Abstract
Introduction
Acetone is a ubiquitous ingredient in many household products (e.g., glue solvents, air fresheners, adhesives, nail polish, and paint) that is putatively abused; however, there is little empirical evidence to suggest that acetone alone has any abuse liability. Therefore, we systematically investigated the conditioned response to inhaled acetone in a place conditioning apparatus.
Method
Three groups of male, Sprague-Dawley rats were exposed to acetone concentrations of 5,000, 10,000 or 20,000 ppm for 1 hour in a conditioned place preference apparatus alternating with air for 6 pairing sessions. A place preference test ensued in an acetone-free environment. To test the preference of acetone as a function of pairings sessions, the 10,000 ppm group received an additional 6 pairings and an additional group received 3 pairings. The control group received air in both compartments. Locomotor activity was recorded by infrared photocells during each pairing session.
Results
We noted a dose response relationship to acetone at levels 5,000-20,000 ppm. However, there was no correlation of place preference as a function of pairing sessions at the 10,000 ppm level. Locomotor activity was markedly decreased in animals on acetone-paired days as compared to air-paired days.
Conclusion
The acetone concentrations we tested for these experiments produced a markedly decreased locomotor activity profile that resemble CNS depressants. Furthermore, a dose response relationship was observed at these pharmacologically active concentrations, however, animals did not exhibit a positive place preference.
Keywords: acetone, inhalant abuse, solvent abuse, conditioned place preference (CPP)
1. Introduction
Solvent abuse continues to be a significant health problem among adolescents in the US and worldwide (Anderson and Loomis, 2003; Basu et al., 2004; Brouette and Anton, 2001; Howard and Jenson, 1999). Considering the prevalence of inhalant abuse and evidence that it precedes poly drug abuse (Dinwiddie, 1994; Schutz et al., 1994), it is critical to establish which inhalants should be of primary concern. When considering inhalants, distinctions are rarely made based on abuse or abuse liability, and very few studies have adequately evaluated which solvents or common properties of classes of solvents might indicate the potential for abuse (Balster, 1987).
Typically, therapeutic drugs intended for humans are thoroughly examined for their safety. However with the exception of volatile anesthetics, abused inhalants are not intended for human exposure and thus much less is known about their safety or potential abuse liability. Since many different classes of chemicals are used on a routine basis as organic solvents, including aliphatic hydrocarbons, aromatic hydrocarbons, ketones, ethanol and tetracloroethylene; it is critical to better understand how the physical and biological properties shared by organic solvents relate to their potential for abuse.
Whether solvent dependence develops as a consequence of occupational exposure or from recreational use, abused volatile compounds are usually inhaled. To mimic this route of administration in an animal model of solvent abuse, we developed a unique inhalant apparatus that allows for controlled amounts of vapors to be delivered into a place conditioning chamber.
Place conditioning is an established model in behavioral pharmacology and drug dependence research in which animals learn to associate a distinct contextual environment with drug administration. Drugs which produce conditioned place preference (CPP) in animals are abused by humans, and drugs which fail to do so appear also do not appear to be abused by humans (Shippenberg and Koob, 2002; Bardo and Bevins, 2000; Tzschentke, 1998). Consistent with the observation that inhaled solvents are primarily abused by adolescents (Wu et al., 2005), we have previously shown that adolescent animals express a preference for an environment paired with toluene vapors (Gerasimov et al., 2003; Lee et al., 2004). Using the CPP paradigm, it has been shown that mice also prefer a toluene-paired environment (Funada et al., 2002), and that adult rats prefer an environment paired with a solvent mixture containing toluene (Yavich et al., 1994). In the present study, we applied the same strategy to a detailed investigation of the place conditioning effects of inhaled acetone.
The concentration, number of pairings and duration of each conditioning session can profoundly influence the development of CPP to any inhaled solvent. Thus, in the service of determining a dose-response relationship for inhaled acetone, we held constant the number of pairings and duration of exposure while we altered the concentration of inhaled acetone. In addition, we examined the number of pairing sessions as a function of preference at the 10,000 ppm level. Horizontal locomotor activity was also monitored during pairings to test the supplementary hypothesis that locomotion would vary as a function of treatment (acetone or air) and pairing concentrations. Our overall goal was to obtain a better understanding of the place conditioning effects of inhaled acetone.
