and muscle pain model was greatly reduced in the the ventral posterolateral nucleus (VPL) of the thalamus (b) and topographic hindlimb regions of primary somatosensory cortex (S1HL) (d) in SNS-PKG-I−/− mice, as compared to PKG-Ifl/fl mice. *indicates statistically significant differences (P < 0.05) between PKG-Ifl/fl mice and SNS-PKG-I−/− mice (ANOVA, post hoc Fisher’s test, n = 4 mice per genotype). Scale bars represent 500 µm.
Somatosensory cortex
The somatosensory cortex is important for the localization of pain. Massive c-Fos positive cells were seen in the topographic hindlimb regions of S1HL in capsaicin-treated PKG-Ifl/fl mice as compared to vehicle-treated group (Figure 7(c)). In comparison with PKG-Ifl/fl littermates, SNS-PKG-I−/− mice showed a much lowered level of c-Fos expression after capsaicin injection (Figure 7(c)). Quantitative analysis of the number of c-Fos positive cells revealed a significant difference between two genotypes (Figure 7(d), P < 0.05, n = 4 mice per group). Similar results were obtained by assessing c-Fos expression from PKG-Ifl/fl and SNS-PKG-I−/− mice upon intramuscular injection of acidic saline (Figure 7(c) and (d)). In addition, the secondary somatosensory cortex (SII) has been shown to be important for the sensory-discriminative dimension of pain as well.34 We observed that SII showed massive c-Fos induction in either capsaicin or muscle pain model in PKG-Ifl/fl mice (Supplementary Figure 2(a)). However, deletion of PKG-I in nociceptors did not alter c-Fos expression in SII region in both pain models (Supplementary Figure 2(a) and (b)).
