Abstract
The construction of the 68Ge/68Ga generator has increased application of radiopharmaceuticals labeled with this isotope in medicine. 68Ga-PET is widely employed in the management of neuroendocrine tumors but favorable chemistry with tri- and tetraaza-ring molecules has opened wide range of 68Ga application in other fields of PET imaging. This review covers the radiopharmaceuticals labeled with gallium in molecular imaging and shows perspectives on the use of gallium-68 as a substitute for technetium-99, fluorine-18 and carbon-11 in some applications.
Keywords: Position emission tomography, 68Ga labeled compounds, Bifunctional chelators, 68Gallium-DOTA-peptides
1. Background
The positron emission tomography (PET) technique is applied in the study of functioning and functional changes in the human body, enabling, among others, precise oncology diagnostics. It is as well an appreciated diagnostic method in neurology and cardiology, allowing to evaluate the condition of the respective systems in a non-invasive way.
The dominant isotope in these types of diagnostics is fluorine-18, widely used in the research and the clinical applications. The source of the isotope is a proton medical cyclotron with particle energies from 10 to 20 MeV range which limits the availability of the isotope to the diagnostic centers, where the operation of such a machine is economically justified and qualified personnel to operate and maintain such an equipment is available. Looking at the history of another isotope, technetium-99m, widely used in the related field of nuclear medicine – scintigraphy, it can be seen that it is critical to the development of the clinical applications to increase the availability of the isotope.
Three isotopes of gallium can be used in nuclear medicine: gallium-66, potentially useful as a PET tracer, gallium-67, for the scintigraphy of the inflammation sites as citrate or with labeled leukocytes, still considered as a routine tool for infection imaging and gallium-68, a fast growing PET radionuclide, especially in cancer diagnostics.
In opposition to fluorine-18 or carbon-11-based PET diagnostics, gallium-68 does not require the use of cyclotrons, thereby reducing costs and increasing flexibility in the practice of nuclear medicine diagnostic imaging. In recent years, gallium-68 has gained importance in molecular imaging by positron emission tomography, due to the advantages of the easy availability both from the 68Ge/68Ga generator and the cyclotrons (natZn(p,n)68Ga) with 7–16 MeV protons,1 good radiation properties and rich Ga3+ coordination chemistry. The quality of imaging is in some applications comparable to the quality of imaging with fluorine-18 isotope. In the nearest future these factors may cause a serious increase in the interest in gallium-68 labeled compounds in biology and medicine.2, 3, 4
This review indicates the growing importance of the radiopharmaceuticals labeled with gallium in molecular imaging and shows the perspectives on the use of gallium-68 as a substitute for technetium-99 in some applications.
2. Gallium chemistry
Gallium is a metal in group 13 of the periodic table. In aqueous acidic solutions, it occurs as a free, hydrated Ga3+ ion, in slightly acidic and neutral pH hydrolyses to insoluble Ga(OH)3 but nanomolar concentrations, it is used for the formulation of radiopharmaceuticals, can be obtained without precipitation. In contrast to Tc(V), where improper reaction conditions lead to insoluble and unreactive TcO2, amphoteric properties of gallium allow the redissolution of the formed hydroxide. If the concentration exceeds the nanomolar level, addition of the carboxylic acids (citrate, oxalate, acetate) prevents precipitation. At higher pH, above 7, gallium hydroxide redissolves as [Ga(OH)4]−.
Gallium is classified as hard Lewis acid and can form octahedral complexes with hard Lewis bases, such as oxygen and nitrogen atoms. Carboxylic, phosphonate, thiol and amino groups form strong six-coordinate complexes, thermodynamically and kinetically stable at physiological conditions. Existing five and four-coordinate gallium complexes are more sensitive to hydrolysis but are enough inert on the time-scale of the gallium applications.
The coordination chemistry and biological properties of Ga are similar to Fe ion (Table 1). Both are 3+ ions with comparable ionic radius and dominating octahedral complex geometry, thus the biomolecules with high Fe affinity could be easily labeled with Ga–ligand exchange between intravenously applied gallium citrate and transferrin, abundant plasma protein, forms a complex used for imaging of inflammatory sites.
Table 1.
Chemical properties of Fe, Ga, Tc and In.
| Iron | Gallium | Technetium | Indium | ||
|---|---|---|---|---|---|
| Atomic number | 26 | 31 | 43 | 49 | |
| Ionic radius [pm] | 64 | 62 | 136 | 80 | |
| Oxidation state | III | III | I, III, IV, V, VII | III | |
| Coordination number | 6 | 4, 5, 6 | 5, 6, 7 | 6, 7, 8 | |
| Complex geometry | Octahedral | Octahedral | Octahedral, trigonal bipyramid, square pyramidal | Octahedral, square antiprismatic | |
| Radionuclide | • | 67Ga | 68Ga | 99mTc | 111In |
| Diagnostic mode | • | SPECT | PET | SPECT | SPECT |
| Half-life | • | 3.56 d | 1.13 h | 6.02 h | 2.83 d |
| Source | • | Cyclotron | Generator | Generator | Cyclotron |
Another element whose coordination chemistry is of great interest for the development of gallium compounds, is indium. As a hard Lewis acid, In3+ prefers hard bases and forms stable complexes with nitrogen and oxygen atoms. Only higher ionic radius (80 pm vs. 60 pm for Ga) differentiates the ligands against a cavity size, where In fits perfectly the tetraaza-macrocyclic ligands (DOTA, Fig. 1a), while Ga prefers smaller triaza rings (NOTA, Fig. 1b).
Fig. 1.
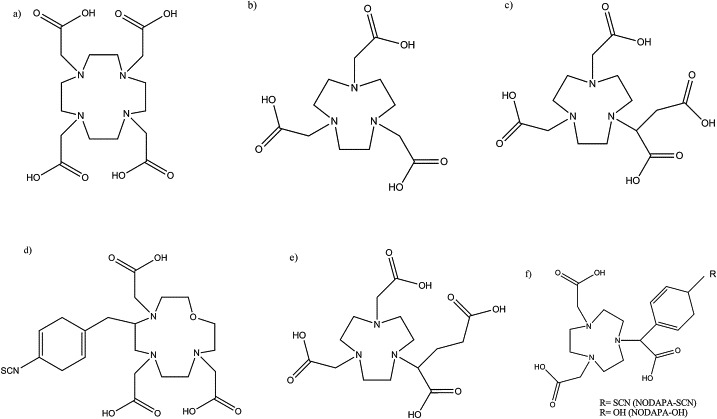
Examples of the ligands DOTA (a), NOTA (b), and derivatives: NODAGA (c), OXO-DO3A (d), NODASA (e) and NODAPA (f).
