Abstract
Uncertainties regarding food location and quality are among the greatest challenges faced by foragers and communal roosting may facilitate success through social foraging. The information centre hypothesis (ICH) suggests that uninformed individuals at shared roosts benefit from following informed individuals to previously visited resources. We tested several key prerequisites of the ICH in a social obligate scavenger, the Eurasian griffon vulture (Gyps fulvus), by tracking movements and behaviour of sympatric individuals over extended periods and across relatively large spatial scales, thereby precluding alternative explanations such as local enhancement. In agreement with the ICH, we found that ‘informed’ individuals returning to previously visited carcasses were followed by ‘uninformed’ vultures that consequently got access to these resources. When a dyad (two individuals that depart from the same roost within 2 min of each other) included an informed individual, they spent a higher proportion of the flight time close to each other at a shorter distance between them than otherwise. Although all individuals occasionally profited from following others, they differed in their tendencies to be informed or uninformed. This study provides evidence for ‘following behaviour’ in natural conditions and demonstrates differential roles and information states among foragers within a population. Moreover, demonstrating the possible reliance of vultures on following behaviour emphasizes that individuals in declining populations may suffer from reduced foraging efficiency.
Keywords: movement ecology, food searching, communal roosting, social information, biotelemetry, group living
1. Introduction
A major challenge in behavioural ecology is assessing the costs and benefits of group living and the distinct roles played by different individuals [1–3]. Social foraging is a widespread phenomenon, and its selective advantages compared to solitary foraging include reduction of uncertainty regarding food location and quality [4]. Foragers choose their search path with respect to external conditions and internal factors [5], which include their personality (e.g. boldness [6]) and their knowledge of available resources [7]. Social foraging is expected to be beneficial when resources are unpredictable [8]. In these cases, foragers (typically conspecifics) can exploit information from other individuals to reduce uncertainty [9,10]. Information sharing was suggested as a possible mechanism promoting aggregations and communal roosting [11] and the information centre hypothesis (ICH; [12]) was suggested as one of the major mechanisms for social foraging.
The ICH asserts that colonies are advantageous because uninformed individuals (those missing accurate information on concurrent resource whereabouts) can benefit from identifying informed individuals (those who know the location of available resources) at a shared roost or a colony and then follow the informed individual on its way to a previously visited resource (hereafter ‘following behaviour’). By contrast, information transfer can occur via local enhancement [13], when individuals encounter each other at random, typically in the vicinity of the food resource (within their perceptual range). The prerequisite of direct following might be unnecessary if resources are nearby the roost [14,15], or in eusocial species that have sophisticated information-sharing methods, such as the honeybee (Apis mellifera) dance or scent trails in ants [16,17]. In most species, however, uninformed individuals are expected to physically follow informed ones over larger scales. Therefore, direct empirical examination of ‘following behaviour’ constitutes the most powerful test of the basic prerequisites of this hypothesis [14,15].
Nevertheless, to date, empirical evidence for ‘following behaviour’, and studies contrasting the ICH with potential alternative social foraging mechanisms, were constrained by methodological limitations; consequently, the ICH remained debatable [11,14,15,18]. It has been criticized as being susceptible to cheating behaviour and should thus be supported by some type of reciprocal altruism in which a successful forager benefits from returning to a shared roost (see Evans et al. [11] for a recent review). Indeed, individuals may differ in their tendency to lead or follow others [1] and can consistently act as leaders (a.k.a. followees) or followers [19,20] as a function of their personality (a.k.a. behavioural type) [6,21], social dominance [22], demographic (age and sex) or morphological traits [23]. On the other hand, variation in an individual's information status and the need to reduce starvation risks [24] may counteract consistency in the role of individuals and favour opportunistic use of conspecific information [25]. Despite the importance of individuals' role for the evolution of social foraging in general and ICH in particular [11,26], to our knowledge no previous study of the ICH has explored aspects of individual consistency.
Communal roosts of avian scavengers are among the most notable examples of animal aggregations that could serve primarily as information centres [12,27,28], fulfilling four prerequisites of the ICH [14]. First, ‘site fidelity’: individuals adhere to specific roost sites, and return to a recently detected carcass. Second, ‘differential success’: individuals differ in the information they have regarding food-locations at any particular moment. Third, ‘signal transfer’: informed individuals may be identified by others through reliable physical or behavioural signals, either intentionally or inadvertently [11]. However, direct evidence for information exchange is rarely available in behavioural studies of wild animals and we can only list some possible mechanisms of information transfer. Signalling modes may include various sensory (e.g. acoustic or visual) modalities and cues [7,29]. For instance, large crop size, blooded feathers and rapid flight upon departure are all typical visible characteristics of ‘informed’ individuals that can served as a signal. Fourth, ‘tolerance’: food resources (carcasses) are typically larger than the feeding capacity of a single individual and rapid decomposition prevents exploitation over long periods; hence, information sharing incurs little costs for followees and high potential benefits for followers. The latter point may also agree with the recruitment centre hypothesis suggesting that higher foraging success of groups (at the feeding site) may favour recruitment (from the roost) and replace or add to benefits driven by information sharing [28]. Nevertheless, since the prediction of high proportion of individuals waiting in the roosts is rarely fulfilled, this is unlikely to be the case for avian scavengers [11].
Previous attempts to support the ICH in birds and avian scavengers specifically found support for several prerequisites for this hypothesis, including ‘synchronous departure’ from roost [30,31] and ‘differential success’ [32]. Markedly, confirmation of the most critical ‘following behaviour’ prerequisite was partial and indirect, relying on departure or arrival timing (e.g. [27,28,30,31]). A few notable exceptions provided circumstantial evidence for ‘following behaviour’, but at relatively small spatial scales [32–34], thus precluding the exclusion of alternative mechanisms such as local enhancement. Recent developments in tracking abilities provide the means to quantify ‘following behaviour’ [35], and examine movement patterns over large spatial scales in which local enhancement is less likely to occur.