Reduced c-Fos expression in the regions responsible for aversive components of pain in SNS-PKG-I−/− mice
Evidence from anatomical, behavioral, and physiological experiments has shown that apart from the spinothalamic tract, a large brain region can also be activated by pain experience, such as ACC, amygdala, PFC, insula, and subcortical areas, e.g., PAG, parabrachial nucleus (PB) which may subserve the information relating to the aversive components of pain. We are therefore interested to know the profile of c-Fos expression induced by capsaicin injection and acidic saline injection in the ACC, amygdala, PFC, insula, PAG, and PB and whether PKG-I in nociceptors exerts any effect on the above c-Fos expression.
Anterior cingulate cortex
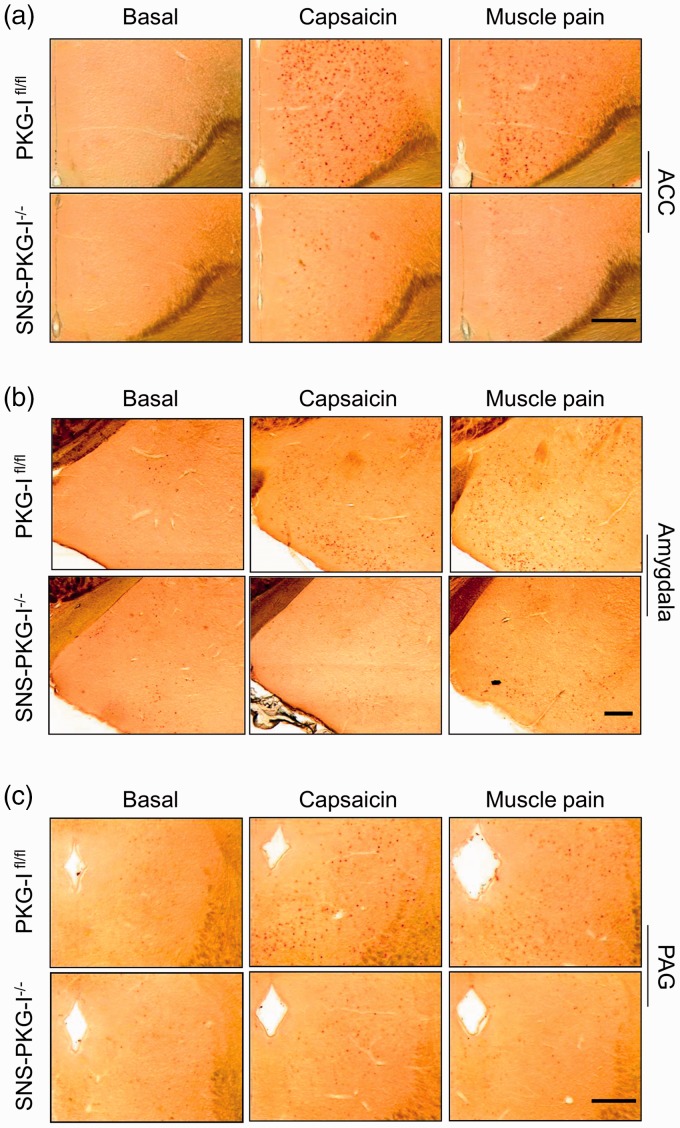
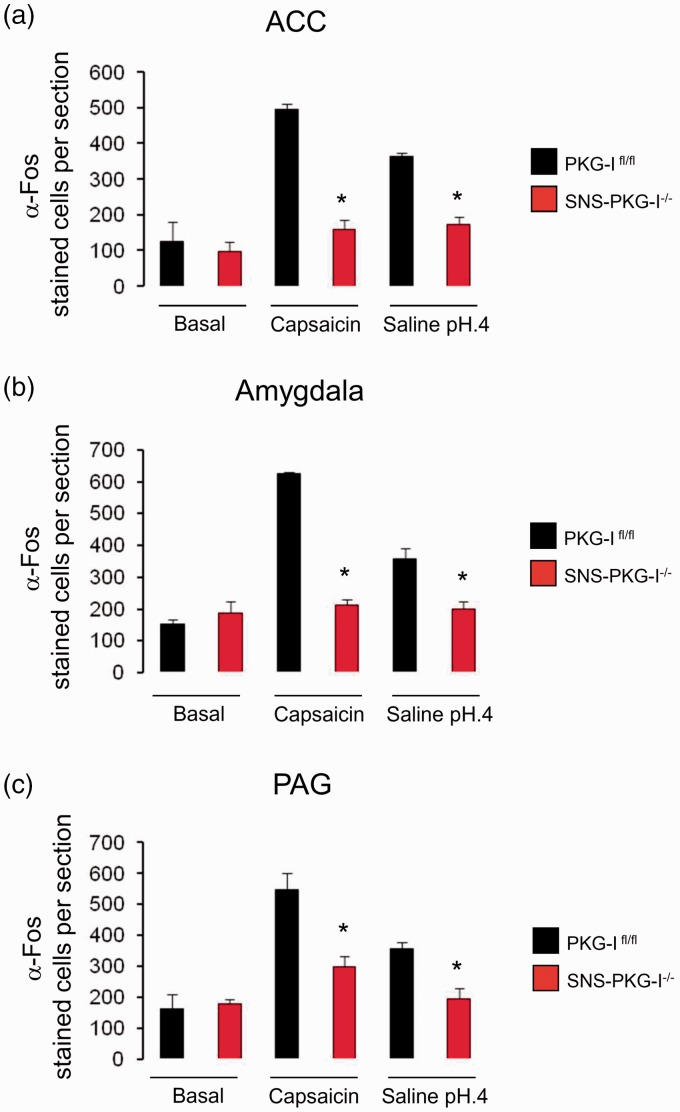
ACC is an important limbic structure which involves the processing of aversive components of pain.4,35,36 As shown in Figure 8, when compared to vehicle group, capsaicin injection evoked a robust increase of c-Fos immunoreactivity in the contralateral ACC derived from PKG-Ifl/fl mice (Figure 8(a), upper panels). Similar c-Fos expression profile was seen after intramuscular injection of acidic saline in PKG-Ifl/fl mice (Figure 8(a), upper panels). In striking contrast, either capsaicin or acidic saline injection produced a much less c-Fos expression in the ACC derived from SNS-PKG-I−/− mice (Figure 8(a), lower panels, P < 0.05, n = 4 mice per group). Quantitative analysis was represented in Figure 9(a).
Figure 8.
