Abstract
Background
HIV point-of-care testing (POCT) was approved for use in Canada in 2005 and provides important public health benefits by providing rapid screening results rather than sending a blood sample to a laboratory and waiting on test results. Access to test results soon after testing (or during the same visit) is believed to increase the likelihood that individuals will receive their results and improve access to confirmatory testing and linkages to care. This paper reviews the literature on the utilization of HIV POCT across Canadian provinces.
Methods
We searched OVID Medline, Embase, EBM Reviews, PsycINFO, CINAHL, and 20 electronic grey literature databases. All empirical studies investigating HIV POCT programs in Canada published in French or English were included.
Results
Searches of academic databases identified a total of 6,091 records. After removing duplicates and screening for eligibility, 27 records were included. Ten studies are peer-reviewed articles, and 17 are grey literature reports. HIV POCT in Canada is both feasible and accepted by Canadians. It is preferred to conventional HIV testing (ranging from 81.1 to 97%), and users are highly satisfied with the testing process (ranging between 96 and 100%).
Conclusion
The majority of studies demonstrate that HIV POCT is feasible, preferred, and accepted by diverse populations in Canada. Losses to follow-up and linkage rates are also good. However, more research is needed to understand how best to scale up HIV POCT in contexts that currently have very limited or no access to testing.
Keywords: HIV, point-of-care testing, utilization, Canada, scoping review
Introduction
HIV testing and diagnosis is the first stage in the HIV continuum of care. Previous studies on HIV-infected individuals suggest that people who are aware of their HIV status are more likely to practice behaviors that lower the risk of HIV transmission, compared to people who are unaware of their HIV status (1). Public health practitioners recommend widespread availability and accessibility of HIV point-of-care testing (POCT) tests, particularly for priority populations (2).
Globally, HIV POCT has been available for use in recent years. Although a low-cost and easy-to-use test such as HIV POCT has great potential for advancing the UNAIDS 90–90–90 targets; the adoption, implementation, and performance of HIV POCT in practice has proved challenging (3). A systematic approach at the national level including the development of proper policies, regulations, and guidelines related to HIV POCT and a stepwise approach including attention to implementation have been recognized as key factors in improving HIV testing and diagnosis rates (4). However, to ensure sustainable quality testing, it is important to recognize the challenges in different settings particularly in relation to regulatory control and quality monitoring (3, 5). The Global Health Strategy on HIV/AIDS has put a strong emphasis on monitoring interventions across the entire continuum of care.
HIV POCT has been approved in Canada since 2005. To date, POCT programs have been implemented primarily in large Canadian cities such as Vancouver, Montreal, Toronto, Edmonton, Winnipeg, and Saskatoon. POCT refers to the practice of providing a rapid preliminary test result within one clinical encounter, rather than sending a blood sample to a laboratory and waiting on test results. HIV POCT provides an important public health benefit for the estimated one quarter of Canadians living with HIV who are unaware of their HIV status (6). This benefit is twofold. First, HIV testing significantly improves the likelihood that clients will receive a preliminary HIV diagnosis as results are conveniently available within minutes of testing (7, 8) Second, it can help to facilitate timely linkages to treatment and care as clients receiving a reactive result are provided with posttest counseling and referrals to care (9, 10). Unlike standard HIV tests, HIV POCT can be performed in any place and has the potential to be more patient-centered and support person-first care (4).
In Canada the only POCT test available: the INSTI HIV-1/HIV-2 Antibody Test has high sensitivity and specificity (>99%). Moreover, current HIV testing guidelines in Canada promote the use of HIV POCT, but these guidelines adhere to strict informed consent and pretest counseling requirements. For example, pretest counseling procedures must clearly convey that test results will be made available within minutes. Moreover, individuals tested must know that results are preliminary, and that confirmatory testing is required for a reactive or indeterminate result (11, 12). HIV POCT programs in Canada will be aware of these guidelines and be required to adapt testing policies accordingly.
The focus of this scoping review was to investigate the utilization of HIV point-of-care-testing in Canadian settings. We sought to understand what is known about the use and implementation of HIV POCT in Canadian settings and to identify gaps in the current knowledge base. The review describes general characteristics of existing POCT programs in Canada and then synthesizes the relationships between HIV POCT programs and acceptability, satisfaction, preference, feasibility, returned results, losses to follow-up, and linkage to care rates. For the purpose of this scoping review, the following terms were operationalized as:
Acceptability: the proportion of testers willing to receive or who received a HIV POCT and/or reasons for acceptance.
Feasibility: a determination that HIV POCT is both easily done and convenient.
Linkage to care: the proportion or number of people who receive confirmatory positive HIV results and are linked to care.
Loss to follow-up: the proportion or number of people tested who receive a reactive POCT but do not receive western blot confirmatory testing results.
Preference: the proportion of testers who favored POCT when compared with conventional testing and/or reasons influencing one’s preference.
Reach: the proportion of individuals who were tested using HIV POCT technology who were previously never or recently tested.
Returned results: the proportion or number of people who receive their POCT result as compared to the number of people who are tested.
Satisfaction: the proportion of testers who were pleased with their POCT experience and/or reasons for their satisfaction.
Sensitivity, specificity and predictive value of HIV POCT.
Previous systematic reviews have investigated the utilization of conventional HIV testing in Canadian settings (13), the barriers associated with HIV POCT in an international context (14), as well as the acceptability of HIV self-testing including participants’ attitudes and testing uptake (15). This scoping review adds to the literature by focusing on the utilization of HIV POCT in Canada.
Methods
Search Strategy
The search strategy using a combination of controlled vocabulary and keyword searching was developed to capture literature relating to HIV POCT. See Table 1 for a sample search strategy. As is recommended by Arksey and O’Malley (16), a wide study selection and database search was conducted to generate breadth of coverage on the research topic.
Table 1.