We studied the Eurasian griffon vulture (Gyps fulvus), a social obligatory scavenger, and applied advanced biotelemetry and behavioural classification methods to examine the use of communal roosts as information centres. By simultaneously tracking multiple individuals over extended periods, we tested information sharing and evidence for or against the ICH across four stages along the foraging track: pre-departure (site fidelity, signal transfer, differential success), departure (synchronous departure), en-route (following) and post-arrival (tolerance). Our goal was not to determine whether social information serve as a primary evolutionary driver for communal roosting. Instead, we recognized that communal roosts do exist, and examined how social information exchanged at these roosts affected foraging decisions. In other words, we investigated the proximate mechanisms underlying the ICH and not the ultimate ones. Furthermore, focusing on long foraging flights in which vultures depart synchronously from a roost allowed us to focus on information transfer at the roost in contrast to information transfer during flight as expected from local enhancement. This enabled examining direct evidence for ‘following behaviour’, the hallmark requirement of the ICH, and exploring, for the first time, its consistency among individuals.
2. Material and methods
(a). Study species and area
The Eurasian griffon vulture (G. fulvus, Hablizl 1783) is an obligate scavenger, travelling long distances while foraging, and possessing a unique ability to fast for long time periods [36,37]. Food search is based on visual identification of the carcass, with an estimated detection range around 4 km [38]. This species is highly social, individuals tend to fly together presumably to facilitate detection of favourable soaring conditions [39,40], and frequently interact at food resources and year-round communal roosts. Roosts are located on high cliffs and commonly also serve as breeding colonies (January to July) [36].
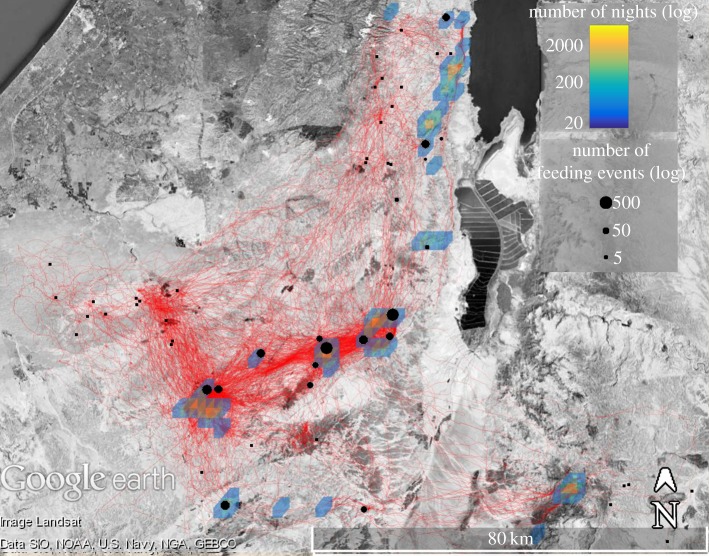
We studied the vulture population in southern Israel (approx. 200 individuals). This population is managed by the Israeli Nature and Park Authority (INPA) due to a decline driven by high rates of adult mortality and breeding failures (O Hatzofe 2016, personal communication). Management includes food deployment at feeding stations (270 ± 201 kg per feeding event, e.g. a few goats or a cow carcass; mean ± s.d.). Because feeding stations (figure 1) were not deployed systematically, food supply was at least partially unpredictable and ephemeral (lasting approx. 4 days; see the electronic supplementary material, figures S1 and S2 and [37,41] for more details on food distribution). About 82% of the feeding events of GPS-tagged vultures during the study period (2009–2014) were at these stations, and the remaining non-station feeding events occurred on occasional local livestock carcasses left in situ.
Figure 1.
A map of the study area with the feeding stations (black circles; size reflect visits of GPS-tagged vultures to each of the stations; log scale), non-station feeding events (black squares), roosting sites (density plot of the sites with more than 20 vulture-nights during the study period with a grid of 2 × 2 km; log scale), and a sample of the foraging tracks (red lines). The distribution of carcasses among feeding stations forces vultures to actively search for currently available food resources.
(b). Data collection and processing
Management also includes capturing vultures during the autumn season (September to December; i.e. outside the breeding season), using a standard walk-in trap (for more details regarding trapping, biometry and molecular sexing, see [24,38]). These captures allowed us to fit 95 vultures with 160 g GPS-Accelerometer tags (E-Obs GmbH; Munich, Germany) in a backpack configuration. Transmitters weighed 2.2 ± 0.2% of the bird's body mass (which is below the recommended 3% for avian telemetry; [42]). Tagged individuals were frequently observed in the vicinity of non-tagged individuals, and no adverse effects of the transmitters on their behaviour, reproduction or movements were observed during the study period. High spatial resolution GPS sampling was set to record locations at 10-min intervals for a 13-h-on/11-h-off diurnal cycle. Accelerometers recorded activity along three perpendicular axes at 10-min intervals, in bouts of approximately 4 and 16–22 s at a frequency of 10 and 3.3 Hz per axis, respectively. Data obtained using other higher frequency sampling protocols were sub-sampled prior to data analysis. Behavioural classifications were obtained from the accelerometer data based on our previous work [37,41].
(c). Data analysis
A key component in testing the ICH is determining individuals' information status before feeding events. Note that for the vast majority of the events, carcass deployment times were known from INPA logs, and therefore the information status was identifiable from the GPS (visits to feeding stations). For non-station feeding events no logs were available and therefore accelerometer data were used to classify feeding events [37,41]. We simplify the uncertainty about the level of information (e.g. individuals may have partial information on resource availability through experience with highly prolific areas) by categorizing individuals as either ‘uninformed’ or ‘informed’. An uninformed individual was assumed to have no knowledge of the location of a currently available resource, if it was not close (more than 10 km) to the relevant resource during the 2 days prior to the focal feeding event (at feeding stations or elsewhere). Vultures often detect a carcass but avoid exploiting it immediately, presumably since landing in a feeding event is energetically costly and might expose them to predation risks [43]; indeed, accelerometer data indicated that in 30 ± 3% of the stops at feeding stations vultures did not eat, corroborating our similar direct observations in the field. Hence, we classified individuals as informed in cases they were within detection range (i.e. less than 4 km) of an existing carcass in the 2 days preceding the feeding event. These included cases in which individuals ate from the carcass at previous days, landed but did not eat or flew above the carcass but did not land. Cases in which individuals were between 4 and 10 km from the food resource were excluded (425 dyads) to avoid false classification of individual's information status.