Induction of c-Fos in the regions responsible for aversive components of pain in the capsaicin and muscle pain model in PKG-Ifl/fl mice and SNS-PKG-I−/− mice. Fos immunoreactivity was induced in the anterior cingulate cortex (ACC), amygdala, and periaqueductal gray (PAG) following capsaicin and acidic saline injection, respectively, in PKG-Ifl/fl mice and SNS-PKG-I−/− mice. Scale bars represent 500 µm.
Figure 9.
Quantification of the number of Fos positive cells revealed that Fos immunoreactivity in both capsaicin and muscle pain model was greatly reduced in the ACC (a), amygdala (b), and PAG in SNS-PKG-I−/− mice, as compared to PKG-Ifl/fl mice. *indicates statistically significant differences (P < 0.05) between PKG-Ifl/fl mice and SNS-PKG-I−/− mice (ANOVA, post hoc Fisher’s test, n = 4 mice per genotype).
Prefrontal cortex
Existing knowledge identifies the PFC as the modulatory area for pain.37 In PKG-Ifl/fl mice, massive c-Fos expression is seen in the prefrontal cortex after either capsaicin injection or acidic saline injection over basal state (Supplementary Figure 3(a), upper panels). In striking contrast, very less c-Fos immunoreactive cells was observed in SNS-PKG-I−/− mice after capsaicin or acidic saline injection (Supplementary Figure 3(a), lower panels). There was significant difference in c-Fos expression between two genotypes (Supplementary Figure 3(b), P < 0.05).
Amygdala
Accumulating evidence indicates that the amygdala is a key structure in the central mechanisms of nociception.38 In PKG-Ifl/fl mice, c-Fos expression is profoundly increased in the amygdala following either capsaicin injection or acidic saline injection over basal state (Figure 8(b), upper panels). As compared to the PKG-Ifl/fl littermates, SNS-PKG-I−/− mice showed very less c-Fos positive cells in the amygdala after capsaicin or acidic saline injection (Figure 8(b), lower panels). The difference of c-Fos expression between PKG-Ifl/fl and SNS-PKG-I−/− mice was significant (Figure 9(b), P < 0.05).
Insular cortex
Evidence from experimental studies in animals and brain imaging studies in human subjects consistently indicated that the insular cortex is prominently activated in acute and chronic pain states.39,40 Unilateral hindlimb injection of capsaicin induced dramatic c-Fos induction in the posterior insular cortex in PKG-Ifl/fl mice, as compared to vehicle-injected group (Supplementary Figure 3(c), upper panels). In contrast, very sparse c-Fos expression can be seen in the posterior insular cortex in mice specifically lacking PKG-I in nociceptors (Supplementary Figure 3(c), lower panels). Quantitative analysis revealed a significant difference in c-Fos expression induced by capsaicin injection between two genotypes (Supplementary Figure 3(d), P < 0.05). Similar results were obtained for intramuscular injection of acidic saline (Supplementary Figure 3(c) and (d), P < 0.05).
Periaquductal gray
The PAG may well be involved in the pain process not only through the descending modulation of nociceptive afferent information but also by its ascending projections to the pain processing centers of the thalamus.41 In PKG-Ifl/fl mice, c-Fos expression is profoundly increased in the PAG following either capsaicin injection or intramuscular acidic saline injection over basal state (Figure 8(c), upper panels). As compared to the PKG-Ifl/fl littermates, SNS-PKG-I−/− mice showed very less c-Fos positive cells in the PAG after capsaicin or acidic saline injection (Figure 8(c), lower panels). The difference of c-Fos expression between PKG-Ifl/fl and SNS-PKG-I−/− mice was significant (Figure 9(c), P < 0.05).
Parabrachial nucleus
As the relay station of sensory information, the PBN is thought to be the critical sites for central perception, integration, and transmission of sensory information especially nociceptive information.42–44 In the present study, we observed massive c-Fos expression in the lateral PBN in PKG-Ifl/fl mice following hindlimb capsaicin injection or intramuscular acidic saline injection (Supplementary Figure 4(a), left panels). In striking contrast, the expression level of c-Fos was much lowered in either capsaicin or muscle pain model after deletion of PKG-I in nociceptors (Supplementary Figure 4(a), right panels). Quantitative summary revealed an obvious difference in c-Fos expression between two genotypes (Supplementary Figure 4(b), P < 0.05, n = 4 mice).
