1. Introduction
In the field of interoception research, one outstanding question is the precise nature of the neural networks underlying interoceptive processing [1]. The anterior insula (AI) is a key component of contemporary interoception models [2]. However, most evidence implicating the AI in interoceptive processing is correlational and it remains unclear what its precise role in interoception is.
This issue was tackled by Pollatos and co-workers [3]. Using an inhibitory form of transcranial magnetic stimulation (TMS), continuous theta burst stimulation (cTBS), they aimed to provide causal evidence for the involvement of the AI in interoception. The main results suggest that stimulation aiming to inhibit the AI or the somatosensory cortex disrupted performance, and confidence in this performance, for interoceptive tasks relative to stimulation applied to an occipital control site. The same stimulation also altered the heartbeat evoked potential (HEP), an EEG measure thought to index cortical processing of one's heartbeats [4]. The authors interpreted these findings as the impairment of certain aspects of interoceptive processing following cTBS to the right AI, concluding that “cTBS is an effective tool to investigate the neural network supporting interoceptive processes” [3, p. 1].
This study should be commended for its originality, the use of a within-subject design, and multiple concurrent multimodal measures of interoceptive processing. However, two critical issues cast doubt on the conclusions that can be drawn from these data.
2. Which brain regions were stimulated?
The AI is a deep cortical region, positioned behind frontal cortical regions (including the inferior frontal gyrus and operculum). Because of the AI's neuroanatomical position and depth, it is doubtful that TMS could directly reach this region with the parameters used by Pollatos and co-workers. While anatomically near regions to the AI such as the auditory cortex have been targeted in other areas of research (e.g. using TMS to modulate tinnitus symptoms [5]), it is unclear whether the observed effects are due to stimulation of deeper regions or more lateral association areas that are more likely to be modulated by TMS (e.g. [6,7]). TMS administered using a figure-of-eight coil is thought to only stimulate brain regions 1.5–3 cm below the scalp [8], while insula depth is estimated at 4–5 cm [9]. Direct stimulation to the cortical depth of the AI may be achieved using double-cone or helmet-shaped coils, although focality of the electrical field is compromised in comparison with superficial cortical stimulation [9].
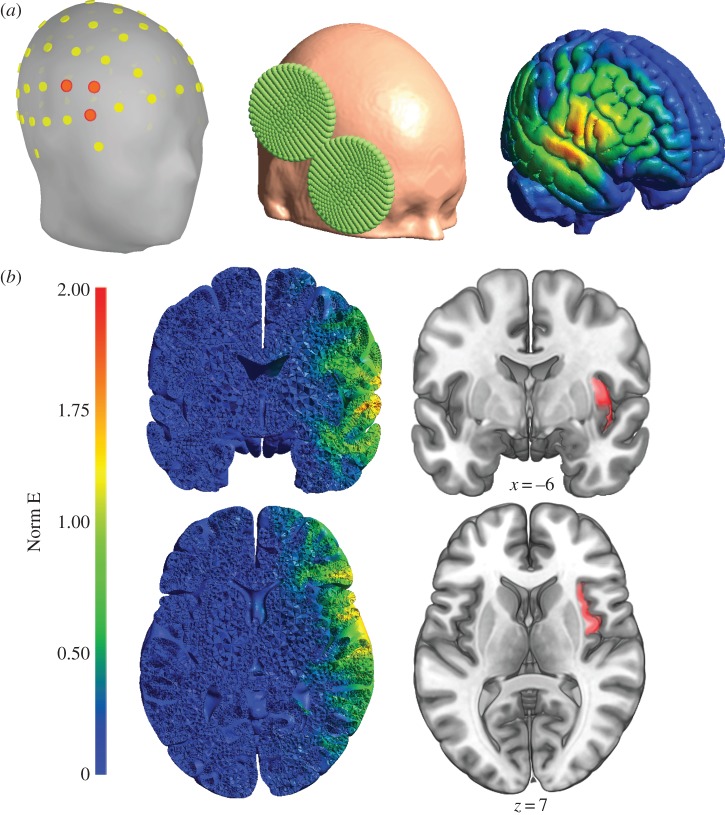
To establish if TMS could reach the AI with the stimulation parameters employed by Pollatos and co-workers, we used the SimNIBS software [10] to run calculations of the electric field induced using these parameters. All parameters and results of this simulation are accessible online (https://osf.io/5qbcs/). As shown in figure 1, the results of this simulation suggest that with these stimulation parameters, only a negligible portion of the electric field reaches the AI.
