Abstract
Background: Health claims regarding the cholesterol-lowering effect of soluble fiber from oat products, approved by food standards agencies worldwide, are based on a diet containing ≥3 g/d of oat β-glucan (OBG). Given the number of recently published randomized controlled trials (RCTs), it is important to update the findings of previous meta-analyses.
Objective: The objective was to quantify the effect of ≥3 g OBG/d on serum cholesterol concentrations in humans and investigate potential effect modifiers.
Design: A meta-analysis was performed on 28 RCTs comparing ≥3 g OBG/d with an appropriate control. Systematic searches were undertaken in PubMed, AGRICOLA, and Scopus between 1 January 1966 and 6 June 2013, plus in-house study reports at CreaNutrition AG. Studies were assessed with regard to inclusion/exclusion criteria, and data were extracted from included studies by reviewers working independently in pairs, reconciling differences by consensus. Estimates of the mean reduction in serum cholesterol from baseline between the OBG and control diets were analyzed by using random-effects meta-analysis models and meta-regression.
Results: OBG in doses of ≥3 g/d reduced low-density lipoprotein (LDL) and total cholesterol relative to control by 0.25 mmol/L (95% CI: 0.20, 0.30; P < 0.0001) and 0.30 mmol/L (95% CI: 0.24, 0.35; P < 0.0001), respectively, with some indication of heterogeneity (P = 0.13 and P = 0.067). There was no significant effect of OBG on high-density lipoprotein (HDL) cholesterol or triglycerides and no evidence that dose (range across trials: 3.0–12.4 g/d) or duration of treatment (range: 2–12 wk) influenced the results. LDL cholesterol lowering was significantly greater with higher baseline LDL cholesterol. There was a significantly greater effect for both LDL and total cholesterol in subjects with diabetes compared with those without (although based on few studies).
Conclusions: Adding ≥3 g OBG/d to the diet reduces LDL and total cholesterol by 0.25 mmol/L and 0.30 mmol/L, respectively, without changing HDL cholesterol or triglycerides.
Keywords: β-glucan, HDL cholesterol, LDL cholesterol, total cholesterol, triglyceride, meta-analysis, oats
INTRODUCTION
European guidelines for the management of dyslipidemias (1) note the strong evidence supporting a relation between total and LDL cholesterol lowering and reduced cardiovascular disease risk and recommend that lifestyle modifications, such as consuming 5–15 g/d soluble fiber from oat products, may be beneficial. U.S. guidelines (2) recommend a multifaceted lifestyle approach, including dietary options for LDL cholesterol lowering, such as consuming 10–25 g/d soluble fiber from oat products and other foods.
Oat β-glucan (OBG)5, the main soluble fiber found in oats, is seen as the main active component responsible for their cholesterol-lowering effect. Health claims regarding the association between cholesterol lowering and soluble fiber from oat products/OBG have been approved by food standards agencies worldwide [United States: U.S. Food and Drug Administration (3); Canada: Health Canada (4); Europe: European Food Safety Authority (5); Australia and New Zealand: Food Standards Australia New Zealand (6); Malaysia: Ministry of Health Malaysia (7)]. Except for Malaysia, these approvals are based on a diet containing at least 3 g OBG/d, although specific conditions may vary; for example, the Food and Drug Administration (3) allows individual servings, including 0.75 g OBG, whereas the European Food Safety Authority (8) requires 1-g portions.
A meta-analysis of 12 randomized controlled trials (RCTs) published between 1985 and 1991 showed that oat products reduced total cholesterol by 0.13 mmol/L, with a higher intake of soluble fiber (≥3 compared with <3 g/d) having a significantly greater effect (9). A meta-analysis of 25 RCTs published between 1966 and 1996 showed that oat products reduced total and LDL cholesterol, respectively, by 0.040 and 0.037 mmol/L per gram of daily soluble fiber intake (10). Kelly et al. (11) included 8 RCTs published between 1991 and 2005 in a meta-analysis showing that oat products reduced total and LDL cholesterol, respectively, by 0.19 and 0.18 mmol/L.
However, an updated meta-analysis is timely and important for several reasons. The previous meta-analyses included studies with intakes of oat soluble fiber <3 g/d; thus, their results do not provide an accurate estimate of the effect on serum cholesterol of complying with the food standards agencies’ requirement that cholesterol-lowering claims for oats relate to daily intakes of ≥3 g oat soluble fiber. Also, we found 10 studies including ∼1200 subjects published since 2005, which more than doubles the amount of data now available for meta-analysis. Finally, previous meta-analyses did not consider the molecular weight (MW) of OBG, but this may be important because high MW may be necessary to obtain a significant cholesterol-lowering effect. Therefore, our primary objective was to quantify the effect of consuming ≥3 g high-MW OBG/d on serum LDL, HDL, and total cholesterol and triglyceride concentrations by using data from RCTs comparing OBG with a control treatment.
METHODS
Literature search and study selection
Three electronic databases (PubMed, www.ncbi.nlm.nih.gov/pubmed; AGRICOLA, agricola.nal.usda.gov; and Scopus, www.elsevier.com/online-tools/scopus) were searched for relevant published articles (papers and abstracts) between 1 January 1966 and 6 June 2013. The reference lists of articles found to be relevant were checked for additional articles of relevance. The in-house collection of study reports at CreaNutrition AG was searched as well (CreaNutrition AG is the Switzerland-based international marketing, sales, and research subsidiary of Swedish Oat Fiber AB). The keywords used for the search were as follows: 1) (oat fibre OR oat fiber OR oat bran OR oat β-glucan OR oats OR oat bran concentrate OR oatmeal) AND cholesterol and 2) (oat fibre OR oat fiber OR oat bran OR oat β-glucan OR oats OR oat bran concentrate OR oatmeal) AND serum lipids. Searches were limited to human studies and publications in English.
An initial screening was undertaken for potentially relevant studies by 2 reviewers (EJB and ST). Articles on studies testing OBG as a dietary intervention for lipid lowering were retrieved and allocated a unique paper identification number. All retrieved studies were assessed with regard to inclusion and exclusion criteria and study quality (Supplemental Tables 1 and 2). Trials investigating the effect of ≥3 g OBG/d (MW ≥100 kDa) for a minimum of 2 wk were to be included.
The increased intake of OBG could be achieved by consumption of a range of food products such as bread, muesli, breakfast cereals, cereal bars, biscuits, cereal drinks, muffins, and powders that contained OBG in 1, 2, or more eating occasions during the day. The control treatment could consist of a comparable food product (bread, muesli, breakfast cereals, cereal bars, biscuits, cereal drinks, muffins, or powders) without soluble fiber (i.e., low in fiber) or a comparable food product high in insoluble fiber. We selected 2 wk as the minimum intervention period in keeping with a previous meta-analysis (10), because the cholesterol-lowering effect is not immediate.
