ABSTRACT
All cells sense and integrate mechanical and biochemical cues from their environment to orchestrate organismal development and maintain tissue homeostasis. Mechanotransduction is the evolutionarily conserved process whereby mechanical force is translated into biochemical signals that can influence cell differentiation, survival, proliferation and migration to change tissue behavior. Not surprisingly, disease develops if these mechanical cues are abnormal or are misinterpreted by the cells – for example, when interstitial pressure or compression force aberrantly increases, or the extracellular matrix (ECM) abnormally stiffens. Disease might also develop if the ability of cells to regulate their contractility becomes corrupted. Consistently, disease states, such as cardiovascular disease, fibrosis and cancer, are characterized by dramatic changes in cell and tissue mechanics, and dysregulation of forces at the cell and tissue level can activate mechanosignaling to compromise tissue integrity and function, and promote disease progression. In this Commentary, we discuss the impact of cell and tissue mechanics on tissue homeostasis and disease, focusing on their role in brain development, homeostasis and neural degeneration, as well as in brain cancer.
KEY WORDS: Glioma, Mechanobiology, Mechanotransduction, Microenvironment, Neurodegeneration
Summary: In this Commentary, the roles of cell and tissue mechanics in brain development, homeostasis and neural degeneration, as well as in brain cancer, are reviewed.
Introduction
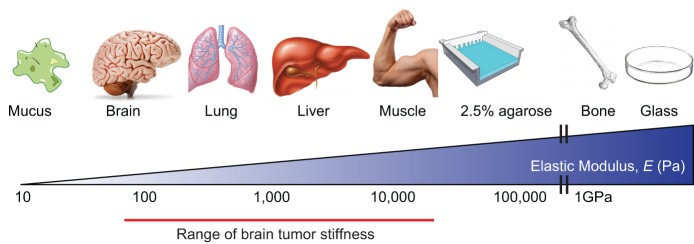
Differentiated tissues exhibit distinct mechanical properties (Fig. 1), which direct their structure and function and reflect the composition and architecture of the extracellular matrix (ECM), the cytoskeleton and tension of the cellular constituents, as well as the fluid dynamics and overall organization of the tissue. In each case, the intrinsic mechanical properties of a tissue impose structural integrity and are also important for tissue function. Cells sense and respond to the mechanical properties of their surrounding tissues through a process termed mechanotransduction that, once activated, regulates tissue-specific differentiation, orchestrates development and maintains tissue homeostasis. Reflecting the specialized cellular constituents and ECM composition and organization, the brain has unique mechanical properties that play a crucial role in neural stem cell behavior, tissue development and homeostasis (Tyler, 2012). Not surprisingly, diseases, such as neurodegeneration and brain cancer (Fig. 1), are accompanied by dramatic changes in the ECM and cellular components that alter the tissues' tensional homeostasis, and contribute to the altered pathology of the tissue (Bonneh-Barkay and Wiley, 2009).
Fig. 1.
Mechanical properties of tissues. Young's, or elastic, modulus (E) describes the amount of force required to deform a substance, with units of force/area (N/m2) or Pascals. E of tissues and cells can be quantified, revealing their relative stiffness. All tissues have distinct intrinsic physical properties, which are important in their structure and function. The stiffest tissues of the body are tooth and bone (E≥109 Pa), muscle tissue is intermediate (E≥104 Pa), and among the softest are lung and brain (E≤4×102 Pa). For reference, a 2.5% agarose gel is approximately 35 kPa, whereas a tissue culture glass is off the scale, in the gigapascal range.
A recent surge of interest in tissue mechanics and mechanotransduction – collectively termed mechanobiology – has emerged from the successful collaborative efforts of physical and life scientists, shedding light on the role that mechanical force plays in embryogenesis, tissue homeostasis and disease. In this Commentary, we review an emerging area in mechanobiology – the regulation of neuronal development, neural stem cell function and differentiation and disease by brain tissue mechanics. We begin with a brief overview of the composition and architecture of the normal brain ECM, then step back to discuss the role of mechanics in brain development and adult stem cell biology, and conclude with a perspective on altered mechanics in the development of glioma and neurodegenerative disease.
Mechanotransduction
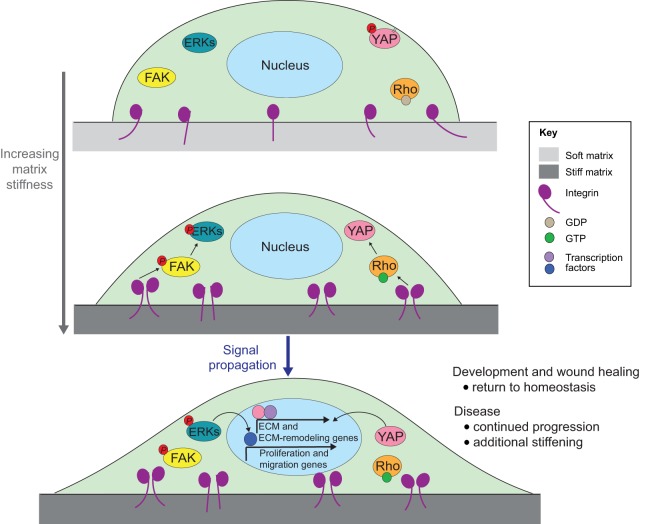
Cells are constantly subjected to physical forces from their microenvironment, and mechanics play an indispensable role in cell phenotype and behavior. Cells continuously monitor their microenvironment in order to respond appropriately to changes in extracellular parameters, such as temperature, oxygen content and nutrient availability. Likewise, cells sense and modify their behavior in response to physical cues, such as osmotic pressure, shear force, compression loading and substrate properties (such as ECM architecture and rigidity) through a process termed mechanotransduction. To transform mechanical information into biochemical signals, cells must be able to detect a force differential through molecular sensors, and then amplify and propagate this mechanical signal to elicit a change in cell behavior (Fig. 2), using processes that are conserved from bacteria to mammals.
Fig. 2.