Figure 1.
Panel (a) illustrates the EEG electrodes (left) used to select the coil position and orientation (centre) and the resulting surface normalized electric field strength (Norm E, right). Panel (b) shows coronal and horizontal slices of the simulation results (left) suggesting that only a negligible portion of the electric field elicited by these stimulation parameters does reach the anterior insula (highlighted in red on the right).
In their discussion, the authors concede that “there is no guarantee of reaching the AI with a TMS coil positioned over the skull” (p. 8), but then argue that their pattern of results seems specific enough to say that the anterior part of the insula was indeed targeted. In the light of our simulation results, we contend that it is unlikely that AI activity was directly influenced by the stimulation. We therefore suggest two possible reinterpretations of Pollatos and co-workers' results. Firstly, it is possible that the AI may have been indirectly disrupted via inhibition of cortical regions it is connected to. Indeed, there is evidence that TMS can affect activity of deeper regions through stimulation of connected cortical areas. For example, cTBS to other frontal regions (dorsolateral prefrontal cortex) has been shown to indirectly suppress insula activation through modulation of fronto-insular connectivity ([11], see also [12]). As direct stimulation of the AI in Pollatos et al.'s experiment was unlikely, one reinterpretation of this data is that worsening of interoceptive performance was the result of indirect modulation of AI activity, via inhibition of more superficial cortical regions.
Alternatively, affected regions in the ventrolateral prefrontal cortex may make a unique contribution to interoceptive processes (indeed, the frontal opercular regions are also activated during interoception tasks; see [13,14]), or during non-interoceptive aspects of interoception tasks. These cortical regions are considered to play an important role in language and decision-making [15,16], disruption to which could feasibly have impacted on the tasks used by Pollatos and co-workers.
3. Are these effects specific to interoception?
Without control tasks however, the nature of this disruption is unclear, leading us onto our second point: it is unclear whether affected performance on the interoception tasks was specific to interoception, or reflective of a more general transient cognitive impairment. Future researchers wishing to use similar methods to examine the role of brain regions in interoceptive processing will of course need to consider which control tasks best suit their experimental question, but it is crucial to use a control task that is similar in nature and difficulty to the interoceptive tasks in order to ensure that decrease in performance is not related to alteration of general processes such as attention, memory or sensation. Such tasks include, but are not limited to, time estimation [17], tone perception [18] and tactile perception [19].
The absence of a control condition is also problematic for the EEG measures used. Indeed, the authors measured the HEP over right fronto-central sites close to the AI and somatosensory stimulation sites, but further from the occipital control site. It is therefore possible that cTBS over the right fronto-central areas altered the electrical brain response of these areas in a non-specific manner. To claim that the disruption of electrocortical potentials caused by the stimulation is specific to the HEP, it is necessary to show that stimulation effects are not generalized to other non-interoceptive evoked potentials (e.g. visual or somatosensory evoked potentials).
In conclusion, non-invasive neurostimulation is arguably one of the most informative tools available in cognitive neuroscience and has certainly been underused in the study of interoception. The recent report of Pollatos and co-workers is in this regard an innovative exploratory study. However, we wish to emphasize that stimulating the AI using this technique is a challenging endeavour, and should be carried out with appropriate cautions and control conditions.
Footnotes
The accompanying reply can be viewed at http://dx.doi.org/10.1098/rstb.2017.0046.
Competing interests
The authors declare no conflict of interest.
Funding
M.-P.C. is funded by a postdoctoral fellowship from the Fonds de Recherche du Québec - Santé.