2. Material and Methods
2.1 Subject
These studies utilized 40 adolescent (1 month old) male Sprague-Dawley rats (100-125 g; n=8 group; Taconic Farms, Germantown, NY). Rats had access to food and water ad libitum. Temperature and humidity were kept at 22 ± 2 ° C and 40 – 60 %, respectively. All animals were housed in pairs and were maintained on a 12/12 light/dark cycle. Handling occred only during the light cycle. Animal procedures were in strict accordance with the National Institute of Health guide for the care and use of all laboratory animals and were approved by the local animal care and use committee.
Animals were divided randomly into five groups (n = 8/group). All experimental animals were exposed to acetone vapors (5,000, 10,000, 20,000 ppm) for 1 hour duration for 6 pairing sessions (1 acetone session, 1 air session). To test the preference of acetone as a function of pairing sessions, the 10,000 ppm group received an additional 6 pairings and an additional group received 3 pairings at 10,000 ppm . Control animals received air for a 30-min period for 6 pairing sessions.
2.2 CPP apparatus
The CPP apparatus used for these experiments is identical to the one previously described (Gerasimov et al., 2003; Lee et al., 2004). Briefly, the place preference box (ENV-013, MED Associates, Inc.) consists of three distinct compartments (white/gray/black) separated by two guillotine doors. The black and white compartments serve as the conditioning chambers and the grey compartment sits between them and is designated the ‘neutral’ compartment. The white compartment is fitted with a textured grate floor and the black compartment with smooth floors. The apparatus is modified to allow acetone vapor flow through an opening on the top of the side of both black and white compartments. Each chamber in the apparatus is equipped with infrared photocells positioned along the walls at the level of animal’s head to automatically record the time spent in each compartment during the test and conditioning sessions. Locomotor activity was recorded during the conditioning phase with these photocells, where forward and backward motion is recorded as successive beam breaks.
2.3 Measurement of acetone concentrations in place conditioning apparatus
An air stream saturated with acetone vapor was generated by bubbling air through a flask containing liquid acetone maintained at 0°C. This air-acetone saturated stream was diluted with compressed air in predefined ratios set by computer-controlled flow regulators (Dyna-Blender, Matheson, PA). For this calibration acetone vapors were introduced at 2 l/min for at least 1 hour so that the chamber volume (∼12 L) was exchanged a minimum of 10 times. In order to independently verify that acetone levels created in the exposure chambers and ensure that the levels were uniform, nine small holes (three for each level: top of the chamber, level of animal’s head, and 2 cm above the floor) were drilled in the walls of both boxes. Air samples were drawn with a gas-tight syringe and were immediately dispensed into vials containing water to trap the acetone vapors.
Acetone vapor concentrations were measured with a gas chromatograph using a 1/8-in Porapak T column. Acetone peaks were analyzed and integrated using a Vision 4 Chromatography Acquisition station. The integrated peaks (in peak-area-units; PAU) were subjected to a linear regression analysis and the resulting equation was used to convert PAU to nM and subsequently parts per million (ppm) of acetone. This resultant standard curve of acetone concentrations was used to derive and maintain the chamber concentration as a function of the combined air and acetone bubbler flow rates. For these studies, we obtained an average acetone vapor concentration of 5,000 ppm with a mixture of 1.80 l/min pure air and 0.20 l/min of acetone. Mixing the gas streams of acetone and air in the proportion of 1.75 l/min of air to 0.25 l/min of acetone produced an average acetone concentration of 10,000 ppm. A mixture of 1.63 l/min. of air and 0.37 l/min of acetone produced an average chamber concentration of acetone of 20,000 ppm. Acetone was maintained at 0°C in an ice bath at all concentrations to retard evaporation.