3. Generators
Gallium-68 is a positron emitter with a half-life of 67.6 min and dominantly decays via 1.92 MeV positron emission (89%) and electron capture (11%). The relatively short half-life and hydrophilic nature are beneficial for the rapid renal clearance and reduce effective dose for the patient. Gallium-68 can be conveniently and economically obtained from the 68Ge/68Ga generator and the long half-life of the parent nuclide 68Ge (t1/2 = 271 days) allows long-term PET imaging on-site. Thereby, the introduction of gallium-68 generator has opened access to the PET radioisotopes for diagnostic sites, located outside production centers equipped with cyclotrons. The availability of the 68Ge/68Ga generator system also provides imaging centers with a PET nuclide that is routinely available in a manner similar to the generator-produced single-photon emission computed tomography nuclide technetium-99m, with added value of better resolution and sensitivity. Thus, the development of gallium radioisotopes and diagnostics procedures combined with on-demand production and operation in the absence of cyclotron facilities provides a reasonable development pathway for gallium-68 labeled compounds in the molecular imaging.
Although the first gallium-68 generators have been used already in the 60s, the chemical forms of the isotope as the inert complexes, prevented the practical application and the development of gallium radiochemistry. The second problem was the presence of significant amounts of parent nuclide and other metallic impurities in eluate. Both of these factors resulted in the temporary loss of interest in applications of gallium-68, but as a complex with EDTA has been used in clinical practice that time and was the β+ source in the development of the PET scanners. Practical applications of the first gallium generators have been described recently.5
Beginning of the 21st century saw new developments in the construction of generators. Various resins, using inorganic oxides (TiO2, IGG100 68Ga generator; Eckert&ZieglerEurope),6 SnO27 and nanoparticles8 or organic polymers, containing N-methylglucamine groups9 or lauryl gallate (ITG GmBH, Germany) were constructed and successfully applied. Contemporary 68Ge/68Ga generator can be used for 1–2 years and the gallium-68 build-up is rapid enough to allow multiple radiotracer preparations daily.
The gallium-68 eluted from the inorganic generator often suffered from the contamination by other metals, which influenced negatively radiolabelling.10 Commonly observed impurities are: Fe(III), Al(III) and Zn(II) at the mg/L levels, Ni(II), Co(II), Cd(II) and Cu(II) at 0.01–0.1 mg/L and traces of Mn, Pb, Cr, V.7, 11 Average gallium-68 breakthrough varies from 10−4 to 10−5%. The most critical impurities are 68Ge, Fe(III), Zn(II), Mn(II), thus additional purification and removal of the metal contaminants has to be applied. The most popular procedures differentiate gallium species (Ga3+ cations and anionic [GaCl4]−) depending on pH and chloride concentration and then separate on the respective ion exchangers. Typically, 68Ga3+ is trapped on the strong cation exchange cartridge (SCX), then converted with HCl, HCl/acetone or NaCl into [68GaCl4]− and transferred with a small volume of concentrated HCl to the strong anion exchange cartridge (SAX) and finally eluted with water or slightly acidic solution.12, 13, 14, 15 The process could be easily automated for the reproducible preparation of ready-to-use 68Ga3+ in optimal activity and chemical form for labeling.16
The generators based on zirconium nanoparticles and organic matrix could be eluted under milder conditions and gallium-68 ions are easier to form suitable complexes for clinical use. Furthermore, they suffer less from the metallic contaminants and the purification processes could be omitted or simplified.17
At the time of this publication, at least four types of the generators with different construction and scalable activity are available commercially and more than 100 centers across the world use this type of generator, both for the basic research on gallium-68 radiochemistry and the clinical trials.18
4. Radiopharmaceuticals
The rapid development of radiopharmaceuticals labeled with gallium is due to the synergy between a growing importance of the PET diagnostics in the molecular imaging and the use of long-term experience with the technetium generators. The trivalent cation 68Ga3+ is eluted from the generator and its chemical form allows simple and universal application in radiopharmaceutical preparations with an appropriate chelator. Ga3+ ion has complexing properties comparable to those of Fe(III) and In(III) but can easily adapt to various Tc targeting concepts (Table 2), resulting in a large group of molecules (vectors), with well-established and proved practical applications in the molecular imaging.
Table 2.
99mTc tracers with their 68Ga analogs.
| Diagnostics | 99mTc | 68Ga |
|---|---|---|
| Peptide receptors | 99mTc-HYNIC-peptide | 68Ga-DOTA-peptide |
| Bone metastases | 99mTc-MDP | 68Ga-phosphonates |
| Renal function | 99mTc-DTPA/MAG3/DMSA | 68Ga-EDTA |
| Cardiac function | 99mTc-RBC/MIBI | 68Ga-BAPEN |
| Lung function | 99mTc-MAA | 68Ga-MAA |
| Hepatobiliary | 99mTc-IDA | 68Ga-IDA |
| Infection | 99mTc-WBC | 68Ga-citrate |
| Brain imaging (perfusion) | 99mTc-ECD | 68Ga-ECD |
Abbreviations: HYNIC, hydrazinonicotinamide; MDP, methylenediphosphonic acid; DTPA, diethylenetriaminepentaacetic acid; MAG3, mercaptoacetyltriglycine; DMSA, dimercaptosuccinic acid; RBC, red blood cell; MIBI, methoxyisobutylisonitrile; MAA, macroaggregated albumin; IDA, iminodiacetic acid; WBC, white blood cell; ECD, ethyl cysteinate dimer; EDTA, ethylenediaminetetraacetic acid; BAPEN, Tris(4,6-dimethoxysalicylaldimine)-N,N′-bis(3-aminopropyl)-N,N′-ethylenediamine.