Example of mechanoreciprocity. In this example (there are many molecular sensors, amplifiers and effectors of mechanics), an adherent cell senses an increase in ECM stiffness through integrins. This leads to an increase in focal adhesion formation and activation of focal adhesion kinase (FAK), which propagates the signal to mitogen-activated kinases, such as extracellular signal-regulated kinase (ERK), and the small GTPase Rho. In response to Rho activation, actomyosin contractility is elevated, causing the cell to become more spread and tightly adhered to its matrix. Additionally, transcription factors such as Yes-associated protein (YAP) are mechanically activated through Rho (Dupont et al., 2011), which induce the expression of ECM and ECM-modifying genes. Signaling downstream of ERK also results in transcriptional activation of proliferation and migration genes. In a physiological context, such as gastrulation or wound healing, this process is eventually resolved. In disease states, such as cancer, this cascade remains active, driving a vicious cycle of matrix stiffening and mechanosignaling, thereby contributing to disease progression.
Stretch-activated ion channels, which appeared early during evolution (Martinac and Kloda, 2003), are the first mechanosensitive molecules to be described and are indispensable across phyla. In bacteria, these mechanosensitive channels (MSC-L and MSC-S) permit ion flux between the cytoplasm and extracellular environment in response to membrane stretching, thereby regulating osmotic homeostasis and cell growth (Kung et al., 2010; Lew et al., 2008; Iida et al., 1994). In mammals, neuronal mechanosensitive transient receptor potential (TRP) ion channels and members of the degenerin/epithelial sodium channel (DEG/ENaC) superfamily play an important role in hearing through detection of sound waves and touch through pressure sensation (Orr et al., 2006; Christensen and Corey, 2007). Downstream of mechanically activated ion channel gating, second messengers, such as kinases and small GTPases, become activated, which propagate these signals to effectors that ultimately modify cell behaviors such as migratory behavior (Ranade et al., 2015). Another type of mechanosensitive molecule that can transduce and amplify physical cues is the integrin family of transmembrane receptors, which are able to bind to the ECM extracellularly and nucleate intercellular adhesion machinery. By sensing cell deformation caused by shear stress, integrins regulate the homeostasis of endothelial cells. Similar to the scenarios discussed below regarding abnormal forces in cancer progression, abnormal shear stress in the vasculature can lead to atherosclerosis in a mechanically dependent fashion (Katsumi et al., 2004; Tzima et al., 2002, 2001).
Many of the signals triggered by mechanical cues also activate feedback mechanisms that ‘hardwire’ phenotypes at the subcellular, cellular and tissue levels. For instance, cells sense increased substrate stiffness through integrins, which can induce the assembly of focal adhesions. Maturation of focal adhesions and integrin clustering can cause activation of focal adhesion kinase (FAK), and adhesion plaque scaffolding proteins can activate the Rho-associated protein kinase (ROCK) cascade to enhance cellular tension through engagement of actomyosin contractility (Humphrey et al., 2014; DuFort et al., 2011). These proteins can go on to activate downstream signals, including mitogen-activated kinases such as extracellular-signal-regulated kinases (ERKs) and the Hippo pathway protein YAP (also known as YAP1) (Fig. 2). When chronically activated, elevated cellular tension reinforces these downstream signaling pathways to potentiate a ‘mechano-circuit’, which can lead to the production of ECM and ECM-remodeling proteins that stiffen the local microenvironment and reinforce mechanosignaling (Samuel et al., 2011). This reciprocal feedback between tissue mechanics and cellular mechanosignaling circuits, referred to as mechanoreciprocity, is fundamental to development, to maintaining tissue homeostasis and to resolving wound healing (Duscher et al., 2014; DuFort et al., 2011). Owing to the elastic nature of the brain and its confinement in the skull, small changes in ECM properties or extracellular fluid pressure in disease states can lead to marked tissue stiffening and compression, resulting in a corruption of the fine-tuned mechano-circuitry. The concept of mechanoreciprocity is illustrated in Fig. 2 and will be a theme in all sections of this Commentary. The illustrated pathways are active in many different cell types and are not specific to neural cells. Although the unique architecture of neurons might result in differences in how mechanical signals are sensed and responded to throughout the cell body – e.g. in the axon verses the soma – such subcellular differences in mechanosensing remain to be characterized in mechanistic detail.
The extracellular matrix and mechanical properties of the normal brain
The adult brain ECM, which occupies an estimated 20% of the organ, is unique in that it is almost entirely composed of glycosaminoglycans (GAGs), including hyaluronic acid, proteoglycans such as lecticans and glycoproteins such as tenascin (Zimmermann and Dours-Zimmermann, 2008; Nicholson and Sykova, 1998; Ruoslahti, 1996). By contrast, the majority of peripheral soft tissues are structurally supported by a network of fibrous proteins, such as fibrillar collagens, and basement membranes, including laminins and non-fibrillar collagens (these types of proteins are restricted to the vasculature and meninges in the brain). The non-fibrillar nature of the ECM components of the brain contribute to the relatively low elastic modulus and high compliance of this organ. Our current understanding of the elastic properties of the brain has been well described previously (Franze et al., 2013) and, as discussed throughout this Commentary, these properties change dramatically during neuronal malignancies (Fig. 1). The viscoelastic nature of the brain contributes to its unique mechanical properties, and different ECM compartments of the brain (Cowman et al., 2015), neuronal cell types and even intercellular compartments could vary greatly in viscoelastic parameters (Lu et al., 2006). Because these differences are just beginning to be understood, and often in the context of repair after traumatic injury (MacManus et al., 2016; Li et al., 2016; Johnson et al., 2016), we do not address viscoelastic properties in great detail in this review.