References
- 1.Schulz SM. 2016. Neural correlates of heart-focused interoception: a functional magnetic resonance imaging meta-analysis. Phil. Trans. R. Soc. B 371, 20160018 ( 10.1098/rstb.2016.0018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. 2004. Neural systems supporting interoceptive awareness. Nat. Neurosci. 7, 189–195. ( 10.1038/nn1176) [DOI] [PubMed] [Google Scholar]
- 3.Pollatos O, Herbert BM, Mai S, Kammer T. 2016. Changes in interoceptive processes following brain stimulation. Phil. Trans. R. Soc. B 371, 20160016 ( 10.1098/rstb.2016.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schandry R, Montoya P. 1996. Event-related brain potentials and the processing of cardiac activity. Biol. Psychol. 42, 75–85. ( 10.1016/0301-0511(95)05147-3) [DOI] [PubMed] [Google Scholar]
- 5.Plewnia C. 2011. Brain stimulation: new vistas for the exploration and treatment of tinnitus. CNS Neurosci. Ther. 17, 449–461. ( 10.1111/j.1755-5949.2010.00169.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plewnia C, Reimold M, Najib A, Brehm B, Reischl G, Plontke SK, Gerloff C. 2007. Dose-dependent attenuation of auditory phantom perception (tinnitus) by PET-guided repetitive transcranial magnetic stimulation. Hum. Brain Mapp. 28, 238–246. ( 10.1002/hbm.20270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andoh J, Zatorre RJ. 2012. Mapping the after-effects of theta burst stimulation on the human auditory cortex with functional imaging. J. Vis. Exp. 12, e3985 ( 10.3791/3985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth Y, Amir A, Levkovitz Y, Zangen A. 2007. Three-dimensional distribution of the electric field induced in the brain by transcranial magnetic stimulation using figure-8 and deep H-coils. J. Clin. Neurophysiol. 24, 31–38. ( 10.1097/WNP.0b013e31802fa393) [DOI] [PubMed] [Google Scholar]
- 9.Downar J, Blumberger DM, Daskalakis ZJ. 2016. The neural crossroads of psychiatric illness: an emerging target for brain stimulation. Trends Cogn. Sci. 20, 107–120. ( 10.1016/j.tics.2015.10.007) [DOI] [PubMed] [Google Scholar]
- 10.Thielscher A, Antunes A, Saturnino GB. 2015. Field modeling for transcranial magnetic stimulation: A useful tool to understand the physiological effects of TMS? In Conf. Proc. IEEE Eng. Med. Biol. Soc., pp. 222–225. Aug. 2015, Milan, Italy.
- 11.Iwabuchi SJ, Raschke F, Auer DP, Liddle PF, Lankappa ST, Palaniyappan L. 2017. Targeted transcranial theta-burst stimulation alters fronto-insular network and prefrontal GABA. Neuroimage 146, 395–403. ( 10.1016/j.neuroimage.2016.09.043) [DOI] [PubMed] [Google Scholar]
- 12.Gratton C, Lee TG, Nomura EM, D'Esposito M. 2013. The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Front. Syst. Neurosci. 30, 124 ( 10.3389/fnsys.2013.00124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaki J, Davis JI, Ochsner KN. 2012. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage 62, 493–499. ( 10.1016/j.neuroimage.2012.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleint NI, Wittchen H-U, Lueken U. 2015. Probing the Interoceptive Network by Listening to Heartbeats: An fMRI Study. PLoS ONE 10, e0133164 ( 10.1371/journal.pone.0133164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartwigsen G, Price CJ, Baumgaertner A, Geiss G, Koehnke M, Ulmer S, Siebner HR. 2010. The right posterior inferior frontal gyrus contributes to phonological word decisions in the healthy brain: evidence from dual-site TMS. Neuropsychologia 48, 3155–3163. ( 10.1016/j.neuropsychologia.2010.06.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark L, Cools R, Robbins TW. 2004. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 55, 41–53. ( 10.1016/S0278-2626(03)00284-7) [DOI] [PubMed] [Google Scholar]
- 17.Shah P, Hall R, Catmur C, Bird G. 2016. Alexithymia, not autism, is associated with impaired interoception. Cortex 81, 215–220. ( 10.1016/j.cortex.2016.03.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terhaar J, Viola FC, Bär KJ, Debener S. 2012. Heartbeat evoked potentials mirror altered body perception in depressed patients. Clin. Neurophysiol. 123, 1950–1957. ( 10.1016/j.clinph.2012.02.086) [DOI] [PubMed] [Google Scholar]
- 19.Ring C, Brener J. 1996. Influence of beliefs about heart rate and actual heart rate on heartbeat counting. Psychophysiology 33, 541–546. ( 10.1111/j.1469-8986.1996.tb02430.x) [DOI] [PubMed] [Google Scholar]