To assess whether the probability of being informed was affected by individual identity, age or sex, we fitted a general linear mixed models (GLMM) with binomial error distribution. These and the following models included age and sex as fixed factors, and the identity of participating individuals as random factors. Each set of models was ranked, based on AICc. Goodness-of-fit of the best model was evaluated using the marginal and conditional R² statistic [44] (more details in the statistical modelling section in the electronic supplementary material).
(i). Pre-departure
‘Site fidelity’ was estimated as the probability of return to a recently visited roost (within a 2 km radius) and to a recently detected food item (within a 2 km radius). The probability of return to a recent detected food item was estimated from days that included foraging movements. Non-foraging days were defined as days in which the individual stayed within 2 km radius from the roost (approx. 25% or tracking days).
(ii). Departure
To test the prediction of more synchronized departures in the presence of informed individuals, while accounting for spatial variation among roosts, we focused on dyads of foraging vultures (2526 dyads) that left the same roost synchronously (while GPS were set to obtain fixes at synchronized times, a 2-min time window was needed to account for variation in time-to-fix when birds were static at roosts with partial satellite coverage). For each departure event, we calculated the mean roost departure time, variance among departure times and variance of departure directions (first GPS measurement outside the roost area). We used LMMs (departure time), and GLMMs with a gamma error distribution (variance in times or directions), taking into account roost and date as random factors.
(iii). En route
We tested the ‘following behaviour’ for the subset of dyads that departed synchronously from the roost and performed long daily flights (daily displacement more than 15 km; 518 dyads; see the electronic supplementary material, table S2 for information on sample sizes), thus excluding events with possible information transfer via local enhancement or direct eye-sight from the roost. Although we also examined groups of larger size (electronic supplementary material, figure S4), we focused on dyad flights because they constituted approximately 90% of the data. We considered four dyad types: (i) two informed individuals, (ii) an informed and an uninformed individual; (iii) two uninformed individuals in the presence of potential resources (i.e. any known carcass in the region, deployed or naturally available during the last 4 days); and (iv) two uninformed individuals in the absence of potential resources. The latter distinction was made because dyads of uninformed individuals may obtain information from a third, non-tagged vulture, departing from the same roost.
We focused on three indices of the ‘following behaviour’, (i) the proportion of the flight time that individuals spent close to each other (within detection range); (ii) the mean distance between individuals during the flight; (iii) whether the informed was leading the dyad, namely closer to the goal site and how this changed along the joint flight. The first two indices were modelled with LMMs with information status, overall flight duration and recent experience (a predictor indicating the individual ate in the focal feeding station during the last 2 days) as fixed effects. For the third index, we calculated the difference in the distances among the individuals relative to the goal for each GPS fix, so positive values indicate that the informed individual was closer to the feeding site and negative values indicate that the uninformed was closer. Here, we used only informed–uninformed dyad flights that ended at the same feeding site on the focal day (104 dyads). Distance to the feeding site was included as a fixed effect and the feeding event as a random factor in the model.
(iv). Post-arrival
‘Tolerance’ at the feeding site was tested by examining the time between arrival to feeding station and initiation of feeding activity (first feeding acceleration measurement) using GLMMs with gamma distribution and the fixed effects, arrival time to feeding event, information status, travel distance and hunger level (defined as the number of days since the last feeding event [37]). The significance of the fixed effects was assessed by their Type II Wald statistics (GLMM) or based on Type II ANOVA (LMM). Post hoc tests with Tukey's style contrasts were used for pairwise comparisons. Directional information was extracted from the trajectory data using Matlab's (MathWorks Inc., Natick, MA, USA) CircStat toolbox [45], and statistical analyses were performed using R 3.2.2 statistical software (R Development Core Team, 2009) with lme4 [46], car and multcomp packages.
3. Results
We followed 76 individuals for 376 ± 329 days (mean ± s.d., range: 30–1600 days), generating a dataset consisting of approximately 30 000 daily tracks. During two-thirds of the study period we tracked at least 10 individuals simultaneously (and up to 35 individuals); 2–11 tagged individuals stayed in the same roost for 30% of the roosting events, constituting 5–30% of birds staying in the focal roost (n = 92 independent counts at roost on-ground surveys). The overall sex ratio of tagged individuals was 0.5, and the ratio of adults (above 5 years), sub-adults (1–5 years) and juveniles (less than 1 year) was 1.2 : 1.0 : 3.4, respectively.
(a). Pre-departure
Overall we identified 45 roosts that had 10 visits or more, and approximately 50% of nights were concentrated in five roosts. Individuals spent 71 ± 2% of the nights in their three primary roosts. They frequently returned to the same roost on consecutive nights (58 ± 3% of the nights) and to the previous day's feeding site area (on 38 ± 3% of the days). While foraging in dyads, models of information status (i.e. tendency to be informed or uninformed) that included identities of both individuals outperformed alternative models missing these factors (binomial GLMM; n = 1710 observations on 76 individuals). Only two individuals showed a significant tendency to being informed across the study period (electronic supplementary material, figure S6). Age (but not sex) improved the predictive power of models (electronic supplementary material, appendix S1).
(b). Departure
Departure timing was highly synchronized among vultures leaving the same roost. In approximately 50% of the instances that included more than one tagged individual in the roost, all tagged dyads departed within a 2-min time window. Models' results, based on 2526 observations in 45 roosts, suggested that roost identity and the focal day explained most of the observed variance in departure times and in variation among individuals in timing and direction of departure, whereas the presence of informed individual was not included in the highest ranked models (electronic supplementary material, appendix S2).