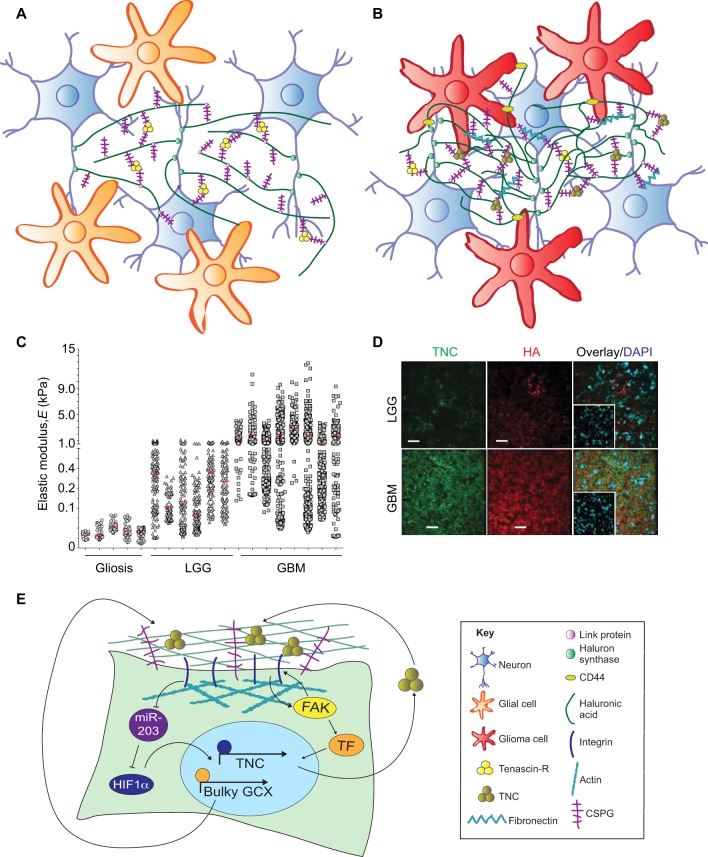
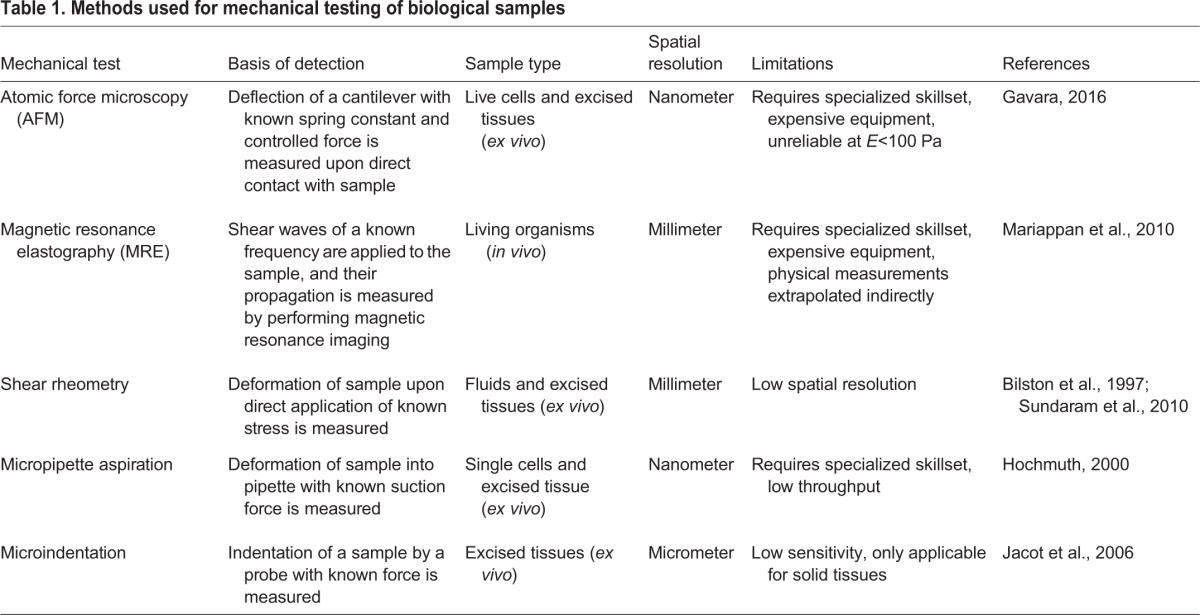
One of the best described functional units of the brain ECM is the perineuronal net (PNN), which serves as a structural scaffold to maintain the integrity of adult neuronal wiring and control of plasticity. These elegant mesh-like structures have been thoroughly reviewed previously (Soleman et al., 2013; Mouw et al., 2014; Kwok et al., 2011). Briefly, long chains of hyaluronic acid project perpendicularly from the neuronal cell membrane at sites where hyaluronan synthases are located (HAS1–HAS3 in mammals) to form the bulk of the net (Fig. 3A). Hyaluronic acid chains are bound along their axis by one end of a lectican (a member of the chondroitin sulfate proteoglycan family, including aggrecan, brevican, neurocan and versican), which are cross-linked to neighboring lecticans at their other end through the glycoprotein tenascin, most often tenascin-R (TNR; Carulli et al., 2006; Zimmermann and Dours-Zimmermann, 2008; Spicer et al., 2003). Alterations within (or mimicry of) PNNs occur in neuronal malignancies, leading to changes in brain tissue mechanics and increased activity of mechanosignaling pathways (Fig. 3B), as discussed below. To fully understand the dysfunctions inherent to glioma and neurodegenerative disorders, it is important to discuss the mechanical properties of tissues (as measured using techniques outlined in Table 1) and the mechanically regulated signals involved in brain development and neural stem cell differentiation, which is addressed in the following section.
Fig. 3.
Tissue mechanics drive glioma aggression. (A) Diagram showing perineuronal nets of the normal brain and (B) the perturbed matrix in the context of glioma. (C) Human glioma samples have been mechanically analyzed by using AFM. Lower-grade gliomas (LGGs) and glioblastomas (GBMs) are progressively stiffer when compared to non-tumor gliotic brain tissue. The red vertical lines indicate the mean elastic modulus, ‘E’, for each sample. (D) Human LGG and GBM sections stained for tenascin C (TNC) and hyaluronic acid (HA) reveal that both factors are elevated in GBMs. Scale bars: 50 µm. Panels C and D are reproduced from Miroshnikova et al., 2016 with permission. (E) Diagram summarizing signaling pathways involved in translating extracellular mechanical and integrin-based signals into cellular responses in the context of glioma progression. FAK, focal adhesion kinase; GCX, glycocalyx genes; HIF1α, hypoxia-inducible factor-1α; TF, transcription factor; TNC, tenascin C.
Table 1.