4.1. Gallium-68 bifunctional chelators (BFC) in the neuroendocrine tumor imaging
The bifunctional chelators are compounds acting as a conjugate between the radioisotope and a vector. The BFC's key features are the ability to quickly and steadily incorporate the isotope and create covalent bonds with biomolecules, responsible for targeting. Over the past decade, the interest in it is inseparably connected with the synthesis and use of radiolabeled peptides for positron emission tomography (PET) in the clinical and preclinical diagnostics, as well as with the radiation-based therapeutics.
The macrocyclic chelators with three or four nitrogen atoms in a ring have been established as frequently considered routes for the introduction of gallium-68. Between them, the molecules with tri- and tetraaza-ring have become the most prevalent since irreversible complexation of the gallium atom, thermodynamic stability and superior kinetic even at room temperature. External substituents, usually in the form of carboxyl groups allow convenient conjugation to various targeting molecules.
DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) remains the most frequently used chelator because of its availability and well-recognized coordination chemistry.2 Its six coordinate triaza-ring analog NOTA ((1,4,7-triazanonane-1,4,7-triyl)triacetic acid) forms slightly deformed octahedral complexes with gallium-68 which display higher stabilities and faster incorporation of Ga(III) at lower temperatures.18 A significant number of compounds, similar to DOTA and NOTA have been used for specific applications (Fig. 2): bifunctional derivatives of NOTA – NODAGA (1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid) and NODASA (1,4,7-triazacyclononane-1-succinic acid-4,7-diacetic acid) are limited to coupling peptides through an amide bond, NODAPA (1,4,7-triazacyclononane-1,4-diacetic acid-7-p-phenyl-acetic acid) with hydroxyl group (NODAPA-OH) or thiocyanate groups (NODAPA-(NCS)n) have been successfully applied as versatile bifunctional chelators.19
Fig. 2.
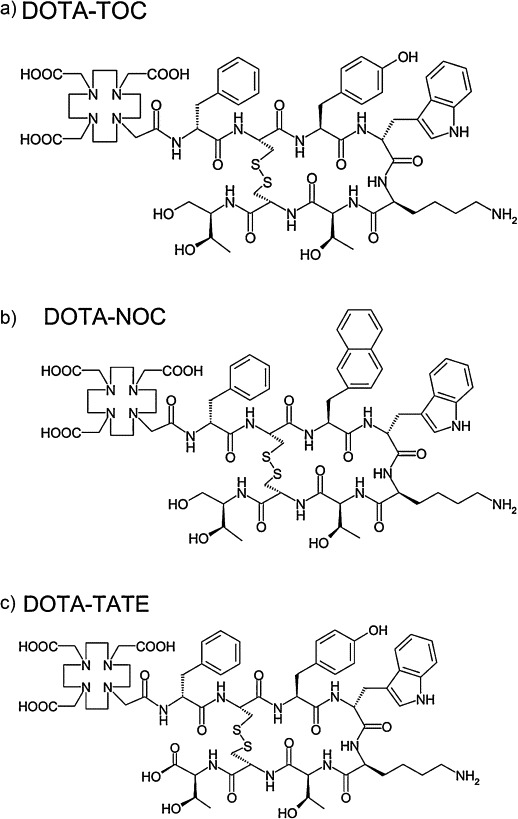
Structures of DOTA-TOC (a), DOTA-NOC (b), and DOTA-TATE (c). The most clinically applied 68Ga-labeled pharmaceuticals in nuclear medicine.
Many receptors are overexpressed in cancer cells, thus the targeted peptides with aminoacids sequences specific for activity centers, labeled with appropriate radioisotope via BFC, have been used for the development of highly specific imaging probes for PET and/or therapeutic agents.
68Ga-DOTA-peptides are a group of PET tracers that specifically bind to somatostatin receptors (SST) that are over-expressed on the neuroendocrine tumor (NET) cells.20 Targeting agents are coupled via the BFC leveraging standard intermediates that enable covalent attachment of peptides, including active octapeptides (octreotride, OC) and related peptide analogs, TOC (Tyr3-OC), TATE (Tyr3-Thr8-OC) and NOC (NaI3-OC) (Fig. 2). Six different SST receptors have been identified (SST1, 2A, 2B, 3, 4, 5) in humans. Of the analogs mentioned above, DOTA-TOC and DOTA-TATE show a high affinity for the SSTR2, although a 10-fold higher affinity for the SSTR2 has been demonstrated for DOTA-TATE as compared to DOTATOC in vitro in the transfected cell cultures. Their binding affinity to SSTR5 is quite low and that to SSTR3 is almost negligible and there is no considerable affinity to SSTR1 and SSTR4. For DOTA-NOC, high affinities for SSTRs’ 2,3 and 5 were reported,21 which improves the binding profile and extends the spectrum of targeted tumors.
Despite the variations in somatostatine receptors affinity, all described compounds (68Ga-DOTA-TOC, -NOC, -TATE) have been reported to be accurate for the localization of well differentiated NET lesions, performing better than CT.22
Comparing to other modalities, studies have shown the superiority of PET over 111In-pentetreotide-SPECT in the detection of metastases and unknown primary neuroendocrine tumors. PET imaging using 68Ga-SSTR has also been found to be more accurate than 18F-FDOPA-PET in non-carcinoid tumors. [68Ga]-DOTA-TATE detected more tumor sites than [123I]-MIBG in patients with the neural crest tumors (including paragangliomas). Only one bone lesion in one patient with adrenal paraganglioma was detected on [123I]-MIBG scan but not in [68Ga]-DOTA-TATE.23, 24, 25, 26, 27, 28, 29
Radionuclide-labeled DOTA-SSTR analogs are used for diagnostics and therapy of the neuroendocrine tumors. Typically, 68Ga-DOTA-TOC is used for diagnosis, while the same compound labeled with high- or medium-energy β-emitters such as yttrium-90 or lutetium-177 is used for therapy. The use of coupled diagnostic and therapeutic isotopes with ligand for treatment of NET allows better planning of therapy and to evaluate the therapeutic outcome as in personalized medicine where the diagnosis is tailored to the subsequent therapy. It influenced radiopharmaceutical sciences with the “theranostics” concept, which combines gallium-68 diagnostics with trivalent therapeutic radiometals (yttrium-90, lutetium-177, bismuth-213)30 with improved personal dosimetry.31
4.2. Behind the NET – applications of 68Ga-BFC-core
Two main trends could be observed in the development of methods and applications of the gallium-68 radiopharmaceuticals, other than NET-focused. First, using the technetium “shake‘n'shoot” concept, has focused attention to the use of gallium-68 as technetium-99m substitute in the part of applications. Recently published papers have presented efficient gallium-68 labeling of commercially available phosphonate kits for scintigraphy of bone lesions,32, 33 MAA (macroaggregated human serum albumin) particulate for perfusion studies,34, 35 68Ga-NOTA-MSA (mannosylated human serum albumin) for immune system imaging,36 and 68Ga-BAPEN (Tris(4,6-dimethoxysalicylaldimine)-N,N′-bis(3-aminopropyl)-N,N′-ethylenediamine) myocardial uptake as substitute for MIBI.37 All these compounds profit from similar affinity of gallium and technetium to some vectors and only slightly differentiate biodistribution and pharmacokinetics (Table 2).