Because the MW of OBG influences its cholesterol-lowering effect (12), we wanted to exclude studies with low MW OBG, which would not be expected to have much effect at a dose of 3 g/d. A MW of 100 kDa was chosen because this represents more than 95% degradation of native OBG (MW ∼2000 kDa), and below 100 kDa OBG displays Newtonian behavior (no entanglements) and has a low viscosity at the relevant concentrations. Even though 3 g OBG/d with a MW of 210 kDa has been reported to have no significant effect on LDL cholesterol, because there is no standard procedure to extract and measure OBG MW, we wanted to err on the side of including studies that may not have used high enough MW rather than excluding those that did. However, studies did not have to report MW to be included. Studies not reporting MW could be included if one or more interventions were considered to contain high MW OBG, based on our knowledge of the effects of different food processing on β-glucan MW. Depolymerization is usually observed during production of OBG concentrates and extracts (13, 14). In general, the production of porridge, muesli, biscuits, cereal bars, and muffins does not greatly affect MW (15–17). Typical extrusion conditions are not severe enough to cause much degradation of β-glucan (12, 16, 17), but high shear can cause significant depolymerization (12).
The study populations included were generally healthy free-living normocholesterolemic or hypercholesterolemic adult men and women from the general population; subjects could be lean, overweight, or obese and could have type 2 diabetes. The quality criteria were based on Appendix H of the European Food Safety Authority guidance for the preparation and presentation of the application for authorization of a health claim (18). Assessors worked in pairs independently to complete assessments for the publications (EJB + ST, EJB + TMSW, ST + TMSW). They then discussed their results and reconciled differences by consensus.
Data extraction and statistical analysis
Assessors worked in pairs to extract data from all included studies into a data collection Excel spreadsheet (AW + EJB, ST, and TMSW). They then discussed their results and reconciled differences by consensus. The following information was extracted from each study whenever available: trial design (parallel group, crossover); number of subjects randomized per treatment arm; health status of study population (healthy, hyperlipidemic, diabetic); mean age; percentage of subjects who are male; details of OBG treatment and control treatment; type of control treatment (diet alone, cereal/low soluble fiber); daily dose of OBG (g/d total); background diet (standard diet or advice on weight maintenance, low fat/cholesterol lowering, energy restriction); treatment duration (wk); mean baseline LDL and total cholesterol (mmol/L); mean LDL, HDL, and total cholesterol and triglyceride concentrations with standard deviations of individual observations or standard errors of means, both at baseline and after treatment and, if available, for change from baseline for each treatment; and mean difference in the change from baseline between the OBG and the control diets with standard deviations of individual observations or standard errors of means.
For trials comparing one dose of OBG with a control treatment, an estimate of the mean difference in cholesterol between OBG and control and its variance was calculated. The difference in the change from baseline between OBG and control was calculated where possible; otherwise, the difference in the mean cholesterol between OBG and control at the end of the trial was used. For trials comparing more than one dose of OBG with a control treatment, estimates and variances were calculated for the differences between each OBG dose and control. Covariance terms were included for studies with more than one estimate of relative treatment effect (see Supplemental Methods). Treatment arms in which the OBG dose was <3 g/d were excluded from the analysis.
Fixed- and random-effects meta-analyses were performed with SAS Proc Mixed (version 9.2; SAS Institute) (19) by using approaches described by van Houwelingen et al. (20). The analyses were based on the study estimates of the relative treatment effect and their variances and covariances. We used χ2 statistics to test the null hypothesis of no treatment difference. Inferences about the effect of OBG were made from the random-effects meta-analyses. Heterogeneity between study estimates of relative treatment effect was tested by using Cochran's Q statistic (21) and quantified by the I2 statistic (22), which measures the proportion of between-trial variation in relative treatment effects that is due to heterogeneity. The threshold for statistical significance was P = 0.05.
Forest plots were used to display the relative treatment effect and its 95% CI for each trial and dose amount and for the overall fixed- and random-effects meta-analyses. In all plots, the area of the circle at the estimate of the relative treatment effect is proportional to the inverse variance of the estimate.
The effect of the following factors on the relative treatment effect was investigated by fitting meta-regression models: trial design, health status, mean age, percentage of subjects who were male, type of control treatment, daily dose of OBG, background diet, treatment duration, and mean baseline LDL and total cholesterol. The statistical significance of the regression coefficients was tested by using a χ2 test.
The effect of the quality of the studies in relation to randomization, blinding, and reporting of subject compliance on the relative treatment effects was also investigated by fitting meta-regressions. Answers to 6 of the quality assessment questions (Q9a: Was allocation to intervention random? Q9b: Was treatment allocation concealed? Q11: Were subjects blinded to intervention received? Q12: Were caregivers blinded to intervention given? Q13: Were outcome assessors blinded to intervention given? Q14: Was compliance of subjects with the intervention reported?) were used to create 5 covariates. Answers to questions Q11–Q14 were dichotomized as “yes” or anything else. A dichotomized score based on both Q9a and Q9b was created with “yes” to both questions or anything else. Funnel plots were presented to display the relationship between the study estimates of mean treatment difference and their precision for LDL and total cholesterol. Estimates were shaded according to the number of “yes” answers to the above 6 quality questions.
