Abstract
Dengue virus (DENV) has emerged as an increasing fitness problem in the world for which no specialized drug is available. The non-structural protein NS3 protease of DENV has already been recognized as a potential therapeutic target for the discovery and development of novel antiviral agents against DENV infections. In this study, we employed the virtual screening technique to explore the potent inhibitors of DENV NS2B/NS3pro from Traditional Chinese Medicine (TCM) database. Total 200 inhibitors from TCM against DENV NS3pro were screened and only five TCM compounds like eriodictyol 7-O-glucuronide, luteolin 8-C-beta-glucopyranoside, (-)-epicatechin-3-O-gallate, 6-O-trans-p-coumaroylgeniposide and luteolin-7-O-glucoside were selected for further analysis which showed binding energies, −7.000, −7.380, −7.380, −7.440 and −7.440 kcal/mol, respectively. The findings of this study suggest that these five TCM compounds can be considered as potent inhibitors for DENV NS2B/NS3pro for the development of anti-dengue drugs.
Keywords: Dengue, NS2B/NS3, TCM, Inhibitors, Drug discovery
Introduction
Dengue virus (DENV), the member of Flaviviridae family, is causative pathogen of dengue fever, which infects people by one of five linked, but different virus serotypes (DEN 1-5) [2, 3, 21, 24, 31, 32]. Dengue hemorrhagic fever [34] and dengue shock syndrome are the very serious cause of this fatal pathogen which is transmitted to individuals by the nibble of Aedes aegypti female mosquito [12, 27–29, 35]. DENV has also emerged as an increasing fitness problem in the world for which no specialized drug is available in the market. The non-structural protein NS3 protease of DENV has already been recognized as a potential therapeutic target for the discovery and development of novel antiviral agents against DENV infection [8, 17, 22, 23, 36, 37]. NS3 molecule of DENV is comprised of a protease (NS3pro) and a helicase (NS3hel) domain, where the NS3pro domain relies on a cofactor, NS2B for their catalytic action and serves as a center for the assembly of the DENV replication complex and also modulates viral pathogenesis and the host immune response [4, 6, 11, 18, 19]. Hence, by targeting the DENV NS2B/NS3pro, the novel inhibitors from a variety of sources using different strategies can be discovered which can pave the way for the development of potential drugs against DENV infection. In the field of drug discovery, natural compounds are the most popular source of effective lead molecules which are used for treatment against different diseases and infections. Over the past decade, various databases of natural compounds have been developed for providing the information about new lead compounds against various diseases and infections. Traditional Chinese medicine (TCM) database is one among those medicinal databases which contains more than 20,000 pure compounds isolated from 453 TCM ingredients [7]. Virtual screening (VS) technique has emerged as a reliable, cost-effective and time-saving technique for discovering new natural lead compounds from the medicinal compounds databases. Screening of the new lead compounds against DENV is very interesting task for developing novel anti dengue drugs.
In the present work, we employed the virtual screening technique to explore the potent inhibitors of DENV NS2B/NS3pro from TCM database.
Materials and methods
Receptor preparation
The crystal structure of complete NS3 molecule fused to 18 residues of the NS2B cofactor was retrieved from Protein Data Bank [5, 30] (PDB-available at http://www.rcsb.org) using the PDB code: 2VBC. This was solved by Luo et al. in [19] at a resolution of 3.15 Å. This structure contains two domains: serine protease N-terminal domain and the ATPase/helicase domain located at the C terminus of NS3. The serine protease N-terminal domain of NS3 was selected for virtual screening of small molecules from TCM database. Prepare Protein tool of iScreen server [38] was used for preparing the protein for virtual screening (http://iscreen.cmu.edu.tw/ligand.php). All the visualizations of molecular structure were performed with Pymol [9] and Ligplot [15, 41] which are very powerful software, for all purpose molecular visualizations.
Virtual screening
In this study, we employed iScreen web server [9] to perform virtual screening of inhibitor molecules for NS3pro of DENV. iScreen allows to dock TCM compounds into a binding site defined by the user. The protein structure file of NS3 serine protease of DENV in PDB format was uploaded into iScreen server and the active site determined in the crystal structure of the protein was defined by providing the list of residues (HIS 51, ASP 75 and SER 135) which form a catalytic triad in binding site of the DENV NS3pro [18].
