Abstract
During the initial months of the 2013–2016 Ebola epidemic, rapid geographical dissemination and intense transmission challenged response efforts across West Africa. Contextual behaviours associated with increased risk of exposure included travel to high-transmission settings, caring for sick and preparing the deceased for traditional funerals. Although such behaviours are widespread in West Africa, high-transmission pockets were observed. Superspreading and clustering are typical phenomena in infectious disease outbreaks, as a relatively small number of transmission chains are often responsible for the majority of events. Determining the characteristics of contacts at greatest risk of developing disease and of cases with greatest transmission potential could therefore help curb propagation of infection. Our analysis of contact tracing data from Montserrado County, Liberia, suggested that the probability of transmission was 4.5 times higher for individuals who were reported as having contact with multiple cases. The probability of individuals developing disease was not significantly associated with age or sex of their source case but was higher when they were in the same household as the infectious case. Surveillance efforts for rapidly identifying symptomatic individuals and effectively messaged campaigns encouraging household members to bring the sick to designated treatment centres without administration of home care could mitigate transmission.
This article is part of the themed issue ‘The 2013–2016 West African Ebola epidemic: data, decision-making and disease control’.
Keywords: Ebola virus disease, West Africa, contact tracing, infectious disease transmission, targeted intervention
1. Introduction
In late March 2014, the first cases of Ebola virus disease (EVD) in Liberia were confirmed, but it was dissemination into Monrovia three months later that fuelled the country's unprecedented epidemic [1]. By September, 350 incident cases were being reported per week in Liberia. Amidst response efforts that ranged from school closures to self-quarantine to community-driven active case finding, the growth of the epidemic curve was reversed by November. The unexpectedly dramatic downturn in incidence was attributed to improved access to resources (e.g. ambulances, personal protective equipment for health workers and pharmaceutical intervention) [2–5]. Willingness to adhere to public health recommendations, including reporting of symptomatic cases and permitting hygienic burials of deceased family members, and other behaviour changes have also been implicated in the successful interruption of transmission chains [2,6].
Higher Ebola case fatality has been associated with healthcare work and age over 45 years [7]. Characteristics of contacts that may heighten their susceptibility, given exposure, to developing symptomatic disease have also been assessed. For instance, age, sex and exposure type, such as direct or indirect contact with bodily fluids, have been implicated as risk factors among household members of EVD cases and healthcare workers treating cases [8–11]. Phylogenetic [12] and spatial analyses [13] have indicated that Ebola transmission is highly clustered, with social factors potentially playing a role [14]. However, the factors characterizing networks of Ebola transmission have been less assessed. An interplay of biological (e.g. viral load and symptom severity), behavioural (e.g. infrequent or delayed care-seeking) and environmental factors (e.g. poor sanitation) is expected to contribute to the extent of transmission originating from a given source case. Identifying such factors could inform the development of interventions targeted at index cases who are at the centre of these clusters and who thus may be associated with higher transmission.
Cultural norms in Liberia and elsewhere in Africa are thought to have exacerbated EVD spread [6,15]. Specifically, caring for the sick at home and preparation of the deceased for traditional funerals result in close contact with highly infectious bodily fluids [16]. Similarly, in the context of poor health literacy and due to the lack of effective, culturally tailored communication about Ebola transmission routes, distrust for top–down response efforts hindered participation in surveillance and case isolation strategies [17,18]. Given the tendency for women to oversee care for sick and funerary preparation of deceased family members, as well as the disparities in both general and health-specific literacy between males and females in Liberia [19], gender roles may have translated into differential transmission risk at the household and community levels [20]. Likewise, young children may be responsible for more transmission since they depend on adults to care for them, they are less likely to consciously restrict their contact with others and their understanding of the mechanisms of transmission may be limited or non-existent [21]. Faye et al. [16] mapped transmission chains for Conakry and neighbouring prefectures in Guinea during the first months of the outbreak. Their findings suggested that 88% of transmission events occurred in community settings, specifically among family members, or at funerals. Males and females were associated with similar numbers of secondary transmission events in their study area. A parallel analysis, however, has not been conducted to evaluate epidemiological and clinical factors affecting transmission potential among cases in Liberia.
Contact tracing facilitates active monitoring of those who had exposure to a known case and thus who are at greatest risk for contracting and further transmitting infectious diseases. In low-incidence settings, the approach has been found to be effective in understanding and controlling disease transmission as well as identifying and initiating early treatment for tuberculosis [22] and sexually transmitted infections, including HIV, gonorrhoea and chlamydia [23,24]. The relative effectiveness of contact tracing compared to such other measures as random screening has been considered for diseases with different transmission routes and levels of incidence. It has been found that the proportion of contacts that must be traced in order to sufficiently interrupt transmission chains and control an outbreak decreases with increasing clustering [25]. Data collected through contact tracing can offer fundamental information about characteristics of cases and their interactions that are most likely to propagate disease and thus facilitate more efficiency in interrupting future transmission chains by identifying potential superspreaders.