Methods used for mechanical testing of biological samples
Mechanical signaling in early neuronal development and neural stem cell biology
Embryonic development requires precise patterning of cells and tissues, which is driven by coordinated signals that include physical and chemical cues. This is abundantly true in brain development, from formation of the ectoderm germ layer during gastrulation to neural crest formation, neural vesicle development and brain maturation. The role of force in embryonic development has been reviewed extensively (Heisenberg and Bellaiche, 2013; Farge, 2011). Nevertheless, much remains to be defined in terms of how forces synchronize with soluble growth factors and morphogens to control cell behavior, particularly in the context of the brain. Although we can gain considerable insight into these processes using animal models of development, a more tractable approach to interrogate the underlying molecular mechanisms involves performing experiments with primary cells from the brain using culture models with defined mechanical properties. In this section, we will review what is known about how mechanical forces and mechanosensitive molecules drive brain development, focusing on vertebrate organisms. We then discuss how defining in vivo mechanical niches combined with stem cell mechanobiology studies have crucially contributed to our understanding of how neural cell types sense and respond to mechanical cues.
Mechanical forces guide brain development
During gastrulation, the dynamic orchestration of cell differentiation and migration causes the physical reorganization of a single sheet of embryonic cells into three distinct tissue, or germ, layers – ectoderm, mesoderm and endoderm (Solnica-Krezel and Sepich, 2012). Organogenesis proceeds after gastrulation, when cells within the three germ layers are further compartmentalized and differentiate to form primitive tissues, then functional organs. Formation of the nervous system (neurulation) is initiated by the migration of cells within the neural plate, an ectodermal layer, giving rise to the neural crest (Mayor and Theveneau, 2013). This U-shaped tissue layer is eventually pinched off into a hollow neural tube, the early central nervous system (CNS), leaving behind neural crest cells outside of this tube that migrate to become the peripheral nervous system (PNS). Many of the cell rearrangements and migrations required for these processes are preceded by an epithelial–mesenchymal transition (EMT), which involves a shift from a collective static epithelial phenotype to an individual migratory phenotype (Przybyla et al., 2016b). Once cells arrive at the appropriate embryonic location, the reverse phenomenon, a mesenchymal–epithelial transition (MET), occurs (Nieto, 2013) as cells re-form an epithelial layer. As cells form more complex tissue structures, their cell–cell and cell–ECM interactions change dynamically, as do the mechanical forces they experience, which can reciprocally drive cell behavior. Throughout neurulation, mechanical changes at the tissue level can initiate and reinforce cycles of EMT and MET by altering cytoskeletal contractility and the ability of cells to bind to ECM components. This can lead to an increase in the production of ECM proteins and ECM-modifying enzymes [digestive enzymes such as matrix metalloproteinases (MMPs) and cross-linking enzymes such as lysyl oxidase (LOX)], which can further alter tissue-level mechanics (Samuel et al., 2011; Levental et al., 2009).
As the embryo progresses through neurulation, regions that will contribute to the brain continue to be shaped by mechanical forces. Actomyosin-driven contraction of cells leads to stiffening of dorsal tissues, which is required for vertebrate neural tube closure (Zhou et al., 2009), and dysregulation of cell adhesion in neural folds, cell migration from the neural crest, or other mechanically regulated processes can result in severe neural tube defects (Greene and Copp, 2009). In the embryonic mesencephalon, β1 integrin activity enhances neurogenesis through a Wnt7a-dependent mechanism (Long et al., 2016). These studies indicate that abundant cellular movements and organizational changes occur during embryogenesis and as the primitive nervous system forms. Therefore, cells in the developing embryo must sense and integrate mechanical cues into their complex signaling microenvironment, and respond by further altering the biophysical environment as development progresses, through mechanisms that we are only just beginning to understand.
Once the brain begins to take shape, neuronal subtype specification and migration occur, which require additional spatiotemporally regulated mechanosensitive pathways. Experimental disruption of ECM, ECM receptors and mechanosignaling proteins in neural cells can dramatically affect early brain development. For example, mutation of the subunits laminin β2 and laminin γ3 causes laminar disruption of the cortex (Radner et al., 2013), and mice lacking FAK in the dorsal forebrain also exhibit cortical lamination defects, neuronal dysplasia and abnormal synapse formation (Beggs et al., 2003; Rico et al., 2004). Although these studies represent manipulations of proteins involved in mechanosignaling, the resulting effects on cell adhesion could also directly contribute to the observed phenotypes. In addition to ECM-based mechanosignals, fluid flow also contributes to neural cell organization and differentiation. The proper orientation of ependymal cells requires forces generated by cerebral spinal fluid (CSF) flow, and coordinated beating of their cilia drives further CSF flow in the developing brain (Ohata and Alvarez-Buylla, 2016; Guirao et al., 2010). The resulting shear forces along the ventricles direct neuroblast alignment and migration (Sawamoto et al., 2006), and consistently, physical obstruction of CSF during development leads to decreased neurogenesis and severe developmental defects (Mashayekhi et al., 2002).
Mechanical signals therefore shape the developing brain throughout morphogenesis, by controlling cell organization within tissues to initiate and reinforce signaling pathways that regulate cell behavior. Next, we will discuss how structural elements of the brain, including the ECM and cells that serve as scaffolds, contribute to developmental programs by providing mechanical inputs to differentiating cells.
ECM in the developing brain
The cell migration patterns and differentiation programs required for proper brain development rely on mechanical cues that are mediated by large-scale changes in ECM composition and physical changes in brain architecture, in addition to soluble signals. ECM proteins in many tissues are synthesized and deposited by fibroblasts and other mesenchymal cells, but in the brain, neuronal cells of all types contribute to ECM production, maturation and structure. The lecticans mentioned above are deposited by neurons, glial cells and neuronal stem cells (Abaskharoun et al., 2010a,b). In addition to ECM, the cells within the developing brain can themselves also act as scaffolds for cell migration. Radial glial cells (RGCs) represent an important progenitor population that gives rise to neurons, glial cells and the progenitor cells of the subventricular zone (SVZ) (Reinhard et al., 2016; Rakic, 2003). RGCs also serve as a scaffold for developing brain architecture and neuronal migration, and have key roles in cerebral cortex folding (Borrell and Götz, 2014). These progenitor cells therefore play major structural and mechanical roles in formation of the brain. Migration of neural precursors along RGCs is effected in part by expression of the glycoprotein tenascin-C (TNC) by the RGCs (Garcion et al., 2001), and TNC expression is also associated with increased proliferation of neurogenic precursors in the developing ventricle (Doetsch et al., 2002). Disruption of other ECM and mechanosignaling proteins in the RGC microenvironment broadly affects their morphology, migration and differentiation (Halfter et al., 2002; Fox et al., 1998; Moore et al., 2002), further indicating that RGC mechanosensing and the mechanics of the neurogenic environment play important roles in brain development.