Summary of systematic search strategy.
| Search strategy |
|---|
| 1. HIV Infections/di [Diagnosis] (12,845) |
| 2. HIV Seropositivity/di [Diagnosis] (2,397) |
| 3. AIDS Serodiagnosis/ (6,158) |
| 4. HIV.ti. (148,826) |
| 5. human immunodeficiency virus.ti. (30,058) |
| 6. or/1–5 (179,146) |
| 7. Point-of-Care Systems/ (7,372) |
| 8. POCT.ti,ab. (588) |
| 9. point of care.ti,ab. (7,517) |
| 10. point of service.ti,ab. (345) |
| 11. ((rapid or instant or home or self) adj3 (test$ or screen$ or kit$)).ti,ab. (28,828) |
| 12. oraquick.ti,ab. (110) |
| 13. clearview.ti,ab. (97) |
| 14. (reveal adj2 rapid).ti,ab. (146) |
| 15. insti.ti,ab. (69) |
| 16. uni-gold recombigen.ti,ab. (7) |
| 17. multispot.ti,ab. (84) |
| 18. (sure adj check).ti,ab. (1) |
| 19. stat-pak.ti,ab. (62) |
| 20. chembio.ti,ab. (29) |
| 21. or/7-20 (40,720) |
| 22. 6 and 21 (2,185) |
| 23. remove duplicates from 22 (2,045) |
Database: Ovid MEDLINE(R) in-process and other non-indexed citations and Ovid MEDLINE(R) <1946 to August 2014>.
The following electronic databases were searched:
Ovid MEDLINE, including in-process and other non-indexed citations (1946–third week, August 2014)
EMBASE (1974–25, August 2014)
EBM reviews (1991–third quarter 2014)
PsycINFO (1806–25, August 2014)
CINAHL (1980–25, August 2014)
No language or date limiters were applied.
To supplement the database search, the review team conducted a search of 19 electronic databases for grey literature. The following sites were searched:
AIDS Committee of Toronto
ASO411
BC Centre for Excellence in HIV/AIDS
Bibliothèque et Archives nationales du Québec
Canadian Agency for Drugs and Technologies in Health
Canadian HIV/AIDS Legal Network
Canadian Nurses Association
CATIE and http://sagecollection.ca
Canadian Health Research Collection
Canadian Institute for Health Information
CIHR Social Research Centre in HIV Prevention
Gay Men’s Sexual Health Alliance
Google custom search: government documents
Google Scholar
Health Nexus
Health Quality Ontario
Institut national d’excellence en santé et en services sociaux
Ontario HIV Treatment Network
Open Grey
Public Health Agency of Canada.
In addition to our online search strategy, we hand-searched the reference lists of included articles for additional items of relevance. We also contacted 65 Canadian researchers who are members of the national CIHR Centre for REACH (Research Evidence into Action for Community Health) in HIV/AIDS POCT Working Group. These contacts provided additional grey literature materials as well as further knowledge of ongoing POCT testing programs in Canada.
Inclusion Criteria and Study Selection
Two members of the scoping review team assessed studies based on information in the title and abstract. As is further recommended by Arksey and O’Malley (16), the inclusion criteria was developed post hoc based on increasing familiarity with the literature and applied to each article to determine their relevance in this scoping review. Studies were included if they met the following criteria:
Empirical study investigating HIV POCT programs, including articles investigating access and uptake to HIV POCT.
Study published in English or French.
Studies that evaluated HIV POCT performance without providing further information about access to testing or testing uptake were excluded. “Access” refers to information about the point of access including structural factors, setting, location, hours, service provider who is offering testing, funding, cost, and time to test. “Uptake” refers to what happens when people are offered a test and whether or not they accept. This concept includes testing rates but is also about acceptability.
A second eligibility stage was completed whereby two members of the scoping review team assessed articles for inclusion in this Canadian-focused scoping review. Literature from both peer-reviewed journals and grey literature sources were included in this review. Items were included that took place in Canadian locations.
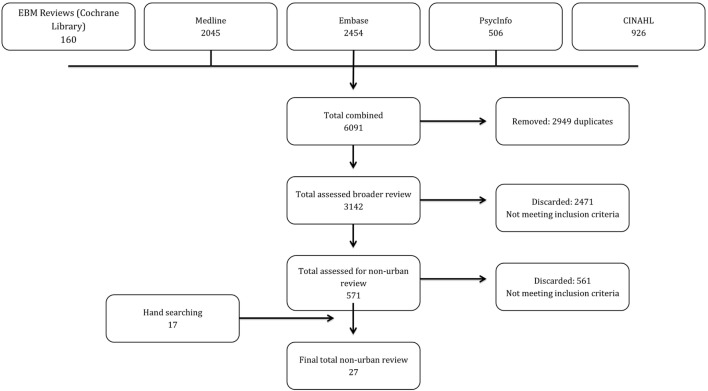
The peer-reviewed database search yielded 6,091 records. After duplicates were removed, 3,142 were screened for eligibility resulting in the identification of 571 items of potential relevance to this review. The grey literature search produced an additional 17 articles. Items were typically excluded because they did not focus on HIV POCT, and/or the HIV POCT program described was not located in a Canadian setting. See Figure 1 for the search strategy decision tree.
Figure 1.
Flow diagram of studies (PRISMA).
Data Extraction and Quality Appraisal
An Excel data extraction sheet, including data extraction guidelines, was prepared to guide the quality appraisal process. Specifically, the tool was designed to organize extracted information relating to citation type, study design and methodology, program participants, and program characteristics such as the test provider, testing combinations, and the site of program delivery. Further information was extracted related to the following outcomes: feasibility, acceptability, preference, satisfaction, and impact including loss to follow-up and linkage to care rates. The data extraction tool was piloted with three articles, revised iteratively, and finalized before the remaining articles were accessed.
Two trained research assistants independently reviewed and extracted the information for each article included in the review. A calibration exercise was undertaken, and eligibility criteria were modified where the agreement between the two reviewers was low (kappa <0.5). The reviewers met biweekly to discuss the extracted information and reach consensus. Discrepancies were adjudicated by a third reviewer.
The quality of each study (both quantitative and qualitative) was also assessed using a scoring system based on the criteria found in “A scoring system for appraising mixed method research, and concomitantly appraising qualitative, quantitative and mixed-methods primary studies in Mixed Studies Reviews” (17). The quality score was not used to exclude studies but rather to identify the overall quality of the evidence base.