To investigate potential differences in the risk of transmission and susceptibility to disease, we analysed contact-tracing data collected in Montserrado County, Liberia, during the 2013–2016 Ebola outbreak in West Africa.
2. Material and methods
(a). Data
The contact-tracing dataset included a line-listing of individuals who were reported to have been in any contact with a case since his or her symptom onset. Data were collected by the Montserrado Health Team as part of the national response under the Liberian Ministry of Health. Upon identification of a suspected case through active surveillance efforts, contact-tracing teams were deployed to interview the case or a proxy, in the event of the case being too ill or already deceased. The contact tracers documented a list of those individuals who were reportedly in contact with the suspected case since his or her symptom onset.
Contact-tracing data were available for cases and their contacts identified between July and October 2014. Date of the report, date of last contact, community and zone of the contact's residence, and individual's status (i.e. became case or not) were included. For cases, age, sex, disease outcome (i.e. dead or alive), community and zone of residence, number of contacts and date of case investigation were also documented. Contacts who developed symptoms and became cases during the study period were also represented in the case database. Information from the contact database, including the source cases reporting them as contacts, and from the case database, including demographic information (i.e. sex, age, disease outcome, number of contacts, and community and zone of residence), were linked through a personal identification code. Open source map databases, including OpenStreetMap and ReliefWeb Map Centre, were used to develop a standardized list of 452 communities, which were located in 21 administrative zones, based on the reported residences of cases and contacts. Prior to analysis, a de-identified dataset was constructed with individuals' assigned surname IDs. All people sharing both a common surname ID and community were assumed to live in the same household. To assess our definition of household, we evaluated the relationship between the number of unique surnames in the contact-tracing database and the number of houses at the zone level. Specifically, we used Pearson's product moment correlation coefficient and tested its significance using a statistic based on a t-distribution to determine whether zones with more houses also had higher numbers of unique surnames. Data for the number of houses were derived from active case finding activities of the Community-Based Initiative, which mapped Montserrado County to facilitate door-to-door surveillance efforts [6].
Data from a second source, the case classification dataset, were analysed to determine sex- and age-specific differences in the likelihood of seeking treatment, time between symptom onset and hospitalization, number of contacts reported, case fatality and reported type of contact with suspect or known Ebola cases or other sick individuals. The dataset consisted of suspected, probable and confirmed Ebola cases only, based on the case definition from the World Health Organization [26]. It comprised responses collected during active surveillance case interviews according to the Viral Haemorrhagic Fever Case Investigation Form [27].
Collection of contact-tracing and case investigation data was authorized by the Liberian incident management system (IMS) as part of the national response to a public health emergency. Raw datasets are available as open access electronic supplementary material [14]. Use of the datasets for the current analysis was approved by the Yale University Human Subjects Committee.
(b). Statistical analysis
Statistical analysis of data from contact-tracing efforts in Montserrado County, Liberia was conducted to evaluate factors that elevated risk of Ebola transmission to contacts. Bivariate logistic regression was performed to assess the association between the odds of a contact developing EVD and each of the following variables: gender of the source case, age of source case, survival status of the infective case, and residential proximity between the contact and source case. The age of the source case was included as a three-level variable: under 15 years, 15–45 years and over 45 years. Residential proximity between cases and their reported contacts was categorized according to four levels: same household, same community but different household, same zone but different community or different zone. Unadjusted odds ratios were calculated by exponentiating the coefficients of the resulting logistic regression models; the corresponding 95% confidence intervals were reported using two-tailed profile-likelihood limits. A multivariate logistic regression model was generated with the four variables to determine adjusted odds ratios and corresponding 95% confidence intervals.
The probability of transmission given sex and age of the source case was determined for each level of residential proximity between case–contact pairs. That is, for example, the probability of a contact of a female or male case developing Ebola disease was evaluated for the entire dataset and separately for case–contact pairs who occupied the same household, the same community but different household, the same zone but different community or different zones.
Between-group differences in continuous and categorical variables were evaluated using t-tests and χ2 or Fisher's exact tests, respectively. p-values less than 0.05 were considered statistically significant.
