Abstract
Incidentally detected, sporadic, nonfunctional pancreatic neuroendocrine tumors are increasingly diagnosed on imaging studies performed for unrelated purposes. Although their resection is usually recommended, controversy still exists regarding their optimal management, due to their highly variable and difficult to predict biologic behavior. Recently, several studies and guidelines advocated an expectant management approach in small size, low grade, incidentally diagnosed nonfunctional pancreatic neuroendocrine tumors. The aim of this study is to review and summarize the available literature addressing nonfunctional pancreatic neuroendocrine tumors, with an emphasis on surgical management controversies.
Keywords: Pancreatic neuroendocrine tumors, Nonfunctional, Incidental, Surgery, Observation
Core tip: Nonfunctional pancreatic neuroendocrine tumors are increasingly diagnosed. Controversy exists regarding their optimal management. Expectant management in small size, low grade, incidentally diagnosed nonfunctional pancreatic neuroendocrine tumors has been suggested as an optional treatment. The aim of this study is to review the available literature addressing nonfunctional pancreatic neuroendocrine tumors, with an emphasis on surgical management controversies.
INTRODUCTION
Pancreatic neuroendocrine tumors (PNETs) are uncommon neoplasms that arise from the islet cells of the pancreas and represent 1%-2% of all pancreatic cancers[1]. PNETs are clinically classified as functional (F-PNETs) and non-functional (NF-PNETs) based on the existence or non-existence of symptoms caused by hormone hypersecretion[2]. F-PNETs can synthesize and produce hormones such as insulin, gastrin, glucagon, somatostatin, and vasoactive intestinal peptide (VIP) resulting in myriad clinical syndromes. NF-PNETs, on the other hand, may secret some peptides such as chromogranin, pancreatic polypeptide, and others, but without clinical syndromes of hypersecretion[3,4]. Historically, F-PNETs were reported to have increased incidence and earlier diagnosis as compared to NF-PNETs due to their symptoms of hypersecretion, although the later accounts for the majority of PNETs[5-7]. With the widespread use and improvement of cross-sectional imaging techniques, NF-PNETs are increasingly discovered incidentally in asymptomatic patients who undergo evaluation for unrelated conditions[8,9]. This has been accompanied by an increase preoperative histologic diagnosis through endoscopic ultrasonography (EUS) and EUS-guided fine needle aspiration[10]. While there is a unanimity consensus that favors surgical resection in F-PNETs, controversy exists among clinicians regarding the optimal management of asymptomatic, small, incidentally discovered NF-PNETs. This article provides an updated review and aims to address the controversies in the management of sporadic small NF-PNETs. In light of article scope limitations, management and treatment of advanced metastatic disease or familial related diseases will not be addressed in this review.
EPIDEMIOLOGY
PNETs are more common in Caucasian and in males, with an incidence that increases with age, reaching a pick in the fifth-sixth decades. Detection is increasing owing to the widespread use of axial imaging, with one retrospective study demonstrating more than 2-fold increase in the incidence of NF-PNETs compared to 16 years ago and that the increase is related to accidental detection of the tumors[8,11]. NF-PNETs are biologically diverse and account for 65% to 90% of PNETs[1,12,13].
While most of PNETs occur sporadically, 10%-30% of them are associated with various inherited disorders including MEN1, Von Hippel-Lindau syndrome, neurofibromatosis 1, tuberous sclerosis, and Mahvash disease[14,15]. The majority of PNETS related to MEN1 and VHL syndromes are non-functioning tumors[1].
CLINICAL PRESENTATION
Patients with F-PNETs have overt clinical symptoms due to their physiologic response to hormone hypersecretion. In contrast, NF-PNETs can remain asymptomatic before they reach a significant tumor burden. Thus, they often present later during the disease with symptoms of local compression or metastatic disease in 21% and 60%, respectively[1,6,16]. When symptomatic, the main complaints observed are abdominal pain (35%-78%), weight loss (20%-35%), and anorexia and nausea (45%). Less frequent signs include icterus (17%-50%), intraabdominal hemorrhage (4%-20%), or a palpable mass (7%-40%)[17]. Up to 50% of non-metastatic NF-PNETs will not show any symptoms being diagnosed incidentally on cross-sectional imaging performed for other indications[8,18].
DIAGNOSIS
The diagnostic approach of patients with NF-PNETs should be thorough and starts with detailed past medical and family history followed by complete physical examination. Then biochemical and imaging studies have uttermost importance for treatment strategy and are performed in order to evaluate the degree of local invasion, lymph node involvement, as well as the presence of metastatic disease.
IMAGING
High-resolution computerized tomography (CT) scan is the initial imaging modality at many institutions due to its noninvasiveness and availability. Studies have reported a sensitivity of more than 80%, with a direct correlation to tumor size[19,20].
Compared to CT, magnetic resonance imaging (MRI) has non-ionized radiation advantage and can be used as an alternative imaging modality. Furthermore, studies reported superiority of MRI over CT in detecting smaller pancreatic lesions and liver metastases[21,22]. One study reported a sensitivity and specificity of up to 85% and 100%, respectively[23].
EUS is an additional imaging modality, and has additional benefits in preoperative diagnosis[24]. Somatostatin receptor imaging (SRI) is a functional imaging modality of choice in the evaluation of neuroendocrine tumors. Besides its utility in the staging of these tumors, SRI may help to select the patients with advanced disease that are suitable for systemic somatostatin-based therapies[25,26].