Results and Discussion
Description of Included Studies
A total of 27 studies met the inclusion criteria. Three articles were written in French, and the remaining documents were written in English. Of the 27 items, 10 were from peer-reviewed journals, and 17 were from grey literature sources. Fourteen studies were quantitative including a mix of cross-sectional (n = 7), cohort (n = 5), and quasi-experimental designs (n = 2). Three studies were qualitative of which two used a narrative design and one used grounded theory. Ten studies used mixed-methods approaches; three were cross-sectional, four were cohort studies, two used participatory action approaches, and one was quasi-experimental (Tables 2 and 3). The review team referenced Creswell’s text (18) on research designs to appropriately categorize the articles by study design.
Table 2.
Research methods of included articles.
| Research methods | Total |
|
|---|---|---|
|
n = 27 | ||
| n | % | |
| Mixed methods | 10 | 37 |
| Qualitative | 3 | 11 |
| Quantitative | 14 | 52 |
Table 3.
Study design of included articles.
| Study design | Total |
|
|---|---|---|
|
n = 27 | ||
| n | % | |
| Cohort | 9 | 33 |
| Cross-sectional | 10 | 37 |
| Grounded theory | 1 | 4 |
| Narrative | 2 | 7.5 |
| Participatory action | 2 | 7.5 |
| Quasi-experimental | 3 | 11 |
The 27 articles included in this literature set represent 20 studies that evaluated or described existing HIV POCT programs in Canada, while 7 articles elicited opinions including preferences for HIV POCT in Canadian settings. Sixteen studies surveyed the recipients of HIV POCT, four studies surveyed health-care providers, and seven studies surveyed both recipients and providers of HIV POCT. The majority of studies (50%) were assessed to be of low quality, 25% were of moderate quality, and 25% were assessed as strong. Table 4 provides an overview of all included studies.
Table 4.
Overview of included studies.
| Reference | Publication type | Language | Type of test offered | Location | Research focus | Study goals |
|---|---|---|---|---|---|---|
| Becker et al. (27) | Peer-reviewed | English | INSTI HIV-1/HIV-2 antibody test | Winnipeg, Manitoba | HIV point-of-care testing (POCT) program | Evaluate success of program |
| Bergman et al. (19, 25) | Peer-reviewed | English | INSTI HIV-1/HIV-2 antibody test | Edmonton, Alberta | HIV POCT and syphilis testing program | Evaluate feasibility |
| Bergman et al. (19, 25) | Grey literature | English | INSTITM HIV-1/HIV-2 rapid antibody test | Edmonton, Alberta | HIV POCT and syphilis testing program | Identify challenges to program implementation |
| Brondani and Chang (23) | Grey literature | English | HIV POCT—not specified | Vancouver, British Colombia | HIV POCT program | Evaluate acceptability |
| Bungay et al. (36) | Peer-reviewed | English | HIV POCT—not specified | Western Canada | HIV POCT program | Evaluate preferences and satisfaction |
| Fielden et al. (31) | Grey literature | English | HIV POCT—not specified | Vancouver and Northern Interior, British Colombia | HIV POCT program | Evaluate preferences |
| Gahagan et al. (32) | Grey literature | English | No test offered | Halifax, Nova Scotia | Research | Evaluate preferences |
| Guenter et al. (30) | Grey literature | English | Fast-check HIV-1/2 whole blood (Fast Check, Biochem Immunosystems Inc., Montreal, QC, Canada) | Toronto, Ontario | HIV POCT program | Evaluate satisfaction |
| Guenter et al. (37) | Peer-reviewed | English | Fast-check HIV-1/2 whole blood | Toronto, Ontario | HIV POCT program | Evaluate satisfaction and predictors of HIV POCT use |
| Halton Region Health Department (20) | Grey literature | English | HIV POCT—not specified | Milton, Ontario | HIV POCT and STI testing program | Evaluate acceptability and satisfaction |
| HIV Counselling and Testing Community Advisory Committee, Nova Scotia Advisory Commission on AIDS (21) | Grey literature | English | No test offered | Nova Scotia | Research | Evaluate acceptability |
| Iqbal et al. (38) | Peer-reviewed | English | No test offered | Toronto, Ontario | Research | Evaluate acceptability |
| Lambert et al. (35) | Grey literature | French | INSTI HIV-1/HIV-2 antibody test | Montréal, Québec | HIV POCT and hepatitis C (HCV) testing program | Evaluate feasibility, acceptability, satisfaction, preference, reach, and impact |
| Lewis et al. (33) | Peer-reviewed | English | No test offered | Halifax, Nova Scotia | Research | Evaluate acceptability |
| Lee et al. (41) | Peer-reviewed | English | INSTITM HIV-1/HIV-2 antibody test | Province-wide, Alberta | HIV POCT program | Performance characteristics of test kits |
| Miller and Martindale (40) | Grey literature | English | HIV POCT—not specified | Canada-wide | HIV POCT program | Evaluate acceptability, satisfaction, and preferences |
| Nine Circles Community Health Centre (29) | Grey literature | English | INSTI HIV-1/HIV-2 antibody test | Winnipeg, Manitoba | HIV POCT and STI testing program | Evaluate satisfaction and preferences |
| Options Clinic (47) | Grey literature | English | INSTI HIV-1/HIV-2 antibody test | London, Ontario | HIV POCT and STI testing program | Determine population served by outreach program |
| Pai et al. (24) | Peer-reviewed | English | Miriad rapid TP/HBV/HIV/HCV antibody test | Montreal, Quebec | HIV POCT program | Evaluate feasibility and preference |
| PHS Community Services Society (28) | Grey literature | English | INSTI HIV-1/HIV-2 antibody test | Vancouver, British Colombia | HIV POCT program | Evaluate impact (returned results and linkage to care rates) |
| Pyra Management Consulting Services Inc. (44) | Grey literature | English | No test offered | Nova Scotia | Research | Understand stakeholder perceptions of POCT |
| Schwandt et al. (34) | Peer-reviewed | English | No test offered | Not reported | Research | Evaluate preferences |
| Thériault et al. (22) | Grey literature | French | HIV POCT—not specified | Québec City, Québec | HIV POCT and STI testing program | Evaluate uptake, feasibility, acceptability, and satisfaction |
| Vancouver STOP Project (46) (dent) | Grey literature | English | HIV POCT—not specified | Vancouver, British Colombia | HIV POCT program | Evaluate acceptability |
| Vancouver STOP Project (26) (out) | Grey literature | English | HIV POCT—not specified | Vancouver, British Colombia | HIV POCT program | Evaluate impact (returned results and linkage to care rates) |
| Veillette-Bourbeau (45) | Grey literature | French | INSTI HIV-1/HIV-2 antibody test | Montreal, Québec | HIV POCT program | Describe implementation process |
| Wertheimer (43) | Grey literature | English | No test offered | Canada-wide | Research | Identify barriers to testing |
Characteristics of POCT Programs in Canada
Of the 20 studies that describe HIV POCT programs in Canada, 12 of the 20 (60%) studies demonstrated the use of rapid finger prick technology, while 8 studies did not specify the exact blood or saliva sampling technology used. Thirteen studies (65%) used HIV POCT technologies alone, while 6 used HIV POCT technologies in combination with STI testing, and 1 study described a multiplex testing strategy whereby POCT technologies were used for HIV, hepatitis C (HCV), and STI testing. In these 20 studies, the majority of tests performed were conducted by nurses (n = 11); followed by HIV testing counselors (n = 3), outreach workers (n = 3), dental professionals (n = 2), and community-based researchers (n = 1).