During embryogenesis, specific cell types develop and differentiate within regions that have distinct matrix mechanical compositions. The same principle holds true for adult stem cells, whose ability to maintain their self-renewal and potency depends upon their tissue-specific environment, or niche. The difficulty in maintaining the ‘stemness’ of isolated stem cells in basic cell culture conditions underscores the necessity of the niche, which provides context-dependent biochemical and physical cues to resident stem cells (Moore and Lemischka, 2006). The ECM composition of the SVZ niche changes over time during development (Campos, 2005), and the relative abundance of collagens, glycoproteins and proteoglycans in the niche regulates the self-renewal and differentiation of neural stem cells (NSCs) (Reinhard et al., 2016). It has been shown that the makeup of the ECM can affect stem cell properties by altering cell adhesion or ligand accessibility but also by mediating biophysical properties, including stiffness. Cell–ECM adhesion in the NSC neurogenic niche of the SVZ is a requirement for homeostasis that is mediated by β1 integrin, VCAM and laminins. Disruption of these molecules leads to unregulated NSC proliferation, resulting in dysregulated self-renewal and differentiation (Alvarez-Buylla and Lim, 2004; Tavazoie et al., 2008). ECM components, including lecticans and heparan sulfate proteoglycans, might directly regulate growth factor binding (Mercier, 2016). For example, the binding of fibroblast growth factor 2 (FGF2), which is involved in NSC maintenance, to its receptor (FGF receptor) is facilitated by heparan sulfate proteoglycans (Rapraeger et al., 1994). In another recent example, the ECM receptor dystroglycan has been found to regulate ECM remodeling in the postnatal SVZ, and to regulate RGC proliferation and differentiation into gliogenic progenitors (McClenahan et al., 2016). The biophysical properties and innate stiffness of niches within the brain are also likely to play a role in cell fate. A recent report noted a pattern of stiffness gradients in the embryonic brain, as measured using in situ atomic force microscopy (AFM) (Koser et al., 2016), and the SVZ specifically is known to stiffen gradually over the course of embryonic development (Iwashita et al., 2014), although the bulk elastic modulus of the brain does not appreciably change during development or postnatally (Majkut et al., 2013). Directly altering brain stiffness or blocking mechanotransduction during development results in aberrant axonal growth and migration (Koser et al., 2016), also implicating mechanical signals as regulators of this process. Defining the mechanical microenvironment of stem cell niches and understanding their contribution to cell behavior will enhance our ability to generate in vitro culture conditions that more faithfully mimic physiological environments. This knowledge will in turn inform approaches for optimizing culture conditions to drive lineage-specific differentiation of distinct neuronal cell types.
Neuronal cell mechanical properties and differentiation
Although model organisms provide valuable insights into neuronal development and stem cell and progenitor niches, our ability to gain molecular information at the level of individual cells is difficult at the organismal scale. This is particularly true in the context of cell and tissue mechanics and mechanosignaling owing to the paucity of tools and the complexity of the physical microenvironment in vivo (see Table 1). To address this issue, researchers often resort to studying neuronal cell mechanosignaling using neuronal cell types that have been differentiated from more naïve cells, neural stem cells or primary neuronal cells grown in culture. With cells in culture, the mechanical responsiveness of cells can be measured and manipulated by controlling the mechanical environment to which cells are exposed. To achieve this, cells can be cultured under perfusion to mimic shear stress, in a bioreactor designed to impart compressive force, or on hydrogels or micropost arrays of tunable stiffness to alter the matrix mechanical environment. For example, studies implementing tunable hydrogels provide evidence that mechanical forces can act as instructive cues for stem cell behavior and lineage commitment by modulating the response to signaling factors that control self-renewal and differentiation (Engler et al., 2006; McBeath et al., 2004; Przybyla et al., 2016a). This technique can also be adapted for use in studying brain development and disease by mimicking physiological tissue properties (Fig. 1).
As might be expected given the importance of the mechanical properties of the in vivo niche (discussed above), pluripotent embryonic stem cells (ESCs) favor neurogenesis when plated on substrates that resemble soft brain tissue (Keung et al., 2012), and mesenchymal stem cells (MSCs) also upregulate neuronal markers when cultured on soft substrates in the Young's modulus range of 0.1–1.0 kPa (Engler et al., 2006). Studies testing the effect of niche mechanics on the behavior of NSCs isolated directly from the SVZ show that NSCs on softer substrates that mimic neurogenic brain regions, such as the dentate gyrus of the hippocampus, tend to differentiate into neurons, whereas cells on substrates of increased stiffness foster glial differentiation (Saha et al., 2008; Georges et al., 2006; Leipzig and Shoichet, 2009). This is seemingly inconsistent with measurements of the intrinsic stiffness of neuronal cells, as neurons at ∼1 kPa are approximately twice as stiff as their neighboring glial cells at 400 Pa (Lu et al., 2006). However, it has been hypothesized that the soft glial cells serve as a compliant substrate for neurons to facilitate neuronal plasticity and provide protection from trauma (Lu et al., 2006). Mechanistically, it appears that NSCs in stiffer tissue inhibit neurogenesis through increased activity of RhoA and contractility, as dominant-negative RhoA prevents stiffness-induced neurogenic suppression in vitro and in vivo (Keung et al., 2011). In another study, the mechanically gated ion channel Piezo1 has been found to be responsible for human NSC neurogenesis versus astrogenesis through a YAP-mediated pathway (Pathak et al., 2014). Differentiation into more specialized neuronal subtypes can also be optimized through mechanical manipulations; for example, motor neuron differentiation of human pluripotent stem cells is most efficient on soft versus stiff micropost arrays, and mediated through a YAP-dependent mechanism (Sun et al., 2014). Functional cellular properties might also rely on mechanical signals; for example, the growth of retinal ganglion axons has recently been found to rely on the ability of cells to sense local tissue stiffness through mechanosensitive ion channels (Koser et al., 2016). These studies indicate that the stiffness on which individual neuronal subtypes are grown could be important to their functionality, so measuring and mimicking this feature of the in vivo environment can be used to direct stem cell fate. Collectively, these studies suggest that mechanotransduction plays an instructive role in stem cell differentiation; when combined with appropriate soluble factors, the forces the cell experiences either permit or restrict exit from self-renewal and commitment to a specific lineage.