RESULTS
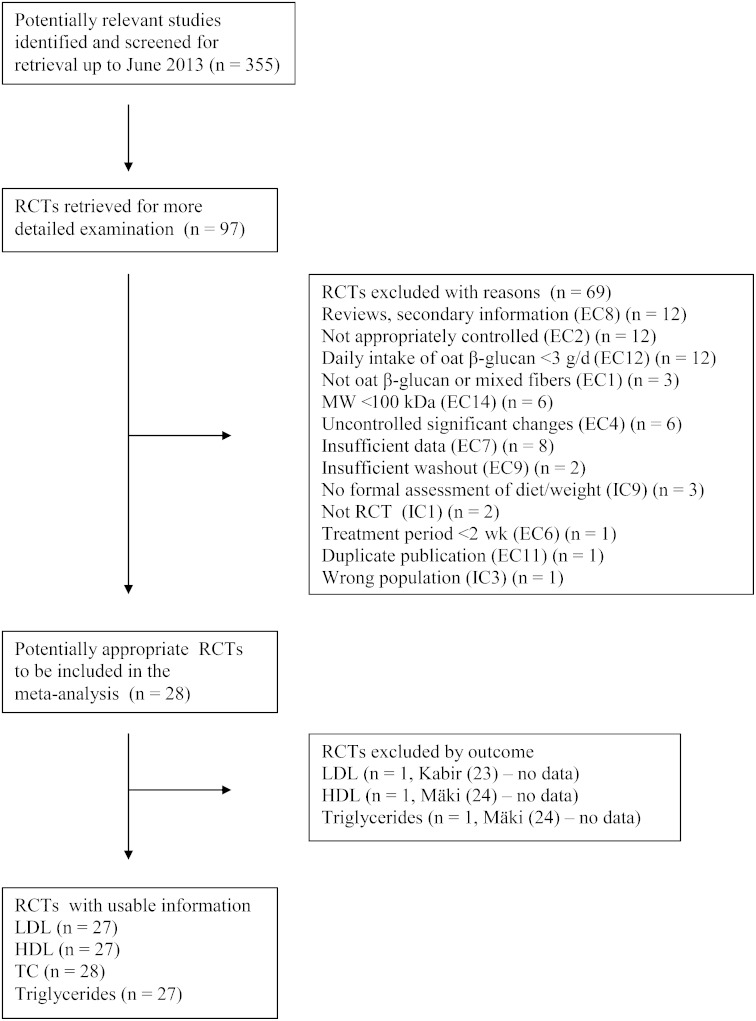
The initial screening yielded 355 publications. Based on titles and abstracts, 97 were reviewed for inclusion in the meta-analysis (Figure 1). Of these, 69 were excluded because they did not meet the inclusion and exclusion criteria. Twelve studies were excluded because the daily consumption of OBG was <3 g/d. These low-dose interventions had the potential to misrepresent the magnitude of the effect of products carrying health claims and the associated health benefit. Of the 97 studies reviewed, information on MW was reported or found in related publications for 14 studies reporting 20 comparisons (some articles included several doses of OBG). Of these 20, 6 comparisons were excluded on this basis. Available data for included studies are given in Table 1. Depolymerization is typically observed during production of bread because of its susceptibility to enzymes during fermentation (41). One study (44) used a combination of bread and muffins, and another (32) used bread alone, but no MW data were provided, so they were included.
FIGURE 1.
Flow diagram. EC2: not sufficiently or appropriately controlled: only baseline data provided and no control group during the treatment period or an inappropriate control group (e.g., another soluble fiber). EC4: any uncontrolled significant changes during the trial known to affect blood lipid concentration—for example, a significant difference in total fat or saturated fat intake between control and intervention groups, diets contain other soluble fibers than soluble nonstarch polysaccharide from oats, or uncontrolled significant body weight change (note: important only if interventions are affected differently). EC7: insufficient information to estimate the magnitude of the effect: no measure or estimate of soluble fiber or oat β-glucan intake or limited amount of information on the outcome measures. IC9: a formal assessment of diet and body weight changes during the trial. MW, molecular weight; RCT, randomized controlled trial; TC, total cholesterol.
TABLE 1.
Study characteristics1
| Study2 | No. of subjects randomized | Duration of treatment, wk | Dose of OBG, g/d | Health status | Treated group | MW of OBG, kDa | Type of control treatment | Background diet | Male, % | Mean age, y | Mean baseline LDL-C, mmol/L | Mean baseline TC, mmol/L |
| Parallel group | ||||||||||||
| Anderson (25) | 21 | 3 | 12.4 | H-chol | Oat bran | Wheat bran | Standard | 100 | 61.0 | 3.47 | 6.57 | |
| Beck 5–6 g (16) | 46 | 12 | 5.5 | Healthy | Oat bran | 1600–3000 | Refined wheat/rice | Energy restr | 0 | 37.4 | 3.04 | 5.10 |
| Beck 8–9 g (16) | 44 | 12 | 8.5 | Healthy | Oat bran | 1600–3000 | Refined wheat/rice | Energy restr | 0 | 37.3 | 2.93 | 5.05 |
| Berg (26) | 235 | 4 | 5.0 | H-chol | Diet + oat bran | Diet alone | Energy restr | 100 | 53.5 | 4.65 | 6.69 | |
| Charlton (17) | 64 | 6 | 3.2 | H-chol | Oat bran | 2100 | Refined wheat/rice/corn | Weight adv | 48 | 51.0 | 3.84 | 6.00 |
| Chen (27) | 110 | 12 | 7.3 | Healthy | Oat bran | Refined wheat/corn | Standard | 40 | 47.9 | 3.27 | 5.15 | |
| Davidson 3.6 g (28) | 44 | 6 | 3.6 | Healthy | Oatmeal | Farina | Low fat/chol | 54 | 51.9 | 4.97 | 6.96 | |
| Davidson 4 g (28) | 44 | 6 | 4.0 | Healthy | Oat bran | Farina | Low fat/chol | 71 | 52.8 | 4.84 | 6.84 | |
| Davidson 6 g (28) | 45 | 6 | 6.0 | Healthy | Oat bran | Farina | Low fat/chol | 62 | 54.1 | 4.74 | 6.81 | |
| Davy (29) | 36 | 12 | 5.5 | Healthy | Oatmeal + oat bran | Whole wheat | Standard | 100 | 62.5 | 3.44 | 5.09 | |
| Donazzolo (up) | 71 | 4 | 3.0 | H-chol | Oat bran | 900 | Wheat bran | Low fat/chol | 68 | 53.0 | 4.17 | 6.60 |
| Gerhardt (30) | 52 | 6 | 3.1 | H-chol | Oat bran | Rice starch | Standard | 52 | 51.7 | 5.13 | 7.22 | |
| Karmally (31) | 152 | 6 | 3.0 | H-chol | Oat flour | Corn flour | Low fat/chol | 32 | 49.0 | 3.55 | 5.27 | |
| Liatis (32) | 46 | 3 | 3.0 | Diabetic | Oat bran | Refined wheat flour | Standard | 56 | 63.0 | 4.13 | 6.09 | |
| Mäki (24) | 204 | 12 | 3.0 | Healthy | Oat flour | Refined wheat/corn | Energy restr | 22 | 48.9 | 4.01 | 5.57 | |
| Pins (33) | 88 | 12 | 5.4 | Healthy | Oatmeal + oat flour | Refined wheat | Standard | 51 | 47.6 | 3.58 | 5.50 | |