While 111Indium-DTPA-octreotide (Octreoscan) has been initially used, with the recent availability of the PET imaging technique, somatostatin analogues have been labeled with positron emitting isotopes, including Gallium-68, to image somatostatin receptor (SSR) expressing tumors[27]. The compounds often used in molecular imaging of NETs with PET are 68Ga-DOTATOC, 68Ga-DOTATATE, and 68Ga-DOTANOC, with a varying affinity to different somatostatin receptors. It has been demonstrated that 68Ga-DOTA-TATE PET CT scan has the highest affinity for SSR2 and can dramatically improve the spatial resolution in parallel with a significantly higher detection rate and accuracy compared to conventional Octreoscan[28,29].
BIOPSY
EUS-guided fine-needle aspiration biopsy can provide preoperative histologic information important for tumor grading. One meta-analysis reported a sensitivity of 87% and a specificity of 98%[30]. The utility of routine preoperative biopsy remains controversial. Some clinicians have argued that the theoretical risk of procedure complications outweighs the benefit, while others, including us, believe in routine biopsy given the importance of characterizing and grading the tumor. Dietrich et al[31] demonstrated in a large study the importance of preoperative diagnosis. Among 394 patients with incidental finding of lesions smaller than ≤15 mm, all were diagnosed by imaging-guided biopsy and/or surgery, 156 (about 40%) were diagnosed with neuroendocrine tumors, 146 pancreatic ductal adenocarcinoma, and 92 with various other etiologies. Although retrospective, approximately 60% did not have pancreatic ductal adenocarcinoma and not necessarily require radical surgery that carries significant risks[31].
BIOCHEMICAL STUDIES
Chromogranin A (CgA) can be used as a nonspecific biochemical marker. It has an approximate sensitivity and specificity of 60% and 80%, respectively[32,33]. False positive elevations of CgA can present in many other conditions such as use of anti-acid drugs (e.g., proton pump inhibitors, H2 blockers, etc.), atrophic gastritis, renal insufficiency, hepatic insufficiency, ect[34].
Pancreatic polypeptide (PP) and neuron specific enolase (NSE) are additional useful NF-PNET markers. As with CgA false positive elevations of pancreatic polypeptide can be postprandial and in renal insufficiency[16,32,35]. Preoperative increased levels of CgA or PP may potentially be helpful in evaluation of response, progression, or recurrence at an early stage[36].
Elevated NSE levels were exclusively associated with poor tumor differentiation[36].
GRADING AND STAGING
From histological point of view, the 2010 World Health Organization (WHO) classification system is the most used grading system. It identifies three categories: Grade 1 tumors (< 2 mitosis/10 HPF and Ki-67 index ≤ 2%), grade 2 (2-20 mitosis/10 HPF and Ki-67 index 3%-20%), and grade 3 (> 20 mitosis/10 HPF and Ki-67 index of > 20%). This classification forms the basis for evaluating prognosis and predicting malignancy[2,37].
Two TNM based staging systems were developed for PNETs, one from the American Joint Committee on Cancer (AJCC) that covers both pancreatic exocrine and neuroendocrine malignancies and the other proposed by the European Neuroendocrine Tumor Society (ENETS) (Table 1)[38,39]. The difference between them is mainly expressed in the soft tissue involvement criteria. While the AJCC characterize T3-T4 using peripancreatic invasion of these tumors (sometimes difficult to assess due to the structure of the pancreas), the ENETS staging system relies on more assessable criteria such as tumor size[40,41]. Despite differences, both staging systems are highly prognostic validated and found to be useful for clinical practice[42-44].
Table 1.
European Neuroendocrine Tumor Society and American Joint Committee on Cancer TNM grading systems for pancreatic tumors[38,39]
| ENETS | AJCC | |
| T Grade (primary tumor) | ||
| Tx | Primary tumor is not assessed | Primary tumor is not assessed |
| T0 | No finding of primary tumor | No finding of a primary tumor |
| Tis | In situ carcinoma | |
| T1 | Tumor is limited to the pancreas and < 2 cm | Tumor is limited to the pancreas and ≤ 2 cm |
| T2 | Tumor is limited to the pancreas and 2 to 4 cm | T2 tumor is limited to the pancreas and > 2 cm |
| T3 | Tumor is limited to the pancreas and > 4 cm or with positive duodenum or biliary tract invasion | Tumor has progressed beyond the pancreas but there is no celiac or mesenteric artery involvement |
| T4 | Tumor has invaded the neighboring organs (stomach, spleen, colon, adrenal gland) or walls of the large vessels (celiac artery or superior mesenteric artery) | Tumor shows celiac or superior mesenteric artery involvement |
| N-lymph node status | ||
| Nx | Regional lymph nodes are not assessed | Regional lymph nodes are not assessed |
| N0 | No regional lymph node metastasis | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis is positive | Regional lymph node metastasis is positive |
| M-distant metastasis | ||
| Mx | Distant metastasis is not assessed | |
| M0 | No distant metastasis | No distant metastasis |
| M1 | Distant metastasis is positive | Distant metastasis is positive |
| Stage | ||
| 0 | Tis, N0, M0 | |
| I | T1, N0, M0 | |
| IA | T1, N0, M0 | |
| IB | T2, N0, M0 | |
| IIA | T2, N0, M0 | T3, N0, M0; T1, N1, M0 |
| IIB | T3, N0, M0 | T2, N1, M0; T3,N1,M0 |
| III | T4, Any N, M0 | |
| IIIA | T4, N0, M0 | |
| IIIB | Any T, N1, M0 | |
| IV | Any T, Any N, M1 | Any T, Any N, M1 |
One retrospective 11-year period report of 425 patients with PNETs demonstrated that the 5-year overall survival rates using the ENETS classification for patients treated in referral neuroendocrine tumor (NET) center for stages I, II, III and IV disease were 100%, 88%, 85%, and 57%, respectively. The corresponding values using the AJCC classification were 92%, 84%, 81%, and 57%, respectively[44]. Another large cohort study of 1072 post-operative patients suggests the ENETS TNM staging system is superior to the AJCC and WHO 2010 TNM staging system and supports its use in clinical practice[42].