The second trend, taking the generator advantages of independence from the cyclotron, much cheaper operation and production, resulted in the synthesis of 68Ga labeled radiopharmaceuticals as an alternative or support to fluorine-18 and carbon-11 compounds. Routine fluorine-18 production runs are bulk and it is hard to scale them for single patient. Therefore, flexibility of gallium-68 generators opens the possibility to produce short series or even single doses. While it is difficult today to imagine the replacement of 18FDG or leading carbon-11 radiopharmaceuticals in routine clinical diagnostics, the use of gallium-68 equivalents in targeted diagnostics seems to be rapidly developing (Table 3).
Table 3.
68Ga analogs to commonly used 18F and 11C PET tracers.
| Diagnostics | 18F/11C | 68Ga |
|---|---|---|
| Angiogenesis | 18F-galacto-RGD | [68Ga]DOTA-RGD, 68Ga-VEGF |
| General cancer imaging | 18FDG | 68Ga-CXCR4 biomarker, 68Ga-uPAR biomarker, 68Ga-SCN-NOTA-BZA |
| Hypoxia | 18F-nitroimidazloes (FAZA, FMISO, FETNIM) | 68Ga-DOTA-imidazoles |
| Proliferation | 18FLT | 68Ga-DO3A-thymidine |
| Glioma | 18FET, 11C-methionine | 68Ga-glutamine, 68Ga-DO3A-alanine, 68Ga-DO2A-tyrosine, |
| Prostate cancer | 18FDG, 11C-acetate, 18F-choline, 11C-choline | 68Ga-DOTA-PSMA |
Abbreviations: RGD, arginylglycylaspartic acid; FAZA, [18F]-1-α-d-(2-deoxy-2-fluoroarabinofuranosyl)-2-nitroimidazole; FMISO, [18F]-fluoromisonidazole; FETNIM, [18F]-fluoroerythronitroimidazole; FLT, [18F]-fluorothymidine; FET, [18F]-fluoroethyl-l-tyrosine; VEGF, vascular endothelial growth factor; CXCR4, chemokine receptor; BZA, benzamide; PSMA, prostate specific membrane antigen; UPAR, urokinase-type plasminogen activator.
4.2.1. Angiogenesis
Receptors playing a key role in angiogenesis are important targets for several experimental drugs. The expression of the αvβ3 integrin has been detected on blood vessels with the intensive angiogenesis and indicates tumors with high metastatic potential. One of the most intensely evaluated compounds so far is [18F]Galacto-RGD38 which specifically binds to the αvβ3 integrin and shows very good pharmacokinetics. Comparable results were received for [68Ga]DOTA-RGD39 or other RGD peptides linked with modified bifunctional ligands: [68Ga]NS3-RGD, [68Ga] Oxo-DO3A-RGD,40 or four nitrogen atoms open chain H2dedpa.41 Effective diagnosis of angiogenesis was reported as well with the VEGF (vascular endothelial growth factor) receptors. The tracer contained single chain VEGF (scVEGF), linked with the polyethylene glycol (PEG) bridge to NOTA, which was suitable for labeling with gallium-68 at ambient temperature. Gallium-68 labeled scVEGFPEG-NOTA was injected intravenously into HT-29 (human colon adenocarcinoma) tumor-bearing mice, and clearly visualized tumor structure.42 Labeling procedure was proposed as a kit-formulated radiopharmaceutical for the targeted imaging of tumor angiogenesis.
4.2.2. General oncology imaging
Mapping of the chemokine receptor CXCR4 in tumors is used for rating tumor aggressiveness and estimation of the metastatic seeding probability in breast, prostate and lung cancer. Specific tracer for the imaging of CXCR4, gallium-68 labeled DOTA-4-FBn-TN14003 (fluorinated 14-aminoacids peptide) was synthesized as a potential PET tracer for this purpose.43
Similar studies were performed with the urokinase-type plasminogen activator receptor (uPAR) which is a well-established biomarker for tumor aggressiveness and metastatic potential. The synthetic peptide (Asp-cyclohexylalanine-Phe-d-Ser-d-Arg-Tyr-Leu-Trp-Ser (AE105)) has been identified to have a high affinity for human uPAR. The use of 68Ga-DOTA-AE105-NH2 and 68Ga-NODAGA-AE105-NH2 as the first gallium-68 labeled uPAR radiotracers for PET imaging were reported, where NODAGA is a favored tracer providing highest tumor-to-background ratio.44 These new tracers constitute an interesting alternative to the 64Cu-labeled version (64Cu-DOTA-AE105 and 64Cu-DOTA-AE105-NH2) for detecting uPAR expression in tumor tissue.45
Radiolabeled benzamides (BZA) have been reported to be the attractive agents for targeting malignant melanoma as they bind melanin and display high accumulation in the melanoma cells. The 68Ga-labeled benzamide via NOTA was presented as a potential PET agent for malignant melanoma. Biodistribution and micro-PET studies of 68Ga-SCN-NOTA-BZA in B16F10-bearing mice showed selective uptake into the tumor. The radiotracer was effectively cleared via renal excretion without further metabolism.46
4.2.3. Hypoxia imaging
Wide range of fluorine-18 nitroimidazloes have been developed such as FETNIM ([18F]-fluoroerythronitroimidazole), FAZA ([18F]-1-α-d-(2-deoxy-2-fluoroarabinofuranosyl)-2-nitroimidazole), FETA ([18F]-fluoroetanidazole) and especially [18F]FMISO, still considered as a gold standard for hypoxia imaging.47 Copper-64 ATSM (diacetyl-bis(N4-methylthiosemicarbazone) was indicated as an alternative, but suffers from less favorable dosimetry, associated with emission of β− (38.5%), and solid target cyclotrone production (64Ni(p,n)64Cu) which limits availability. Several 68Ga-complexes with nitroimidazoles were proposed for hypoxic tumor imaging: DOTA-derivative with one or two metronidazole moieties,48 imidazole coupled to DOTA by conjugating via an amide or thiourea bond.49
The results obtained were comparable with reference images obtained with [18F]FMISO and [18F]FAZA, but the usage of methylated 5-nitroimidazole improved performance of 68Ga-DOTA-nitroimidazoles in hypoxia imaging.50
4.2.4. Proliferation
Radiolabeled thymidine analogs specifically addressing DNA synthesis have significant potential for imaging of tissue proliferation in vivo. [18F]-fluoro-3′-deoxy-3′-l-fluorothymidine ([18F]FLT) has been developed and well-established as a proliferation tracer.