In the entire article set (n = 27), a number of priority populations were reached. Men who have sex with men (MSM) and lesbian, gay, bisexual, transgender, and queer (LGBTQ) populations were the focus in six and four studies, respectively. People with a history of substance use were a priority population in seven studies, while Aboriginal people were the focus of four, commercial sex workers of five, and incarcerated men and women of two.
Utilization of HIV POCT in Canada
The included studies described HIV POCT programs currently operating or piloted in the following provinces:
Alberta (n = 3)
British Colombia (n = 7)
Manitoba (n = 2)
Ontario (n = 4)
Québec (n = 4).
For the studies in our review, HIV POCT programs were offered in the following Canadian settings:
Aboriginal health/friendship centers (n = 2)
Addictions facilities (n = 2)
Community-based organizations (n = 5)
Community health centers (n = 4)
Dental offices (n = 3)
Hospital (n = 4)
Indoor commercial sex markets (n = 1)
Primary care centers (n = 1)
Prisons or correctional facilities (n = 5)
Sexual health/HIV clinics (n = 4)
Street outreach (n = 4).
The Impact of HIV POCT Programs in Canada
The following section focuses on the relationships between HIV POCT and acceptability, feasibility, satisfaction, preference, returned results, losses to follow-up, and linkage to care rates. Table 5 summarizes the findings described below.
Table 5.
Summary of findings relevant to utilization of point-of-care testing (POCT) in Canada.
| Reference | Study design | Study setting | Study population | Sample size | Data collection instrument | Feasibility | Acceptability | Satisfaction | Preference |
|---|---|---|---|---|---|---|---|---|---|
| Becker et al. (27) | Cross-sectional | Emergency department at hospital | Emergency department patients | 501 | Posttest questionnaire and INSTI HIV-1/HIV-2 antibody test | – | – | 96% satisfaction | – |
| Bergman et al. (19, 25) | Cohort | Community health center, bathhouses, gay bars, drop-in center prisons, addictions facilities | Men and women | 1,031 | INSTI HIV-1/HIV-2 antibody test | 81.5% | Highest acceptance among testing sites for MSM and the lowest acceptance at community-based organizations | – | – |
| Men who have sex with men (MSM) | |||||||||
| People who use or have history of injection drug use | |||||||||
| Commercial sex workers | |||||||||
| Bergman et al. (19, 25) | Narrative | Community health centers, community centers, prisons, drop-in centers | Not reported | Not reported | INSTITM HIV-1/HIV-2 rapid antibody test | – | – | – | – |
| Brondani and Chang (23) | Cross-sectional | Community dental clinics | Men and women | 32 | Self-administered questionnaire and HIV test | – | 92% | – | – |
| Bungay et al. (36) | Participatory action research design | Indoor commercial sex markets | Women | 113 | Survey | – | – | Satisfaction was high for women tested due to flexibility of POCT | POCT preferred as it is less invasive, more comfortable, and less painful than standard test |
| Commercial sex workers | Focus group | ||||||||
| Fielden et al. (31) | Cross-sectional | Primary care clinic, sexual health clinic, community health center, hospital, street outreach, aboriginal friendship centers, prisons, dental office, addiction facilities | Men and women, aboriginal peoples | 243 | Survey | – | – | – | 40% preferred POCT to standard |
| Interviews | |||||||||
| HIV test results | |||||||||
| Gahagan et al. (32) | Cross-sectional | Sexual health clinic | Not reported | 258 | Survey | – | – | – | 90% prefer rapid to standard test |
| Guenter et al. (30) | Cohort | Sexual health clinics | Men and women | 1,257 | Posttest questionnaire or interview and fast-check HIV-1/2 whole blood test | – | – | 98.9% satisfaction (non-reactive testers) | – |
| 100% satisfaction with reactive testers | |||||||||
| Guenter et al. (37) | Cohort | Sexual health clinic | Men and women | 1,257 | Posttest questionnaire or interview and fast-check HIV-1/2 whole blood test | – | – | 99% satisfaction | – |
| Halton Region Health Department (20) | Observational | Correctional facilities | Incarcerated men and women | 156 | Survey | HIV POCT was accepted because results were available immediately | 98% satisfaction | ||
| HIV testing data | |||||||||
| HIV Counselling and Testing Community Advisory Committee, Nova Scotia Advisory Commission on AIDS (21) | Cohort study | Not reported | Men, women, transgender people, aboriginal peoples | 50 | Interview | – | Acceptability was related to lessening the waiting period, and that rapid testing might be an effective way to reach communities that do not know or do not want to know their HIV status | – | – |
| African, Nova Scotians | HIV incidence data | ||||||||
| PWAs | Policy scan | ||||||||
| People living with hepatitis C (HCV) | |||||||||
| Iqbal et al. (38) | Cross-sectional | Hospital | Pregnant women | 92 | Survey | – | 59% of women were willing to be tested. Willingness was significantly associated with an interest in learning about HIV treatment options, access to health-care services, and the partner notification process | – | – |
| Lambert et al. (35) | Before and after | Correctional facilities | Men and women, MSM, people who use injection drugs, commercial sex workers, incarcerated men and women, people from endemic countries | 478 | Survey | – | 72.4% | 97.1% satisfaction | 93% prefer rapid to standard testing |
| Interview | |||||||||
| HIV testing data | |||||||||
| Lewis et al. (33) | Cross-sectional | Sexual health clinic | Men and women, lesbian, gay, bisexual, transgender, and queer (LGBTQ) individuals | 258 | Survey | – | – | – | 90.3% prefer rapid to standard |
| Lee et al. (41) | Observational | Hospital | Pregnant women, health-care workers with occupational exposures, acutely ill patients | 1,737 | INSTITM HIV-1/HIV-2 antibody test | – | – | – | – |