3. Results
(a). Characteristics of Ebola cases
The case classification dataset included 4373 suspected, probable and confirmed cases and was analysed to assess characteristics of source cases that may have enhanced or reduced transmission potential. Specifically, treatment-seeking behaviour and numbers of reported contacts were evaluated for males and females and for adults and children. Data on gender were available for 95.8% or 4190 cases, with 53% male (1965 females and 2225 males). Male cases were 26% more likely to die than female cases (OR: 1.26; 95% CI: 1.10, 1.43; p = 0.001). Females were 1.2 times as likely to seek treatment as males (OR: 1.21; 95% CI: 1.05, 1.39, p = 0.008); however, male and female cases who sought care did not significantly differ in terms of average number of days between symptom onset and hospitalization (males = 4.68 ± 3.88 days; females = 4.61 ± 3.93 days, p = 0.758). Moreover, there were no significant differences between males and females in terms of the average number of contacts reported (males = 7.92 ± 8.05 contacts; females = 8.21 ± 10.34 contacts, p = 0.487).
Data on age were available for 87.6% or 3829 cases. On average, cases were 33.1 years old (s.d.: 17.5). Although being almost half of the country's population, children under 15 years accounted for only 15.8% (605/3829) of cases. Additionally, children were 52% less likely to die than adult cases (15 years or older; OR: 0.48; 95% CI: 0.39, 0.58; p < 0.001). Adults and children sought treatment at similar rates (153/605 or 25.3% for children and 869/3224 or 27.0% for adults, p = 0.424), yet time between symptom onset and treatment-seeking was significantly lower for children than adults (children = 3.93 ± 3.36 days; adults = 4.78 ± 3.98 days, p = 0.003). Moreover, adults tended to report more contacts than children (children = 6.13 ± 6.22 contacts; adults = 8.40 ± 9.55 contacts, p < 0.001).
During one month prior to symptom onset, 1262 cases reported contact with a confirmed or suspected case, or with any sick person. Of these contacts, 29.6% (373/1262) involved touching the case's bodily fluids, such as blood, vomit or saliva; 51.0% (644/1262) involved direct contact with the case's body; 36.9% (466/1262) involved touching the linen, clothes or eating utensils of the case; and 35.3% (446/1262) spent time in the same physical space as the case. Between July and October 2014, the frequency with which all contact types were reported increased (electronic supplementary material, table S1), with the greatest increase in the frequency of newly symptomatic individuals indicating they had ‘touched or shared the linens, clothes or dishes/eating utensils of a case.’ Contact types were not found to significantly differ between male and female cases (all p > 0.05). Children under 15 years were more likely than adults to have spent time in the same household or room as a case in the past month (children = 4 6.1%; adults = 32.7%, p < 0.001). Likewise, children were in contact with the linen, clothes or dishes/eating utensils of cases more often than adults (children = 45.3%; adults = 35.0%, p = 0.003).
Accordingly, sex- and age-related differences in care-seeking, time to isolation and number and type of contacts suggested possible differences in transmission potential. In particular, males had a higher case fatality, which could affect their likelihood of transmission as non-survivors of Ebola virus disease tended to harbour greater viraemia than survivors [27,28]. It was found that adults sought treatment later in terms of time from symptom onset than children and thus likely remained symptomatic in their communities for a longer period of time. Children tended to have been in the same household or shared household items with a suspect or known Ebola case more often than adults.
(b). Probability of transmission by sex, age and geographical proximity
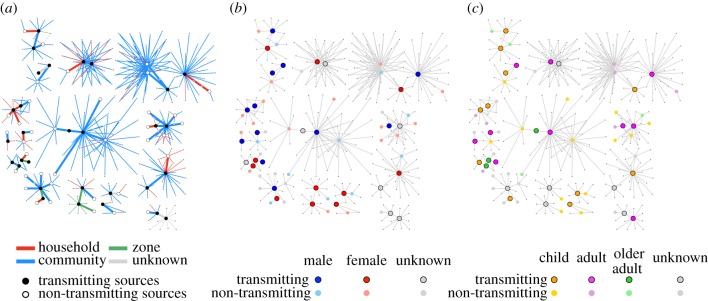
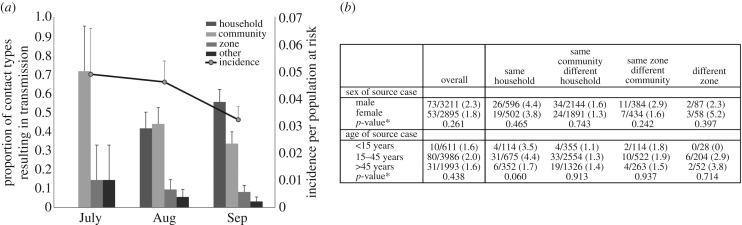
The contact tracing dataset included 1585 cases and 9056 contact–case pairs reported between 7 July 2014 and 28 October 2014. Within this dataset, 749 individuals were reported as contacts of multiple cases and 284 (284/7933, 3.6%) of unique contacts became cases (figure 1). The proportion of investigated contacts developing disease decreased over time (figure 2a). In July, 4.6% (29/611) of contacts became cases after developing Ebola disease; 4.0% (100/2509) and 1.8% (53/2935) of contacts developed disease in August and September, respectively. Contacts who developed disease tended to be female (118/209, 56.5%) and adults between 15 and 45 years (107/206, 51.9%; table 1). The probability of developing EVD among contacts who were reported by multiple cases was 4.5 times greater than the probability of disease among contacts reported by a single case (table 1; 12.1% (91/749) versus 2.7% (193/7184)). Among individuals who were reported by multiple sources, with each additional source, the odds of developing EVD increased by 63% (OR: 1.63, 95% CI: 1.35, 1.99, p < 0.001). No significant differences in sex and age were observed between individuals who became symptomatic after contact with a single case or with multiple cases (table 1).