(c). En route
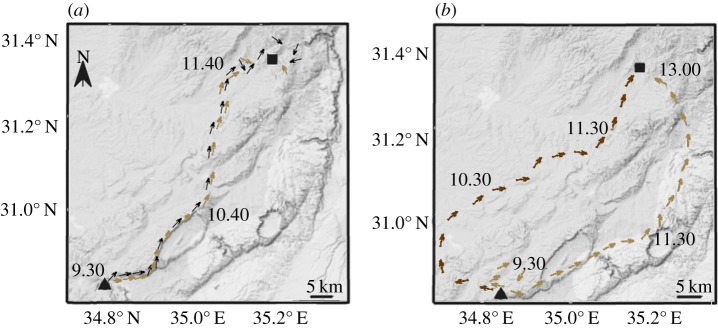
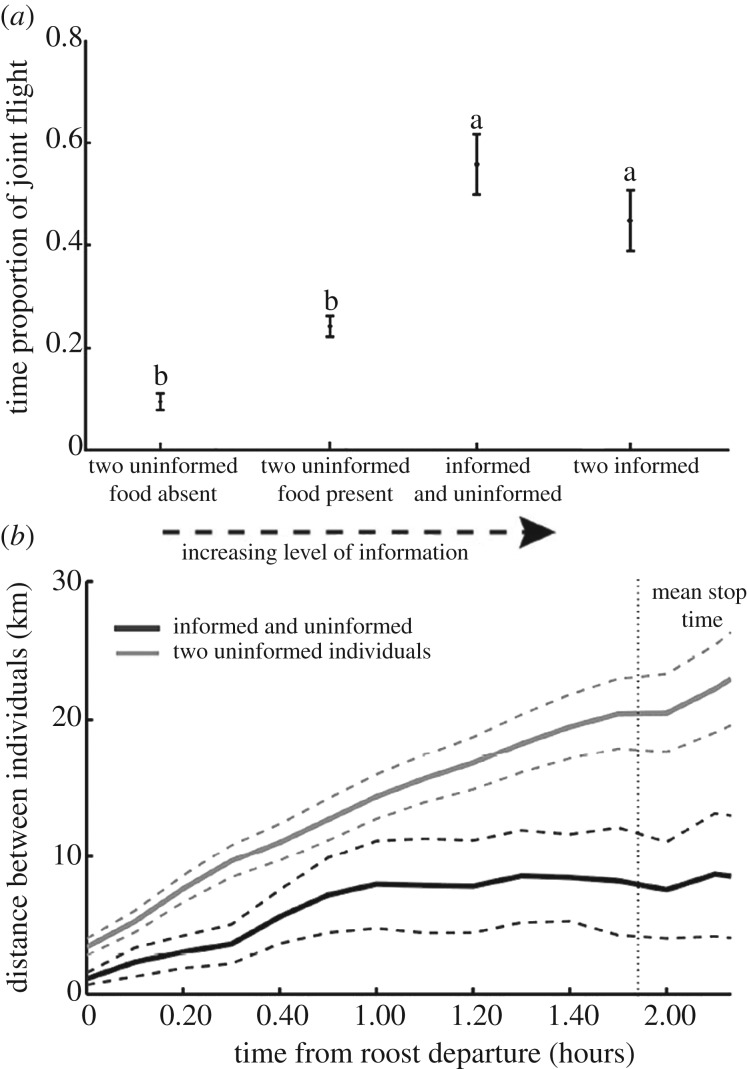
In agreement with the predictions from the ICH for the ‘following behaviour’, we found that the presence of an informed individual increased the probability that a dyad remained together over the foraging track. This occurred at spatial scales that are well beyond vultures' perceptual range (see example trajectories in figure 2), as demonstrated by the higher proportion of flight time that individuals spent close to each other (LMM; F518,4 = 7.3, p < 0.001, figure 3a), and lower mean distance between individuals along the track (LMM; F518,4 = 12.7, p < 0.001, figure 3b). The calculated mean distance between individuals during the flight (at the typical time to reach a food resource; approx. 1.5 h from departure) was approximately 20 km for uninformed–uninformed dyads, approximately 8 km for informed–uninformed dyads and only approximately 1.5 km for informed–uninformed dyads which ended at the same feeding site (about 40% of informed–uninformed dyads). When food was present in the area during the last 4 days, dyads of uninformed individuals showed that individuals spent a higher proportion of the flight time close to each other and a lower mean distance compared with cases in which no food was present in feeding stations, yet these were not significant. Informed individuals flew ahead of uninformed ones (1.61 ± 0.83 km, d.f. = 17.55, Wald t = 2.05, p = 0.05, electronic supplementary material, appendix S3; mean ± s.e.), especially in the beginning and at the end of the joint flights (figure 4).
Figure 2.
Foraging tracks of two independent vulture dyads over different days. (a) A joint track of an informed individual (black) followed by an uninformed individual (brown). (b) Simultaneous tracks of two uninformed individuals (light and dark brown). Arrows represent GPS fixes at 10-min intervals with heading direction. Black triangles represent the morning roost site and black squares represent the feeding site. See electronic supplementary material, figure S5 for an additional example of a dyad flight presenting the movement pattern of two individuals over successive days. (Online version in colour.)
Figure 3.
Joint flight and ‘following behaviour’ during dyad flights. (a) Mean proportion of joint flight (defined as two birds being within a detection range of 4 km) for four dyad types, representing a gradient of increasing information about location of available resources. (b) Mean distance between dyads of birds differed markedly in the presence (dark grey) and absence (light grey) of an informed individual in the dyad. The dotted vertical line marks the average track duration until the first stop. Mean and CI are presented in both panels. Letters represent significant differences between dyad types. See the electronic supplementary material for statistical modelling.
Figure 4.