Discussion
Clinical pain associated with tissue damage or nerve injury is characterized by persistent pain hypersensitivity. This includes spontaneous pain (pain experienced in the absence of any obvious peripheral stimulus), hyperalgesia (an increased responsiveness to noxious stimuli), and allodynia (pain in response to normally innocuous stimuli). The cellular and molecular mechanism underlying spontaneous pain and hyperalgesia as well as allodynia is not well understood. The present study unravels a peripheral target, PKG-I localized in primary afferent nociceptors, which mediates spontaneous pain and hyperalgesia as well as allodynia associated with peripheral injury. Furthermore, this PKG-I-mediated pain hypersensitivity is mediated by its modulation of the nociceptive plasticity from the periphery to the cortex along the ascending pain pathways.
PKG-I localized in nociceptors facilitates spontaneous pain and hyperalgesia as well as allodynia following peripheral injury
Immunohistochemical studies have shown that PKG-I is widely expressed in various regions along the somatosensory pain pathways, including DRG, spinal cord, and several regions in the brain.12,45,46 So far, regarding the role of PKG-I in pain modulation, pharmacological inhibitors of PKG-I, diverse cGMP analogs, as well as global deletion mutants of PKG-I have yielded ambiguous results and have failed to clarify the locus of PKG-I action.15,18,47–52 For example, pharmacological studies have suggested that PKG-I contributes to spinal hyperalgesia. Delivery of PKG inhibitors reduces formalin-induced nociceptive behavior and abrogates thermal hyperalgesia induced by injection of the NO donors.18 Paradoxically, however, the cGMP-PKG-I pathway has also been implicated in inhibition of sensitization via activation of diverse K+ channels and in mediating hypoalgesia caused by drugs, such as Gabapentin.49,52,53 Whereas some electrophysiological studies ascribed a role for PKG-I in long-term hyperexcitability in sensory neurons in Aplysia14 and rodents,15 others reported lack of effects or even depressive functions for PKG-I on cultured sensory neurons.50 Dose-dependent reciprocal modulation of pain-associated behaviors by cGMP analogs has also been reported.47 The most plausible explanation for these contradictory findings is the serious lack of specificity of commonly used pharmacological modulators of the cGMP-PKG pathway; for example, KT5823, which was used a “specific” PKG-I inhibitor in several studies, has been shown to block several other kinases and only unreliably block PKG-I in a context-dependent manner.54,55 Furthermore, a recent study which systematically elucidated the selectivity and cross-reactivity of commonly used cyclic nucleotide analogs has revealed that most cAMP and cGMP analogs have multiple molecular targets and that conventional PKG-I inhibitors cross-react with the cAMP system by profoundly affecting activity of diverse phosphodiesterase.56 Mice genetically engineered to constitutively lack PKG-I show reduced nociceptive behaviors evoked by injection of irritants like formalin, suggesting that PKG-I plays a pro-nociceptive role in sensory processing.18 However, an unambiguous interpretation of these results is occluded by the multiple developmental deficits in sensorimotor circuits18,57 and other organs as well as early post-natal lethality, observed in these mutants.10,58
In the present study, using viable, developmentally normal transgenic mice lacking the PKG-I specifically in primary afferent nociceptors with preserved expression in spinal neurons, brain, and all other organs, we show here that PKG-I localized in primary afferent nociceptors constitutes a key mediator of pain hypersensitivity in sub-acute and chronic inflammatory pain states. We took intraplantar formalin model to test the role of PKG-I in nociceptors in the development of spontaneous pain behavior. Following intraplantar formalin injection, biphasic spontaneous pain responses were greatly reduced by the deletion of PKG-I in nociceptors. This inhibitory effect in SNS-PKG-I−/− mice is comparable to that produced in global PKG-I knockout mice,18 suggesting that a large component of spontaneous nociception is mediated by PKG-I expression in primary afferent nociceptors. To explore the contribution of PKG-I in nociceptors in the development of hyperalgesia and allodynia, we utilized two models in which lower thigh injection of capsaicin or intramuscular injection of acidic saline produces dramatic hyperalgesia and allodynia in the hindpaws of mice. We found that the extent of hyperalgesia and allodynia in either pain model was much lowered in SNS-PKG-I−/− mice in comparison with their littermate controls. In support of this result, our recent study demonstrated that SNS-PKG-I−/− mice showed a much loss of spinal long-term potentiation, which is assumed to serve as a cellular and molecular mechanism for pain hypersensitivity caused by injury or inflammation.19 In striking contrast, PKG-I is not required in the basal nociception. We infer therefore that the main function of PKG-I in primary sensory neurons is to amplify nociceptive signals into the CNS when nociceptors are persistently activated in pathological states.