CURRENT GUIDELINES
Several guidelines for the management of PNETs have been established in order to help physicians treating these complex patients. The 2012 and 2016 European Neuroendocrine Tumor Society (ENETS) guidelines, the National Comprehensive Cancer Network 2016 (NCCN), North American Neuroendocrine Tumor Society-2013 (NANETS), and European Society of Medical Oncology-2012 (ESMO) have published diagnostic and therapeutic guidelines[3,12,45-47].
For initial biochemical workup the ENETS, ESMO, and NANETS guidelines all recommend measuring CgA and PP serum levels as a useful tool for reaching a diagnosis in a fraction of NF-PNETs.
The first imaging modality recommended is multiphasic CT/MRI with contrast agents imaging modality, while octreotide scintigraphy (planar and SPECT) but mainly 68Ga-labeled somatostatin analogues with PET/CT are also recommended, if available.
All four guidelines recommend using the 2010 WHO grading system as the grading of choice and generally advocate surgical resection as the preferred option as long as there are no surgical limiting contraindications, highly diffuse metastatic disease, or selected cases that can be observed discussed in the next sections.
The surgical options for locoregional NF-PNETs mentioned in all guidelines range between simple enucleation, central pancreatectomy, distal pancreatectomy with or without splenectomy, and pancreatoduodenectomy (Whipple’s operation). The extent and type of surgery mainly depends on the location of the primary tumor (head, body, or tail). Tumors larger than 2 cm that are locally invasive or have positive lymph node involvement in preoperative evaluation should all include regional lymph node dissection. In patients with smaller than 2 cm NF-PNETs, lymph node sampling is not always mandatory. While both NCCN and ENETS recognize the role of laparoscopic approach in PNETs resections, the EMCO guidelines do not recommend this approach due to the need for thorough intraoperative lymph node inspection.
Currently, updated ENETS and NCCN guidelines both acknowledge nonoperational options, with different tumor size-cutoff (NCCN < 1 cm, ENETS < 2 cm), as suitable for managing small NF-PNETs while taking into account factors such as incidental discovery, lack of clinical syndromes and radiological signs suspicious for malignancy, as well as patient’s characteristics (surgical risk, comorbidities, and personal wishes)[12,45,47]. However, data supporting this non-operational option are controversial and will be reviewed in the next section. Figure 1 offers a suggested algorithm for patient management.
Figure 1.
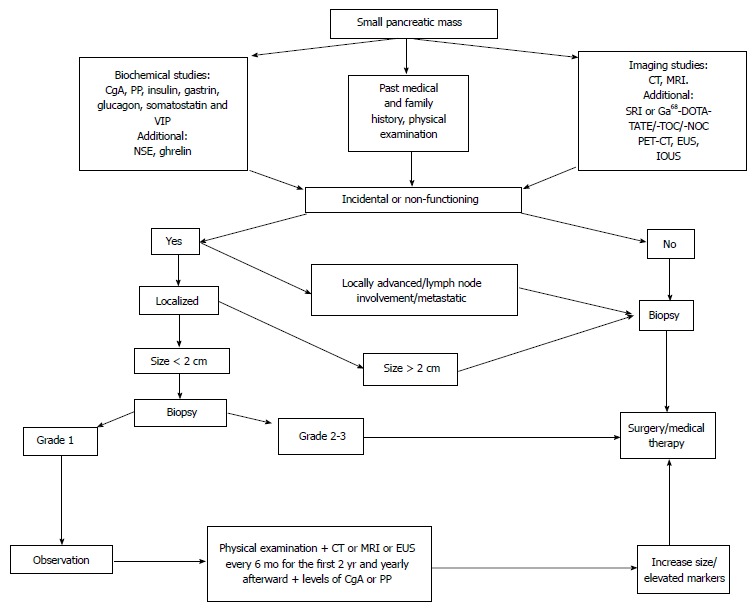
Suggested algorithm for the management of small pancreatic mass. CgA: Chromogranin A; PP: Pancreatic polypeptide; VIP: Vasoactive intestinal peptide; NSE: Neuron-specific enolase; CT: Computed tomography; MRI: Magnetic resonance imaging; SRI: Somatostatin-receptor imaging; EUS: Endoscopic ultrasonography; IOUS: Intraoperatively ultrasonography; PET: Positron emission tomography; Ga68-DOTA-TATE/-TOC/-NOC: 68Gallium-DOTA-TATE, 68Gallium-DOTA-TOC68 and Gallium-DOTA-NOC respectively.
SURGICAL MANAGEMENT CONTROVERSY
In most cases surgery remains the curative modality of choice for NF-PNETs, with preliminary evidence demonstrating improved survival especially with localized disease[48,49]. However, as previously mentioned, during the last recent years there is a significant increase in the detection of small, incidentally discovered, asymptomatic NF-PNETs, that may be managed conservatively by observation. At present there are no RCTs or meta-analyses that can assist to outline the optimal approach for the management of such small NF-PNETs. Nevertheless, there are 12 retrospective series that may shed some light on this controversy.