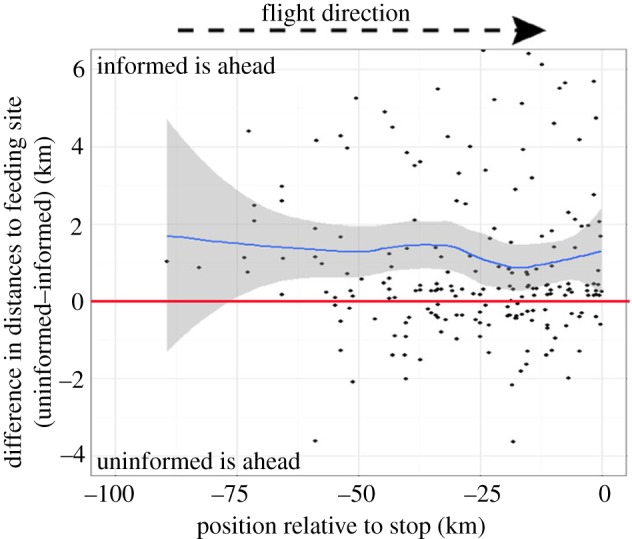
Informed individuals tend to fly ahead of the uninformed individual along the joint flights. Values represent the difference in distances to the goal; positive values indicate that the informed is closer to the goal site (typically a carcass) and negative values indicate measurements in which the uninformed is flying ahead. Lines indicate locally weighted smoothing of the data (blue) with confidence intervals and null expectation of zero difference (red). Overall the informed led the dyad most of the way, especially at the beginning (roost departure) and the end of the joint flight (arrival at the carcass). Even when flying ahead, uninformed individuals remained close to their informed partner (negative deviations are smaller than the positive ones). (Online version in colour.)
(d). Post-arrival
Upon reaching the food resource, the probability that an individual showed feeding behaviour (GLMM; binomial distributed; N = 1064) was affected by arrival time (0.88 ± 0.28, Wald z = −5.58, p < 0.001) and information status (0.70 ± 0.12, Wald z = 3.16, p < 0.001). The time to initiate feeding (GLMM; gamma distributed; N = 477 on 62 individuals) was negatively associated with age (shorter in adults compared with sub-adults and juveniles; Wald t = 8.56, p < 0.0001) and with hunger level (−3.75 ± 1.10, Wald t = −3.39, p = 0.0007), and positively associated with arrival time (5.47 ± 1.59, Wald t = 3.42, p = 0.0006). Information status and the distance individual passed in order to reach the food item did not exhibit significant effects (electronic supplementary material, appendix S4).
4. Discussion
High-resolution tracking of movements and behaviours of free-ranging vultures allowed us to test several key prerequisites of the ICH [11,12]. Our findings are consistent with the use of roosts as information centres. Although we cannot overrule other adaptive benefits of communal roosting, by determining individuals' information status and focusing on long tracks we can exclude other social foraging explanations and the local enhancement hypothesis in particular. Moreover, we found that almost all tracked individuals, when uninformed, followed informed individuals (electronic supplementary material, figure S6). Nevertheless, some individuals spent significantly more time than average in a specific information state: that is, some individuals were, on average, much less informed than others. This demonstrates the role of phenotypic variation in social foraging in free-ranging animals, and undermines the critics of the ICH as being susceptible to cheaters [18]. Regarding ‘signal transfer’, the 10-min sampling resolution did not allow for identification of fine-scale differences in departure that may serve as viable signals for information.
(a). Departure
In raptors that rely on soaring flight, ‘synchronous departure’ may result from environmental constraints, such as thermal availability and wind direction (e.g. [39,40,47]), irrespective of information sharing. Therefore, ‘synchronous departure’ should serve as a necessary but not sufficient condition for ICH in cases foraging trips span relatively large spatial scales [15]. As we observed, roost characteristics appear to explain most of the variance in the timing of departure and the presence of an informed individual did not affect departure timing of long flights.
(b). En route
Our results show that vultures in a dyad with an informed bird spent more time within the estimated detection range, compared to dyads of uninformed individuals (figure 3a). While a highly spatial and temporal-wise synchronized movement may be a product of other factors, the observed difference between the types of dyads supports ICH and does not support the alternative explanation, asserting that vultures just tend to fly together along favourable soaring routes. We also found that when food was locally absent, dyads of two uninformed individuals had a lower tendency to fly together (compared with instances when food was present). This may reflect the presence of untagged informed individuals in the roost (figure 3a), or situations of local enhancement in which following was not initiated in the roost, but later on during flight. In addition, the observed differences in the mean distance between informed–uninformed dyads during flight (those that left the roost and the subset that arrived to the same feeding event) may result from variation in the tendency to fly with or to avoid specific individuals, or from the availability of several feeding opportunities.
Previous support for the occurrence of ‘following behaviour’ from roosts to food resources was indirect [32–34], and limited to local scales that cannot reasonably exclude local enhancement. In contrast, in the current study we show for the first time how differences in individuals' information on resource whereabouts can lead to coarse-grained synchronization of forager movements over relatively large spatial scales. Moreover, the results show a significant tendency of informed birds to precede uninformed ones when both travel from the same roost to the same feeding site. These patterns arise despite the variance expected given the partial representation of the population (approx. 10%) and the observation that only 38% of the informed individuals return to the feeding site. The partial sampling of the population and of informed individuals, in particular, are expected to result in deviations from the actual distances. Consequently, our study probably underestimates the importance of following because an informed individual could have been followed by untagged informed individuals or vice versa (an uninformed individual could have been following untagged informed individual). However, the observed difference between the dyad types (figure 3a) is not likely to be sensitive to the partial representation of the vulture population. Therefore, whereas our results likely underestimate the extent of following behaviour due to partial sampling, our dataset was still sufficient to yield strong evidence for following behaviour in this population. This motivates future efforts to dissect the relative importance and selective consequences of self-information and the alternative social information mechanisms (local enhancement, ICH and hetero-specific information; [7]) for vultures' performance and its dynamics across habitats and species. Yet, addressing this intriguing question [48] requires both simultaneous tracking of a considerable proportion of the population and full knowledge of resource locations—two major practical challenges that have yet to be achieved.