2.4 Procedures
In the pre-conditioning phase, animals were habituated for three days to the conditioning room and handled for 4 hours on each day. On the fourth day, a pre-conditioning test was conducted to measure baseline chamber preference. As a group, animals exhibited no bias for either black or white chambers; however, on the occasion that an individual animal exhibited a chamber bias, acetone was paired in its least preferred chamber and air in the preferred chamber, other wise, animals were assigned randomly. Cage mates were exposed at the same time in their respective chambers so that on any given day both conditioning chambers were filled with either air or acetone vapors.
During the conditioning phase animals received either compressed air or acetone vapors on alternate days for 1 hour sessions. The conditioning chambers were cleaned with warm water following each animal’s conditioning session. On acetone-paired days, this was accomplished by opening the lid, cleaning the cages, then immediately closing the lid and resealing the chamber while acetone vapors continued to be introduced.
On the test day (the day immediately following the last acetone vapor exposure) animals were placed in the middle compartment for a 5 min acclimation period. Animals were then allowed free access to all three compartments for 15 min while their activity was monitored electronically.
2.5 Statistical Analysis
The preference score (mean time spent in acetone-paired chamber — mean time spent in air-paired chamber) data were analyzed using one-way repeated measures analysis of variance (ANOVA) using the factor acetone concentration. The 10,000 ppm groups’ preference scores were also analyzed by one-way repeated measures ANOVA using the factor number of pairing sessions. All pairwise multiple comparisons utilized Student-Newman-Keuls Method. Activity, expressed as area under curve (AUC), was collected in 1 min intervals during the conditioning sessions. These data were analyzed by three-way ANOVA using factors; treatment (acetone or air), exposure session (1-6) and concentration.
Results
1. General Results
We did not observe a gradient in the measured acetone concentration as a function of the vertical sampling position in the chamber, regardless of the target concentration. However, we intentionally waited 60 minutes prior to sampling, to avoid such gradients in vapor concentration and to ensure that the chamber volume was thoroughly exchanged.
2. Locomotor Results
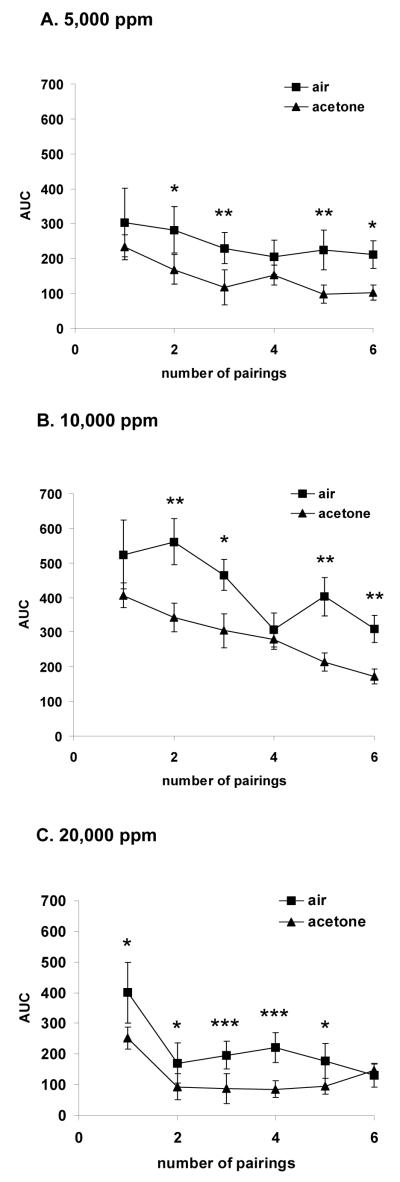
Acetone effects on locomotor activity for each concentration are depicted in Figure 1 (A, B, C). A three-way ANOVA yield a significant effect of treatment (F[1,287]=73, p < 0.001), exposure session (F[5,287]=23, p < 0.001) and concentration (F[2,287]=72, p < 0.001). Additionally, there was a significant interaction between exposure session and concentration [F(10,287)=2, p < 0.05].
Figure 1.