PKG-I localized in nociceptors modulates nociceptive neuronal activity along the somatosensory pain pathways after injury or inflammation
Numerous studies have consistently shown that injury or inflammation produces increased neuronal excitability in the regions that are involved in the processing of painful information from the periphery to CNS.1,2,59,60 However, the mechanism underlying this neuronal plasticity is not well understood. Several lines of evidence have suggested that c-Fos and pERK1/2 may be useful as a sensitive marker of the neuronal activation and plasticity following noxious stimuli.20–22,23 In the present study, we systemically demonstrated that populated neurons along the somatosensory pain pathways exhibited increased activity after various peripheral irritants challenge, displaying as an up-regulation of c-Fos and pERK1/2 in various regions from DRG to cerebral cortex. More importantly, this increased nociceptive neuronal activity along the somatosensory pain pathways is dependent upon activation of PKG-I localized in nociceptors.
PKG-I activation in nociceptors is involved in the neuronal plasticity in DRG and spinal cord in pathological states
Previous electrophysiological studies by use of inhibitors of cGMP-PKG pathway have demonstrated that peripheral PKG-I contributes to DRG neuronal hyperexcitability caused by peripheral injury.13–16 Since PKG-I is also expressed in Schwann cells in the DRG,17 the use of pharmacological agents above cannot fully dissect the PKG-I contribution expressed in primary nociceptive neurons in DRG neuronal hyperexcitability. To this end, we in the present study utilized PKG-I mutant mice specifically in nociceptors to clarify the functional relevance of PKG-I in injury-related neuronal hyperexcitability. Following hindlimb irritants challenge with formalin, capsaicin, or acidic saline, a dramatic increase of c-Fos and pERK1/2 induction was seen in the DRG and spinal dorsal horn, especially in lamina I and II, and lamina V and VI of the dorsal horn, indicating an increased neuronal activity of the DRG and spinal dorsal horn after peripheral irritants challenge. Deletion of PKG-I in nociceptors eliminated this c-Fos and pERK1/2 induction, indicating a key role of PKG-I in nociceptive primary sensory neurons on the increased neuronal activity of DRG and spinal dorsal horn caused by peripheral injury.
PKG-I activation in nociceptors is involved in the increased cortical neuronal activity along the somatosensory pain pathways in pathological states
The spinal dorsal horn neurons receive major projections from nociceptive DRG neurons and ascend incoming pain signals to the cortex via several ascending pathways so that pain is ultimately perceived in its multiple dimensions. For instance, the lateral spinothalamic tract projects multimodal sensory inputs from spinal dorsal horn to the lateral thalamus, which in turn send sensory signals to the somatosensory cortex; this pathway has been implicated in processing sensory and discriminative aspects of pain. By contrast, the medial spinothalamic tract and the spinoparabrachial as well as spinomesencephalic tract project nociceptive signals to the medial thalamus, which send sensory signals further to widespread for brain regions such as ACC, PFC, amygdala, and insula; this pathway are believed to mediate the emotional and aversive components of pain.4,61 The present study systemically demonstrated that peripheral noxious stimuli (e.g., lower thigh injection of capsaicin or intramuscular injection of acidic saline) caused an increase in neuronal activity along the spinothalamic tract, manifesting as dramatic c-Fos expression in various regions including spinal dorsal horn, ventral posterolateral nucleus of thalamus, and topographic hindlimb regions of S1HL. More importantly, this increase of c-Fos expression along the spinothalamic tract is greatly reduced by the deletion of PKG-I in nociceptors, suggesting that the increased cortical neuronal activity involved in the sensory-discriminative components of pain is dependent on PKG-I activation in primary nociceptors.