Tumor size as criteria for treatment decision
Bettini et al[50] demonstrated a distinct correlation between tumor size and lower malignancy potential on 177 patients, who were divided into three groups depending on tumor size (≤ 2 cm, 2-4 cm, > 4 cm), all underwent curative resection. Patients with tumor ≤ 2 cm (n = 51) had higher frequency of incidental diagnosis compared with patients with > 4 cm (57% vs 32%, P = 0.014). Among those who were incidentally discovered, only 6% were malignant and none died from the disease. In addition, a correlation between tumor size and Ki67 was demonstrated. Patients with tumors ≤ 2 cm had lower Ki67 median values compared with patients with tumors > 2 cm ≤ 4 cm and > 4 cm (1% vs 2% and 3%, respectively). The authors suggested that nonsurgical management could be advocated in selected cases for low-grade tumors less than 2 cm, due to their indolent course. In an attempt to determine the prognostic value of indicators of malignancy in NF-PNETs ≤ 2 cm, Regenet et al[51] demonstrated, by using multivariate analysis, that tumor size is a significant indicator of malignancy, and even proposed a new 1.7 cm cutoff as more accurate for prediction of malignancy potential with a sensitivity of 92% and specificity of 75%. Despite these findings, Ki67 was not found to be a significant indicator of malignancy probably due to large number of patients without complete histologic assessment and Ki67 evaluation[51]. In a larger population study by Gratian et al[52] among 1854 patients with NF-PNETs ≤ 2 cm, who were identified from the National Cancer Data Base (NCDB), 309 patients (29%) presented with regional lymph node involvement and 180 patients (10%) presented with distant metastases[52]. In contrast to Bettini et al findings, they conclude that tumors smaller than 2 cm have a significant risk of malignancy. It is worth mentioning that the study was limited by missing data of several variables, including Ki67.
Incidental vs non-incidental diagnosis as criteria for treatment decision
Different studies have recently tried to distinguish between incidental and non-incidental NF-PNETs, especially those discovered at early age, in terms of prognosis and treatment approach. Cheema et al[18] identified 143 nonmetastatic PNETs, 40% were diagnosed incidentally. They demonstrated that 5-year progression free survival (PFS) was significantly prolonged in patients with incidental diagnosed vs symptomatic tumors (86% vs 59%, P = 0.007).
Tumor grading as criteria for treatment decision
It should be noted that histopathologic grade was another statistically significant factor for progression on multivariate analysis (hazard ratio of 3.0 for Grade 2 vs Grade 1, P = 0.007), though Ki67 proliferation index was only evaluated in 25% of cases[18]. As opposed to Cheema et al[18], Haynes at el[53] described 139 patients who all underwent surgery and identified no large difference in tumor size (3.0 cm vs 3.5 cm, P = 0.48), frequency of malignant histopathologic findings (28% vs 30%), or 5-year PFS (83% vs 82%, P = 0.27) between incidental and non-incidental groups[18,53]. Of the 39 patients with tumors ≤ 2 cm, 3 patients (7.7%) had late metastases or recurrence. Though problematic due to lack of observational group, they concluded that all patients should undergo tumor resection, even in incidentally discovered NF-PNETs smaller than 2 cm. From a staging point of view Crippa et al[54] demonstrated in a larger (n = 355) retrospective study that NF-PNETs diagnosed incidentally have greater 5-year PFS rates in all stages than symptomatic tumors: Stage I (97% vs 78%, P = 0.013), stage II (93% vs 74%, P = 0.036), stage III (69% vs 27%, P < 0.0001), and stage IV (60% vs 17%, P = 0.112). On multivariate analysis Grade 2 NF-PNETs was found to be a predictor of PFS among 124 incidentally diagnosed patients, with a hazard ratio of 3.402 (95%CI: 0.92-12.57, P = 0.066). In addition, they reported that 12 excluded patients, who underwent non-operative management of incidental NF-PNETs and had no tumor progression after median follow up of 36 mo. In this small group of patients the median tumor size at diagnosis was 1.4 cm (range 1.0-2.9 cm), and was stable throughout the surveillance period. Similar PFS rates were demonstrated in another retrospective study by Birnbaum et al[55] that included 106 patients, 65 discovered incidentally. These patients demonstrated both higher incidence of tumors smaller than 2 cm (65% vs 42%, P = 0.019) and lower Ki67 proliferation index (1% vs 4%, P = 0.004) compared to symptomatic patients. The authors concluded that pancreas sparing surgery is recommended as an optional treatment for these incidental NF-PNETs, due to less aggressive characteristics compared with symptomatic tumors (Table 2).
Table 2.