Results
Virtual screening
Virtual screening is applied to discover new potent inhibitors for target protein which involves docking of inhibitor structures into active site of the target, and scoring and ranking of each compound [13]. Molecular docking of small molecules into the binding site of structures of protein targets is a technique commonly used in the area of drug discovery and molecular interaction studies [42]. We used such virtual screening technique to predict the inhibitory potential of 200 compounds from TCM against DENV NS3pro. Total 5 TCM compounds having the highest docking score were selected for further analysis.
Molecular interaction studies of selected compounds
Molecular interaction mode analysis of protein–ligand complexes is essential research in the area of structure-based discovery for the development of new efficient drugs against different diseases [33]. It is pivotal for complete comprehension of the molecular mechanisms of biological frameworks [1]. Five selected TCM compounds (eriodictyol 7-O-glucuronide, luteolin 8-C-beta-glucopyranoside, (-)-epicatechin-3-O-gallate, 6-O-trans-p-coumaroylgeniposide and luteolin-7-O-glucoside) were docked again into the receptor structure to form complex structures using MTiAutoDock server [14]. The molecular docking results showed that all the selected inhibitors showed good binding affinity with DENV NS3pro. It was observed that the DENV NS3pro binding energies for luteolin 8-C-beta-glucopyranoside, (-)-eriodictyol 7-O-glucuronide, epicatechin-3-O-gallate, 6-O-trans-p-coumaroylgeniposide and luteolin-7-O-glucoside were −7.000, −7.380, −7.380, −7.440 and −7.440 kcal/mol respectively.
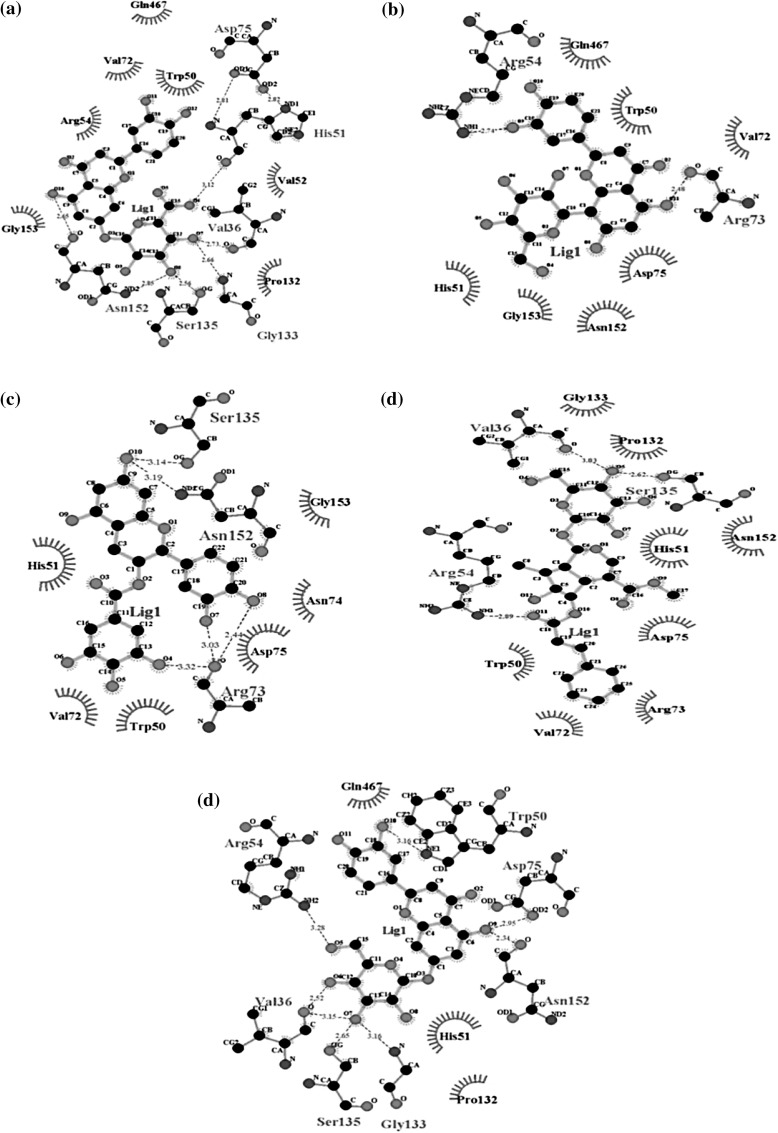
Hydrogen bonding and hydrophobic interactions play a very important role to fit the ligands into the active site of the target [26]. Hence, to understand the binding patterns between DENV NS2B-NS3pro and above described ligands associated with these interactions (Fig. 1) are described separately as follows.