(A-C) Mean (± S.E.M.) area under curve (AUC) of horizontal movement counts during 1 hour acetone inhalation period, separated into concentrations (5,000, 10,000 and 20,000 ppm; n=8/group), and air during a 60-min period on alternate days for a total of 6 pairings. The three-way ANOVA yield a significant effect of treatment F(1,287)=73, p < 0.001, exposure session F(5,287)=23, p < 0.001 and concentration F(2,287)=72, p < 0.001. Additionally, there was a significant interaction between exposure session and concentration F(10,287)=2, p < 0.05. Lastly, A pairwise multiple comparisons (Student-Newman-Keuls Method) revealed significant, * p<0.05, ** p<0.01, *** p<0.001, differences between each air session to each acetone session within the pairing regimen.
3. Place Conditioning Results
The combined pretest data indicate that there was not a baseline chamber bias (mean ± S.E.M; black: 281 ± 22 sec; white: 271 ± 26 sec, p = 0.4). More detailed analysis indicated that animals which received 5,000 ppm spent 310 ± 35 sec on the black and 323 ± 26 sec on the white sides (p = 0.8). Animals in the 10,000 ppm group that received 6 pairings spent 217 ± 19 sec and 176 ± 32 sec in the black and white chambers, respectively (p = 0.3). This same group of animals received an additional 6 pairings. Additionally at the 10,000 ppm level, animals paired with 3 pairing sessions spent 202 ± 25 sec and 204 ± 18 sec in the black and white chambers, respectively (p = 1.0). Further, animals in the 20,000 ppm group spent 315 ± 13 sec in the black and 316 ± 19 sec in the white compartments, p = 1.0. The pre-test data were similar to control animals that received air in both the black and white compartments on the test day (241 ± 33 sec and 234 ± 34 sec, respectively). Animals spent approximately 40-50 % of their time in the middle acclimation chamber on the pre-test and test day.
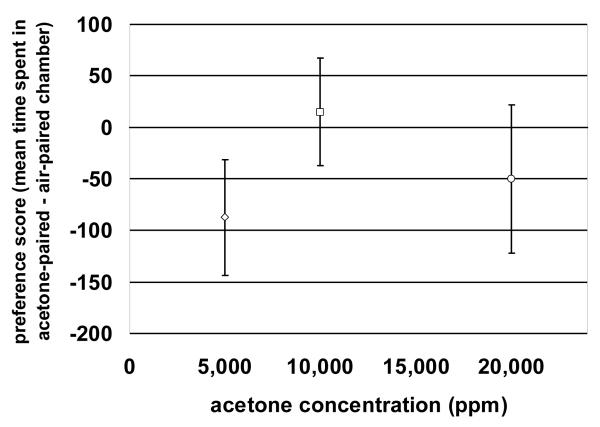
A one-way repeated measures analysis of variance ANOVA comparison of preference score vs. concentration yielded a significant (F[1,47] = 81, p < 0.001; Fig. 2) effect; however, no statistically significant difference was observed between preference score and the number of pairings at the 10,000 ppm level F(1,47) = 1.9, p = 0.17
Figure 2.
Dose-effect relationship of acetone place preference conditioning. Preference scores (mean time spent in the acetone-paired chamber — mean time spent in the air-paired chamber) measured in an acetone-free environment (n = 8/group) after 6 pairing sessions for 1 hour duration. The preference scores are plotted on the y-axis as a function of the acetone concentration (5,000, 10,000, and 20,000 ppm) on the x-axis. A one-way repeated measures analysis of variance ANOVA comparison of preference score vs. concentration yielded a significant F(1,47) = 81, p < 0.001 dose-relationship effect.
Conclusion
Acetone and other ketones are inhaled through nail polish removers and some paint thinners. Bruckner and Peterson (1981a) demonstrated that acetone is a much less potent CNS depressant than toluene, the prototypic abused solvent (Balster, 1997). Pharmacological and behavioral effects shared by the abused solvents toluene, trichloroethane and CNS depressant drugs, such as ethanol, barbiturates and or benzodiazepines have been carefully documented (Bowen et al., 1999; Hinman, 1987).