Retrospective studies regarding incidental discovery
| Ref. | Study period | Patients (n) | Group | Number of patients n (%) | 5-yr PFS rates (%) | P value | Median follow-up time (mo) |
| Cheema et al[18] | 1999-2010 | 143 | Incidental | 56 (40) | 86 | 0.07 | 67 (mean) |
| Non-incidental | 87 (60) | 59 | |||||
| Crippa et al[54] | 1990-2009 | 355 | Incidental | 124 (35) | 83 | < 0.001 | 44 |
| Non-incidental | 231 (65) | 32 | |||||
| Haynes et al[53] | 1997-2009 | 139 | Incidental | 109 (82) | 82.8 | 0.27 | 34.2 |
| Non-incidental | 30 (18) | 81.7 | |||||
| Birnbaum et al[55] | 1994-2010 | 108 | Incidental | 65 (61) | 92 | 0.03 | 42 |
| Non-incidental | 43 (39) | 82 |
Observation for selected patients
Several retrospectively designed studies tried to answer the question whether observational management is suitable for NF-PNETs smaller than 2 cm and to assess the risk-benefit balance of this approach. Gaujoux et al[56] published a series of 46 patients who were followed for at least 18 mo (median 34, range 24-52 mo) with an average of four (range 4-6) serial imaging sessions or followed up after resection[56]. Among the resection group (n = 8), all grade 1 and without lymph node involvement, 5 were resected upon initial diagnosis and only 3 were resected due to tumor enlargement under imaging observations. The remaining 38 patients, who were managed without surgery, did not show any significant characteristics of malignancy such as distant metastases, nodal involvement, or significant increase in tumor size. In this study the overall median tumor growth was 0.12 mm per year. Both Lee et al[57] and Rosenberg et al[58] published similar results where small NF-PNETs in either the operative or non-operative groups demonstrated no evidence of progression, with lower, though important, operational-morbidity related rates (46% and 35%, respectively)[57,58]. They both conclude that non-operative management may be advocated and safe in selected patients. In the Lee et al[57] study, both surgical and nonsurgical group’s tumors had low or intermediate grade and Ki67 values smaller than 5%, in all patients with available results[57]. Rosenberg et al[58] published a 35 patients series divided into operative and non-operative groups as well: Ki67 proliferation index rates of < 2% and 3%-20% were 65% vs 0% and 30% vs 27%, respectively. Ki67 data was not available in 1 (5%) patient in the operative group vs 11 (73%) in the non-operative group[58].
The observational approach for certain tumors was reinforced by another recently published matched case-control study by Sadot et al[59] who demonstrated that 5-year PFS was 95% and 91% (P = 0.3) for observational and resection only groups, respectively. A quarter (n = 26) of the observation group crossed over to resection group, due to different reasons. After a median follow-up of 7 years, none of these patients developed malignant features (node involvement or metastases). These data imply that initially observational approach and delayed surgical intervention may not compromise long-term outcomes.
Contrary to this claim, Sharpe et al[60] performed a population based study and demonstrated that patients who were managed with observation had nearly three times the risk of mortality in comparison to those who underwent resection[60]. Their study was large and based on patients collected from NCDB, all with NF-PNETs smaller than 2 cm. The authors concluded that surgical resection provides a benefit regardless of tumor grade, though it wasn’t statistically proven at poorly differentiated/undifferentiated tumor. A summary of the studies regarding surgical vs observational approach in NF-PNETs is presented in Table 3.
Table 3.
Retrospective studies regarding surgery vs observational management
| Ref. | Study period | Patients n | Group | Number of patients n (%) | Median follow-up time (mo) | Surgery morbidity rate (%) |
| Gaujoux et al[56] | 2000-2011 | 46 | Observational | 38 (83) | > 18 | 62 |
| Surgery | 8 (17) | 27 | ||||
| Lee et al[57] | 2000-2011 | 133 | Observational | 77 (57) | 44 (Mean) | 46 |
| Surgery | 56 (43) | 52 (Mean) | ||||
| Rosenberg et al[58] | 1999-2014 | 35 | Observational | 15 (42) | 28 | 35 |
| Surgery | 20 (58) | 34 | ||||
| Sharpe et al[60] | 1998-2006 | 380 | Observational | 71 (19) | 60 | N/A |
| Surgery | 309 (81) | 60 | ||||
| Sadot et al[59] | 1993-2013 | 181 | Observational | 1042 (57) | 44 | N/A |
| Surgery | 771 (43) | 57 |
Matched group;
Before cross over; N/A: Not available.
OBSERVATION PROTOCOL
History and physical examination, as well as biochemical markers and conventional trans-sectional high-resolution imaging should be used for both non-operative and postoperative surveillance. Postoperatively in patients with NF-PNETs grade 1 and 2, imaging is indicated every 3-9 mo (CT, MRI, or EUS), while more frequent imaging (up to 2-3 mo intervals periods) is indicated in Grade 3 or recurrent symptomatic patients, during the first year following surgery[12,45,47,61]. Either Octreoscan or PET/CT using 68Ga-DOTA-TOC/-NOC/-TATE should be repeated every 18-24 mo for grades 1-2[61]. In non-surgical patients with less than 2 cm NF-PNETs, Gaujoux et al[56] recommend conventional contrast enhanced CT or MRI every 6 mo for the first 2 years and yearly afterward[56]. In patients who underwent surgical resection of the tumor, imaging at 6-12 mo intervals should be performed between one and ten years post resection, although the optimal duration surveillance time for either non-operative nor postoperative patients is unknown[46].
CONCLUSION
In the last two decades the incidence of small NF-PNETs neoplasms has been steadily increasing. Unfortunately, there are still no clear prognostic factors that can enable us to distinguish between tumors suitable for observation and tumors with greater malignant potential that should be treated more aggressively. Several retrospective population based studies were reviewed in this article in an attempt to reduce the uncertainty. However, issues of selection bias, small sampling, and lack of data that are inherent in this type of studies limit our ability to conclude valid recommendations. In our NET center, the decision on treatment approach (follow-up vs surgical excision) for incidental NF-PNETs patients is based on tumor size (less or more than 2 cm), tumor grading, intensity of uptake on functional imaging (68GaDOTATATE-PET/CT), on the stage of the disease, as well as on patient’s desire. Larger scale, preferably multicenter randomized control trials, are needed in order to clarify the optimal management strategy and treatment for these rare small incidentally discovered tumors.