| Miller and Martindale (40) | Before and after | Not reported | Young gay and bisexual men | 300 | Survey | – | 90% | 66% satisfied with testing experience | 97% preferred rapid to standard test |
| HIV test | |||||||||
| Nine Circles Community Health Centre (29) | Cross-sectional | Community health center | Men and women | 54 | Survey | – | – | 96.6% of clients satisfied with testing experience | Preference for POCT related to benefits of an immediate result |
| LGBTQ | Focus group | ||||||||
| MSM | Document review | ||||||||
| People who use injection drugs, aboriginal peoples, Asian and African Canadian people, commercial sex workers | |||||||||
| Options clinic (47) | Cohort | Sexual health clinic, youth drop-in center, bathhouses, London Pride, Aboriginal friendship centers, needle exchange programs, university health clinics | MSM | 945 | Document review | – | – | – | – |
| LGBTQ | |||||||||
| People who use injection drugs, aboriginal peoples, students | |||||||||
| Pai et al. (24) | Cross-sectional | Hospital | Men and women, people who use injection drugs | 109 | Semi-structured questionnaire and Miriad Rapid TP/HBV/HIV/HCV antibody test | 92.4% completion rate | – | – | 97.2% preferred multiplex to conventional testing |
| PHS Community Services Society (28) | Cohort | Community centers, street fairs, single-room occupancy hotels | People who use injection drugs | 4,773 | Survey | – | – | – | – |
| HIV testing data | |||||||||
| Pyra Management Consulting Services Inc. (44) | Narrative research | Not reported | Not reported | 22 | Interview | – | – | – | – |
| Schwandt et al. (34) | Cross-sectional | Primary care clinics | Women | 100 | Self-administered questionnaire | – | – | – | 81% prefer rapid to standard |
| Thériault et al. (22) | Cross-sectional | Sexual health clinics | MSM, people who use injection drugs, commercial sex workers, people who inhale drugs | 249 | Interviews | Nurses had skills to adopt rapid testing easily into clinical practice | 95.4% chose rapid test | All people were either satisfied or very satisfied | – |
| Surveys | |||||||||
| Focus groups | |||||||||
| Document review | |||||||||
| HIV testing data | |||||||||
| Vancouver STOP Project (26, 46) | Cross-sectional | Dental clinic | Not reported | 22 | Survey | – | Acceptability was high among clients tested | – | – |
| Vancouver STOP Project (26, 46) | Cohort | AIDS service organization, bathhouses, pride parade, parks, single-occupancy hotel rooms | Not reported | Not reported | Not reported | – | – | – | – |
| Veillette-Bourbeau (45) | Grounded theory | Community health center | MSM | 10 | Interviews | – | – | – | – |
| Observation | |||||||||
| Document review | |||||||||
| Wertheimer (43) | Participatory action research design | Sexual health clinics, community centers | Women | 90 | Interviews | – | – | – | – |
| Surveys | |||||||||
Acceptability
HIV POCT participant acceptability rates were measured in seven studies and ranged from 52 to 92%. Higher acceptability rates were reported among MSM (19). Participants also reported higher acceptability due to the availability of rapid HIV POCT results lessening wait times (20–23).
Feasibility
Three studies measured feasibility and determined that HIV POCT was feasible in hospitals (24), sexual health and HIV clinics (22), and outreach settings (25).
Linkages to Care
Linkages to care rates were 89% in one study (26) and 100% in two studies (19, 27). A third study demonstrated that peer HIV POCT helped relink 324 previously diagnosed individuals to care (28). While linkage to care rates are high, they reflect linkages to confirmatory HIV testing only, which is just one small step in the HIV care cascade; more information regarding linkage to counseling and retention to care is needed.
Loss to Follow-Up
Losses to follow-up were generally very low ranging from no loss (29) to a loss of 1.1% (30) and a loss of 3% (31).
Preferences
When compared to standard testing, participant preferences for HIV POCT ranged from 81.1 to 97%. A multiplex strategy in which individuals were tested for HIV, HCV, and other STIs was preferred by 97% (n = 109) of those enrolled in the study (24). Preferences for HIV POCT were reported by study participants in multiple settings including sexual health clinics (32, 33), primary care clinics (34), hospitals (24), community health centers (29), detention centers (35), and community-based organizations (31). For commercial sex workers in British Columbia, HIV POCT was preferred due to its flexibility and less invasive procedures (36).
Reach (To Those Who Have Never Tested)
Four programs were successful in reaching those who have never been tested. Forty-two percent of participants tested in two provincial correctional facilities in Ontario (20) and 61% of women tested in a correctional facility in Montréal, Québec were never-testers (35). Twelve and a half percent of commercial sex workers were also reached for the first time by trained outreach staff (36). Finally, 12.5% of participants reached in a sexual health clinic in Québec city had not previously been tested for HIV (22).
Reach (To Those Who Are Previously Tested)
In five studies (24, 27, 34, 37, 38), there were a large percentage of individuals who had previously been tested for HIV ranging from 50 to 96% of the total sample.
Returned Results (Confirmatory Testing)
Two studies measured rates of returned results. In one study, 100% of participants in a correctional facility in Montréal received test results (35). In the other study, 98% of testers at a sexual health clinic in Toronto received test results, of which 22 (1.5%) were reactive. Four of the 22 individuals who were tested with a rapid HIV test did not receive their results from confirmatory testing (37). Previous studies show that a high percentage of people with reactive (70–100%) results seek confirmatory testing (39).