It is becoming increasingly clear that mechanical cues integrate with other signals in the cellular microenvironment to drive differentiation and migration during embryonic development, including during neural specification and brain organogenesis. Obtaining a better understanding of how mechanical forces contribute to cell differentiation is important for advancing fundamental knowledge of brain development, and should also enhance our ability to culture and differentiate naïve and adult neuronal cell types. Because regenerative medicine requires the production of specialized cell types from more-proliferative progenitor populations, optimizing the differentiation protocols for neuronal subtypes will be important for clinical development of stem-cell-based therapies. Understanding how mechanical signals cause cells to organize into tissue-level structures that are crucial to proper development will also inform studies of how de-differentiated cancer cells contribute to tumorigenesis, as discussed in detail in the following section.
Tissue mechanics in brain cancer microenvironment
The physical stiffening of tissues during cancer progression is an ancient clinical observation, and is the basis for modern palpation-based diagnostic methodologies. Physical changes in the tumor microenvironment occur on many levels, including elevated fluid pressure (subsequent to edema), cell compression, stiffening of the ECM, increased cellular contractility and changes in cell membrane tension (fluidity). These can collectively drive tumor progression and impede treatment (i) through sustained activation of pro-tumorigenic mechanosignaling pathways, (ii) or by providing new ‘tracks’ on which tumor cells can migrate and (iii) by compromising blood vessel integrity, which can influence both the recruitment of inflammatory cells and the permeability of macromolecules, including therapeutic compounds (Netti et al., 2000; Jain, 1999; Pickup et al., 2014; Padera et al., 2004). During metastasis, cancer cells experience a wide range of forces when moving from one microenvironment to the next, and the ability to navigate and endure these forces greatly influences the successful survival and colonization of the metastatic cell. Although tissue-level stiffening of tumors is common, the increased compliance of individual cells is associated with metastatic progression and tumor aggression due to an enhanced ability to invade through basement membranes and ECM, and pass through the circulatory system (Barnes et al., 2012; Cross et al., 2007). Convincing evidence for mechanical regulation of solid tumors is quickly accumulating, particularly in the context of breast cancer (Pickup et al., 2014), and studies on the interplay between brain tissue mechanics and tumor biology are increasing as well.
Mechanical properties of brain tumor subtypes
The World Health Organization (WHO) defines the criteria for the clinical classification of brain tumors, which is updated periodically. Traditionally, brain tumors have been diagnosed primarily upon examination of hematoxylin and eosin (H&E)-stained biopsies, and the scoring of mitotic events, necrosis and microvascular proliferation. In the 2016 WHO update, molecular factors, such as mutations and chromosomal abnormalities, were added to brain tumor classification, improving the accuracy of diagnosis and prognosis (Louis et al., 2016). This section focuses on gliomas, which are primary (originating in the brain rather than from a metastasis) brain tumors with a glial phenotype, occurring most frequently in adults. Gliomas are scored as WHO grades I through IV; grade I tumors are typically well managed with surgery, whereas grades II through IV are progressively more difficult to treat and have worse prognosis, with the median survival of grade IV at less than two years (Louis et al., 2016). For simplicity, grades II and III will be referred to as lower grade gliomas (LGGs) and grade IV as glioblastoma (GBM). Details of the morbidity and mortality of these tumors can be found on the American Brain Tumor Association (www.abta.org) and National Brain Tumor Society (www.braintumor.org) websites.
Early investigations of the physical properties of brain tumors were conducted in individuals using elastography (see Table 1). Although many of these studies have shown that tumors are stiffer than normal brain (Xu et al., 2007; Scholz et al., 2007, 2005; Chauvet et al., 2015), some have shown the opposite (Reiss-Zimmermann et al., 2015; Streitberger et al., 2014), and determination of stiffness differences between tumor grades has been inconclusive using this technique. Consistent with observations of stiffening of brain tumors, increased diffusion of water within a tumor, as measured by magnetic resonance imaging (MRI), is prognostic of poor outcome and is correlated with increased expression of ECM genes whose products are expected to stiffen a tissue (Pope et al., 2012, 2009). Recently, direct mechanical testing of fresh biopsies has revealed that stiffness of the associated brain tumor ECM correlates with poor prognosis (Miroshnikova et al., 2016). Overall, non-tumor gliotic tissue exhibits the lowest level of ECM stiffness, whereas LGGs and GBMs were progressively stiffer, although individual-to-individual heterogeneity was documented (Fig. 3C). This mechanical heterogeneity has been reconciled by categorizing GBMs by their isocitrate dehydrogenase-1 (IDH1) status, as IDH1 is a metabolic enzyme whose mutation is associated with greater progression-free survival (Cancer Genome Atlas Research et al., 2015; Reitman et al., 2010). The majority of LGGs are characterized by mutant IDH1, and the few LGGs with wild-type IDH1 had a stiffness that resembled that of average GBMs. Conversely, the rare GBMs that had the mutant form of IDH1 exhibited a stiffness similar to that of the average LGGs. Nevertheless, once IDH1-mutant LGG or GBM recurred, they presented with a striking increase in ECM stiffness that was markedly heterogeneous, emphasizing the need for further analysis of phenotypic heterogeneity in glioma behavior. It is important to note that the discrepancy between magnetic-resonance-elastography- and AFM-based measurements could be due to the nature of the detection method (imaging versus contact) and/or the context of the tissue (in vivo versus ex vivo; see Table 1).