Footnotes
Conflict-of-interest statement: The authors report no financial or ethical conflicts of interest.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
Peer-review started: August 30, 2016
First decision: September 27, 2016
Article in press: January 12, 2017
P- Reviewer: Run Y, Tamagno G, Yoshitomi H S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
References
- 1.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosman FT, Carneiro F, Hruban RH, Theise N. WHO Classification of Tumor of the Digestive System. Lyon, France: IARC Press; 2010. [Google Scholar]
- 3.Kulke MH, Anthony LB, Bushnell DL, de Herder WW, Goldsmith SJ, Klimstra DS, Marx SJ, Pasieka JL, Pommier RF, Yao JC, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735–752. doi: 10.1097/MPA.0b013e3181ebb168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krampitz GW, Norton JA. Pancreatic neuroendocrine tumors. Curr Probl Surg. 2013;50:509–545. doi: 10.1067/j.cpsurg.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Bilimoria KY, Tomlinson JS, Merkow RP, Stewart AK, Ko CY, Talamonti MS, Bentrem DJ. Clinicopathologic features and treatment trends of pancreatic neuroendocrine tumors: analysis of 9,821 patients. J Gastrointest Surg. 2007;11:1460–1467; discussion 1467-1469. doi: 10.1007/s11605-007-0263-3. [DOI] [PubMed] [Google Scholar]
- 6.Franko J, Feng W, Yip L, Genovese E, Moser AJ. Non-functional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2,158 patients. J Gastrointest Surg. 2010;14:541–548. doi: 10.1007/s11605-009-1115-0. [DOI] [PubMed] [Google Scholar]
- 7.Fraenkel M, Kim MK, Faggiano A, Valk GD. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012;26:691–703. doi: 10.1016/j.bpg.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Vagefi PA, Razo O, Deshpande V, McGrath DJ, Lauwers GY, Thayer SP, Warshaw AL, Fernández-Del Castillo C. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: the Massachusetts General Hospital experience from 1977 to 2005. Arch Surg. 2007;142:347–354. doi: 10.1001/archsurg.142.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald TL, Hickner ZJ, Schmitz M, Kort EJ. Changing incidence of pancreatic neoplasms: a 16-year review of statewide tumor registry. Pancreas. 2008;37:134–138. doi: 10.1097/MPA.0b013e318163a329. [DOI] [PubMed] [Google Scholar]
- 10.Ashkar M, Gardner TB. Role of endoscopic ultrasound in pancreatic diseases: a systematic review. Minerva Gastroenterol Dietol. 2014;60:227–245. [PubMed] [Google Scholar]
- 11.Kuo JH, Lee JA, Chabot JA. Nonfunctional pancreatic neuroendocrine tumors. Surg Clin North Am. 2014;94:689–708. doi: 10.1016/j.suc.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153–171. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18, vii. doi: 10.1016/j.ecl.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Ro C, Chai W, Yu VE, Yu R. Pancreatic neuroendocrine tumors: biology, diagnosis,and treatment. Chin J Cancer. 2013;32:312–324. doi: 10.5732/cjc.012.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhyu J, Yu R. Mahvash disease: an autosomal recessive hereditary pancreatic neuroendocrine tumor syndrome. International Journal of Endocrine Oncology. 2016;3:235–243. [Google Scholar]
- 16.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–1492. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falconi M, Plockinger U, Kwekkeboom DJ, Manfredi R, Korner M, Kvols L, Pape UF, Ricke J, Goretzki PE, Wildi S, et al. Well-differentiated pancreatic nonfunctioning tumors/carcinoma. Neuroendocrinology. 2006;84:196–211. doi: 10.1159/000098012. [DOI] [PubMed] [Google Scholar]
- 18.Cheema A, Weber J, Strosberg JR. Incidental detection of pancreatic neuroendocrine tumors: an analysis of incidence and outcomes. Ann Surg Oncol. 2012;19:2932–2936. doi: 10.1245/s10434-012-2285-7. [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa T, Peterson MS, Federle MP, Baron RL, Haradome H, Kawamori Y, Nawano S, Araki T. Islet cell tumor of the pancreas: biphasic CT versus MR imaging in tumor detection. Radiology. 2000;216:163–171. doi: 10.1148/radiology.216.1.r00jl26163. [DOI] [PubMed] [Google Scholar]
- 20.Khashab MA, Yong E, Lennon AM, Shin EJ, Amateau S, Hruban RH, Olino K, Giday S, Fishman EK, Wolfgang CL, et al. EUS is still superior to multidetector computerized tomography for detection of pancreatic neuroendocrine tumors. Gastrointest Endosc. 2011;73:691–696. doi: 10.1016/j.gie.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Sundin A, Vullierme MP, Kaltsas G, Plöckinger U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: radiological examinations. Neuroendocrinology. 2009;90:167–183. doi: 10.1159/000184855. [DOI] [PubMed] [Google Scholar]