Satisfaction
Satisfaction with HIV POCT was high among program participants with satisfaction levels between 96 and 100%. HIV POCT was reported to be less invasive, less stressful, and less painful than traditional models of HIV testing leading to increased satisfaction (22, 36, 40).
Sensitivity, Specificity, and Predictive Value of HIV POCT
Two studies (19, 41) compared the sensitivity of the HIV POCT to conventional HIV testing. In both studies, the sensitivity value of the HIV POCT was 100%.
Three studies (19, 31, 41) compared the specificity of the HIV POCT to conventional HIV testing. The specificity of the HIV POCT ranged from 99.8 to 99.9%.
Three studies (19, 31, 41) compared the positive predictive and negative predictive values of the HIV POCT with the predictive values of the standard serological test. The positive predictive value ranged from 66.7 to 96%, and the negative predictive value was 100% in all three studies.
Discussion
Our scoping review investigated the utilization of HIV POCT in Canada. Our scoping review findings found evidence that HIV POCT has been implemented in five provinces and in a number of settings including community health centers and sexual health clinics, hospitals, primary care clinics, community organizations, correctional facilities, and outreach settings such as parks and gay pride parades. Moreover, HIV POCT programs have targeted the following populations: indigenous peoples, incarcerated individuals, LGBTQ individuals, MSM, people who use injection drugs, and pregnant women. The evidence in this review suggests that HIV POCT has broadened access to testing services for both those who have never tested and for return testers across much of Canada.
Overall, our scoping review found very high acceptance and satisfaction rates with HIV POCT programs in Canada. A large majority surveyed in these studies reported a preference for HIV POCT compared to conventional standard testing. Reasons commonly expressed to support these findings are that HIV POCT is more flexible, less invasive, and less stressful (due to a shortened wait period) than conventional testing. Moreover, losses to follow-up rates were generally very low for the HIV POCT programs identified in this review, while linkages to care rates were nearly perfect. In keeping with results from two other systematic reviews investigating the use and implementation of rapid HIV testing in North America (39) and among youth (42), the evidence in this review suggests that HIV POCT in Canada is feasible, preferred, and accepted by diverse populations.
The literature in this scoping review raises two important knowledge and policy gaps that should be addressed. First, HIV POCT services are not universally accessible across Canada (43, 44). In fact, there is little to no availability in the Northern Territories (Yukon, Northwest Territories, and Nunavut) and no availability in any of the four Atlantic Provinces. Second, HIV POCT is also unavailable in many rural and remote communities across Canada, some of which are First Nations, Inuit, and Métis communities. Further research and POCT services are required in these communities to understand how best to scale up HIV POCT in contexts that currently have very limited or no access to testing.
Despite these knowledge gaps, the evidence in this scoping review provides a number of actions to consider when implementing an HIV POCT program. First, program organizers must find qualified health-care professionals to offer HIV POCT (45), consider how to address confidentiality concerns, informed consent, and pretest counseling procedures (31, 33, 46), as well as ensure that confirmatory lab services are available and able to process additional POCT test kits (41). Program administrators also need to foster trusting relationships between participants and health-care providers (47) while providing multilingual programs and services that aim to enhance cultural safety (26, 36). Table 6 presents a summary of the implications for practice.
Table 6.
Implications for practice.
| 1 | HIV point-of-care testing (POCT) is more flexible, less invasive, and less stressful (due to a shortened wait period) than conventional testing |
| 2 | Program organizers must find qualified health-care professionals to offer HIV POCT |
| 3 | Concerns of confidentiality must be addressed |
| 4 | Confirmatory lab services must be available and able to process additional POCT test kits |
| 5 | Program organizers must develop trust between participants and health-care providers while providing multilingual and culturally safe services |
Moreover, the approval and implementation of HIV POCT programs in any country will have a substantial impact on screening and public health programs as it raises questions related to costs, equitable access to testing services, uptake, streamlined counseling services, and timely linkages to care. Public health providers thinking about implementing HIV POCT programs can learn from the experiences of others who have already implemented these programs.
There are two limitations of our search strategy and evidence base that must be noted. First, while the analysis includes both peer-reviewed and grey literature sources, we relied primarily on electronic sources rather than both electronic and print sources, which may contribute to publication bias. However, we did search the reference lists of all included articles as well as contact well-known researchers who work on POCT in Canada. Moreover, based on the low quality assessment of the articles in this review, the findings, while overwhelming supportive of HIV POCT, are from a low evidence base. This evidence base is low as most studies were observational in nature, thus the results should be interpreted with appropriate cautions. These findings do suggest, however, that HIV POCT is widely accepted by the Canadian population, including among key populations, and has high satisfaction rates. However, we did not compare the impact of HIV POCT among different population or geographies, and our findings must be interpreted with appropriate considerations. In addition, narrowing the scope of this review to Canadian literature may affect the generalizability of this review; however, it increases its practicality by controlling for variations in national policies and regulations with respect to country-level HIV POCT. The practical and technical challenges identified at the local level in one nation may be beneficial to decision-makers in other places who are planning to scale up similar testing in other geographic locations or to prepare for implementation in other settings.
Conclusion
In the process of this scoping review, we investigated 20 HIV POCT programs and 7 studies that elicited opinions including preferences for HIV POCT in Canadian settings. Our analysis focused on the utilization of HIV POCT including the feasibility, acceptability, satisfaction, preference for, and impacts of HIV POCT programs in Canada. Scoping reviews such as this one provide strong evidence of the benefits, reach, and acceptance of HIV POCT in Canadian provinces. They also identify important considerations for researchers, service providers, and policy makers when implementing new programs. The findings of this review will be useful to decision-makers both in Canada and globally as it distinguishes various barriers and enablers of successful implementation. These findings will help service providers anticipate potential challenges and maximize the benefits of HIV POCT in any setting.