Fig. 1.
2D molecular interaction mode diagrams of DENV NS2B-NS3pro domain with the bound ligands, a eriodictyol 7-O-glucuronide, b luteolin 8-C-beta-glucopyranoside, c (-)-epicatechin-3-O-gallate, d 6-O-trans-p-coumaroylgeniposide and e luteolin-7-O-glucoside. In all the figures, dashes represent hydrogen bonds while arcs represent hydrophobic interactions. Other highlights: carbon—C; oxygen—O; nitrogen—N
DENV NS2B-NS3pro and eriodictyol 7-O-glucuronide complex
The interaction analysis of NS2B-NS3pro and eriodictyol 7-O-glucuronide complex (Fig. 1a) revealed that total six residues (Val36, His51, Asp75, Gly133, Ser135 and Asn152) of DENV NS2B-NS3pro including three of catalytic triad were found to involved in the formation of hydrogen bonds with eriodictyol 7-O-glucuronide, while Trp50, Val52, Arg54, Val72, Pro132, Gly153 and Gln467 residues were involved in the hydrophobic interactions.
DENV NS2B-NS3pro and luteolin 8-C-beta-glucopyranoside complex
The interaction of DENV NS2B-NS3pro and luteolin 8-C-beta-glucopyranoside complex, as shown in Fig. 1b, illustrated that total two hydrogen bonds were found to be involved between luteolin 8-C-beta-glucopyranoside and NS2B-NS3pro residues Arg54 and Arg73 in which both residues formed only one hydrogen bond. Total eight amino acid residues; Trp50, His51, Val72, Asp75, Asn152, Gly153, Gln467, of NS2B-NS3pro were found to be involved in hydrophobic interaction with luteolin 8-C-beta-glucopyranoside.
DENV NS2B-NS3pro and (-)-epicatechin-3-O-gallate complex
From the Fig. 1c, showing DENV NS2B-NS3pro and (-)-epicatechin-3-O-gallate complex, it can be deduced that total five hydrogen bonds were found to be involved between (-)-epicatechin-3-O-gallate and NS2B-NS3pro residues Arg73, Ser135 and Asn152, in which all residues formed only one hydrogen bond except Arg73 which formed three hydrogen bonds. Total six amino acid residues; Trp50, His51, Val72, Asn74, Asp75 and Gly153 of NS2B-NS3pro were found to be involved in hydrophobic interaction with (-)-epicatechin-3-O-gallate complex.
DENV NS2B-NS3pro and 6-O-trans-p-coumaroylgeniposide complex
The interaction diagram of DENV NS2B-NS3pro and 6-O-trans-p-coumaroylgeniposide complex as shown in Fig. 1d illustrated that total three hydrogen bonds were found to be involved between 6-O-trans-p-coumaroylgeniposide and NS2B-NS3pro residues Val36, Arg54 and Ser135 in which both residues formed only one hydrogen bond. Total eight amino acid residues; Trp50, His51, Val72, Arg73, Asp75, Pro132, Gly133 and Asn152 of NS2B-NS3pro were found to be involved in hydrophobic interaction with 6-O-trans-p-coumaroylgeniposide.
DENV NS2B-NS3pro and luteolin-7-O-glucoside complex
The interaction diagram of DENV NS2B-NS3pro and luteolin-7-O-glucoside complex as shown in Fig. 1e illustrated that total eight hydrogen bonds were found to be involved between luteolin-7-O-glucoside and NS2B-NS3pro residues Val36, Trp50, Arg54, Asp75, Gly133, Ser135 and Asn152 in which both residues formed only one hydrogen bond except Val36 which formed two hydrogen bonds. Total three amino acid residues; His51, Pro132 and Gln467 of NS2B-NS3pro were found to be involved in hydrophobic interaction with luteolin-7-O-glucoside.
Discussion
Among the family of Flaviviridae, DENV is being considered as an increasing medical issue in many countries, for which neither a specialized vaccine nor an efficient drug is available. NS2B-NS3 protease of DENV plays a vital role in genome replication and viral RNA synthesis, through the cleavage of the viral polyprotein precursor to discharge individual non-structural macromolecules and a C-terminal NTPase-dependent RNA helicase [16, 18, 37]. Hence, NS2B-NS3 protease is considered as a therapeutic target for the discovery and designing of potential lead compounds to combat the DENV infection.