However, animal models of acetone abuse have been scarce and difficult to replicate. For example, a study conducted by Goldberg and colleagues (Goldberg et al., 1964), suggests that tolerance to the acute behavioral effects of acetone vapors (6000 ppm) develops after a few sessions in rats. Further, Glowa and colleagues (Glowa, 1987; Glowa et al., 1986) reported that acute exposure to 3000 ppm of acetone vapors reduced the rate of responding under an FI (fixed interval) schedule in rats, and Geller et al., (1979) reported variable differences in FR (fixed ratio) and FI response rates of rats exposed to 150 ppm. More recently Christoph et al., (2003) demonstrated no effect on operant performance during or after subchronic exposures up to 4000 ppm acetone vapors. Similarly, no observable differences were made in an electrical self-stimulation study at equipotent exposures (Bespalov et al., 2003). Although, some of these studies used different strains of rodents and food restriction protocols, the somewhat contradictory literature adds to this complicated area of scientific inquiry.
As observed in our previous study (Gerasimov et al., 2003) the magnitude of preference was greatest for the middle chamber (40 - 50 %) on the pretest day, presumably related to the more enclosed nature of this chamber than the larger, more open, side chambers. This observation agrees with other reports using a similar apparatus to assess place conditioning with CNS depressant drugs (Cunningham et al., 2003; Roma and Riley, 2005). Although animals spend the most time in the middle chamber, they exhibit no bias (27 % in the white and 28 % in the black) as a combined group to either conditioning chambers. In fact, Roma and Riley (2005) observed that the apparatus in our laboratory (MED Associates, Inc.; three-chamber), under the same conditions (dim light) does not produce a chamber bias.
On the test day, animals again preferred the middle chamber (40 - 50%) over the two conditioning chambers. This has been a general concern in the CPP literature; that is, to what extent does reaction and habituation to novelty influence the outcome of the conditioning experiment (Reid et al., 1989). While it has been shown that rats prefer a relatively novel chamber to a familiar chamber (Bardo et al., 1990; Bardo et al., 1995), Parker (1992) previously demonstrated that animals preferred amphetamine, apomorphine, and/or morphine-paired compartments over both the novel and saline-paired compartments. Results from our study and others using a similar apparatus (Gerasimov et al., 2003; Rice et al., 2002; Thanos et al., 2005) indicate the contrary; rats prefer the novel compartment over the familiar conditioning chambers.
It appears that the motor suppressant effects induced by inhaled-acetone at the doses we measured are consistent with inhalants like toluene that share considerable overlap with other CNS depressants (Bruckner and Peterson, 1981a, 1981b) including barbiturates and benzodiazepines.
This is the first study in which a dose response relationship was established using inhaled acetone in freely moving animals. Using the experimental conditions we detailed, our place preference results suggest an inverted-u-shaped curve at the three concentrations we tested, 5,000, 10,000 and 20,000 ppm. These data are consistent with our previous findings using inhaled toluene (Lee et al., 2006). However, at a concentration of 10,000 ppm, we did not observe any correlation between preference score and the number of pairings. Thus, while inhaled acetone produced a marked decrease in locomotor activity similiar to those reported using CNS depressants (Bowen and Balster, 1998; Bowen et al., 1996) these doses and pairing conditions did not produce a rewarding affect.