Figure 1.
Contact networks of Ebola cases. Secondary transmission was more probable among contacts who were reported by multiple sources. (a) The odds of transmission were higher for within-household contacts between an individual and an infectious case. The probability of transmission was not found to vary significantly between (b) male and female source cases or across (c) age categories. However, transmission within households tended to be more probable when infectious cases were children (less than 15 years) and adults (15–45 years) than when they were older adults (more than 45 years).
Figure 2.
Temporal changes in the geographical proximity relationships between cases and their contacts. (a) Contact-tracing data were used to categorize case–contact relationships according to residential proximity (same household, same community but different household, same zone but different community, or different zone). Over three months of the second phase (starting June 2014) of Liberia's outbreak, cases reported increasing contact with individuals in their same households and communities and decreasing contact with people in different communities. There were 611 contacts of incident cases investigated in July, 2509 in August and 2935 in September. Incidence, as the proportion of reported contacts developing disease, decreased over time, probably due to heightened contact-tracing efforts and higher numbers of contacts being reported. Error bars represent the upper limits of the 95% confidence intervals calculated using the normal approximation for the binomial proportion. (b) Overall, the probability of a case–contact interaction resulting in transmission was not significantly related to the sex or age of the symptomatic source, although the transmission probabilities associated with children (less than 15 years) and adult cases (15–45 years) were higher than that associated with older adults (more than 45 years). In particular, when considering the residential proximity between the case and contact, it was observed that the probability of transmission from symptomatic children (under 15 years) and adults (15–45 years) in households was over twice (35/789, 4.4%) that of transmission from older adults (more than 45 years) (6/352, 1.7%). *p-values calculated using χ2 or Fisher's exact tests.
Table 1.
Characteristics of individuals developing symptomatic disease given reported contact with an Ebola case.
| reported by single source (n = 193) n (%) | reported by multiple sources (n = 91) n (%) | p-value | |
|---|---|---|---|
| sex of contact becoming case | 0.803 | ||
| male | 61 (44.4) | 30 (41.7) | |
| female | 76 (55.5) | 42 (58.3) | |
| age of contact becoming case | 0.798 | ||
| <15 years | 29 (21.5) | 18 (25.4) | |
| 15–45 years | 72 (53.3) | 35 (49.3) | |
| >45 years | 34 (25.2) | 18 (25.4) |
Owing to the small number of transmission events for which data are available, a statistical evaluation of temporal trends in the age and sex of contacts becoming cases is limited. However, we note that the proportion of contacts developing disease which was female was higher than that which was male during July, August and September, irrespective of whether the contact was reported by a single or by multiple potential source cases. Children under 15 years constituted an increasing proportion of contacts becoming cases over time (electronic supplementary material, table S2).
Lack of identifiability and causality (i.e. case–contact pairs reporting each other as sources and contacts) was found among 26.4% (24/91) of recorded pairs for which the contact had been associated with multiple cases. This was observed in 8.8% (17/193) of individuals among those reported by a single source. The case whose infective dose led to disease could not be determined for contacts reported by multiple cases. Therefore, analyses on source traits were limited to case–contact pairs for which each contact who developed symptoms was reported by a single case only and for which contacts were not listed as potential sources of the cases reporting them. Overall, no significant relationships were found between the sex and age of the source case and the odds with which a contact developed symptomatic disease (table 2).
Table 2.