Thus, thymidine analogs substituted by BFC with gallium-68, were proposed as alternative to [18F]FLT. DO3A macrocycle in N-3 position was attached to thymidine and then labeled with gallium-68.51
Amino acids labeled with carbon-11 or fluorine-18 play an important role in clinical diagnostics and understanding the basic processes in biochemistry and physiology.52, 53, 54 They are involved in the synthesis of peptides and proteins, thus tumor cells concentrate labeled amino acids at a high rate, while the uptake in normal cerebral tissue is relatively low. 11C-methionine (MET) and 18F-fluoroethyl-l-tyrosine (FET) are routinely applied for gross tumor volume delineation in brain gliomas and for the differentiation between treatment-related changes and residual/recurrent tumor.
Radiolabeled with gallium-68 glutamine was used for tracing glutamine for imaging and for determination of protein synthesis rates in tumors. Glutamine was conjugated to DOTA and labeled with gallium-68 by mixing the Ga3+ species with the conjugate in ethanol and reacting at 65 °C.55, 56
Alanine derivatives of DO2A and DO3A were synthesized and labeled with gallium-68. Cell uptake assays were carried out in Hep3B (human hepatoma) and U87MG (human glioma) cell lines at 37 °C. Positron emission tomography (PET) imaging studies were performed using balb/c mice xenografted with CT-26 (mouse colon cancer).
68Ga-DO3A-homoalanine showed the highest uptake value ratio, followed by 68Ga-DO2A-alanine, 68Ga-DO3A-alanine and 68Ga-DO2A-homoalanine, but all derivatives were found to have high tumor cell uptakes, high tumor/nontumor ratio and low nonspecific uptake in normal organs, except for the kidneys.57
The novel tyrosine chelate (68Ga-DO2A-(OBu-l-tyr)2) (68Ga-1,4,7,10-tetraazacyclododecane-1,7-diacetic acid-4,10-di-(O-butyl)-l-tyrosine) was proposed as an approach which uses the biological amino acid transporter targeting properties of l-tyrosine.
In vitro studies utilizing the F98-glioblastoma cell line revealed specific uptake of [68Ga]Ga-DO2A-(OBu-l-tyr)2 that was comparable to that of the reference [18F]fluoroethyl-l-tyrosine (FET). These promising results indicate a high potential of tyrosine analogs labeled with GaDOTA for molecular imaging of tumor-driven amino acid uptake by PET.58
4.2.5. Prostate cancer
Imaging of prostate cancer was dominated by 3 tracers: 18FFDG, acetate and choline, both labeled with fluorine-18 or carbon-11.59 The prostate specific membrane antigen (PSMA) is a unique membrane bound glycoprotein, which is overexpressed in prostate cancer and is well-established in the diagnosis as highly specific prostate cancer cell-surface protein. Recently, theranostics concept had been applied to label PSMA-ligands with gallium-68 and lutetium-177.60 Successful synthesis of 68Ga-DOTA-PSMA61 and 68Ga(HBED-CC)-PSMA62 was followed by promising results of clinical trials, where at least one lesion suspicious for cancer was indicated with 100% detection ratio at PSA >2.2 ng/ml. Lesions suspicious for prostate cancer were presented with excellent contrast as early as 1 h post injection, with high detection rates even at low PSA levels.63
5. Availability
The effective synthesis of DOTA-TOC and DOTA-TATE were presented64 and numerous strategies of automated65, 66 or semi-automated67 purification and synthesis were proposed. One of the recent developments influenced radiopharmaceuticals sciences with a new term “autoclabeling”,68 describing convenient procedure for gallium-68-labeling by combining the labeling reaction and the steam sterilization into one single step to get the final, sterile ready-to-use product. Increase in clinical and radioprotection demands have boosted for extensive automation of the production process and resulted in a number of commercial synthetic modules, but regulatory restrictions, both requesting GMP-compliant generator and GMP regulations for labeling as for the standard drugs, has retarded the implementation in clinical practice. Both these obstacles have been overcome and at least one pharmaceutical 68Ge/68Ga generator was officially approved for the use in clinical studies and some modules were registered as GMP-compliant.69 Additional positive impact could be caused by the debate on adopting GMP-regulations for microdoses to PET-radiopharmaceuticals production specificity,70 intending to maintain the required quality aspects but not to hamper innovation and dynamic development of gallium-68 radiopharmaceuticals in clinical trials.
6. Conclusions
Construction of the 68Ge/68Ga generator increased the applications of radiopharmaceuticals labeled with this isotope in medicine. The availability of the commercial, pharmaceutical grade 68Ga/68Ge generator for routine applications and a favorable chemistry using DOTA, NOTA-derived bifunctional chelators have opened a brilliant future for the application of gallium-68 in all fields of PET imaging. Wide range of the PET radiopharmaceuticals based on gallium-68 labeling can be an alternative or complement to the already well-established radiopharmaceuticals based on fluorine-18 or carbon-11.