| Queenan (34) | 90 | 6 | 6.0 | H-chol | Oat bran | Dextrose | Standard | 33 | 44.9 | 4.15 | 6.20 | |
| Saltzman (35) | 43 | 6 | 6.7 | Healthy | Oatmeal | Mixed refined grains | Energy restr | 47 | 44.6 | 2.98 | 4.65 | |
| Uusitupa (36) | 41 | 8 | 10.3 | H-chol | Oat bran | Wheat bran | Low fat/chol | 56 | 47.8 | 5.05 | 7.14 | |
| Wolever 4L (12) | 150 | 4 | 4.0 | Healthy | Oat bran | 210 | Wheat bran | Standard | 39 | 52.4 | 3.82 | |
| Wolever 3M (12) | 151 | 4 | 3.0 | Healthy | Oat bran | 530 | Wheat bran | Standard | 42 | 52.0 | 3.85 | |
| Wolever 4M (12) | 154 | 4 | 4.0 | Healthy | Oat bran | 850 | Wheat bran | Standard | 45 | 52.0 | 3.82 | |
| Wolever 3H (12) | 173 | 4 | 3.0 | Healthy | Oat bran | 2210 | Wheat bran | Standard | 46 | 52.0 | 3.81 | |
| Zhang (37) | 182 | 6 | 3.3 | H-chol | Oatmeal | Wheat noodles | Weight adv | 39 | 53.2 | 4.24 | 6.18 | |
| Crossover | ||||||||||||
| Abrahamsson (38) | 31 | 5 | 7.6 | Healthy | Oat bran | Wheat bran | Standard | 0 | 26.6 | 2.90 | 4.51 | |
| Amundsen (39) | 20 | 3 | 5.1 | H-chol | Diet + oat bran | Diet alone | Low fat/chol | 56 | 57.0 | 5.15 | 7.65 | |
| Braaten (40) | 20 | 4 | 5.8 | H-chol | Diet + oat gum | ∼1000 | Diet alone | Standard | 45 | 54.1 | ||
| Kabir (23) | 13 | 4 | 3.0 | Diabetic | Oat bran | Wheat fiber | Weight adv | 100 | 58.4 | 5.20 | ||
| Kerckhoffs (41) | 26 | 2 | 5.0 | H-chol | Oat bran | 72% >250 | Wheat fiber | Standard | 40 | 53.4 | 3.99 | 6.00 |
| Kestin (42) | 24 | 4 | 11.8 | H-chol | Oat bran | Wheat bran | Standard | 100 | 46.0 | 4.55 | 6.34 | |
| Kristensen (43) | 24 | 2 | 7.6 | Healthy | Diet + oat bran | Diet alone | Weight adv | 25.2 | 2.70 | 4.03 | ||
| Pick (44) | 8 | 12 | 9.0 | Diabetic | Oat bran | Refined wheat | Weight adv | 100 | 45.5 | 4.65 | ||
| Theuwissen (45) | 43 | 4 | 5.0 | Healthy | Oat fiber | Wheat fiber | Standard | 48 | 52.4 | |||
| Whyte (46) | 24 | 4 | 9.5 | Healthy | Oat bran | Wheat fiber | Standard | 100 | 45.0 | 4.11 | 5.84 |
H-chol, hypercholesterolemic; LDL-C, LDL cholesterol; low fat/chol, low fat/cholesterol lowering; MW, molecular weight; OBG, oat β-glucan; restr, restricted; TC, total cholesterol; up, unpublished; Weight adv, weight maintenance advice.
For studies with more than one OBG arm, the number of subjects randomized equals those randomly allocated to the particular OBG arm plus those randomly allocated to the control arm. For Beck et al. (16), the 2 OBG doses were 5–6 and 8–9 g/d. For Davidson et al. (28), the 3 OBG doses were 84 g oatmeal (3.6 g/d), 56 g oat bran (4 g/d), and 84 g oat bran (6 g/d). For Wolever et al. (12), the 4 OBG doses were 4 g/d low MW (4L), 3 g/d medium MW (3M), 4 g/d medium MW (4M), and 3 g/d high-MW (3H).
The characteristics of the 27 published (12, 16, 17, 23–46) and 1 unpublished (Y. Donazzolo, M. Latreille-Barbier, C. Ruel, S. Layre, R. Alken, M. Macmahon, unpublished results, 2006) RCTs included in the meta-analysis are described in Table 1. Eighteen RCTs had a parallel group design and 10 a crossover design. The dose of OBG ranged from 3.0 to 12.4 g/d, and treatment duration ranged from 2 to 12 wk. Twelve studies recruited healthy subjects, 13 recruited subjects with hypercholesterolemia, and 3 recruited subjects with type 2 diabetes. The study quality scores are provided in Supplemental Table 3.
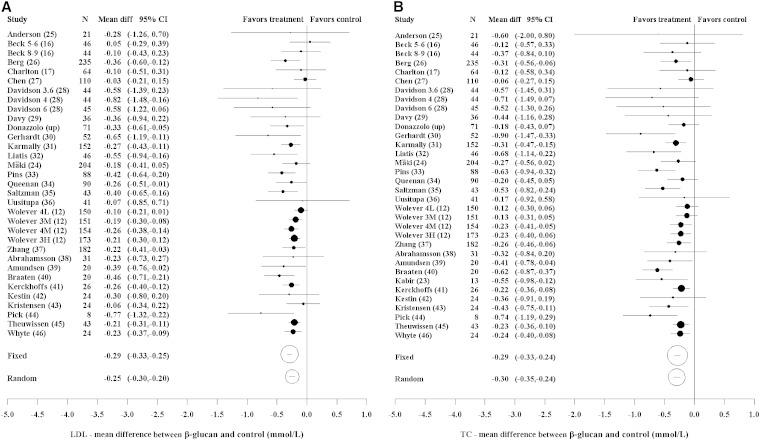
The effect of OBG relative to control on LDL cholesterol for all studies and dose amounts is shown in Figure 2A. There was some indication of heterogeneity among the studies, although this did not reach statistical significance (P = 0.13). The random-effects analysis showed a statistically significant difference for OBG relative to control of −0.25 mmol/L (95% CI: −0.30, −0.20; P < 0.0001). There was evidence of an increasing effect with baseline LDL cholesterol (P = 0.039) and a significant, increased effect for subjects with diabetes relative to healthy subjects (P = 0.013) (Supplemental Table 4 and Supplemental Figure 1). There was an indication of an increasing effect with baseline total cholesterol (P = 0.074), age (P = 0.080), percentage of subjects who were male (P = 0.054), and for hypercholesterolemic subjects relative to healthy subjects (P = 0.079), although effects did not reach statistical significance. No other factors showed a significant effect.
FIGURE 2.