Effects of mechanical changes on glioma progression
Stiff GBM tumors with wild-type IDH1 have cores that are usually necrotic and present with an abnormal and compromised vasculature, leading to oxygen tension and signaling through hypoxia-inducible factor-1α (HIF1α), a transcription factor that acts as a master effector of hypoxia. HIF1α directly binds to the promoter of TNC, inducing its transcription (Reitman et al., 2010; Miroshnikova et al., 2016). TNC acts as an ECM modifier by cross-linking lecticans, which are non-covalently bound to hyaluronic acid (Fig. 3B). This hyaluronic-acid–lectican–TNC complex (a corrupted version of the PNN structure described above and shown in Fig. 3A) stiffens the tumor tissue relative to normal brain by limiting the flexibility of the ECM (Mouw et al., 2014; Day et al., 2004; Kim and Kumar, 2014). Because increased amounts of hyaluronic acid are produced in GBMs (Fig. 3D), tissue stiffening is exacerbated in the disease state (Kim and Kumar, 2014). The ability of IDH1-mutant GBMs to sense hypoxia is blunted, which leads to dramatically reduced production of HIF1α and TNC, thus contributing to the softer nature of IDH1-mutant GBMs (Miroshnikova et al., 2016). Interestingly, ECM stiffening is able to override this protective effect of blunted hypoxia signaling by downregulating the HIF1α-targeting microRNA miR-203 (Miroshnikova et al., 2016) (Fig. 3E).
Alterations in extracellular fluid flow and pressure also influence glioma cell behavior. In the normal brain, the interstitial fluid pressure is low, below 15 mmHg (Narayan et al., 1982; Stocchetti and Maas, 2014). During glioma progression, alterations in ECM and elevated hypoxia signaling lead to a compromised and leaky vasculature with poor perfusion. As extracellular fluid accumulates, interstitial fluid pressures can rise dramatically (Alberti et al., 1978; Kullberg and West, 1965; Narayan et al., 1982; Stocchetti and Maas, 2014) and, as this fluid moves down its pressure gradient, into the healthy brain, local increases in fluid shear forces are experienced by tumor cells (Munson and Shieh, 2014). Increased fluid pressure causes tissue compression, which leads to increased migration and transcriptional changes in cancer cells (Butcher et al., 2009). Elevated shear forces have been shown to induce GBM migration, in the direction of fluid flow, through mechanical activation of chemokine receptors (Munson et al., 2013). Changes in fluid pressure and flow are difficult to study in vivo and have thus been overlooked in glioma research. A better understanding of the combined effects of ECM- and fluid-based tissue mechanics promises to reveal therapeutic targets to improve treatment outcome.
Tissue mechanics drive tumor progression through regulation of cellular plasticity
ECM stiffness influences GBM invasion by facilitating the binding between CD44 and hyaluronic acid, which results in pro-migration signaling downstream and also influences the binding of integrins to their ECM substrates (Kim and Kumar, 2014; Knupfer et al., 1999). Enhanced ECM stiffness also drives GBM cell proliferation and a phenotype reminiscent of EMT, which further enhances GBM invasion (Ulrich et al., 2009; Cancer Genome Atlas Research et al., 2015). GBMs exhibit extreme cellular and molecular heterogeneity, and in an effort to tailor therapy to individuals, molecular profiling has been used to stratify GBMs into subclasses, which represent different stages of neuronal development (Phillips et al., 2006; Verhaak et al., 2010). Mesenchymal GBM cells, which resemble migratory cells of the neural crest, are associated with increased treatment resistance, and when GBM recurs after standard-of-care therapy, mesenchymal cells are observed at a higher frequency (Lu et al., 2012). Cells which have undergone EMT often present with an altered glycocalyx, the saccharide–protein network on the cell surface (Roy et al., 2011; Porsch et al., 2013; Moustakas and Heldin, 2014). A strong glycocalyx elicits pro-tumorigenic effects by enhancing focal adhesion formation and downstream signaling (Paszek et al., 2014), and has also been implicated in resistance to small-molecule and antibody therapies because it provides a physical shield to the cell membrane and its associated receptors (Yang et al., 2013; Thompson et al., 2010; Singha et al., 2015). Indeed, in GBMs, we find that glycocalyx bulk augments mechanical signaling (unpublished data). Examples of tumor mechanoreciprocity, whereby mechanosignaling drives ECM production to stiffen the microenvironment and reinforce mechanical activation of pro-survival and -invasion pathways, have been demonstrated in other tumors (Samuel et al., 2011), perhaps revealing a fundamental principle in tumor progression (illustrated in Fig. 3E) that could be exploited in the treatment of GBM. For instance, targeting FAK, effectors of EMT or components of the glycocalyx, each of which are being examined as targets in clinical trials (Hingorani et al., 2016; Traber et al., 2013; Serrels et al., 2015), could improve disease outcome when combined with standard-of-care treatment. These findings also suggest that, similar to what has been shown for solid tumors (Acerbi et al., 2015), mechanical testing or assessment of mechanosignaling of gliomas can extend the repertoire of conventional molecular biomarkers, thereby improving predictions of response to therapy.
Altered tissue mechanics in neurodegenerative diseases
Because mechanics play important roles in brain development and homeostasis, acute changes in forces sensed by brain cells might have far-reaching consequences on brain function. This can be a consequence of physical damage to cells and tissues after brain trauma but also of disruption to fluid flow and mechanical regulation of the niches required to replenish damaged cells. The inability of cells to return to homeostasis can result in problems, such as dementia and neurodegeneration that can manifest several years later (Smith et al., 2013). Brain degeneration is associated with problems in protein folding and clearance (Hetz and Mollereau, 2014), and recent findings also demonstrate a correlation between ECM composition and/or mechanical changes, altered CSF dynamics and the onset of neurodegenerative diseases, such as Alzheimer's disease and Parkinson's Disease (Bonneh-Barkay and Wiley, 2009; Tyler, 2012; Simon and Iliff, 2016).