Acknowledgements
Supported by National Institute of Health grant DA 22346 DA15082, DA16025, DA15041 and performed under Brookhaven Science Associates contract No. DE-AC02-98CH10886 with the U.S. Department of Energy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CE, Loomis GA. Recognition and prevention of inhalant abuse. Am Fam Physician. 2003;68:869–874. [PubMed] [Google Scholar]
- Balster RL. Abuse potential evaluation of inhalants. Drug Alcohol Depend. 1987;19:7–15. doi: 10.1016/0376-8716(87)90082-2. [DOI] [PubMed] [Google Scholar]
- Balster RL. College on problems of drug dependence presidential address 1996: inhalant abuse, a forgotten drug abuse problem. NIDA Res Monogr. 1997;174:3–8. [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Lacy M, Mattingly BA. Effects of apomorphine on novelty-induced place preference behavior in rats. Pharmacol Biochem Behav. 1990;37:89–93. doi: 10.1016/0091-3057(90)90046-k. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Basu D, Jhirwal OP, Singh J, Kumar S, Mattoo SK. Inhalant abuse by adolescents: a new challenge for Indian physicians. Indian J Med Sci. 2004;58:245–249. [PubMed] [Google Scholar]
- Bespalov A, Sukhotina I, Medvedev I, Malyshkin A, Belozertseva I, Balster R, Zvartau E. Facilitation of electrical brain self-stimulation behavior by abused solvents. Pharmacol Biochem Behav. 2003;75:199–208. doi: 10.1016/s0091-3057(03)00071-6. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Balster RL. A direct comparison of inhalant effects on locomotor activity and schedule-controlled behavior in mice. Exp Clin Psychopharmacol. 1998;6:235–247. doi: 10.1037//1064-1297.6.3.235. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Wiley JL, Balster RL. The effects of abused inhalants on mouse behavior in an elevated plus-maze. Eur J Pharmacol. 1996;312:131–136. doi: 10.1016/0014-2999(96)00459-1. [DOI] [PubMed] [Google Scholar]
- Bowen SE, Wiley JL, Jones HE, Balster RL. Phencyclidine- and diazepam-like discriminative stimulus effects of inhalants in mice. Exp Clin Psychopharmacol. 1999;7:28–37. doi: 10.1037//1064-1297.7.1.28. [DOI] [PubMed] [Google Scholar]
- Brouette T, Anton R. Clinical review of inhalants. American Journal On Addictions. 2001;10:79–94. doi: 10.1080/105504901750160529. [DOI] [PubMed] [Google Scholar]
- Bruckner JV, Peterson RG. Evaluation of toluene and acetone inhalant abuse. I. Pharmacology and pharmacodynamics. Toxicol Appl Pharmacol. 1981a;61:27–38. doi: 10.1016/0041-008x(81)90004-1. [DOI] [PubMed] [Google Scholar]
- Bruckner JV, Peterson RG. Evaluation of toluene and acetone inhalant abuse. II. Model development and toxicology. Toxicol Appl Pharmacol. 1981b;61:302–312. doi: 10.1016/0041-008x(81)90351-3. [DOI] [PubMed] [Google Scholar]
- Christoph GR, Malley LA, Stadler JC. Subchronic inhalation exposure to acetone vapor and scheduled controlled operant performance in male rats. Inhal Toxicol. 2003;15:781–798. doi: 10.1080/08958370390217846. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard MA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Dinwiddie SH. Abuse of inhalants: a review. Addiction. 1994;89:925–939. doi: 10.1111/j.1360-0443.1994.tb03348.x. [DOI] [PubMed] [Google Scholar]
- DiVincenzo GD, Yanno FJ, Astill BD. Exposure of man and dog to low concentrations of acetone vapor. Am Ind Hyg Assoc J. 1973;34:329–336. doi: 10.1080/0002889738506857. [DOI] [PubMed] [Google Scholar]
- Funada M, Sato M, Makino Y, Wada K. Evaluation of rewarding effect of toluene by the conditioned place preference procedure in mice. Brain Res Brain Res Protoc. 2002;10:47–54. doi: 10.1016/s1385-299x(02)00182-4. [DOI] [PubMed] [Google Scholar]