Odds of developing disease given characteristics of and residential proximity to source case. ‘ref’ indicates the reference group for the odds ratio calculations.
| unadjusted OR (95% CI) | p-value | adjusted OR (95% CI) | p-value | |
|---|---|---|---|---|
| sex of source | ||||
| male | ref | — | ref | — |
| female | 0.80 (0.56, 1.15) | 0.225 | 0.96 (0.64, 1.44) | 0.841 |
| age of source | ||||
| <15 years | ref | — | ref | — |
| 15–45 years | 1.23 (0.63, 2.39) | 0.539 | 1.31 (0.64, 2.67) | 0.455 |
| >45 years | 0.95 (0.46, 1.95) | 0.888 | 0.84 (0.38, 1.86) | 0.667 |
| residential proximity with source case | ||||
| same household | ref | — | ref | — |
| same zone, different community | 0.46 (0.32, 0.65) | <0.001** | 0.27 (0.17, 0.42) | <0.001** |
| same community, different household | 0.43 (0.26, 0.70) | 0.001** | 0.42 (0.22, 0.80) | 0.008** |
| different zone | 1.06 (0.60, 1.88) | 0.833 | 0.74 (0.26, 2.11) | 0.571 |
| disease outcome of source case | ||||
| survived | ref | — | ref | — |
| deceased | 1.34 (0.96, 1.87) | 0.089 | 1.14 (0.76, 1.72) | 0.517 |
**p < 0.05.
Since exposure type was expected to vary across different transmission settings and interaction types, the probability of transmission over different categories of proximity between residences for case and contacts was investigated. Individuals were assumed to reside in the same household if they shared a surname and reported living in the same community. On average, 2.54 (range: 1.68–5.23, s.d.: 0.74) contacts in a given zone shared the same surname (electronic supplementary material, figure S1). Using data from the Community-Based Initiative [6], it was determined that zones consisted of 1891.85 houses on average (range: 381–5616, s.d.: 1291.80). In Liberia, a house may consist of multiple households. A positive correlation was observed between the number of unique surnames and the number of houses across zones (Pearson's correlation coefficient = 0.668, p-value = 0.001). Between July and September 2014, the proportions of contacts that were between communities and between zones decreased, while the proportion of contacts that were within communities and within households increased (figure 2a). Individuals who resided in the same community but different households as a source case were about half as likely to develop disease than individuals living in the same households as their source cases (table 2; OR: 0.46, 95% CI: 0.32, 0.65, p < 0.001). Transmission was not more likely from male or female sources, irrespective of whether they resided in the same household, same community or different community as their contacts (figure 2b). When residing in the same household as their reported contacts, the probability of transmission was higher for children (less than 15 years) and adults (15–45 years) than for older adults (more than 45 years), although the trend between age categories and transmission probability was not statistically significant (figure 2b; p = 0.060). Symptomatic children under 15 years were found to be associated with transmission in 3.5% (4/114) of their contacts, while the probability of transmission among older adults was 1.7% (6/352).
4. Discussion
Our findings suggest that during a three-month period of peak Ebola virus transmission in Liberia, the probability of contact with symptomatic cases resulting in transmission increased with exposure. Specifically, being in contact with multiple cases, and thus multiple potential sources of transmission, was associated with a higher probability of developing disease. Additionally, exposures between people in different households and different communities were significantly less likely to result in transmission. Most mathematical modelling studies on Ebola interventions (e.g. [5,28,29]) have evaluated the effectiveness of the WHO-recommended strategies [5]. These include the use of personal protective equipment among healthcare workers, implementation of sanitary burial practices, contact tracing and case isolation. However, while the effectiveness of these interventions has been demonstrated, they require specialized training and/or equipment and large-scale human resources. Our study suggests that prevention strategies, such as social mobilization focused on messaging campaigns to interrupt within-household transmission, could be complementary in curtailing transmission. In particular, efforts to educate caregivers and encourage prompt treatment-seeking, especially for children and adults, could minimize infectious contacts.
The high risk of disease given contact with multiple infectious individuals could explain the high degree of clustering observed during the outbreak [12]. If an individual were part of a network with multiple cases who had not sought care, this could implicate both biology (i.e. cumulative exposure to virus) and behaviour (i.e. lack of care-seeking and isolation) in heightened susceptibility among certain households. The finding also provides insight into the high case fatalities and infection rates among healthcare workers and offers support for hypotheses of nosocomial transmission to suspected but not infected individuals undergoing testing [30], who would have been in contact with multiple infectious cases and thus prone to cumulative exposures.
Previous studies have indicated that individuals susceptible to highest viraemia and thus most prone to advanced disease and death transmit to more secondary cases than those with lower viraemia and a higher chance of survival [28,31]. It has also been demonstrated that male and female Ebola cases have similar viral loads [32], although males have been shown to be at a slightly greater risk of death [13]. Gender roles in the affected countries may have led to behaviours that increased risk of exposure and subsequent transmission for women in particular. The involvement of gender roles in spreading infectious disease has been broadly discussed [20]. Although we observed sex-based differences in care-seeking behaviour and case fatality, the probability of Ebola transmission was similar for male and female sources. These results are consistent with findings by the WHO Ebola Response Team, which evaluated sex-based differences across all three countries and reported that the time between symptom onset and hospitalization was shorter for females than males and that case fatality was lower among females when compared with males [11].