Forest plots of estimates of mean differences (95% CIs). LDL cholesterol: heterogeneity (χ2, P = 0.13; I2 = 22%) (A). TC: heterogeneity (χ2, P = 0.067; I2 = 28%) (B). The area of the circle is proportional to the inverse variance of the estimate of mean difference. For studies with more than one OBG arm, the number of subjects randomized equals those randomly allocated to the particular OBG arm plus those to the control arm. For Beck et al. (16), the 2 OBG doses were 5–6 and 8–9 g/d. For Davidson et al. (28), the 3 OBG doses were 84 g oatmeal (3.6 g/d), 56 g oat bran (4 g/d), and 84 g oat bran (6 g/d). For Wolever et al. (12), the 4 OBG doses were 4 g/d low MW (4L), 3 g/d medium MW (3M), 4 g/d medium MW (4M), and 3 g/d high MW (3H). diff, difference; MW, molecular weight; OBG, oat β-glucan; TC, total cholesterol; up, unpublished.
The effect of OBG relative to control on total cholesterol for all studies and dose amounts is shown in Figure 2B. There was some indication of heterogeneity among the studies (P = 0.067). The random-effects meta-analysis showed a statistically significant difference for OBG relative to control of −0.30 mmol/L (95% CI: −0.35, −0.24; P < 0.0001). There was a significantly greater effect for subjects with type 2 diabetes relative to those without diabetes (P = 0.004) (Supplemental Table 4). No other factor showed a significant effect.
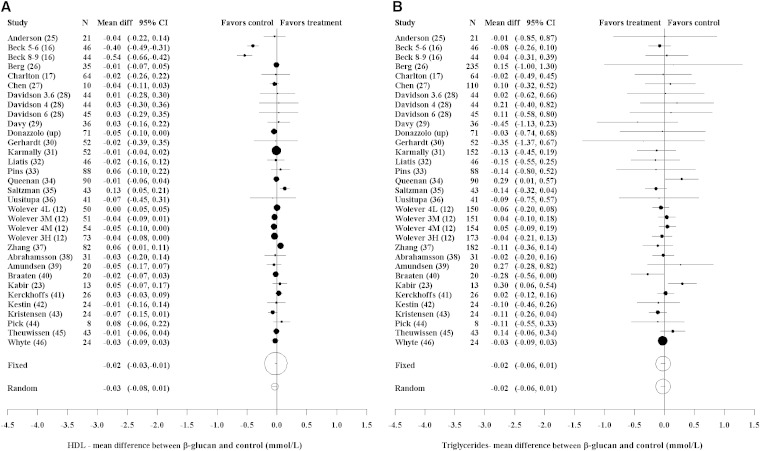
The effect of OBG relative to control on HDL cholesterol for all studies and dose amounts is shown in Figure 3A. There was significant evidence of heterogeneity among the studies (P < 0.001). The random-effects meta-analysis of the effect of OBG relative to control showed no significant difference (−0.035 mmol/L; 95% CI: −0.078, 0.008; P = 0.12). The results from the Beck et al. (16) study are quite different from the rest. In a post hoc analysis that omitted this study, there was little evidence of heterogeneity (P = 0.18). Excluding the Beck et al. study, the random-effects meta-analysis showed no significant difference between the OBG and control treatments (−0.007 mmol/L; 95% CI: −0.025, 0.010; P = 0.41).
FIGURE 3.
Forest plots of estimates of mean difference (95% CIs). HDL cholesterol: heterogeneity (χ2, P < 0.001; I2 = 81%) (A). Triglycerides: heterogeneity (χ2, P = 0.58; I2 = 0%) (B). The area of the circle is proportional to the inverse variance of the estimate of mean difference. For studies with more than one OBG arm, the number of subjects randomized equals those randomly allocated to the particular OBG arm plus those to the control arm. For Beck et al. (16), the 2 OBG doses were 5–6 and 8–9 g/d. For Davidson et al. (28), the 3 OBG doses were 84 g oatmeal (3.6 g/d), 56 g oat bran (4 g/d), and 84 g oat bran (6 g/d). For Wolever et al. (12), the 4 OBG doses were 4 g/d low MW (4L), 3 g/d medium MW (3M), 4 g/d medium MW (4M), and 3 g/d high MW (3H). diff, difference; MW, molecular weight; OBG, oat β-glucan; up, unpublished.
Because of the influence of the Beck et al. (16) study on the HDL cholesterol results, the LDL and total cholesterol meta-analyses were repeated without this study. Omitting the Beck et al. study had little effect on the results for LDL and total cholesterol.
OBG tended to reduce serum triglyceride concentrations slightly compared with control, but the difference was not significant (−0.023 mmol/L; 95% CI: −0.060, 0.015; P = 0.23) (Figure 3B). There was little evidence of heterogeneity (P = 0.58).
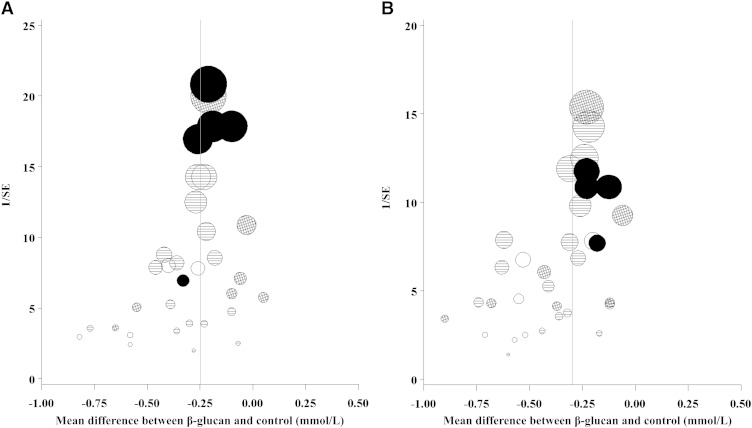
To assess the effect of study quality on the results, we undertook meta-regressions on each of the dichotomized quality questions (Q11–Q14), as well as a composite of Q9a and Q9b, for LDL and total cholesterol. These indicated a reduction in the OBG effect between studies that met the quality criterion compared with those that did not (Supplemental Table 4). However, these differences were not statistically significant apart from Q13. The effect of OBG was significantly less by 0.11 mmol/L (P = 0.024) on LDL cholesterol and 0.13 mmol/L (P = 0.021) on total cholesterol for trials in which blinding of outcome assessors was specifically mentioned relative to the other trials. Larger effects of OBG on total cholesterol were associated with studies of a lower precision (i.e., larger 95% CI), although this was less obvious for LDL cholesterol (Figure 4).
FIGURE 4.
Funnel plots of inverse SE compared with the estimate of mean difference for LDL cholesterol (A) and TC (B). Shading of circles indicates the number of 6 quality questions (Q9a, random sequence generation; Q9b, treatment allocation concealed randomization; Q11, blinding of subjects; Q12, blinding of caregivers; Q13, blinding of outcome assessors; and Q14, reporting of subject compliance) with “yes”: empty, 0; lines, 1–3; hashed, 4–5; and solid, 6. The area of a circle is proportional to the inverse variance of estimate of mean difference. Q, question; TC, total cholesterol.