- Geller I, Hartmann RJ, Randle SR, Gause EM. Effects of acetone and toluene vapors on multiple schedule performance of rats. Pharmacol Biochem Behav. 1979;11:395–399. doi: 10.1016/0091-3057(79)90114-x. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Collier L, Ferrieri A, Alexoff D, Lee D, Gifford AN, Balster RL. Toluene inhalation produces a conditioned place preference in rats. Eur J Pharmacol. 2003;477:45–52. doi: 10.1016/j.ejphar.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Glowa JR. Comparisons of some behavioral effects of d-amphetamine and toluene. Neurotoxicology. 1987;8:237–247. [PubMed] [Google Scholar]
- Glowa JR, DeWeese J, Natale ME, Holland JJ, Dews PB. Behavioral toxicology of volatile organic solvents. I. Methods: acute effects of toluene. J Environ Pathol Toxicol Oncol. 1986;6:153–168. [PubMed] [Google Scholar]
- Goldberg ME, Johnson HE, Pozzani UC, Smyth HF., Jr. Effect Of Repeated Inhalation Of Vapors Of Industrial Solvents On Animal Behavior. I. Evaluation Of Nine Solvent Vapors On Pole-Climb Performance In Rats. Am Ind Hyg Assoc J. 1964;25:369–375. doi: 10.1080/00028896409342606. [DOI] [PubMed] [Google Scholar]
- Hinman DJ. Biphasic dose-response relationship for effects of toluene inhalation on locomotor activity. Pharmacol Biochem Behav. 1987;26:65–69. doi: 10.1016/0091-3057(87)90535-1. [DOI] [PubMed] [Google Scholar]
- Howard MO, Jenson JM. Inhalant use among antisocial youth: prevalence and correlates. Addictive Behaviors. 1999;24:59–74. doi: 10.1016/s0306-4603(98)00039-2. [DOI] [PubMed] [Google Scholar]
- Lee DE, Schiffer WK, Dewey SL. Gamma-vinyl GABA (vigabatrin) blocks the expression of toluene-induced conditioned place preference (CPP) Synapse. 2004;54:183–185. doi: 10.1002/syn.20072. [DOI] [PubMed] [Google Scholar]
- Lee DE, Gerasimov MR, Schiffer WK, Gifford AN. Concentration-dependent conditioned place preference to inhaled toluene vapors in rats. Drug Alcohol Depend. 2006;85:87–9.0. doi: 10.1016/j.drugalcdep.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Parker LA. Place conditioning in a three-or four-choice apparatus: role of stimulus novelty in drug-induced place conditioning. Behav Neurosci. 1992;106:294–306. doi: 10.1037//0735-7044.106.2.294. [DOI] [PubMed] [Google Scholar]
- Reid LD, Marglin SH, Mattie ME, Hubbell CL. Measuring morphine’s capacity to establish a place preference. Pharmacol Biochem Behav. 1989;33:765–775. doi: 10.1016/0091-3057(89)90468-1. [DOI] [PubMed] [Google Scholar]
- Rice OV, Gordon N, Gifford AN. Conditioned place preference to morphine in cannabinoid CB1 receptor knockout mice. Brain Res. 2002;945:135–138. doi: 10.1016/s0006-8993(02)02890-1. [DOI] [PubMed] [Google Scholar]
- Roma PG, Riley AL. Apparatus bias and the use of light and texture in place conditioning. Pharmacol Biochem Behav. 2005;82:163–169. doi: 10.1016/j.pbb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Koob GF. Neuropharmacology. Lippincott Williams and Wilkins; New York: 2002. Animal models of drug addiction 5th generation of progress; pp. 1381–1397. 2002. [Google Scholar]
- Schutz CG, Chilcoat HD, Anthony JC. The association between sniffing inhalants and injecting drugs. Compr Psychiatry. 1994;35:99–105. doi: 10.1016/0010-440x(94)90053-k. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Dimitrakakis ES, Rice O, Giffrod A, Volkow N. Ethanol self-administration and ethanol conditioned place preference are reduced in mice lacking cannabinoid CB1 receptors. Behav Brain Res. 2005;164:206–213. doi: 10.1016/j.bbr.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Wu LT, Pilowsky DJ, Schlenger WE. High prevalence of substance use disorders among adolescents who use marijuana and inhalants. Drug Alcohol Depend. 2005;78:23–32. doi: 10.1016/j.drugalcdep.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Yavich L, Patkina N, Zvartau E. Experimental estimation of addictive potential of a mixture of organic solvents. Eur Neuropsychopharmacol. 1994;4:111–118. doi: 10.1016/0924-977x(94)90004-3. [DOI] [PubMed] [Google Scholar]