Abstract
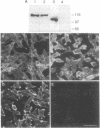
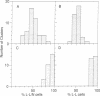
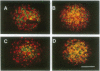
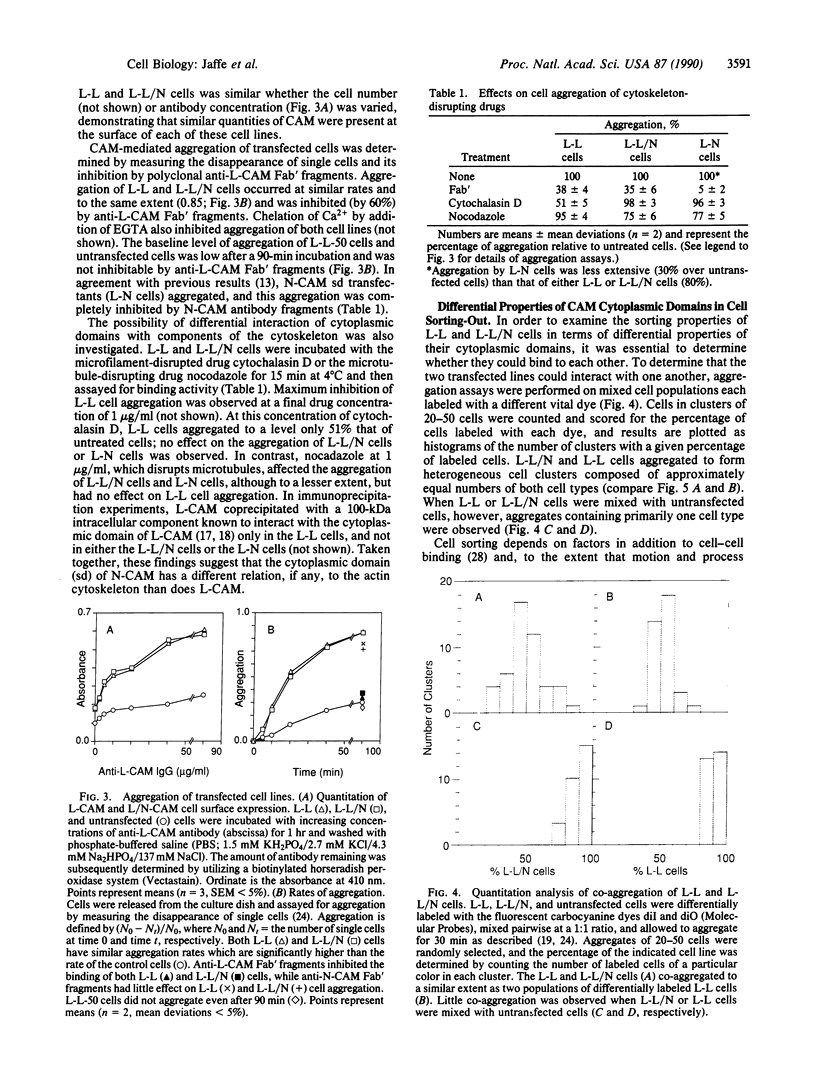
Cell adhesion molecules (CAMs) are cell surface glycoproteins that play important roles in morphogenesis and histogenesis, particularly in defining discrete borders between cell populations. Previous studies have suggested that the cytoplasmic domains of CAMs play a significant role in their adhesion properties. These domains may also be involved in regulating other cellular interactions, such as those involved in the sorting-out of cells to form tissues. In the present studies, we have compared the effects of replacing the cytoplasmic domain of one CAM with that of another CAM of different homophilic binding specificity on cell adhesion and cell sorting-out. The molecules studied were liver CAM (L-CAM) and the neural CAM (N-CAM) sd polypeptide. One cDNA was constructed that encodes a chimeric molecule composed of the extracellular domain of L-CAM and the cytoplasmic plus transmembrane domains of the sd polypeptide of chicken N-CAM (called L/N-CAM). Another was constructed encoding a truncated L-CAM missing the last 50 residues of the cytoplasmic domain. Permanently transfected lines of mouse L cells were obtained expressing the truncated L-CAM ("L-L-50 cells") or the chimeric L/N-CAM ("L-L/N cells") and were compared with cells expressing intact L-CAM ("L-L cells"). Immunoblotting and ELISA analyses demonstrated that these various cell lines expressed similar amounts of CAMs at the cell surface. Aggregation of L-L and L-L/N cells occurred at similar rates in short-term aggregation assays and was inhibited by antibodies to the extracellular L-CAM binding domain. In contrast, L-L-50 cells did not aggregate. Incubation of transfected cells with cytochalasin D, which disrupts microfilaments, markedly inhibited aggregation of L-L cells but had no effect on L-L/N cell aggregation. Mixed L-L and L-L/N cells co-aggregated in short-term assays; in the longer-term sorting-out assays, however, they behaved differently: L-L cells sorted out from both L-L/N and untransfected cells, whereas L-L/N cells did not sort out from untransfected cells. These studies not only suggest that interactions of cytoplasmic domains of different CAMs with the cytoskeleton can modulate cell adhesion but also suggest that specific interactions with certain cytoskeletal components are required for events such as cell sorting and cell patterning.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong P. B. Cell sorting out: the self-assembly of tissues in vitro. Crit Rev Biochem Mol Biol. 1989;24(2):119–149. doi: 10.3109/10409238909086396. [DOI] [PubMed] [Google Scholar]
- Becker J. W., Erickson H. P., Hoffman S., Cunningham B. A., Edelman G. M. Topology of cell adhesion molecules. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1088–1092. doi: 10.1073/pnas.86.3.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti R., Rutishauser U., Edelman G. M. A cell surface molecule involved in aggregation of embryonic liver cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4831–4835. doi: 10.1073/pnas.77.8.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Hemperly J. J., Murray B. A., Prediger E. A., Brackenbury R., Edelman G. M. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987 May 15;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- Duband J. L., Rocher S., Chen W. T., Yamada K. M., Thiery J. P. Cell adhesion and migration in the early vertebrate embryo: location and possible role of the putative fibronectin receptor complex. J Cell Biol. 1986 Jan;102(1):160–178. doi: 10.1083/jcb.102.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. CAMs and Igs: cell adhesion and the evolutionary origins of immunity. Immunol Rev. 1987 Dec;100:11–45. doi: 10.1111/j.1600-065x.1987.