The contact-tracing data represent disease dynamics during the first months of widespread transmission in Montserrado County. Until mid-August 2014, there were approximately 20 designated Ebola treatment unit beds in the county [33]. The opening of the Médecins Sans Frontières Ebola treatment unit (ETU) in mid-August expanded capacity by 120 beds [34]. However, daily incidence was over 50 suspected, probable and confirmed cases in September [35,36], still far surpassing the number of available isolation beds. By the end of October, after the peak of the epidemic in Liberia, about 620 ETU beds were available; daily admission rates steadied between 10 and 20 new cases in isolation throughout November [37]. While increases in bed capacity were essential to isolating and treating Ebola patients [3,28], the plateauing and ultimate reduction in incidence in Liberia have been attributed to community mobilization through active case finding led by community members and effective messaging campaigns facilitated through the Ministry of Health [6,33].
We showed that changes occurred in the relative proportions of contacts that took place within versus between households and communities across Montserrado County's epidemic period. Such geographical relationships are probably representative of the type of contact, with shared household contacts resulting in more frequent or intimate interactions than different household contacts. Direct contact with the body or bodily fluids of suspected or confirmed Ebola cases as well as indirect contact via shared items, such as linen or clothes, or via shared space were increasingly reported over time (electronic supplementary material, table S1). Interestingly, when compared with between-zone contacts, contacts with sources in the same community but different households were less likely to contract Ebola. It was observed that within-community behaviour change involved formal social distancing measures, such as school closures and self-quarantining measures by household members of sick individuals, and informal measures, such as reduced numbers of passengers within shared vehicles. These led to less frequent and probably shorter contacts between community members in different households. Furthermore, it is anticipated that out-of-zone contacts included friends or family members visiting one another and possibly staying as household guests, while within-community contacts would tend to include meeting in public spaces for casual conversations. Moreover, transmission probabilities within households tended to be associated with age of the source case. Contact with symptomatic children under 15 years resulted in 3.5% chance of transmission, whereas contact with adults older than 45 years resulted in transmission with a probability of 1.7%. Children who developed disease visited the ETU with the same frequency as adults who developed disease, suggesting that prior to treatment-seeking, administering care to children within a household may have occurred more frequently than that for adults who were likely to care for themselves or be isolated.
A limitation of our study is our inexact definition of household, as explicit information on whether individuals lived at the same address was unavailable. However, using this method, individuals within the contact-tracing data were found to, on average, report 3.6 contacts whom we classified as being in the same household. Since the average household size in urban areas of Liberia is 4.9 individuals [38,39], we consider our approach to have generally reflected the number of individuals in a household and underestimation would have made our findings conservative. Another limitation involved the timeframe of the available contact-tracing data. Significant shifts in behaviour and increases in intervention implementation, such as community-mandated reporting of visitors from different zones and the use of hygiene kits by female caregivers, were occurring in late September and October 2014. Changes in transmission potential that may have been associated with these changes in behaviour could not be assessed, given the sparse data available during October as methods of documentation and databases were transitioning. Furthermore, our analysis depends on the completeness and correctness of the data collected. Underreporting of both cases and/or contacts could be associated with fear of stigmatization, distrust for the governmental and/or international response, and insufficient human and other resources in the early months of the epidemic. Data on children, in particular, may be underreported if caregivers were wary of the response and sought to protect symptomatic children. Additionally, cases diagnosed post-mortem may not have been traced if a surrogate interviewee were not available. The absence of these contacts, however, is expected to have also rendered our findings conservative.
5. Conclusion
Contact-tracing data from a period of intense Ebola spread in Montserrado County, Liberia provide evidence for elevated risk of disease among individuals exposed to multiple infectious cases. Age-based differences in transmission probability were observed for within-household contacts. Our results were most pronounced for within-household contacts. Our results suggest that interventions encouraging immediate reporting of symptomatic cases to limit household exposures would have the potential for significant reductions in the incidence of Ebola.
Supplementary Material
Supplementary Material
Supplementary Material
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
M.P.F., T.N. and L.B. oversaw data collection. L.B. coordinated database assembly. L.A.S., A.H., R.Y. and D.Y. were involved in conceiving the research question and designing the analysis plan. L.A.S. and S.G.G. cleaned and analysed the data and created the figures. L.A.S. drafted the manuscript, and A.H., D.Y., M.P.F., A.P.G. and R.Y. contributed substantially to revisions.
Competing interests
We have no competing interests.
Funding
A.P.G. acknowledges grant support from NIH U01 GM15627 and MIDAS U01GM087719.