An increasing body of evidence from anatomical, neurochemical, electrophysiological, and behavioral studies strongly supports the concept of ACC, PFC, amygdala, and insula as important players in the emotional-affective dimension of pain.59,62 Consistent with this, we demonstrated that Fos-positive cells were also observed in several limbic brain areas, such as ACC, PFC, amygdala, and insula after peripheral irritants challenge, in addition to the regions directly linked to processing sensory and discriminative information of pain. More importantly, c-Fos upregulation in limbic brain areas is dependent on PKG-I activation in primary nociceptors as well, suggesting a pivotal role of PKG-I localized in nociceptors in modulation of increased neuronal activity in the cortical areas involved in the aversive component of pain.
Conclusions
In summary, our results show that PKG-I expressed in nociceptors is not only a key determinant of DRG hyperexcitability and spinal potentiation but also an important modulator of increased cortical neuronal activity observed in pathological pain states. This study presents a strong basis for the PKG-I as a new class of targets in the periphery for pain therapeutics.
Supplementary Material
Acknowledgments
The authors are grateful towards Rohini Kuner for her critical comments on the manuscript, towards Feil Robert and Franz Hofmann for the kind gift of PKG-I flox mice.
Author contributions
VG, XW, and CL conceived of the project and conducted the experiments. CL drafted and finished the final manuscript. VG and XW contributed to this work equally.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Natural Science Foundation of China (NSFC) Grants (No. 31471059, 31671088) to C.L. and by the State Key Laboratory of Neuroscience (No. SKLN-2015B02) to C.L.
Supplementary Material
The supplementary material is available at http://journals.sagepub.com/doi/suppl/DOI10.1177/1744806917701743.
References
- 1.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science 2000; 288: 1765–1769. [DOI] [PubMed] [Google Scholar]
- 2.Woolf CJ, Ma Q. Nociceptors–noxious stimulus detectors. Neuron 2007; 55: 353–364. [DOI] [PubMed] [Google Scholar]
- 3.Luo C, Kuner T, Kuner R. Synaptic plasticity in pathological pain. Trends Neurosci 2014; 37: 343–355. [DOI] [PubMed] [Google Scholar]
- 4.Bliss TV, Collingridge GL, Kaang BK, et al. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci 2016; 17: 485–496. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda H, Heinke B, Ruscheweyh R, et al. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 2003; 299: 1237–1240. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda H, Stark J, Fischer H, et al. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science 2006; 312: 1659–1662. [DOI] [PubMed] [Google Scholar]
- 7.Baranano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trends Neurosci 2001; 24: 99–106. [DOI] [PubMed] [Google Scholar]
- 8.Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain 1993; 52: 127–136. [DOI] [PubMed] [Google Scholar]
- 9.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev 2002; 82: 769–824. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann F, Feil R, Kleppisch T, et al. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 2006; 86: 1–23. [DOI] [PubMed] [Google Scholar]
- 11.Schmidtko A, Gao W, Sausbier M, et al. Cysteine-rich protein 2, a novel downstream effector of cGMP/cGMP-dependent protein kinase I-mediated persistent inflammatory pain. J Neurosci 2008; 28: 1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian Y, Chao DS, Santillano DR, et al. cGMP-dependent protein kinase in dorsal root ganglion: relationship with nitric oxide synthase and nociceptive neurons. J Neurosci 1996; 16: 3130–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang ZJ, Li HC, Liu S, et al. Activation of cGMP-PKG signaling pathway contributes to neuronal hyperexcitability and hyperalgesia after in vivo prolonged compression or in vitro acute dissociation of dorsal root ganglion in rats. Sheng Li Xue Bao 2012; 64: 563–576. [PubMed] [Google Scholar]
- 14.Lewin MR, Walters ET. Cyclic GMP pathway is critical for inducing long-term sensitization of nociceptive sensory neurons. Nat Neurosci 1999; 2: 18–23. [DOI] [PubMed] [Google Scholar]