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Murray B. A., Mege R. M., Cunningham B. A., Gallin W. J. Cellular expression of liver and neural cell adhesion molecules after transfection with their cDNAs results in specific cell-cell binding. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8502–8506. doi: 10.1073/pnas.84.23.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Friedlander D. R., Mège R. M., Cunningham B. A., Edelman G. M. Cell sorting-out is modulated by both the specificity and amount of different cell adhesion molecules (CAMs) expressed on cell surfaces. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7043–7047. doi: 10.1073/pnas.86.18.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin W. J., Edelman G. M., Cunningham B. A. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1038–1042. doi: 10.1073/pnas.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin W. J., Sorkin B. C., Edelman G. M., Cunningham B. A. Sequence analysis of a cDNA clone encoding the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci U S A. 1987 May;84(9):2808–2812. doi: 10.1073/pnas.84.9.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M., Edelman G. M. Heterotypic binding between neuronal membrane vesicles and glial cells is mediated by a specific cell adhesion molecule. J Cell Biol. 1984 May;98(5):1746–1756. doi: 10.1083/jcb.98.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol. 1986 Feb;102(2):457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Sorkin B. C., White P. C., Brackenbury R., Mailhammer R., Rutishauser U., Cunningham B. A., Edelman G. M. Chemical characterization of a neural cell adhesion molecule purified from embryonic brain membranes. J Biol Chem. 1982 Jul 10;257(13):7720–7729. [PubMed] [Google Scholar]
- Mege R. M., Matsuzaki F., Gallin W. J., Goldberg J. I., Cunningham B. A., Edelman G. M. Construction of epithelioid sheets by transfection of mouse sarcoma cells with cDNAs for chicken cell adhesion molecules. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7274–7278. doi: 10.1073/pnas.85.19.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Shirayoshi Y., Okazaki K., Yasuda K., Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987 Sep 24;329(6137):341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988 Dec 1;7(12):3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Transmembrane control of cadherin-mediated cell adhesion: a 94 kDa protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1989 Nov;1(1):37–44. doi: 10.1091/mbc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A., Nagafuchi A., Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988 Sep 23;54(7):993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Baribault H., Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989 Jun;8(6):1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prediger E. A., Hoffman S., Edelman G. M., Cunningham B. A. Four exons encode a 93-base-pair insert in three neural cell adhesion molecule mRNAs specific for chicken heart and skeletal muscle. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9616–9620. doi: 10.1073/pnas.85.24.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto A. L., Crossin K. L., Cunningham B. A., Edelman G. M. Localization of mRNA for neural cell adhesion molecule (N-CAM) polypeptides in neural and nonneural tissues by in situ hybridization. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9579–9583. doi: 10.1073/pnas.86.23.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988 Apr;102(4):639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. P., Edwards B. F., Erickson C. A. Tension in the culture dish: microfilament organization and migratory behavior of quail neural crest cells. Cell Motil. 1985;5(3):225–237. doi: 10.1002/cm.970050305. [DOI] [PubMed] [Google Scholar]
- Volk T., Geiger B. A 135-kd membrane protein of intercellular adherens junctions. EMBO J. 1984 Oct;3(10):2249–2260. doi: 10.1002/j.1460-2075.1984.tb02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]