References
- 1.Infographics: timeline of Ebola virus disease progress in West Africa. 2016. The Lancet. See http://www.thelancet.com/pb/assets/raw/Lancet/infographics/ebola-timeline.pdf. (accessed 15 November 2016).
- 2.Funk S, Knight GM, Jansen VAA. 2014. Ebola: the power of behaviour change. Nature 515, 492 ( 10.1038/515492b) [DOI] [PubMed] [Google Scholar]
- 3.Kucharski AJ, Camacho A, Flasche S, Glover RE, Edmunds WJ, Funk S. 2015. Measuring the impact of Ebola control measures in Sierra Leone. Proc. Natl Acad. Sci. USA 112, 14 366–14 371. ( 10.1073/pnas.1508814112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsend JP, Skrip LA, Galvani AP. 2015. Impact of bed capacity on spatiotemporal shifts in Ebola transmission. Proc. Natl Acad. Sci. USA 112, 14 125–14 126. ( 10.1073/pnas.1518484112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandey A, Atkins KE, Medlock J, Wenzel N, Townsend JP, Childs JE, Nyenswah TG, Ndeffo-Mbah ML, Galvani AP. 2014. Strategies for containing Ebola in West Africa. Science 346, 991–995. ( 10.1126/science.1260612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fallah M, et al. 2016. Interrupting Ebola transmission in Liberia through community-based initiatives. Ann. Intern. Med. 164, 367–369. ( 10.7326/M15-1464) [DOI] [PubMed] [Google Scholar]
- 7.WHO Ebola Response Team 2014. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N. Engl. J. Med. 371, 1481–1495. ( 10.1056/NEJMoa1411100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bower H, et al. 2016. Exposure-specific and age-specific attack rates for Ebola virus disease in Ebola-affected households, Sierra Leone. Emerg. Infect. Dis. J. 22, 1403 ( 10.3201/eid2208.160163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francesconi P, et al. 2003. Ebola hemorrhagic fever transmission and risk factors of contacts, Uganda. Emerg. Infect. Dis. 9, 1430–1437. ( 10.3201/eid0911.030339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bausch DG, Towner JS, Dowell SF, Kaducu F, Lukwiya M, Sanchez A, Nichol ST, Ksiazek TG, Rollin PE. 2007. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J. Infect. Dis. 196(Suppl 2), S142–S147. ( 10.1086/520545) [DOI] [PubMed] [Google Scholar]
- 11.WHO Ebola Response Team. 2016. Ebola virus disease among male and female persons in West Africa. N. Engl. J. Med. 374, 96–98. ( 10.1056/NEJMc1510305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarpino SV, et al. 2015. Epidemiological and viral genomic sequence analysis of the 2014 Ebola outbreak reveals clustered transmission. Clin. Infect. Dis. 60, 1079–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curran KG, et al. 2016. Cluster of Ebola virus disease linked to a single funeral - Moyamba District, Sierra Leone, 2014. MMWR Morb. Mortal. Wkly. Rep. 65, 202–205. ( 10.15585/mmwr.mm6508a2) [DOI] [PubMed] [Google Scholar]
- 14.Fallah MP, Skrip LA, Gertler S, Yamin D, Galvani AP. 2015. Quantifying poverty as a driver of Ebola transmission. PLoS Negl. Trop. Dis. 9, e0004260 ( 10.1371/journal.pntd.0004260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewlett BS, Amola RP. 2003. Cultural contexts of Ebola in Northern Uganda. Emerg. Infect. Dis. J. 9, 1242 ( 10.3201/eid0910.020493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faye O, et al. 2015. Chains of transmission and control of Ebola virus disease in Conakry, Guinea, in 2014: an observational study. Lancet Infect. Dis. 15, 320–326. ( 10.1016/S1473-3099(14)71075-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansumana R, Bonwitt J, Stenger DA, Jacobsen KH. 2014. Ebola in West Africa: gaining community trust and confidence. Lancet 383, 1946. [DOI] [PubMed] [Google Scholar]
- 18.Greiner AL, Angelo KM, McCollum AM, Mirkovic K, Arthur R, Angulo FJ. 2015. Addressing contact tracing challenges—critical to halting Ebola virus disease transmission. Int. J. Infect. Dis. 41, 53–55. ( 10.1016/j.ijid.2015.10.025) [DOI] [PubMed] [Google Scholar]
- 19.At a Glance: Liberia, Statistics. 2016. UNICEF. See https://www.unicef.org/infobycountry/liberia_statistics.html. (Accessed 15 November 2016.)
- 20.Anker M, et al. 2007. Addressing sex and gender in epidemic-prone infectious diseases. Geneva, Switzerland: World Health Organization Report.