- 15.Song XJ, Wang ZB, Gan Q, et al. cAMP and cGMP contribute to sensory neuron hyperexcitability and hyperalgesia in rats with dorsal root ganglia compression. J Neurophysiol 2006; 95: 479–492. [DOI] [PubMed] [Google Scholar]
- 16.Zheng JH, Walters ET, Song XJ. Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP. J Neurophysiol 2007; 97: 15–25. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Chopp M, Szalad A, et al. Phosphodiesterase-5 is a therapeutic target for peripheral neuropathy in diabetic mice. Neuroscience 2011; 193: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tegeder I, Del TD, Schmidtko A, et al. Reduced inflammatory hyperalgesia with preservation of acute thermal nociception in mice lacking cGMP-dependent protein kinase I. Proc Natl Acad Sci U S A 2004; 101: 3253–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo C, Gangadharan V, Bali KK, et al. Presynaptically localized cyclic GMP-dependent protein kinase 1 is a key determinant of spinal synaptic potentiation and pain hypersensitivity. PLoS Biol 2012; 10: e1001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature 1987; 328: 632–634. [DOI] [PubMed] [Google Scholar]
- 21.Luo C, Chen J, Li HL, et al. Spatial and temporal expression of c-Fos protein in the spinal cord of anesthetized rat induced by subcutaneous bee venom injection. Brain Res 1998; 806: 175–185. [DOI] [PubMed] [Google Scholar]
- 22.Coggeshall RE. Fos, nociception and the dorsal horn. Prog Neurobiol 2005; 77: 299–352. [DOI] [PubMed] [Google Scholar]
- 23.Ji RR, Baba H, Brenner GJ, et al. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci 1999; 2: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 24.Wegener JW, Nawrath H, Wolfsgruber W, et al. cGMPdependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ Res 2002; 90: 18–20. [DOI] [PubMed]
- 25.Agarwal N, Offermanns S, Kuner R. Conditional gene deletion in primary nociceptive neurons of trigeminal ganglia and dorsal root ganglia. Genesis 2004; 38: 122–129. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Luo C, Li H, et al. Primary hyperalgesia to mechanical and heat stimuli following subcutaneous bee venom injection into the plantar surface of hindpaw in the conscious rat: a comparative study with the formalin test. Pain 1999; 83: 67–76. [DOI] [PubMed] [Google Scholar]
- 27.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 2001; 24: 37–46. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal N, Pacher P, Tegeder I, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 2007; 10: 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tjolsen A, Berge OG, Hunskaar S, et al. The formalin test: an evaluation of the method. Pain 1992; 51: 5–17. [DOI] [PubMed] [Google Scholar]
- 30.Coderre TJ, Vaccarino AL, Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res 1990; 535: 155–158. [DOI] [PubMed] [Google Scholar]
- 31.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983; 306: 686–688. [DOI] [PubMed] [Google Scholar]
- 32.Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J 2009; 2: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer RA, Ringkamp M, Campbell JN, et al. Peripheral mechanism of cutaneous nociception. In: McMahon SB, Koltzenburg M. (eds). Wall and Melzack’s textbook of pain, London: Elsevier Churchill Livingstone, 2006, pp. 3–34. [Google Scholar]
- 34.Maihöfner C, Herzner B, Otto Handwerker H. Secondary somatosensory cortex is important for the sensory-discriminative dimension of pain: a functional MRI study. Eur J Neurosci 2006; 23: 1377–1383. [DOI] [PubMed] [Google Scholar]
- 35.Gao YJ, Ren WH, Zhang YQ, et al. Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain 2004; 110: 343–353. [DOI] [PubMed] [Google Scholar]
- 36.Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci 2001; 98: 8077–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitsi V, Zachariou V. Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience 2016; 338: 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neugebauer V, Li W, Bird GC, et al. The amygdala and persistent pain. Neuroscientist 2004; 10: 221–234. [DOI] [PubMed] [Google Scholar]
- 39.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 2011; 152: S49–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson LA, Gandevia SC, Macefield VG. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain 2007; 128: 20–30. [DOI] [PubMed] [Google Scholar]