In this study, we performed virtual screening of natural compounds available in the TCM database by docking them into the active site of the DENV NS2B-NS3 protease. Total 200 TCM compounds were found as inhibitor molecules at the end of virtual screening experiment. Five compounds (luteolin8-C-beta-glucopyranoside, (-)-eriodictyol 7-O-glucuronide, epicatechin-3-O-gallate, 6-O-trans-p-coumaroylgeniposide and luteolin-7-O-glucoside) were selected for detail molecular interaction studies. The binding energies for the selected lead compounds such as luteolin8-C-beta-glucopyranoside, (-)-eriodictyol 7-O-glucuronide, epicatechin-3-O-gallate, 6-O-trans-p-coumaroylgeniposide and luteolin-7-O-glucoside were observed as −7.000, −7.380, −7.380, −7.440 and −7.440 kcal/mol respectively. The hydrogen bonding patterns between the lead molecule and one of the catalytic triad (His-51, Asp-75, and Ser-135) amino acid residue of NS3 protease might disrupt the electron exchange between the carboxyl group of Asp-75 and nitrogen atom on imidazole group of His-51, probably disturbing the capability of His-51 to trigger nucleophilic assault of hydroxyl group (β-OH) of Ser-135, which is crucial for the initiation of proteolysis [20, 25, 40]. 5 conserved amino acid residues (Asp-129, Phe-130, Tyr-150, Asn-152 and Gly-153) of NS3 protease, found among all the proteases of flavivirus and form the small part in the β-sheet are well recognized for their crucial role in substrate binding [10, 16, 25, 39, 40]. The ligand molecules such as eriodictyol 7-O-glucuronide, (-)-epicatechin-3-O-gallate, 6-O-trans-p-coumaroylgeniposide and luteolin-7-O-glucoside show hydrogen bond interactions with the catalytic triad except luteolin 8-C-beta-glucopyranoside. But all the ligands including luteolin 8-C-beta-glucopyranoside show interaction with some of the substrate binding residues.
The hydrogen bonding and hydrophobic interactions between these lead molecules and one of the catalytic triad (His-51, Asp-75, and Ser-135) amino acid residues of NS3 protease (as described in 2D molecular interaction diagrams) can alter the functional attribute of DENV NS2B-NS3 protein by changing their conformation. Therefore, our findings suggests that luteolin8-C-beta-glucopyranoside, (-)-eriodictyol 7-O-glucuronide, epicatechin-3-O-gallate, 6-O-trans-p-coumaroylgeniposide and luteolin-7-O-glucoside can be evaluated in further in vitro and in vivo studies for the development of effective DENV drugs.
Acknowledgments
Authors acknowledge the support provided by Department of Biotechnology, D.D.U. Gorakhpur University, Gorakhpur.
References
- 1.Anand P, Nagarajan D, Mukherjee S, Chandra N. PLIC: protein–ligand interaction clusters. Database, 2014, bau029. [DOI] [PMC free article] [PubMed]
- 2.Anciotti RS, Gubler DJ, Trent DW. Molecular evolution and phylogeny of dengue-4 viruses. J Gen Virol. 1997;78:2279–2286. doi: 10.1099/0022-1317-78-9-2279. [DOI] [PubMed] [Google Scholar]
- 3.Anderson CR, Downs WG, Hill AE. Isolation of dengue virus from a human being in Trinidad. Science. 1956;124:224–225. doi: 10.1126/science.124.3214.224. [DOI] [PubMed] [Google Scholar]
- 4.Arias CF, Preugschat F, Strauss JH. Dengue 2 virus NS2B and NS3 form a stable complex that can cleave NS3 within the helicase domain. Virology. 1993;193:888–899. doi: 10.1006/viro.1993.1198. [DOI] [PubMed] [Google Scholar]
- 5.Berman HM, Rose PW, Dutta S, Zardecki C, Prlić A. The Protein Data Bank: overview and tools for drug discovery. In: Multifaceted roles of crystallography in modern drug discovery. 2015. pp. 93–106.