Nowadays, a complete replacement of these radioisotopes is hard to imagine, but the extension of diagnostic procedures by on-request, short series synthesis of targeted radiopharmaceuticals labeled with gallium-68, seems to be a very reasonable option in the nearest future.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Engle J.W., Lopez-Rodriguez V., Gaspar-Carcamo R.E. Very high specific activity 66/68Ga from zinc targets for PET. Appl Radiat Isot. 2012;70:1792–1796. doi: 10.1016/j.apradiso.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breeman W.A.P., de Blois E., Chan H., Konijnenberg M., Kwekkeboom D.J., Krenning E.P. 68Ga-labeled DOTA-peptides and 68Ga-labeled radiopharmaceuticals for positron emission tomography: current status of research, clinical applications, and future perspectives. Semin Nucl Med. 2011;41:314–321. doi: 10.1053/j.semnuclmed.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Khan M.U., Khan S., El-Refaie S., Win Z., Rubello D., Al-Nahhas A. Clinical indications for gallium-68 positron emission tomography imaging. EJSO. 2009;35:561–567. doi: 10.1016/j.ejso.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Smith D.L., Breeman W.A.P., Sims-Mourtada J. The untapped potential of Gallium68-PET: the next wave of 68Ga-agents. Appl Radiat Isot. 2013;76:14–23. doi: 10.1016/j.apradiso.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Rösch F. Past, present and future of 68Ge/68Ga generators. Appl Radiat Isot. 2013;76:24–30. doi: 10.1016/j.apradiso.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Lin M., Ranganathan D., Mori T. Long-term evaluation of TiO2-based 68Ge/68Ga generators and optimized automation of [68Ga]DOTATOC radiosynthesis. Appl Radiat Isot. 2012;70:2539–2544. doi: 10.1016/j.apradiso.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw D.D., Breeman W.A.P. Scaled-up radiolabelling of DOTATATE with 68Ga eluted from a SnO2-based 68Ge/68Ga generator. Appl Radiat Isot. 2012;70:1741–1750. doi: 10.1016/j.apradiso.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarty R., Shukla R., Ram R., Tyagi A.K., Dash A., Venkatesh M. Development of a nano-zirconia based 68Ge/68Ga generator for biomedical application. Nucl Med Biol. 2011;38:575–583. doi: 10.1016/j.nucmedbio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama M., Haratake M., Ono M. A new 68Ge/68Ga generator system using an organic polymer containing N-methylglucamine groups as adsorbent for 68Ge. Appl Radiat Isot. 2003;58:9–14. doi: 10.1016/s0969-8043(02)00268-3. [DOI] [PubMed] [Google Scholar]
- 10.Chakravarty R., Chakraborty S., Dash A., Pillai M.R.A. Detailed evaluation on the effect of metal ion impurities on complexation of generator eluted 68Ga with different bifunctional chelators. Nucl Med Biol. 2013;40:197–205. doi: 10.1016/j.nucmedbio.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Astia M., De Pietri G., Fraternali A. Validation of 68Ge/68Ga generator processing by chemical purification for routine clinical application of 68Ga-DOTATOC. Nucl Med Biol. 2008;35:721–724. doi: 10.1016/j.nucmedbio.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Zhernosekov K.P., Filosofov D.V., Baum R.P. Processing of generator-produced 68Ga for medical application. J Nucl Med. 2007;48:1741–1748. doi: 10.2967/jnumed.107.040378. [DOI] [PubMed] [Google Scholar]
- 13.Mueller D., Klette I., Baum R.P., Gottschaldt M., Schultz M.K., Breeman W.A.P. Simplified NaCl based (68)Ga concentration and labeling procedure for rapid synthesis of (68)Ga radiopharmaceuticals in high radiochemical purity. Bioconjug Chem. 2012;23(8):1712–1717. doi: 10.1021/bc300103t. [DOI] [PubMed] [Google Scholar]
- 14.Schultz M.K., Mueller D., Baum R.P., Watkins G.L., Breeman W.A.P. A new automated NaCl based robust method for routine production of gallium-68 labeled peptides. Appl Radiat Isot. 2013;76:46–54. doi: 10.1016/j.apradiso.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loktionova N.S., Belozub A.N., Filosofov D.V. Improved column-based radiochemical processing of the generator produced 68Ga. Appl Radiat Isot. 2011;69:942–946. doi: 10.1016/j.apradiso.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 16.Gebhardt P., Opfermann T., Saluz H.P. Computer controlled 68Ga milking and concentration system. Appl Radiat Isot. 2010;68:1057–1059. doi: 10.1016/j.apradiso.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Maecke H.R., Andre J.P. In: 68 Ga-PET radiopharmacy: a generator-based alternative to 18F-radiopharmacy. Chemistry P.E.T., Schubiger P.A., Lehmann L., Friebe M., editors. Springer-Verlag; Berlin, Heidelberg: 2007. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee S.R., Pomper M.G. Clinical applications of gallium-68. Appl Radiat Isot. 2013;76:2–13. doi: 10.1016/j.apradiso.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riss P.J., Kroll C., Nagel V., Rösch F. NODAPA-OH and NODAPA-(NCS)n: synthesis 68Ga-radiolabelling and in vitro characterisation of novel versatile bifunctional chelators for molecular imaging. Bioorg Med Chem Lett. 2008;18:5364–5367. doi: 10.1016/j.bmcl.2008.09.054. [DOI] [PubMed] [Google Scholar]
- 20.Cambioli S., Ambrosini V., Morigi J.J., Tabacchi E., Fanti S. 68Ga-labelled peptides for diagnosis of neuroendocrine tumours. Méd Nucl. 2013;37:66–70. [Google Scholar]
- 21.Wild D., Schmitt J.S., Ginj M. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging. 2003;30(10):1338–1347. doi: 10.1007/s00259-003-1255-5. [DOI] [PubMed] [Google Scholar]
- 22.Ambrosini V., Campana D., Tomassetti P., Grassetto G., Rubello D., Fanti S. PET/CT with 68Gallium-DOTA-peptides in NET: an overview. Eur J Radiol. 2011;80:e116–e119. doi: 10.1016/j.ejrad.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Naji M., Zhao C., Welsh S.J. 68Ga-DOTA-TATE PET vs. 123I-MIBG in identifying malignant neural crest tumours. Mol Imaging Biol. 2011;13(4):769–775. doi: 10.1007/s11307-010-0396-8. [DOI] [PubMed] [Google Scholar]