- 41.Linnman C, Moulton EA, Barmettler G, et al. Neuroimaging of the periaqueductal gray: state of the field. Neuroimage 2012; 60: 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aicher SA, Hermes SM, Hegarty DM. Corneal afferents differentially targetthalamic- and parabrachial-projecting neurons in spinal trigeminal nucleuscaudalis. Neuroscience 2013; 232: 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu YC, Chen YZ, Wei YY, et al. Neurochemical properties of the synapses between theparabrachial nucleus-derived CGRP-positive axonal terminals and the GABAergic neurons in the lateral capsular division of central nucleus ofamygdala. Mol Neurobiol 2015; 51: 105–118. [DOI] [PubMed] [Google Scholar]
- 44.Missig G, Roman CW, Vizzard MA, et al. Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide(PACAP) signaling in the amygdala: implication for the sensory andbehavioral effects of pain. Neuropharmacology 2014; 86: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feil R, Kleppisch T. NO/cGMP-dependent modulation of synaptic transmission. Handb Exp Pharmacol 2008; 184: 529–560. [DOI] [PubMed] [Google Scholar]
- 46.Feil S, Zimmermann P, Knorn A, et al. Distribution of cyclic GMP-dependent protein kinase type I and its isoforms in the mouse brain and retina. Neuroscience 2005; 135: 863–868. [DOI] [PubMed] [Google Scholar]
- 47.Tegeder I, Schmidtko A, Niederberger E, et al. Dual effects of spinally delivered 8-bromo-cyclic guanosine mono-phosphate (8-bromo-cGMP) in formalin-induced nociception in rats. Neurosci Lett 2002; 332: 146–150. [DOI] [PubMed] [Google Scholar]
- 48.Tao YX, Hassan A, Haddad E, et al. Expression and action of cyclic GMP-dependent protein kinase Ialpha in inflammatory hyperalgesia in rat spinal cord. Neuroscience 2000; 95: 525–533. [DOI] [PubMed] [Google Scholar]
- 49.Sachs D, Cunha FQ, Ferreira SH. Peripheral analgesic blockade of hypernociception: activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc Natl Acad Sci USA 2004; 101: 3680–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kress M, Rodl J, Reeh PW. Stable analogues of cyclic AMP but not cyclic GMP sensitize unmyelinated primary afferents in rat skin to heat stimulation but not to inflammatory mediators, in vitro. Neuroscience 1996; 74: 609–617. [DOI] [PubMed] [Google Scholar]
- 51.Salter M, Strijbos PJ, Neale S, et al. The nitric oxide-cyclic GMP pathway is required for nociceptive signalling at specific loci within the somatosensory pathway. Neuroscience 1996; 73: 649–655. [DOI] [PubMed] [Google Scholar]
- 52.Jain NK, Patil CS, Singh A, et al. Sildenafil-induced peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Brain Res 2001; 909(1–2): 170–178. [DOI] [PubMed] [Google Scholar]
- 53.Mixcoatl-Zecuatl T, Flores-Murrieta FJ, Granados-Soto V. The nitric oxide-cyclic GMP-protein kinase G-K+ channel pathway participates in the antiallodynic effect of spinal gabapentin. Eur J Pharmacol 2006; 531(1–3): 87–95. [DOI] [PubMed] [Google Scholar]
- 54.Bain J, McLauchlan H, Elliott M, et al. The specificities of protein kinase inhibitors: an update. Biochem J 2003; 371: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burkhardt M, Glazova M, Gambaryan S, et al. KT5823 inhibits cGMP-dependent protein kinase activity in vitro but not in intact human platelets and rat mesangial cells. Biol Chem 2000; 275: 33536–33541. [DOI] [PubMed] [Google Scholar]
- 56.Poppe H, Rybalkin SD, Rehmann H, et al. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods 2008; 4: 277–278. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt H, Werner M, Heppenstall PA, et al. cGMP-mediated signaling via cGKIalpha is required for the guidance and connectivity of sensory axons. J Cell Biol 2002; 159: 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfeifer A, Klatt P, Massberg S, et al. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J 1998; 17: 3045–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhuo M. Cortical excitation and chronic pain. Trends Neurosci 2008; 31: 199–207. [DOI] [PubMed] [Google Scholar]
- 60.Kuner R. Central mechanisms of pathological pain. Nat Med 2010; 16: 1258–1266. [DOI] [PubMed] [Google Scholar]
- 61.Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol 2015; 227: 261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neugebauer V, Galhardo V, Maione S, et al. Forebrain pain mechanisms. Brain Res Rev 2009; 60: 226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.