(c). Post-arrival
Resource monopolization and competitive exclusion from the carcass are common among vultures both at the interspecific [49,50] and intraspecific levels [51], resulting in more timid species or juvenile griffon vultures being excluded from the carcass. Accordingly, we found that age and hunger level had a negative effect on the time from arrival to initiation of feeding activity. Information status did not affect waiting duration, suggesting that following others to feeding sites may pay off. Yet, we note that current methodology (acceleration-based behavioural identification) does not permit quantifying the quantity and quality of the food consumed. Future studies may develop new methods to test whether uninformed individuals suffer from lower intake despite similar waiting times and probability of eating, and test whether foraging benefits from ‘following behaviour’ outperform those of alternative foraging strategies. This will improve the link to the adaptive benefits of ICH, and its role in communal roosts.
5. Intra-population heterogeneity and the potential adaptive value of information sharing
We found that most individuals did not show a significant tendency to be informed or uninformed and occasionally following others. These findings, along with vultures' long lifespan and strong tendency to return to the same roost sites, suggest that reciprocity among roost mates in exchanging information may serve as another selective promoter of information transfer (either passive or active) [14,18]. The fact that a few individuals did show a consistent tendency to be informed while flying in groups may be attributed to variation in unmeasured phenotypic factors such as personality, social dominance or morphology [6,19,21,52]. Alternatively, such consistent capacities in obtaining self-information and exploiting social information may be the drivers for the emergence of differential personalities. However, this intriguing hypothesis remains to be tested with theoretical models and with datasets directly measuring additional individual traits. Regardless of the causes of differential social foraging roles and behavioural differences, intra-population heterogeneity may contribute to its persistence through a better division of labour, space use, and capacity of coping with environmental changes [3,6,53].
A major cost of information transfer for the informed individual is the enhanced level of intraspecific competition at feeding sites [4]. Yet, for avian scavengers in general and for griffon vultures in particular (especially at low densities), this cost is probably minimal because carcasses are ephemeral and nearly always many-fold larger than the total feeding capacity of the local foragers. The extensive size of foraging areas (exceeding inter-roost distances) of vultures and their high resistance to pathogens, may further minimize costs of communal living, in contrast to the adaptive benefits of information transfer. These benefits include reduction of various risks, such as the risk of starvation due to more efficient search by multiple individuals [24] and interspecific competitive exclusion [50,51]. Possible benefits are not limited to social foraging, and may include (here or in other systems) enhanced predator avoidance, brood parasitism reduction, migratory phenology synchronization, mate and habitat choice [11]. Here, we observed that followers fed on the resource and therefore may benefit from following behaviour and from reducing search times via ICH. Yet, a genuine positive adaptive value (or payoff) for ICH depends also on enhanced foraging efficiency compared to other search/foraging strategies [24], a challenge that will require further studies. The strength (or subsistence) of potential benefits may also vary with roosting patterns, food distribution patterns and population density, and therefore affect the revisitation rates to food items.
Vulture populations around the world have decreased dramatically during recent decades [54]. A few modelling studies have compared the importance of self- and social-information for vultures' foraging efficiency and population persistence [55–57]. They suggested that this acute reduction may impair the efficiency of social foraging, resulting in an Allee effect for the population. For instance, Jackson et al. [55] focused mostly on local enhancement and argued that efficient foraging can be achieved only when the density of searchers is above a critical threshold. Cortés-Avizanda et al. [57] confronted vulture counts at carcasses with simulation models and concluded that social information transfer better matches the observed data compared to non-social foraging. Whereas these general findings agree with our results, the importance of ICH was largely overlooked in these studies. For instance, in the latter study vultures started their daily search from random locations rather than designated colonies (and simulated tracking followed simplified movement rules), thereby precluding consideration of information sharing through ICH. This different perspective may be driven due to great differences among regions in food distribution patterns and vulture density, which likely affect the way individuals use social information.
Supplementary Material
Acknowledgements
We are grateful to Ohad Hatzofe, Ygal Miller, Amiram Cohen, Yossi Sinai and Amram Zabari from the Israel Nature and Parks Authority for their help in fieldwork; Marian Segal and Sondra Feldman for English editing; Yoav Bartan and other members of the Movement Ecology Lab for their help during the research. We thank the editor, Juan Manuel Morales, Maria Delgado and four anonymous reviewers for their valuable suggestions that helped improve this manuscript.
Ethics
The research was conducted with permission from the Israel Nature and Parks Authority and the ethics committee of the Hebrew University of Israel (NS-07-11063-2).
Data accessibility
The data used in this study are available from the Movebank Data Repository (www.movebank.org).
Authors' contributions
All authors designed the study. O.S. and R.H. performed the fieldwork, collected and analysed the data. R.H. wrote the first draft of the manuscript, and all authors contributed substantially to revisions and gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
This Israeli project was funded by the U.S.–Israel Bi-national Science Foundation and by the special BSF Multiplier Grant Award from the Rosalinde and Arthur Gilbert Foundation (BSF 255/2008 to R.N. and W.M.G.), and by the Adelina and Massimo Della Pergola Chair of Life Sciences and the Minerva Center for Movement Ecology (to R.N.). We also acknowledge scholarships from the Israeli Ministry of Science, Technology & Space (to R.H. and O.S.).