DISCUSSION
We found that diets containing ≥3 g OBG/d reduce serum total and LDL cholesterol relative to control by 0.30 and 0.25 mmol/L, respectively, with no effect on HDL cholesterol or triglycerides. Although generally confirming the results of previous meta-analyses that oat products reduce serum cholesterol, the present results differ in that the magnitude of the effects seen are 50–100% greater than those reported in previous meta-analyses. This is important because our study provides a more accurate assessment of the effect on serum cholesterol of following the recommendations of food standards agencies to consume ≥3 g OBG/d than do previous meta-analyses that included studies in which subjects consumed <3 g OBG/d and studies in which OBG was more than 95% degraded.
We found no significant effect of dose or duration of treatment of the range of OBG doses (3.0–12.4 g/d) and durations of treatment (2–12 wk) used in the RCTs included in the meta-analysis. The former suggests that a cholesterol-lowering effect of oats can be achieved with the minimum dose (3 g/d) considered effective by regulatory agencies and that consuming more may not have any additional effect. The lack of dose response is curious and somewhat unexpected because a significant effect of dose was found in both previous meta-analyses that tested for it (9, 10). However, the previous meta-analyses included studies with intakes of OBG <3 g/d, and one (10) also assumed a linear dose-response relation. Also, the ability of our meta-analysis to detect an effect of dose may be limited because of unknown effects of unmeasured confounding variables, such as the source of oats or the nature of the food products used to deliver OBG, which may have affected the physicochemical properties and, hence, viscosity of OBG (47). It is believed that the cholesterol-lowering effect of OBG depends on its viscosity in the small intestine, which, in turn, is affected by the MW and amount of β-glucan in solution. The MW of β-glucan may be reduced by β-glucanase before being incorporated into food products by exposure to β-glucanase naturally present in foods to which it is added (e.g., wheat flour) (41) or by heat and pressure exerted on foods during processing. Viscosity is inversely related to log (MW). Wolever et al. (12) showed a reduced cholesterol-lowering effect of OBG with low MW (210 kDa) relative to a medium MW (530 kDa) or high MW (2210 kDa). We restricted our meta-analysis to studies including OBG with MW ≥100 kDa. However, because there is no standardized method for measuring the MW of β-glucan, this could have been a source of confounding in our analysis. The amount of β-glucan in solution in the small intestine depends on its ability to be solubilized and released from the food matrix (i.e., bioaccessibility); β-glucan solubility is known to be reduced by low water availability in a food and storage of hydrated β-glucan at cool temperatures. Because we had no way of assessing the bioaccessibility of the β-glucan in most studies included in our analysis, it also is a potential source of confounding.
The lack of effect of study duration on the results suggests that the effect of oats on serum cholesterol is durable, as found by Bazzano et al. (48), but none of the studies we included lasted for longer than 12 wk.
We found evidence that the LDL cholesterol-lowering effect of oats was greater in subjects with type 2 diabetes and subjects with higher baseline LDL cholesterol. Previous studies suggest that the cholesterol-lowering effect of OBG is greater in nonwhites than in whites (24, 31, 49); this, taken together with our results suggesting that OBG has a greater effect in subjects with type 2 diabetes, might indicate that the OBG reduces serum cholesterol via a mechanism or mechanisms related in some way to dysglycemia, insulin resistance, and/or insulin secretion. Although type 2 diabetes generally is not associated with increased LDL cholesterol, it is associated with increased secretion of VLDL particles, which, after interaction with lipoprotein-lipase, hepatic-lipase, and cholesterol-ester transfer protein, eventually are metabolized to become LDL particles. In addition, this suggests that those with increased risk for cardiovascular disease due to high cholesterol or diabetes will obtain at least as much, if not more, benefit from the cholesterol-lowering effect of oats as individuals without these risk factors; however, these conclusions are based on a limited number of studies and should be interpreted with caution.
The studies included a wide range of subjects, including healthy individuals and those with hypercholesterolemia and type 2 diabetes. The studies were conducted in Europe, North America, Asia, and Australia. A wide range of common food products were used to study the effect of β-glucan, including rolled oats, whole oat flour, oat bran, bread, muffins, muesli, breakfast cereals, cereal bars, and biscuits. Therefore, it would appear that the results are applicable to the general population and that benefits could be achieved eating regularly consumed foods.
The funnel plots indicated that the studies with a lower precision had a tendency to show a more beneficial effect of OBG on total cholesterol, although this effect was less obvious for LDL cholesterol. This is sometimes taken to infer that there is publication bias—that is, a number of small studies have been undertaken and not published because the effect is not statistically significant or is negative. However, there may be other reasons, such as larger studies being undertaken after promising results from small studies. As suggested by the meta-regressions on the quality questions, the funnel plots show some association between OBG effect and study quality, with lower study quality being associated with a larger effect. However, this might be explained by study quality improving over time.
We conclude that there is robust evidence that consuming oats or oat-containing food products containing at least 3 g OBG/d with MW ≥100 kDa reduces serum cholesterol in lean, overweight, or obese male and female adults without diabetes and those with type 2 diabetes. Directions for future research include the need for high-quality studies to determine whether there is a dose-response effect of OBG and whether the effect persists in the long term (i.e., longer than 3 mo). Because the purported mechanisms of cholesterol lowering require a significant viscosity of the gastrointestinal contents, and this is supported by recent work (12, 41), future studies need to consider the physicochemical properties of β-glucan in food products targeted at lowering total and LDL cholesterol to ensure the intended physiologic effects.
Supplementary Material
Acknowledgments
We thank Ruedi Duss (previously, CreaNutrition AG; currently, DSM Nutritional Products) for providing in-house study reports and information regarding oat β-glucan and helpful discussions.
The authors’ responsibilities were as follows—AW, EJB, ST, and TMSW: contributed to the study concept and design; EJB, ST, and TMSW: reviewed the literature, screened the records, assessed the quality of studies, and extracted data; and AW: supervised the study, performed the statistical analysis, and wrote the manuscript. All authors read and approved the final version of the manuscript. AW's institution received funding from DSM Nutritional Products for her participation in the study. EJB has acted as a consultant for the Grains and Legumes Nutrition Council. ST's institution has received funds to support her research activities outside the submitted work from CreaNutrition and Pepsico. TMSW is president and part owner of Glycemic Index Laboratories Inc., a contract research organization from which he receives payment as medical director, management board member, and principal investigator. TMSW has a patent for Solid Oral Diagnostic Test Meal and Methods of Use Thereof licensed to Ceapro Inc. and has received consultancy fees from Bunge Inc. for activities outside the submitted work. The authors had no other relevant financial conflicts of interest to report.