- 21.Lado M, Howlett P. 2016. Ebola virus disease in children: towards a better clinical picture and improved management. Lancet Glob. Health 4, e436–e437. ( 10.1016/S2214-109X(16)30111-5) [DOI] [PubMed] [Google Scholar]
- 22.Tian Y, Osgood ND, Al-Azem A, Hoeppner VH. 2013. Evaluating the effectiveness of contact tracing on tuberculosis outcomes in Saskatchewan using individual-based modeling. Health Educ. Behav. 40, 98S–110S. ( 10.1177/1090198113493910) [DOI] [PubMed] [Google Scholar]
- 23.Oh MK, Boker JR, Genuardi FJ, Cloud GA, Reynolds J, Hodgens JB. 1996. Sexual contact tracing outcome in adolescent chlamydial and gonococcal cervicitis cases. J. Adolesc. Health 18, 4–9. ( 10.1016/1054-139X(95)00109-6) [DOI] [PubMed] [Google Scholar]
- 24.Ramstedt K, Forssman L, Johannisson G. 1991. Contact tracing in the control of genital Chlamydia trachomatis infection. Int. J. STD AIDS 2, 116–118. ( 10.1177/095646249100200208) [DOI] [PubMed] [Google Scholar]
- 25.Eames KTD, Keeling MJ. 2003. Contact tracing and disease control. Proc. R. Soc. Lond. B 270, 2565–2571. ( 10.1098/rspb.2003.2554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. 2002. Regional Office for Africa: Technical Guidelines for Integrated Disease Surveillance and Response in the African Region. Harare, Zimbabwe: WHO. [Google Scholar]
- 27.Office of Information And Regulatory Affairs. 2017. Viral Hemorrhagic Fever Case Investigation Form (Liberia). See https://www.reginfo.gov/public/do/PRAViewIC?ref_nbr=201410-0920-027&icID=213611. (Accessed 9 January 2017.)
- 28.Yamin D, Gertler S, Ndeffo-Mbah ML, Skrip LA, Fallah M, Nyenswah TG, Altice FL, Galvani AP. 2015. Effect of Ebola progression on transmission and control in Liberia. Ann. Intern. Med. 162, 11–17. ( 10.7326/M14-2255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chretien J-P, Riley S, George DB. 2015. Mathematical modeling of the West Africa Ebola epidemic. Elife 4, e09186 ( 10.7554/eLife.09186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zachariah R, Harries AD. 2015. The WHO clinical case definition for suspected cases of Ebola virus disease arriving at Ebola holding units: reason to worry? Lancet Infect. Dis 15, 989–990. ( 10.1016/S1473-3099(15)00160-7) [DOI] [PubMed] [Google Scholar]
- 31.Towner JS, et al. 2004. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J. Virol. 78, 4330–4341. ( 10.1128/JVI.78.8.4330-4341.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de La Vega M-A, et al. 2015. Ebola viral load at diagnosis associates with patient outcome and outbreak evolution. J. Clin. Invest. 125, 4421–4428. ( 10.1172/JCI83162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyenswah T, et al. 2016. Ebola and its control in Liberia, 2014–2015. Emerg. Infect. Dis. J. 22, 169 ( 10.3201/eid2202.151456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Médecins Sans Frontières. 2015. Pushed to the limit and beyond: A critical analysis of the global Ebola response one year into the deadliest outbreak in history, 23 March 2015. See http://www.msf.org/en/article/ebola-pushed-limit-and-beyond.
- 35.Ministry of Health And Social Welfare. 2014. Liberia Ebola SitRep no. 130. 22 September 2014. See http://ilabliberia.org/wp-content/uploads/2014/08/Liberia-Ebola-SitRep-130-Sept-22-2014.pptx-New.pdf.
- 36.Ministry of Health And Social Welfare. 2014. Liberia Ebola SitRep no. 123. 15 September 2014. See http://ilabliberia.org/wp-content/uploads/2014/08/Liberia-Ebola-SitRep-123Sept-15-2014.pdf.
- 37.Ministry of Health And Social Welfare. 2014. Liberia Ebola Daily Sitrep no. 184. See https://www.humanitarianresponse.info/system/files/documents/files/SITRep%20184%20Nov%2015th%202014.pdf.
- 38.World Health Organization. 2016. Liberia: Analytical summary - Social determinants - AHO. See http://www.aho.afro.who.int/profiles_information/index.php/Liberia:Analytical_summary_-_Social_determinants. (Accessed 15 November 2016.)
- 39.Liberia Institute of Statistics and Geo-Information Services (LISGIS). 2008. 2008 national population and housing census: preliminary results. Monrovia, Liberia: Liberia Institute of Statistics and Geo-Information Services (LISGIS). See unstats.un.org/unsd/dnss/docViewer.aspx?docID=2075.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.