References
- 1.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 3.Farine DR, Montiglio PO, Spiegel O. 2015. From individuals to groups and back: the evolutionary implications of group phenotypic composition. Trends Ecol. Evol. 30, 609–621. ( 10.1016/j.tree.2015.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giraldeau LA, Caraco T. 2000. Social foraging theory. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. 2008. A movement ecology paradigm for unifying organismal movement research. Proc. Natl Acad. Sci. USA 105, 19 052–19 059. ( 10.1073/pnas.0800375105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurvers RH, Van Oers K, Nolet BA, Jonker RM, Van Wieren SE, Prins HH, Ydenberg RC. 2010. Personality predicts the use of social information. Ecol. Lett. 13, 829–837. ( 10.1111/j.1461-0248.2010.01473.x) [DOI] [PubMed] [Google Scholar]
- 7.Spiegel O, Crofoot MC. 2016. The feedback between where we go and what we know—information shapes movement, but movement also impacts information acquisition. Curr. Opin. Behav. Sci. 12, 90–96. ( 10.1016/j.cobeha.2016.09.009) [DOI] [Google Scholar]
- 8.Klaassen RHG, Nolet BA, Van Gils JA, Bauer S. 2006. Optimal movement between patches under incomplete information about the spatial distribution of food items. Theor. Popul. Biol. 70, 452–463. ( 10.1016/j.tpb.2006.04.002) [DOI] [PubMed] [Google Scholar]
- 9.Danchin E, Giraldeau LA, Valone TJ, Wagner RH. 2004. Public information: from nosy neighbors to cultural evolution. Science 305, 487–491. ( 10.1126/science.1098254) [DOI] [PubMed] [Google Scholar]
- 10.Bonnie KE, Earley RL. 2007. Expanding the scope for social information use. Anim. Behav. 74, 171–181. ( 10.1016/j.anbehav.2006.12.009) [DOI] [Google Scholar]
- 11.Evans JC, Votier SC, Dall SR. 2015. Information use in colonial living. Biol. Rev. 91, 658–672. ( 10.1111/brv.12188) [DOI] [PubMed] [Google Scholar]
- 12.Ward P, Zahavi A. 1973. Importance of certain assemblages of birds as information-centers for food-finding. Ibis 115, 517–534. ( 10.1111/j.1474-919X.1973.tb01990.x) [DOI] [Google Scholar]
- 13.Krebs JR. 1974. Colonial nesting and social feeding as strategies for exploiting food resources in great blue heron (Ardea herodias). Behaviour 51, 99–134. ( 10.1163/156853974X00165) [DOI] [Google Scholar]
- 14.Mock DW, Lamey TC, Thompson DBA. 1988. Falsifiability and the information-center hypothesis. Ornis. Scand. 19, 231–248. ( 10.2307/3676564) [DOI] [Google Scholar]
- 15.Bijleveld AI, Egas M, van Gils JA, Piersma T. 2010. Beyond the information centre hypothesis: communal roosting for information on food, predators, travel companions and mates? Oikos 119, 277–285. ( 10.1111/j.1600-0706.2009.17892.x) [DOI] [Google Scholar]
- 16.Von Frisch K. 1967. The dance language and orientation of bees. Cambridge, MA: Harvard University Press. [Google Scholar]
- 17.Hölldobler B. 1990. The ants. Cambridge, MA: Harvard University Press. [Google Scholar]
- 18.Richner H, Heeb P. 1996. Communal life: honest signaling and the recruitment center hypothesis. Behav. Ecol. 7, 115–118. ( 10.1093/beheco/7.1.115) [DOI] [Google Scholar]
- 19.Beauchamp G. 2000. Individual differences in activity and exploration influence leadership in pairs of foraging zebra finches. Behaviour 137, 301–314. ( 10.1163/156853900502097) [DOI] [Google Scholar]
- 20.Hodgkin LK, Symonds MRE, Elgar MA. 2014. Leaders benefit followers in the collective movement of a social sawfly. Proc. R. Soc. B 281, 20141700 ( 10.1098/rspb.2014.1700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurvers RH, Eijkelenkamp B, van Oers K, van Lith B, van Wieren SE, Ydenberg RC, Prins HH. 2009. Personality differences explain leadership in barnacle geese. Anim. Behav. 78, 447–453. ( 10.1016/j.anbehav.2009.06.002) [DOI] [Google Scholar]
- 22.Leca JB, Gunst N, Thierry B, Petit O. 2003. Distributed leadership in semifree-ranging white-faced capuchin monkeys. Anim. Behav. 66, 1045–1052. ( 10.1006/anbe.2003.2276) [DOI] [Google Scholar]
- 23.Krause J, Reeves P, Hoare D. 1998. Positioning behaviour in roach shoals: the role of body length and nutritional state. Behaviour 135, 1031–1039. ( 10.1163/156853998792913519) [DOI] [Google Scholar]
- 24.Caraco T. 1981. Risk-sensitivity and foraging groups. Ecology 62, 527–531. ( 10.2307/1937716) [DOI] [Google Scholar]
- 25.Kendal RL, Coolen I, Laland KN. 2004. The role of conformity in foraging when personal and social information conflict. Behav. Ecol. 15, 269–277. ( 10.1093/beheco/arh008) [DOI] [Google Scholar]
- 26.Barta Z, Giraldeau LA. 2001. Breeding colonies as information centers: a reappraisal of information-based hypotheses using the producer–scrounger game. Behav. Ecol. 12, 121–127. ( 10.1093/beheco/12.2.121) [DOI] [Google Scholar]
- 27.Prior KA, Weatherhead PJ. 2004. Turkey vultures foraging at experimental food patches: a test of information transfer at communal roosts. Behav. Ecol. Sociobiol. 28, 385–390. ( 10.1007/BF00164119) [DOI] [Google Scholar]
- 28.Buckley NJ. 1997. Experimental tests of the information-center hypothesis with black vultures (Coragyps atratus) and turkey vultures (Cathartes aura). Behav. Ecol. Sociobiol. 41, 267–279. ( 10.1007/s002650050388) [DOI] [Google Scholar]
- 29.Galef BG, Giraldeau LA. 2001. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 61, 3–15. ( 10.1006/anbe.2000.1557) [DOI] [PubMed] [Google Scholar]
- 30.Rabenold PP. 1987. Recruitment to food in black vultures: evidence for following from communal roosts. Anim. Behav. 35, 1775–1785. ( 10.1016/S0003-3472(87)80070-2) [DOI] [Google Scholar]