- 24.Maurice J.B., Troke R., Win Z. A comparison of the performance of 68Ga-DOTATATE PET/CT and 123I-MIBG SPECT in the diagnosis and follow-up of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39(August (8)):1266–1270. doi: 10.1007/s00259-012-2119-7. [DOI] [PubMed] [Google Scholar]
- 25.Taieb D., Rubello D., Al-Nahhas A. Imaging for paragangliomas: relation to genetic mutations. EJSO. 2011;37:662–668. doi: 10.1016/j.ejso.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Kowalski J., Henze M., Schuhmacher J., Macke H.R., Hoffmann M., Haberkom U. Evaluation of positron emission tomography imaging using [68Ga]-DOTA-d-Phe1-Tyr3-octreotide in comparison to [111In]-DTPA-OC SPECT. First results in patients with neuroendocrine tumours. Mol Imaging Biol. 2003;5:42–48. doi: 10.1016/s1536-1632(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 27.Gabriel M., Decristoforo C., Kendler D. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 28.Buchmann I., Henze M., Engelbrecht S. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34:1617–1634. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 29.Krausz Y., Freedman N., Rubinstein R. 68Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: comparison with 111In-DTPA-octreotide (OctreoScan®) Mol Imaging Biol. 2011;13(June (3)):583–593. doi: 10.1007/s11307-010-0374-1. [DOI] [PubMed] [Google Scholar]
- 30.Roesch F., Baum R.P. Generator-based PET radiopharmaceuticals for molecular imaging of tumours: on the way to THERANOSTICS. Dalton Trans. 2011;40(23):6104–6111. doi: 10.1039/c0dt01504k. [DOI] [PubMed] [Google Scholar]
- 31.Velikyan I., Sundin A., Eriksson B. In vivo binding of [68Ga]-DOTATOC to somatostatin receptors in neuroendocrine tumours – impact of peptide mass. Nucl Med Biol. 2010;37:265–275. doi: 10.1016/j.nucmedbio.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Fellner M., Biesalski B., Bausbacher N. 68Ga-BPAMD: PET-imaging of bone metastases with a generator based positron emitter. Nucl Med Biol. 2012;39:993–999. doi: 10.1016/j.nucmedbio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Toegel S., Wadsak W., Mien L.K. Preparation and pre-vivo evaluation of no-carrier-added, carrier-added and cross-complexed [68Ga]-EDTMP formulations. Eur J Pharm Biopharm. 2008;68:406–412. doi: 10.1016/j.ejpb.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Mathias C.J., Green M.A. A convenient route to [68Ga]Ga-MAA for use as a particulate PET perfusion tracer. Appl Radiat Isot. 2008;66:1910–1912. doi: 10.1016/j.apradiso.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maus S., Buchholz H.G., Ament S., Brochhausen C., Bausbacher N., Schreckenberger N. Labelling of commercially available human serum albumin kits with 68Ga a surrogates for 99mTc-MAA microspheres. Appl Radiat Isot. 2011;69:171–175. doi: 10.1016/j.apradiso.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Choi J.Y., Jeong J.M., Yoo B.C. Development of 68Ga-labeled mannosylated human serum albumin (MSA) as a lymph node imaging agent for positron emission tomography. Nucl Med Biol. 2011;38(April (3)):371–379. doi: 10.1016/j.nucmedbio.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Yang B.Y., Jeong J.M., Kim Y.J. Formulation of 68Ga BAPEN kit for myocardial positron emission tomography imaging and biodistribution study. Nucl Med Biol. 2010;37:149–155. doi: 10.1016/j.nucmedbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Beer A.J., Grosu A.L., Carlsen J. [18F]Galacto-RGD positron emission tomography for imaging of αvβ3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6610–6616. doi: 10.1158/1078-0432.CCR-07-0528. [DOI] [PubMed] [Google Scholar]
- 39.Dijkgraaf I., Yim C.B., Franssen G.M. PET imaging of αvβ3 integrin expression in tumours with 68Ga-labelled mono-, di- and tetrameric RGD peptides. Eur J Nucl Med Mol Imaging. 2011;38:128–137. doi: 10.1007/s00259-010-1615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knetsch P.A., Petrik M., Rangger Ch. [68Ga]NS3-RGD and [68Ga] Oxo-DO3A-RGD for imaging αvβ3 integrin expression: synthesis, evaluation, and comparison. Nucl Med Biol. 2013;40:65–72. doi: 10.1016/j.nucmedbio.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Boros E., Ferreira C.L., Patrick B.O., Adam M.J., Orvig C. RGD conjugates of the H2dedpa scaffold: synthesis, labeling and imaging with 68Ga. Nucl Med Biol. 2011;38(8):1165–1174. doi: 10.1016/j.nucmedbio.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Eder M., Krivoshein A.V., Backer M., Backer J.M., Haberkorn U., Eisenhut M. ScVEGF-PEG-HBED-CC and scVEGF-PEG-NOTA conjugates: comparison of easy-to-label recombinant proteins for [68Ga]PET imaging of VEGF receptors in angiogenic vasculature. Nucl Med Biol. 2010;37:405–412. doi: 10.1016/j.nucmedbio.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Hennrich U., Seyler L., Schäfer M. Synthesis and in vitro evaluation of 68Ga-DOTA-4-FBn-TN14003, a novel tracer for the imaging of CXCR4 expression. Bioorg Med Chem. 2012;20(February (4)):1502–1510. doi: 10.1016/j.bmc.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 44.Persson M., Madsen J., Ostergaard S., Ploug M., Kjaer A. 68Ga-labeling and in vivo evaluation of a uPAR binding DOTA- and NODAGA-conjugated peptide for PET imaging of invasive cancers. Nucl Med Biol. 2012;39:560–569. doi: 10.1016/j.nucmedbio.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Li D., Liu S., Shan H., Conti P., Li Z. Urokinase plasminogen activator receptor (uPAR) targeted nuclear imaging and radionuclide therapy. Theranostics. 2013;3(7):507–515. doi: 10.7150/thno.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H.J., Kim D.Y., Park J.H. Synthesis and characterization of a 68Ga-labeled N-(2-diethylaminoethyl)benzamide derivative as potential PET probe for malignant melanoma. Bioorg Med Chem. 2012;20(August (16)):4915–4920. doi: 10.1016/j.bmc.2012.06.047. [DOI] [PubMed] [Google Scholar]