- 6.Chambers TJ, Nestorowicz A, Amberg SM, Rice CM. Mutagenesis of the yellow fever virus NS2B protein: effects on proteolytic processing, NS2B-NS3 complex formation, and viral replication. J Virol. 1993;67:6797–6807. doi: 10.1128/jvi.67.11.6797-6807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CYC. TCM Database@ Taiwan: the world’s largest traditional Chinese medicine database for drug screening. PLoS One. 2011;6:e15939. doi: 10.1371/journal.pone.0015939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Almeida H, Bastos IM, Ribeiro BM, Maigret B, Santana JM. New binding site conformations of the dengue virus NS2B-NS3 protease accessed by molecular dynamics simulation. PLoS One. 2013;8:e72402. doi: 10.1371/journal.pone.0072402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLano WL. Pymol: an open-source molecular graphics tool. CCP4 Newslett Protein Crystallogr. 2002;40:82–92. [Google Scholar]
- 10.Dwivedi VD, Tripathi IP, Mishra SK. In silico evaluation of inhibitory potential of triterpenoids from Azadirachta indica against therapeutic target of dengue virus, NS2B-NS3 protease. J Vector Borne Dis. 2016;53:156–161. [PubMed] [Google Scholar]
- 11.Falgout B, Pethel M, Zhang YM, Lai CJ. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus 651 nonstructural proteins. J Virol. 1991;65:2467–2475. doi: 10.1128/jvi.65.5.2467-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev. 1998;11:480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitchen DB, Decornez H, Furr JR, Bajorath J. Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov. 2004;3:935–949. doi: 10.1038/nrd1549. [DOI] [PubMed] [Google Scholar]
- 14.Labbé CM, Rey J, Lagorce D, Vavruša M, Becot, J, et al. MTiOpenScreen: a web server for structure-based virtual screening. Nucleic Acids Res 2015; gkv306. [DOI] [PMC free article] [PubMed]
- 15.Laskowski RA, Swindells MB. LigPlot + : multiple ligand–protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- 16.Lee YK, Tan SK, Wahab HA, Rohana Y. Nonsubstrate based inhibitors of dengue virus serine protease: a molecular docking approach to study binding interactions between protease and inhibitors. Asia Pac J Mol Biol Biotechnol. 2007;15:53–59. [Google Scholar]
- 17.Lescar J, Luo D, Xu T, Sampath A, Lim SP, Canard B, Vasudevan SG. Towards the design of antiviral inhibitors against flaviviruses: the case for the multifunctional NS2B-NS3 protein from Dengue virus as a target. Antivir Res. 2008;80:94–101. doi: 10.1016/j.antiviral.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Luo D, Vasudevan S, Lescar J. The flavivirus NS2B–NS3 protease–helicase as a target for antiviral drug. Development. 2015;6:7. doi: 10.1016/j.antiviral.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Luo D, Xu T, Hunke C, Grüber G, Vasudevan SG, Lescar J. Crystal structure of the NS3 protease-helicase from dengue virus. J Virol. 2008;82:173–183. doi: 10.1128/JVI.01788-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matusan AE, Pryor MJ, Davidson AD, Wright PJ. Mutagenesis of the dengue virus type 2 NS3 protein within and outside helicase motifs: effects on enzyme activity and virus replication. J Virol. 2001;75(20):9633–9643. doi: 10.1128/JVI.75.20.9633-9643.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mustafa MS, Rasotgi V, Jain S, Gupta V. Discovery of fifth serotype of dengue virus (DENV-5): a new public health dilemma in dengue control. Med J Armed Forces India. 2015;71:67–70. doi: 10.1016/j.mjafi.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natarajan S. NS2B-NS3 protease from flavivirus as a target for designing antiviral inhibitors against dengue virus. Genet Mol Biol. 2010;33:214–219. doi: 10.1590/S1415-47572010000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nitsche C, Behnam MM, Steuer C, Klein CD. Retro peptide-hybrids as selective inhibitors of the Dengue virus NS2B- NS2B-NS3 protease. Antivir Res. 2012;94:72–79. doi: 10.1016/j.antiviral.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Normile D. Surprising new dengue virus throws a spanner in disease control efforts. Science. 2013;342(6157):415. doi: 10.1126/science.342.6157.415. [DOI] [PubMed] [Google Scholar]