- 22.Dromain C, de Baere T, Lumbroso J, Caillet H, Laplanche A, Boige V, Ducreux M, Duvillard P, Elias D, Schlumberger M, et al. Detection of liver metastases from endocrine tumors: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J Clin Oncol. 2005;23:70–78. doi: 10.1200/JCO.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Thoeni RF, Mueller-Lisse UG, Chan R, Do NK, Shyn PB. Detection of small, functional islet cell tumors in the pancreas: selection of MR imaging sequences for optimal sensitivity. Radiology. 2000;214:483–490. doi: 10.1148/radiology.214.2.r00fe32483. [DOI] [PubMed] [Google Scholar]
- 24.James PD, Tsolakis AV, Zhang M, Belletrutti PJ, Mohamed R, Roberts DJ, Heitman SJ. Incremental benefit of preoperative EUS for the detection of pancreatic neuroendocrine tumors: a meta-analysis. Gastrointest Endosc. 2015;81:848–856.e1. doi: 10.1016/j.gie.2014.12.031. [DOI] [PubMed] [Google Scholar]
- 25.Schillaci O, Spanu A, Scopinaro F, Falchi A, Corleto V, Danieli R, Marongiu P, Pisu N, Madeddu G, Delle Fave G, et al. Somatostatin receptor scintigraphy with 111In-pentetreotide in non-functioning gastroenteropancreatic neuroendocrine tumors. Int J Oncol. 2003;23:1687–1695. [PubMed] [Google Scholar]
- 26.Lebtahi R, Cadiot G, Sarda L, Daou D, Faraggi M, Petegnief Y, Mignon M, le Guludec D. Clinical impact of somatostatin receptor scintigraphy in the management of patients with neuroendocrine gastroenteropancreatic tumors. J Nucl Med. 1997;38:853–858. [PubMed] [Google Scholar]
- 27.Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40–47. doi: 10.1111/j.1754-9485.2011.02327.x. [DOI] [PubMed] [Google Scholar]
- 28.Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, Kovacs P, Von Guggenberg E, Bale R, Virgolini IJ. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Kan Y, Ge BH, Yuan L, Li C, Zhao W. Diagnostic role of Gallium-68 DOTATOC and Gallium-68 DOTATATE PET in patients with neuroendocrine tumors: a meta-analysis. Acta Radiol. 2014;55:389–398. doi: 10.1177/0284185113496679. [DOI] [PubMed] [Google Scholar]
- 30.Puli SR, Kalva N, Bechtold ML, Pamulaparthy SR, Cashman MD, Estes NC, Pearl RH, Volmar FH, Dillon S, Shekleton MF, et al. Diagnostic accuracy of endoscopic ultrasound in pancreatic neuroendocrine tumors: a systematic review and meta analysis. World J Gastroenterol. 2013;19:3678–3684. doi: 10.3748/wjg.v19.i23.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietrich CF, Sahai AV, D’Onofrio M, Will U, Arcidiacono PG, Petrone MC, Hocke M, Braden B, Burmester E, Möller K, et al. Differential diagnosis of small solid pancreatic lesions. Gastrointest Endosc. 2016;84:933–940. doi: 10.1016/j.gie.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 32.Panzuto F, Severi C, Cannizzaro R, Falconi M, Angeletti S, Pasquali A, Corleto VD, Annibale B, Buonadonna A, Pederzoli P, et al. Utility of combined use of plasma levels of chromogranin A and pancreatic polypeptide in the diagnosis of gastrointestinal and pancreatic endocrine tumors. J Endocrinol Invest. 2004;27:6–11. doi: 10.1007/BF03350903. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence B, Gustafsson BI, Kidd M, Pavel M, Svejda B, Modlin IM. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:111–134, viii. doi: 10.1016/j.ecl.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Gut P, Czarnywojtek A, Fischbach J, Bączyk M, Ziemnicka K, Wrotkowska E, Gryczyńska M, Ruchała M. Chromogranin A - unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch Med Sci. 2016;12:1–9. doi: 10.5114/aoms.2016.57577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinik AI, Silva MP, Woltering EA, Go VL, Warner R, Caplin M. Biochemical testing for neuroendocrine tumors. Pancreas. 2009;38:876–889. doi: 10.1097/MPA.0b013e3181bc0e77. [DOI] [PubMed] [Google Scholar]
- 36.Oberg K, Modlin IM, De Herder W, Pavel M, Klimstra D, Frilling A, Metz DC, Heaney A, Kwekkeboom D, Strosberg J, et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015;16:e435–e446. doi: 10.1016/S1470-2045(15)00186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang M, Tian BL, Zhang Y, Su AP, Yue PJ, Xu S, Wang L. Evaluation of the World Health Organization 2010 grading system in surgical outcome and prognosis of pancreatic neuroendocrine tumors. Pancreas. 2014;43:1003–1008. doi: 10.1097/MPA.0000000000000153. [DOI] [PubMed] [Google Scholar]
- 38.Edge S, Byrd D, Compton C. AJCC Cancer Staging Manual, vol. 7th ed. New York: Springer; 2010. [Google Scholar]
- 39.Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adsay NV, Bagci P, Tajiri T, Oliva I, Ohike N, Balci S, Gonzalez RS, Basturk O, Jang KT, Roa JC. Pathologic staging of pancreatic, ampullary, biliary, and gallbladder cancers: pitfalls and practical limitations of the current AJCC/UICC TNM staging system and opportunities for improvement. Semin Diagn Pathol. 2012;29:127–141. doi: 10.1053/j.semdp.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Reid MD, Balci S, Saka B, Adsay NV. Neuroendocrine tumors of the pancreas: current concepts and controversies. Endocr Pathol. 2014;25:65–79. doi: 10.1007/s12022-013-9295-2. [DOI] [PubMed] [Google Scholar]