- 47.Kurihara H., Honda N., Kono Y., Arai Y. Radiolabelled agents for PET imaging of tumor hypoxia. Curr Med Chem. 2012;19(20):3282–3289. doi: 10.2174/092986712801215964. [DOI] [PubMed] [Google Scholar]
- 48.Sano K., Okada M., Hisada H. In vivo evaluation of a radiogallium-labeled bifunctional radiopharmaceutical, Ga-DOTA-MN2, for hypoxic tumor imaging. Biol Pharm Bull. 2013;36(4):602–608. doi: 10.1248/bpb.b12-00982. [DOI] [PubMed] [Google Scholar]
- 49.Hoigebazar L., Jeong J.M., Hong M.K. Synthesis of 68Ga-labeled DOTA-nitroimidazole derivatives and their feasibilities as hypoxia imaging PET tracers. Bioorg Med Chem. 2011;19(April (7)):2176–2181. doi: 10.1016/j.bmc.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 50.Fernández S., Dematteis S., Giglio J., Cerecetto H., Rey A. Synthesis, in vitro and in vivo characterization of two novel 68Ga-labelled 5-nitroimidazole derivatives as potential agents for imaging hypoxia. Nucl Med Biol. 2013;40:273–279. doi: 10.1016/j.nucmedbio.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 51.Schmid M., Neumaier B., Vogg A.T. Synthesis and evaluation of a radiometal-labeled macrocyclic chelator-derivatised thymidine analog. Nucl Med Biol. 2006;33(April (3)):359–366. doi: 10.1016/j.nucmedbio.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 52.Ermert J., Coenen H.H. Methods for 11C- and 18F-labelling of amino acids and derivatives for positron emission tomography imaging. J Label Compd Radiopharm. 2013;56:225–236. doi: 10.1002/jlcr.2996. [DOI] [PubMed] [Google Scholar]
- 53.Dunet V., Rossier C., Buck A., Stupp R., Prior J.O. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and metaanalysis. J Nucl Med. 2012;53(February (2)):207–214. doi: 10.2967/jnumed.111.096859. [DOI] [PubMed] [Google Scholar]
- 54.Walter F., la Fougère C., Belka C., Niyazi M. Technical issues of [(18)F]FET-PET imaging for radiation therapy planning in malignant glioma patients – a review. Front Oncol. 2012;2(October):130. doi: 10.3389/fonc.2012.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pellegrini P.A., Howell N.R., Shepherd R.K. Synthesis and radiolabelling of DOTA-linked glutamine analogues with 67,68Ga as markers for increased glutamine metabolism in tumour cells. Molecules. 2013;18(June (6)):7160–7178. doi: 10.3390/molecules18067160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kou Y.W., Chen W.J., Lee T.W., Lo J.M. Synthesis and evaluation of 67Ga- and 68Ga-DOTA-glutamine. Ann Nucl Med Sci. 2009;22:35–42. [Google Scholar]
- 57.Shetty D., Jeong J.M., Ju C.H. Synthesis of novel 68Ga-labeled amino acid derivatives for positron emission tomography of cancer cells. Nucl Med Biol. 2010;37(November (8)):893–902. doi: 10.1016/j.nucmedbio.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 58.Burchardt C., Riss P.J., Zoller F. [68Ga]Ga-DO(2)A-(OBu-l-tyr)(2): synthesis, 68Ga-radiolabeling and in vitro studies of a novel 68Ga-DO(2)A-tyrosine conjugate as potential tumor tracer for PET. Bioorg Med Chem Lett. 2009;19(July (13)):3498–3501. doi: 10.1016/j.bmcl.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52(January (1)):81–89. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Behe M., Alt K., Deininger F. In vivo testing of 177Lu-labelled anti-PSMA antibody as a new radioimmunotherapeutic agent against prostate cancer. In Vivo. 2011;25(1):55–59. [PubMed] [Google Scholar]
- 61.Banerjee S.R., Pullambhatla M., Byun Y. 68Ga-labeled inhibitors of prostate-specific membrane antigen (PSMA) for imaging prostate cancer. J Med Chem. 2010;53(July (14)):5333–5341. doi: 10.1021/jm100623e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Afshar-Oromieh A., Malcher A., Eder M. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40(April (4)):486–495. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 63.Afshar-Oromieh A., Haberkorn U., Eder M., Eisenhut M., Zechmann C.M. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imaging. 2012;39(June (6)):1085–1086. doi: 10.1007/s00259-012-2069-0. [DOI] [PubMed] [Google Scholar]
- 64.Velikyan I., Xu H., Nair M., Hall H. Robust labeling and comparative preclinical characterization of DOTA-TOC and DOTA-TATE. Nucl Med Biol. 2012;39(July (5)):628–639. doi: 10.1016/j.nucmedbio.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 65.Ocak M., Antretter M., Knopp R. Full automation of 68Ga labelling of DOTA-peptides including cation exchange prepurification. Appl Radiat Isot. 2010;68(February (2)):297–302. doi: 10.1016/j.apradiso.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 66.Di Pierro D., Rizzello A., Cicoria G. Radiolabelling, quality control and radiochemical purity assessment of the Octreotide analogue 68Ga DOTA NOC. Appl Radiat Isot. 2008;66(August (8)):1091–1096. doi: 10.1016/j.apradiso.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Azhdarinia A., Yang D.J., Chao C., Mourtada F. Infrared-based module for the synthesis of 68Ga-labeled radiotracers. Nucl Med Biol. 2007;34(January (1)):121–127. doi: 10.1016/j.nucmedbio.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 68.Blom E., Koziorowski J. 68Ga-autoclabeling of DOTA-TATE and DOTA-NOC. Appl Radiat Isot. 2012;70(6):980–983. doi: 10.1016/j.apradiso.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 69.Boschi S., Lodi F., Malizia C., Cicoria G., Marengo M. Automation synthesis modules review. Appl Radiat Isot. 2013;76:38–45. doi: 10.1016/j.apradiso.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 70.Verbruggen A., Coenen H.H., Deverre J.R. Guideline to regulations for radiopharmaceuticals in early phase clinical trials in the EU. Eur J Nucl Med Mol Imaging. 2008;35(November (11)):2144–2151. doi: 10.1007/s00259-008-0853-7. [DOI] [PubMed] [Google Scholar]