- 25.Othman R, Kiat TS, Khalid N, Yusof R, Irene NE, Newhouse JS, et al. Docking of noncompetitive inhibitors into dengue virus type 2 protease: understanding the interactions with allosteric binding sites. J Chem Inf Model. 2008;48:1582–1591. doi: 10.1021/ci700388k. [DOI] [PubMed] [Google Scholar]
- 26.Patil R, Das S, Stanley A, Yadav L, Sudhakar A, Varma AK. Optimized hydrophobic interactions and hydrogen bonding at the target-ligand interface leads the pathways of drug-designing. PLoS One. 2010;5:e12029. doi: 10.1371/journal.pone.0012029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranjit S, Kissoon N. Dengue hemorrhagic fever and shock syndromes*. Pediatr Crit Care Med. 2011;12:90–100. doi: 10.1097/PCC.0b013e3181e911a7. [DOI] [PubMed] [Google Scholar]
- 28.Rigau-Pérez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV. Dengue and dengue haemorrhagic fever. Lancet. 1998;352(9132):971–977. doi: 10.1016/S0140-6736(97)12483-7. [DOI] [PubMed] [Google Scholar]
- 29.Rodenhuis-Zybert IA, Wilschut J, Smit JM. Dengue virus life cycle: viral and host factors modulating infectivity. Cell Mol Life Sci. 2010;67(16):2773–2786. doi: 10.1007/s00018-010-0357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose PW, Prlić A, Bi C, Bluhm WF, Christie CH, Dutta S, et al. The RCSB Protein Data Bank: views of structural biology for basic and applied research and education. Nucleic Acids Res. 2015;43(D1):345–356. doi: 10.1093/nar/gku1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell PK, Buescher EL, McCown JM, Ordoñez J. Recovery of dengue viruses from patients during epidemics in Puerto Rico and East Pakistan. Am J Trop Med Hyg. 1966;15:573–579. doi: 10.4269/ajtmh.1966.15.573. [DOI] [PubMed] [Google Scholar]
- 32.Sabin AB, Schlesinger RW. Production of immunity to dengue with virus modified by propagation in mice. Science. 1945;101(2634):640–642. doi: 10.1126/science.101.2634.640. [DOI] [PubMed] [Google Scholar]
- 33.Salentin S, Schreiber S, Haupt VJ, Adasme MF, Schroeder M. PLIP: fully automated protein–ligand interaction profiler. Nucleic Acids Res 2015; gkv315. [DOI] [PMC free article] [PubMed]
- 34.Sarkar JK, Chatterjee SN, Chakravarty SK. Haemorrhagic fever in calcutta: some epidemiological observations. Indian J Med Res. 1964;52:651–659. [PubMed] [Google Scholar]
- 35.Simmons CP, Farrar JJ, Van VCN, Wills B. Dengue. N Engl J Med. 2012;366(15):1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 36.Tiew KC, Dou D, Teramoto T, Lai H, Alliston KR, et al. Inhibition of dengue virus and west nile virus proteases by click chemistry-derived benz [d]isothiazol-3(2H)-one derivatives. Bioorg Med Chem. 2012;20:1213–1221. doi: 10.1016/j.bmc.2011.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomlinson SM, Watowich SJ. Use of parallel validation high-throughput screens to reduce false positives and identify novel dengue NS2B-NS2B-NS3 protease inhibitors. Antivir Res. 2012;93:245–252. doi: 10.1016/j.antiviral.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai TY, Chang KW, Chen CYC. IScreen: world’s first cloud-computing web server for virtual screening and de novo drug design based on TCM database@ Taiwan. J Comput Aided Mol Des. 2011;25:525–531. doi: 10.1007/s10822-011-9438-9. [DOI] [PubMed] [Google Scholar]
- 39.Valle RP, Falgout B. Mutagenesis of the NS3 protease of dengue virus type 2. J Virol. 1998;72:624–632. doi: 10.1128/jvi.72.1.624-632.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velmurugan D, Mythily U, Rao K. Design and docking studies of peptide inhibitors as potential antiviral drugs for dengue virus ns2b/ns3 protease. Protein Pept Lett. 2014;21:815–827. doi: 10.2174/09298665113209990062. [DOI] [PubMed] [Google Scholar]
- 41.Wallace AC, Laskowski RA. Thornton JM LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995;8:127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Q, Bang TH, Ohnuki K, Sawai T, Sawai K, Shimizu K. Inhibition of neuraminidase by Ganoderma triterpenoids and implications for neuraminidase inhibitor design. Sci Rep 2015;5. [DOI] [PMC free article] [PubMed]