- 31.Sonerud GA, Smedshaug CA, Bråthen Ø. 2001. Ignorant hooded crows follow knowledgeable roost-mates to food: support for the information centre hypothesis. Proc. R. Soc. Lond. B 268, 827–831. ( 10.1098/rspb.2001.1586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown CR. 1986. Cliff swallow colonies as information centers. Science 234, 83–85. ( 10.1126/science.234.4772.83) [DOI] [PubMed] [Google Scholar]
- 33.Marzluff JM, Heinrich B, Marzluff CS. 1996. Raven roosts are mobile information centres. Anim. Behav. 51, 89–103. ( 10.1006/anbe.1996.0008) [DOI] [Google Scholar]
- 34.Wright J, Stone RE, Brown N. 2003. Communal roosts as structured information centres in the raven, Corvus corax. J. Anim. Ecol. 72, 1003–1014. ( 10.1046/j.1365-2656.2003.00771.x) [DOI] [Google Scholar]
- 35.Thiebault A, Mullers R, Pistorius P, Meza-Torres MA, Dubroca L, Green D, Tremblay Y. 2014. From colony to first patch: processes of prey searching and social information in Cape gannets. Auk 131, 595–609. ( 10.1642/AUK-13-209.1) [DOI] [Google Scholar]
- 36.Mundy P, Butchart D, Ledger J, Piper S. 1992. The vultures of Africa. London, UK: Academic Press. [Google Scholar]
- 37.Spiegel O, Harel R, Getz WM, Nathan R. 2013. Mixed strategies of griffon vultures’ (Gyps fulvus) response to food deprivation lead to a hump-shaped movement pattern. Move. Ecol. 1, 5 ( 10.1186/2051-3933-1-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pennycuick C. 1972. Soaring behaviour and performance of some East African birds observed from a motorglider. Ibis 114, 178–218. ( 10.1111/j.1474-919X.1972.tb02603.x) [DOI] [Google Scholar]
- 39.Harel R, Horvitz N, Nathan R. 2016. Adult vultures outperform juveniles in challenging thermal soaring conditions. Sci. Rep. 6, 1–8. ( 10.1038/srep27865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harel R, et al. 2016. Decision-making by a soaring bird: time, energy and risk considerations at different spatio-temporal scales. Phil. Trans. R. Soc. B 371, 20150397 ( 10.1098/rstb.2015.0397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nathan R, Spiegel O, Fortmann-Roe S, Harel R, Wikelski M, Getz WM. 2012. Using tri-axial acceleration data to identify behavioral modes of free-ranging animals: general concepts and tools illustrated for griffon vultures. J. Exp. Biol. 215, 986–996. ( 10.1242/jeb.058602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenward R. 2001. A manual for wildlife radio tagging. London, UK: Academic Press. [Google Scholar]
- 43.Spiegel O, Getz WM, Nathan R. 2013. Factors influencing foraging search efficiency: why do scarce lappet-faced vultures outperform ubiquitous white-backed vultures? Am. Nat. 181, E102–E115. ( 10.1086/670009) [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. ( 10.1111/j.2041-210x.2012.00261.x) [DOI] [Google Scholar]
- 45.Berens P. 2009. CircStat: a MATLAB toolbox for circular statistics. J. Stat. Softw. 31, 1–21. [Google Scholar]
- 46.Bates D, Maechler M, Bolker B, Walker S. 2013. lme4: Linear mixed-effects models using Eigen and S4. R package version 1. [Google Scholar]
- 47.Xirouchakis SM, Andreou G. 2009. Foraging behaviour and flight characteristics of Eurasian griffons Gyps fulvus in the island of Crete, Greece. Wildlife Biol. 15, 37–52. ( 10.2981/07-090) [DOI] [Google Scholar]
- 48.Kane A, Jackson AL, Ogada DL, Monadjem A, McNally L. 2014. Vultures acquire information on carcass location from scavenging eagles. Proc. R. Soc. B 281, 20141072 ( 10.1098/rspb.2014.1072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greene E. 1987. Individuals in an osprey colony discriminate between high and low quality information. Nature 329, 239–241. ( 10.1038/329239a0) [DOI] [Google Scholar]
- 50.Spiegel O, Harel R, Centeno-Cuadros A, Hatzofe O, Getz WM, Nathan R. 2015. Moving beyond curve fitting: using complementary data to assess alternative explanations for long movements of three vulture species. Am. Nat. 185, E44–E54. ( 10.1086/679314) [DOI] [Google Scholar]
- 51.Donázar JA, Cortés-Avizanda A, Carrete M. 2010. Dietary shifts in two vultures after the demise of supplementary feeding stations: consequences of the EU sanitary legislation. Eur. J. Wildlife. Res. 56, 613–621. ( 10.1007/s10344-009-0358-0) [DOI] [Google Scholar]
- 52.Spiegel O, Leu ST, Bull CM, Sih A. 2017. What's your move? Movement as a link between personality and spatial dynamics in animal populations. Ecol. Lett. 20, 3–18. ( 10.1111/ele.12708) [DOI] [PubMed] [Google Scholar]
- 53.Spiegel O, Leu ST, Sih A, Godfrey SS, Bull CM. 2015. When the going gets tough: behavioural type-dependent space use in the sleepy lizard changes as the season dries. Proc. R. Soc. B 282, 20151768 ( 10.1098/rspb.2015.1768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sekercioğlu C, Daily GC, Ehrlich PR. 2004. Ecosystem consequences of bird declines. Proc. Natl Acad. Sci. USA 101, 18 042–18 047. ( 10.1073/pnas.0408049101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson AL, Ruxton GD, Houston DC. 2008. The effect of social facilitation on foraging success in vultures: a modelling study. Biol. Lett. 4, 311–313. ( 10.1098/rsbl.2008.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deygout C, Gault A, Duriez O, Sarrazin F, Bessa-Gomes C. 2010. Impact of food predictability on social facilitation by foraging scavengers. Behav. Ecol. 21, 1131–1139. ( 10.1093/beheco/arq120) [DOI] [Google Scholar]
- 57.Cortés-Avizanda A, Jovani R, Donázar JA, Grimm V. 2014. Bird sky networks: how do avian scavengers use social information to find carrion? Ecology 95, 1799–1808. ( 10.1890/13-0574.1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are available from the Movebank Data Repository (www.movebank.org).