Author Contributions
A Minichiello, M Swab, MC, ZM, JG, A Maybank, AH, M Schwandt, SG, OH, and SA were involved in all stages of the project including design, screening, review, and analysis. M Swab designed the search strategy and coordinated the initial screening of all the references. SA, A Minichiello, M Swab, MC, A Maybank, and AH conducted screening, and data extraction. All authors contributed to and have approved the final manuscript. OH reviewed and provided comments for manuscript editing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was led by the CIHR Centre for REACH in HIV/AIDS (REACH 2.0), a national partnership among people living with HIV, community-based organizations, and other front-line service providers, health researchers from over 25 academic institutions across Canada, and federal, provincial, and regional policy makers. REACH is spearheading research on point-of-care testing (POCT) across all regions with the goal of generating a set of evidence-based best practices that can be used to develop and implement effective POCT interventions across the country.
References
- 1.Johnston B, Conly J. Point-of-care testing for HIV: HIV counselling and testing. Can J Infect Dis (2002) 13(2):85. 10.1155/2002/480403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens W, Gous N, Ford N, Scott L. Feasibility of HIV point-of-care tests for resource-limited settings: challenges and solutions. BMC Med (2014) 12:173. 10.1186/s12916-014-0173-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global Health Sector Strategy on HIV 2016–2021. Towards Ending AIDS. World Health Organization; (2016). [Google Scholar]
- 4.World Health Organization. Improving the Quality of HIV-Related Point-of-Care Testing: Ensuring the Reliability and Accuracy of Test Results. World Health Organization; (2015). [Google Scholar]
- 5.Arora DR, Maheshwari M, Arora B. Rapid point-of-care testing for detection of HIV and clinical monitoring. ISRN AIDS (2013) 2013:287269. 10.1155/2013/287269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Public Health Agency of Canada. Summary: Estimates of HIV Prevalence and Incidence in Canada. (2011). Available from: http://www.phac-aspc.gc.ca/aidssida/publication/survreport/estimat-2011-eng.php
- 7.Carey MP, Coury-Doniger P, Senn TE, Vanable PA, Urban MA. Improving HIV rapid testing rates among STD clinic patients: a randomized controlled trial. Health Psychol (2008) 27(6):833. 10.1037/0278-6133.27.6.833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weis KE, Liese AD, Hussey J, Coleman J, Powell P, Gibson JJ, et al. A routine HIV screening program in a South Carolina Community Health Center in an area of low HIV prevalence. AIDS Patient Care STDS (2009) 23(4):251–8. 10.1089/apc.2008.0167 [DOI] [PubMed] [Google Scholar]
- 9.Basta T, Stambaugh T, Fisher C. Efficacy of an educational intervention to increase consent for HIV testing in rural Appalachia. Ethics Behav (2015) 25(2):129. 10.1080/10508422.2014.948958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryder PT, Meyerson BE, Coy KC, Von Hippel CDJ. Pharmacists’ perspectives on HIV testing in community pharmacies. J Am Pharm Assoc (2013) 53(6):595–600. 10.1331/JAPhA.2013.12240 [DOI] [PubMed] [Google Scholar]
- 11.Winnipeg Regional Health Authority. HIV Point-of-Care-Testing (POCT) Clinical Practice Guideline. (2013). Available from: http://www.wrha.mb.ca/extranet/publichealth/files/services/healthy-sexuality/9.8.HIVPoint-of-CareTestingPOCT.pdf
- 12.BC Centre for Disease Control. Communicable Disease Control Manual – Chapter 5 Point of Care HIV Test Guidelines for Health Care Settings. (2014). Available from: http://www.bccdc.ca/NR/rdonlyres/8D50320D-9175-4FCD-81AB623E9C051277/0/Point_of_Care_HIV_Test_Guidelines_for_HealthCareSettings_May2014.pdf
- 13.Ha S, Paquette D, Tarasuk J, Dodds J, Gale-Rowe M, Brooks J, et al. A systematic review of HIV testing among Canadian populations. Can J Public Health (2014) 105(1):E53–62. 10.17269/cjph.105.4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai NP, Wilkinson S, Deli-Houssein R, Vijh R, Vadnais C, Behlim T, et al. Barriers to implementation of rapid and point-of-care tests for human immunodeficiency virus infection: findings from a systematic review (1996–2014). Point Care (2015) 14(3):81–7. 10.1097/POC.0000000000000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krause J, Subklew-Sehume F, Kenyon C, Colebunders R. Acceptability of HIV self-testing: a systematic literature review. BMC Public Health (2013) 13:735. 10.1186/1471-2458-13-735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol (2005) 8(1):19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 17.Pluye P, Gagnon M, Griffiths F, Johnson-Lafleur JA. Scoring system for appraising mixed methods research, and concomitantly appraising qualitative, quantitative and mixed methods primary studies in mixed studies reviews. Int J Nurs Stud (2009) 46(4):529–46. 10.1016/j.ijnurstu.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 18.Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. Fourth ed Thousand Oaks, CA: SAGE; (2014). [Google Scholar]
- 19.Bergman J, Gratrix J, Plitt S, Fenton J, Archibald C, Wong T, et al. Feasibility and field performance of a simultaneous syphilis and HIV point-of-care test based screening strategy in at risk populations in Edmonton, Canada. AIDS Res Treat (2013) 2013:819593. 10.1155/2013/819593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halton Region Health Department. Pilot to Offer Anonymous, Rapid Point-of-Care HIV Testing Prisons: Programming Connection. (2014). Available from: http://www.catie.ca/en/pc/program/poc-prisons
- 21.HIV Counselling and Testing Community Advisory Committee, Nova Scotia Advisory Commission on AIDS. HIV Counselling and Testing in Nova Scotia: Implications for Policy and Practice. (2010). Available from: http://novascotia.ca/aids/documents/HIV-Counselling-and-Testing-Nova-Scotia