- 42.Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, Capella C, Caplin M, Couvelard A, Doglioni C, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104:764–777. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 43.Strosberg JR, Cheema A, Weber JM, Ghayouri M, Han G, Hodul PJ, Kvols LK. Relapse-free survival in patients with nonmetastatic, surgically resected pancreatic neuroendocrine tumors: an analysis of the AJCC and ENETS staging classifications. Ann Surg. 2012;256:321–325. doi: 10.1097/SLA.0b013e31824e6108. [DOI] [PubMed] [Google Scholar]
- 44.Strosberg JR, Cheema A, Weber J, Han G, Coppola D, Kvols LK. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol. 2011;29:3044–3049. doi: 10.1200/JCO.2011.35.1817. [DOI] [PubMed] [Google Scholar]
- 45.Falconi M, Bartsch DK, Eriksson B, Klöppel G, Lopes JM, O’Connor JM, Salazar R, Taal BG, Vullierme MP, O’Toole D. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120–134. doi: 10.1159/000335587. [DOI] [PubMed] [Google Scholar]
- 46.Kunz PL, Reidy-Lagunes D, Anthony LB, Bertino EM, Brendtro K, Chan JA, Chen H, Jensen RT, Kim MK, Klimstra DS, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013;42:557–577. doi: 10.1097/MPA.0b013e31828e34a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Cancer Comprehensive Network (NCCN) NCCN neuroendocrine tumors version 2. 2016. [Google Scholar]
- 48.Keutgen XM, Nilubol N, Glanville J, Sadowski SM, Liewehr DJ, Venzon DJ, Steinberg SM, Kebebew E. Resection of primary tumor site is associated with prolonged survival in metastatic nonfunctioning pancreatic neuroendocrine tumors. Surgery. 2016;159:311–318. doi: 10.1016/j.surg.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, Tseng JF. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer. 2009;115:741–751. doi: 10.1002/cncr.24065. [DOI] [PubMed] [Google Scholar]
- 50.Bettini R, Partelli S, Boninsegna L, Capelli P, Crippa S, Pederzoli P, Scarpa A, Falconi M. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150:75–82. doi: 10.1016/j.surg.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Regenet N, Carrere N, Boulanger G, de Calan L, Humeau M, Arnault V, Kraimps JL, Mathonnet M, Pessaux P, Donatini G, et al. Is the 2-cm size cutoff relevant for small nonfunctioning pancreatic neuroendocrine tumors: A French multicenter study. Surgery. 2016;159:901–907. doi: 10.1016/j.surg.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 52.Gratian L, Pura J, Dinan M, Roman S, Reed S, Sosa JA. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol. 2014;21:3515–3521. doi: 10.1245/s10434-014-3769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haynes AB, Deshpande V, Ingkakul T, Vagefi PA, Szymonifka J, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, Fernández-del Castillo C. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg. 2011;146:534–538. doi: 10.1001/archsurg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crippa S, Partelli S, Zamboni G, Scarpa A, Tamburrino D, Bassi C, Pederzoli P, Falconi M. Incidental diagnosis as prognostic factor in different tumor stages of nonfunctioning pancreatic endocrine tumors. Surgery. 2014;155:145–153. doi: 10.1016/j.surg.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Birnbaum DJ, Gaujoux S, Cherif R, Dokmak S, Fuks D, Couvelard A, Vullierme MP, Ronot M, Ruszniewski P, Belghiti J, et al. Sporadic nonfunctioning pancreatic neuroendocrine tumors: prognostic significance of incidental diagnosis. Surgery. 2014;155:13–21. doi: 10.1016/j.surg.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Gaujoux S, Partelli S, Maire F, D’Onofrio M, Larroque B, Tamburrino D, Sauvanet A, Falconi M, Ruszniewski P. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2013;98:4784–4789. doi: 10.1210/jc.2013-2604. [DOI] [PubMed] [Google Scholar]
- 57.Lee LC, Grant CS, Salomao DR, Fletcher JG, Takahashi N, Fidler JL, Levy MJ, Huebner M. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery. 2012;152:965–974. doi: 10.1016/j.surg.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg AM, Friedmann P, Del Rivero J, Libutti SK, Laird AM. Resection versus expectant management of small incidentally discovered nonfunctional pancreatic neuroendocrine tumors. Surgery. 2016;159:302–309. doi: 10.1016/j.surg.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 59.Sadot E, Reidy-Lagunes DL, Tang LH, Do RK, Gonen M, D’Angelica MI, DeMatteo RP, Kingham TP, Groot Koerkamp B, Untch BR, et al. Observation versus Resection for Small Asymptomatic Pancreatic Neuroendocrine Tumors: A Matched Case-Control Study. Ann Surg Oncol. 2016;23:1361–1370. doi: 10.1245/s10434-015-4986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharpe SM, In H, Winchester DJ, Talamonti MS, Baker MS. Surgical resection provides an overall survival benefit for patients with small pancreatic neuroendocrine tumors. J Gastrointest Surg. 2015;19:117–123; discussion 123. doi: 10.1007/s11605-014-2615-0. [DOI] [PubMed] [Google Scholar]
- 61.Öberg K, Knigge U, Kwekkeboom D, Perren A. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii124–vii130. doi: 10.1093/annonc/mds295. [DOI] [PubMed] [Google Scholar]