Footnotes
Abbreviations used: MW, molecular weight; OBG, oat β-glucan; RCT, randomized controlled trial.
REFERENCES
- 1.The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2011;32:1769–818. [DOI] [PubMed] [Google Scholar]
- 2.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection. JAMA 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration (FDA). Health claims: soluble fiber from certain foods and the risk of coronary heart disease (CHD). Code of Federal Regulations Title 2; Section 101.81 [Internet]. Silver Spring (MD): FDA. 1997 [updated 2014 Sep 1; cited 2013 Nov 14]. Available from: www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=101.81.
- 4.Bureau of Nutritional Sciences, Food Directorate, Health Products and Food Branch, Health Canada. Oat products and blood cholesterol lowering: summary of assessment of a health claim about oat products and blood cholesterol lowering [Internet]. Ottawa, Ontario: Health Canada. 2010 [cited 2013 Nov 14]. Available from: www.hc-sc.gc.ca/fn-an/label-etiquet/claims-reclam/assess-evalu/oat-avoine-eng.php.
- 5.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the substantiation of a health claim related to oat beta-glucan and lowering blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J 2010;8:1885– 1900. [Google Scholar]
- 6.Food Standards Australia New Zealand. Standard 1.2.7: Nutrition, health and related claims. Food Standards Gazette [Internet]. 2013;80. Available from: www.foodstandards.gov.au/code/changes/gazette/documents/gazette_138.pdf.
- 7.Ministry of Health Malaysia. Malaysian dietary guidelines—Key Message 14—make effective use of nutrition information on food labels [Internet]. Putrajaya (Malaysia): Ministry of Health Malaysia. 2010 [cited 2013 Nov 14]. Available from: www.moh.gov.my/images/gallery/Garispanduan/diet/km14.pdf.
- 8.Commission Regulation (EU) No 1160/2011 of 14 November 2011 on the authorisation and refusal of authorisation of certain health claims made on foods and referring to the reduction of disease risk [Internet]. : Brussels (Belgium): Eur-Lex. 2011 [cited 2013 Nov 14]. Available from: http://eur-lex.europa.eu/JOHtml.do?uri=OJ:L:2011:296:SOM:EN:HTML.
- 9.Ripsin CM, Keenan JM, Jacobs DR, Jr, Elmer PJ, Welch RR, Van Horn L, Liu K, Turnbull WH, Thye FW, Kestin M, et al. Oat products and lipid lowering: a meta-analysis. JAMA 1992;267:3317–25. Erratum in: JAMA 1992;268:3074. [PubMed] [Google Scholar]
- 10.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999;69:30–42. [DOI] [PubMed] [Google Scholar]
- 11.Kelly SAM, Summerbell CD, Byrnes A, Whittaker V, Frost G. Wholegrain cereals for coronary heart disease. Cochrane Database Syst Rev 2007;(2):CD005051. [DOI] [PubMed] [Google Scholar]
- 12.Wolever TM, Tosh SM, Gibbs AL, Brand-Miller J, Duncan AM, Hart V, Lamarche B, Thomson BA, Duss R, Wood PJ. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: a randomized clinical trial. Am J Clin Nutr 2010;92:723–32. [DOI] [PubMed] [Google Scholar]
- 13.Biörklund M, van Rees A, Mensink RP, Önning G. Changes in serum lipids and postprandial glucose and insulin concentrations after consumption of beverages with β-glucans from oats or barley: a randomised dose-controlled trial. Eur J Clin Nutr 2005;59:1272–81. [DOI] [PubMed] [Google Scholar]
- 14.Lazaridou A, Biliaderis CG, Micha-Screttas M, Steele BR. A comparative study on structure-function relations of mixed-linkage (1→3), (1→4) linear β-D-glucans. Food Hydrocoll 2004;18:837–55. [Google Scholar]
- 15.Åman P, Rimsten L, Andersson R. Molecular weight distribution of β-glucan in oat-based foods. Cereal Chem 2004;81:356–60. [Google Scholar]
- 16.Beck EJ, Tapsell LC, Batterham MJ, Tosh SM, Huang XF. Oat beta-glucan supplementation does not enhance the effectiveness of an energy-restricted diet in overweight women. Br J Nutr 2010;103:1212–22. [DOI] [PubMed] [Google Scholar]
- 17.Charlton KE, Tapsell LC, Batterham MJ, O'Shea J, Thorne R, Beck E, Tosh SM. Effect of 6 weeks’ consumption of β-glucan-rich oat products on cholesterol levels in mildly hypercholesterolaemic overweight adults. Br J Nutr 2012;107:1037–47. [DOI] [PubMed] [Google Scholar]
- 18.European Food Safety Authority. Opinion of the panel on dietetic products, nutrition and allergies (NDA) on a request from the commission related to scientific and technical guidance for the preparation and presentation of the application for authorisation of a health claim. EFSA J 2007;5:530–574. [Google Scholar]
- 19.SAS Institute. The Mixed procedure. In: SAS/STAT 9.2 User's Guide. Cary (NC): SAS Institute; 2008. [Google Scholar]
- 20.van Houwelingen HC, Arends LR, Stijnen T. Tutorial in biostatistics: advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589–624. [DOI] [PubMed] [Google Scholar]
- 21.Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–29. [Google Scholar]
- 22.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 23.Kabir M, Oppert JM, Vidal H, Bruzzo F, Fiquet C, Wursch P, Slama G, Rizkalla SW. Four-week low-glycemic index breakfast with a modest amount of soluble fibers in type 2 diabetic men. Metabolism 2002;51:819–26. [DOI] [PubMed] [Google Scholar]
- 24.Mäki KC, Beiseigel JM, Jonnalagadda SS, Gugger CK, Reeves MS, Farmer MV, Kaden VN, Rains TM. Whole-grain ready-to-eat oat cereal, as part of a dietary program for weight loss, reduces low-density lipoprotein cholesterol in adults with overweight and obesity more than a dietary program including low-fiber control foods. J Am Diet Assoc 2010;110:205–14. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JW, Gilinsky NH, Deakins DA, Smith SF, Spencer O'Neal D, Dillon DW, Oeltgen PR. Lipid responses of hypercholesterolemic men to oat-bran and wheat-bran intake. Am J Clin Nutr 1991;54:678–83. [DOI] [PubMed] [Google Scholar]