- 22.Thériault N, Noël L, Gagnon D. Évaluation de l’implantation de la trousse de dépistage rapide du VIH dans le réseau des SIDEP du CSSS de la Vieille-Capitale. Agence de la santé et des services sociaux de la Capitale-Nationale; (2013). [Google Scholar]
- 23.Brondani MA, Chang SM. Are we ready for HIV screening in dental clinics? J Can Dent Assoc (2014) 80:e58. [PubMed] [Google Scholar]
- 24.Pai NP, Dhurat R, Potter M, Behlim T, Landry G, Vadnais C, et al. Will a quadruple multiplexed point-of-care screening strategy for HIV-related co-infections be feasible and impact detection of new co-infections in at-risk populations? Results from cross-sectional studies. BMJ Open (2014) 4(12):e005040. 10.1136/bmjopen-2014-005040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergman J, Anderson B, Singh A, Poetz A. Syphilis and HIV Point-of-Care Testing Pilot Project. National Collaborating Centre for Infectious Diseases; (2013). [Google Scholar]
- 26.Vancouver STOP Project. STOP Outreach Team: Programming Connection. (2013). Available from: http://www.catie.ca/en/pc/program/stop-outreach
- 27.Becker M, Thompson L, Pindera C, Bridger N, Lopez C, Keynan Y, et al. Feasibility and success of HIV point-of-care testing in an emergency department in an urban Canadian setting/La faisabilité et le succès du test de dépistage du VIH au point de service au sein du département d’urgence d’un établissement canadien en milieu urbain. Can J Infect Dis Med Microbiol (2013) 24(1):27–31. 10.1155/2013/164797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.PHS Community Services Society. Peer HIV Testing: Programming Connection. (2013). Available from: http://www.catie.ca/en/pc/program/peer-testing-project
- 29.Nine Circles Community Health Centre. Rapid Point-of-Care HIV Testing (POCT) Demonstration Program – Evaluation Report. (2009). Available from: http://ninecircles.ca/images/stories/Research/POCT_Full.pdf
- 30.Guenter D, Trow R, Robinson G. The Effects of a Rapid Point-of-Care HIV Testing Program Hassle Free Clinic, Toronto. (2003). Available at: http://fhs.mcmaster.ca/slru/clear/paper/PaperC03-3.pdf
- 31.Fielden S, Lindegger M, Pedersen H, McAloney C, Krajden M, Ogilvie G, et al. Evaluation Findings from the Pilot Phase of BC’s Provincial Point of Care HIV Testing Program: The first 18 Months. Provincial Point of Care HIV Testing Program Evaluation Report. BC Centre for Disease Control; (2014). [Google Scholar]
- 32.Gahagan J, Stein C, Campbell A. Report on Anonymous HIV Testing Program and Perceptions of Acceptability of Rapid Point-of-Care Testing at Halifax Sexual Health Center. Halifax Sexual Health Centre; (2012). [Google Scholar]
- 33.Lewis NM, Gahagan JC, Stein C. Preferences for rapid point-of-care HIV testing in Nova Scotia, Canada. Sex Health (2013) 10(2):124–32. 10.1071/SH12100 [DOI] [PubMed] [Google Scholar]
- 34.Schwandt M, Nicolle E, Dunn S. Preferences for rapid point-of-care HIV testing in primary care. J Int Assoc Physicians AIDS Care (2012) 11(3):157–63. 10.1177/1545109711427605 [DOI] [PubMed] [Google Scholar]
- 35.Lambert G, Fall A, Chalifoux S. Évaluation de l’implantation d’un test de détection du VIH à résultat rapide dans les centres de détention de la région de Montréal: rapport final. Agence de la santé et des services sociaux de Montréal; (2014). [Google Scholar]
- 36.Bungay V, Kolar K, Thindal S, Remple VP, Johnston CL, Ogilvie G. Community-based HIV and STI prevention in women working in indoor sex markets. Health Promot Pract (2013) 14(2):247–55. 10.1177/1524839912447189 [DOI] [PubMed] [Google Scholar]
- 37.Guenter D, Greer J, Barbara A, Robinson G, Roberts J, Browne G. Rapid point-of-care HIV testing in community-based anonymous testing program: a valuable alternative to conventional testing. AIDS Patient Care STDS (2008) 22(3):195. 10.1089/apc.2007.0137 [DOI] [PubMed] [Google Scholar]
- 38.Iqbal S, De Souza L, Yudin M. Acceptability, predictors and attitudes of Canadian women in labour toward point-of-care HIV testing at a single labour and delivery unit. Can J Infect Dis Med Microbiol (2014) 25(4):201–6. 10.1155/2014/160370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broeckaert L, Challacombe L. Rapid Point-of-Care HIV Testing: A Review of the Evidence. (2015). Available from: http://www.catie.ca/en/pif/spring-2015/rapid-point-care-hiv-testing-review-evidence
- 40.Miller ML, Martindale S. “Rapid Testing Proves Popular.” – Conference Paper/Oral Presentation (Peer-Reviewed). (2000). Available from: http://www.cfenet.ubc.ca/vanguard/publications_journal/talk_5yr.pdf
- 41.Lee BE, Plitt S, Fenton J, Preiksaitis JK, Singh AE. Rapid HIV tests in acute care settings in an area of low HIV prevalence in Canada. J Virol Methods (2011) 172(1):66–71. 10.1016/j.jviromet.2010.12.022 [DOI] [PubMed] [Google Scholar]
- 42.Turner SD, Anderson K, Slater M, Quigley L, Dyck M, Guiang CB. Rapid point-of-care HIV testing in youth: a systematic review. J Adolesc Health (2013) 53(6):683–91. 10.1016/j.jadohealth.2013.07.029 [DOI] [PubMed] [Google Scholar]
- 43.Wertheimer S. Women and HIV Testing in Canada: Barriers and Recommendations as Identified by Service Providers: A Summary of Key Research Findings. (2011). Available from: http://www.cdnaids.ca/wp-content/uploads/Women-and-HIV-Testing-in-Canada-A-Summary-of-Key-Research-Findings.pdf
- 44.Pyra Management Consulting Services Inc. A Strategic Approach to Increase HIV Testing in Nova Scotia. (2008). Available from: http://novascotia.ca/aids/documents/Strategic%20Approach%20to%20Increase%20HIV%20Testing%202008.pdf
- 45.Veillette-Bourbeau L. Analyse de l’implantation d’une innovation en prévention du VIH: le dépistage rapide en milieu communautaire gai (Unpublished Dissertation). (2013). Available from: http://papyrus.bib.umontreal.ca/xmlui/handle/1866/9854
- 46.Vancouver STOP Project. HIV Screening in Dental Clinics: Programming Connection. (2013). Available from: http://www.catie.ca/en/pc/program/hiv-screening-dental-clinics
- 47.Options Clinic. Anonymous HIV Testing Program: Programming Connection. (2014). Available from: http://www.catie.ca/en/pc/program/anon