- 26.Berg A, König D, Deibert P, Grathwohl D, Berg A, Baumstark MW, Franz I-W. Effect of an oat bran enriched diet on the atherogenic lipid profile in patients with an increased coronary heart disease risk: a controlled randomized lifestyle intervention study. Ann Nutr Metab 2003;47:306–11. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, He J, Wildman RP, Reynolds K, Streiffer RH, Whelton PK. A randomized controlled trial of dietary fiber intake on serum lipids. Eur J Clin Nutr 2006;60:62–8. [DOI] [PubMed] [Google Scholar]
- 28.Davidson MH, Dugan LD, Burns JH, Bova J, Story K, Drennan KB. The hypocholesterolemic effects of beta-glucan in oatmeal and oat bran: a dose-controlled study. JAMA 1991;265:1833–9. [PubMed] [Google Scholar]
- 29.Davy BM, Davy KP, Ho RC, Beske SD, Davrath LR, Melby CL. High-fiber oat cereal compared with wheat cereal consumption favorably alters LDL-cholesterol subclass and particle numbers in middle-aged and older men. Am J Clin Nutr 2002;76:351–8. [DOI] [PubMed] [Google Scholar]
- 30.Gerhardt AL, Gallo NB. Full-fat rice bran and oat bran similarly reduce hypercholesterolemia in humans. J Nutr 1998;128:865–9. [DOI] [PubMed] [Google Scholar]
- 31.Karmally W, Montez MG, Palmas W, Martinez W, Branstetter A, Ramakrishnan R, Holleran SF, Haffner SM, Ginsberg HN. Cholesterol-lowering benefits of oat-containing cereal in Hispanic Americans. J Am Diet Assoc 2005;105:967–70. [DOI] [PubMed] [Google Scholar]
- 32.Liatis S, Tsapogas P, Chala E, Dimosthenopoulos C, Kyriakopolous K, Kapantais E, Katsilambros N. The consumption of bread enriched with betaglucan reduces LDL-cholesterol and improves insulin resistance in patients with type 2 diabetes. Diabete Metab 2009;35:115–20. [DOI] [PubMed] [Google Scholar]
- 33.Pins JJ, Geleva D, Keenan JM, Frazel C, O'Connor PJ, Cherney LM. Do whole-grain oat cereals reduce the need for antihypertensive medications and improve blood pressure control? J Fam Pract 2002;51:353–9. [PubMed] [Google Scholar]
- 34.Queenan KM, Stewart ML, Smith KN, Thomas W, Fulcher RG, Slavin JL. Concentrated oat, a fermentable fiber, lowers serum cholesterol in hypercholesterolemic adults in a randomized controlled trial. Nutr J 2007;6:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saltzman E, Das SK, Lichtenstein AH, Dallal GE, Corrales A, Schaefer EJ, Greenberg AS, Roberts SB. An oat-containing hypocaloric diet reduces systolic blood pressure and improves lipid profile beyond effects of weight loss in men and women. J Nutr 2001;131:1465–70. [DOI] [PubMed] [Google Scholar]
- 36.Uusitupa MI, Ruuskanen E, Mäkinen E, Laitinen J, Toskala E, Kervinen K, Kesäniemi YA. A controlled study on the effect of beta-glucan-rich oat bran on serum lipids in hypercholesterolemic subjects: relation to apolipoprotein E phenotype. J Am Coll Nutr 1992;11:651–9. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Li L, Song P, Wang C, Man Q, Meng L, Cai J, Kurilich A. Randomized controlled trial of oatmeal consumption versus noodle consumption on blood lipids of urban Chinese adults with hypercholesterolemia. Nutr J 2012;11:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrahamsson L, Göranzon H, Karlström B, Vessby B, Åman P. Metabolic effects of oat bran and wheat bran in healthy women. Scand J Nutr 1994;38:5–10. [Google Scholar]
- 39.Amundsen ÅL, Haugum B, Andersson H. Changes in serum cholesterol and sterol metabolites after intake of products enriched with an oat bran concentrate within a controlled diet. Scand J Nutr 2003;47:68–74. [Google Scholar]
- 40.Braaten JT, Wood PJ, Scott FW, Wolynetz MS, Lowe MK, Bradley-White P, Collins MW. Oat beta-glucan reduces blood cholesterol concentration in hypercholesterolemic subjects. Eur J Clin Nutr 1994;48:465–74. [PubMed] [Google Scholar]
- 41.Kerckhoffs DA, Hornstra G, Mensink RP. Cholesterol-lowering effect of beta-glucan from oat bran in mildly hypercholesterolemic subjects may decrease when beta-glucan is incorporated into bread and cookies. Am J Clin Nutr 2003;78:221–7. [DOI] [PubMed] [Google Scholar]
- 42.Kestin M, Moss R, Clifton PM, Nestel PJ. Comparative effects of three cereal brans on plasma lipids, blood pressure, and glucose metabolism in mildly hypercholesterolemic men. Am J Clin Nutr 1990;52:661–6. [DOI] [PubMed] [Google Scholar]
- 43.Kristensen M, Bügel S. A diet rich in oat bran improves blood lipids and hemostatic factors, and reduces apparent energy digestibility in young healthy volunteers. Eur J Clin Nutr 2011;65:1053–8. [DOI] [PubMed] [Google Scholar]
- 44.Pick ME, Hawrysh ZJ, Gee MI, Toth E, Garg ML, Hardin RT. Oat bran concentrate bread products improve long-term control of diabetes: a pilot study. J Am Diet Assoc 1996;96:1254–61. [DOI] [PubMed] [Google Scholar]
- 45.Theuwissen E, Mensink RP. Simultaneous intake of beta-glucan and plant stanol esters affects lipid metabolism in slightly hypercholesterolemic subjects. J Nutr 2007;137:583–8. [DOI] [PubMed] [Google Scholar]
- 46.Whyte JL, McArthur R, Topping D, Nestel P. Oat bran lowers plasma cholesterol levels in mildly hypercholesterolemic men. J Am Diet Assoc 1992;92:446–9. [PubMed] [Google Scholar]
- 47.Tosh SM, Brummer Y, Miller SS, Regand A, Defelice C, Duss R, Wolever TMS, Wood PJ. Processing affects the physicochemical properties of β-glucan in oat bran cereal. J Agric Food Chem 2010;58:7723–30. [DOI] [PubMed] [Google Scholar]
- 48.Bazzano LA, He J, Ogden LG, Loria CM, Whelton PK. Dietary fiber intake and reduced risk of coronary heart disease in US men and women: the National Health and Nutrition Examination Survey I epidemiologic follow-up study. Arch Intern Med 2003;163:1897–904. [DOI] [PubMed] [Google Scholar]
- 49.Wolever TMS, Gibbs AL, Brand-Miller J, Duncan AM, Hart V, Lamarche B, Tosh SM, Duss R. Bioactive oat β-glucan reduces LDL cholesterol in Caucasians and non-Caucasians. Nutr J 2011;10:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.