Mechanical changes resulting from traumatic brain injury
Traumatic brain injury (TBI) is associated with irreversible cognitive dysfunction and progressive neurodegeneration, but little is known about how TBI leads to the upregulation of mechanosensitive signaling pathways, or how cellular mechanics are altered downstream of injury. On a large scale, interruption of CSF flow can lead to an increase in intracranial pressure, resulting in tissue ischemia and brain herniation (Smith et al., 2013). This is further exacerbated by inflammation resulting from neutrophil recruitment due to a loss of endothelial cell adhesion integrity (Carlos et al., 1997), which dramatically affects cell and tissue function. The breakdown and remodeling of the ECM has been implicated in modulating injury responses to brain trauma (Lo et al., 2002), but the molecular pathways involved are unknown. In one example, mechanically mediated Na+ channel activation downstream of acute mechanical injury has been shown to lead to Ca2+-mediated excitotoxicity (Wolf et al., 2001), but that study did not address how this might lead to long-term effects on brain function. Importantly, damage that results in remodeling and activation of the adult SVZ neurogenic niche after injury can contribute substantially to long-term problems with neuronal replenishment and brain functionality (Chang et al., 2016), which provides clues as to how acute brain injury can lead to chronic disease. There is also evidence that TNC is upregulated in a discrete region around sites of brain lesions (Laywell et al., 1992) and that it is induced in CSF after injury-mediated aneurysmal subarachnoid hemorrhage (Suzuki et al., 2015). Because TNC is able to stiffen the ECM and cause cells to reciprocally remodel their mechanical environment (as discussed above), TNC upregulation as a result of injury could contribute to long-term deleterious effects on brain structure and function.
Mechanotransduction breakdown in neurodegenerative disorders
Although brain stiffness generally increases with age, it decreases in neurodegenerative disorders, which could be related to a loss of adult neurogenesis (Klein et al., 2014), although it is likely that changes in the ECM or a loss in myelin content also contribute. Slowing of CSF flux through the brain is also associated with increasing age (Kress et al., 2014), which might affect cell functions that are dependent on sensing shear flow. However, although mechanical changes are associated with age and disease states, the extent to which they drive pathology is unclear. The ECM composition is changed in the brains of individuals with Alzheimer’s disease (Lau et al., 2013), and such changes can either directly or indirectly lead to synaptic and neural loss (Bonneh-Barkay and Wiley, 2009), which could be due to a loss of matrix molecules that are necessary to maintain the progenitor cell niches. Regional changes in brain stiffness in individuals with Alzheimer's disease have also been documented (Murphy et al., 2016), and in this context, the overall decrease in stiffness, as measured by performing three-dimensional magnetic resonance elastography, could serve as a noninvasive diagnostic tool (Murphy et al., 2011). Similarly, during Parkinson's disease progression, changes in the stiffness or elasticity of the substantia nigra can be detected by using ultrasound hyperechogenecity, even before individuals develop any motor impairment (Berg, 2011). Clearly, gaining additional insights into how these changes occur and what they mean for cellular and organ function could enhance our ability to diagnose and treat these diseases. Indeed, preliminary results indicate that mechanical stimulation might improve autonomic control in individuals with Parkinson's disease (Bassani et al., 2014). During development of multiple sclerosis, which is characterized by loss of neuronal myelination, CNS basement membranes become discontinuous and abnormal, and levels of fibrillar collagens increase, leading to perivascular fibrosis (Mohan et al., 2010). This is associated with increased deposition of several ECM components, including chondroitin sulfate proteoglycans and hyaluronic acid (Yiu and He, 2006), which impede remyelination and oligodendrocyte progenitor cell proliferation (Back et al., 2005). Neurodegenerative diseases and brain injury are therefore associated with dramatic changes in ECM properties, and the fact that either an increase or decrease in ECM deposition or stiffness can lead to severe neurological defects highlights the importance of maintaining a mechanically homeostatic equilibrium.
Conclusions and future perspectives
The conservation of mechanotransduction from bacteria to mammals underscores the necessity of cells to convert mechanical cues into physiological information that dictates phenotype and behavior. As discussed throughout this Commentary, tissue mechanical forces are generated by processes that include collective cell tractions, fluid movement and changes in ECM composition. Through mechanotransduction, these forces regulate the development of the brain by orchestrating the compartmentalization of cells and shaping of tissues, and by instructing fate decisions of neural stem and progenitor cells. In the developed brain, tissue mechanics contribute to homeostasis and function by regulating neurotransmission and stem cell renewal and differentiation in specialized niches. As with any important cell-signaling regulator, perturbation of tissue mechanics can lead to changes in tissue function and development of disease. In this review, we discussed how physical cues reciprocally contribute to the progression of malignant brain tumors, and how acute mechanical perturbations can result in chronic disruption of homeostasis. An emerging theme is that physical changes in the tumor microenvironment activate signaling pathways that lead to transcriptional changes and ECM remodeling that positively feed back to enhance pro-tumorigenic mechanosignaling, leading to therapy resistance and poor prognosis. A closer examination of this mechanoreciprocity circuit will allow researchers to identify new biomarkers and therapeutic targets. Although the role of mechanobiology in neurodegenerative diseases is less understood, it is becoming clear that physical changes to brain tissue and corresponding mechanosignaling pathways are intimately involved in the progression of Alzheimer's disease, multiple sclerosis and Parkinson's disease. Finally, changes to brain tissue mechanics resulting from traumatic brain injury are likely to be crucial to the development of cognitive and motor defects associated with sports and combat-related injuries. The extent to which mechanosignaling coordinates brain development and function is only beginning to be fully appreciated and many questions remain. For example, it would be of great value to better understand how ECM mechanics, fluid flow and mechanosensitive ion channels synchronize with soluble growth factors and cytokines to control cell migration and direct differentiation in the embryonic and adult brains. Such knowledge would aid in the development of neural regenerative medicine approaches and in the battle against brain cancer and neurodegenerative diseases.
Acknowledgements
We thank Jon Muncie and Janna Mouw (both of University of California, San Francisco) for helpful comments on the Commentary.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Grant support was provided by National Institutes of Health [grant numbers U54CA163155, R01GM059907 and U01 CA202241 to V.M.W.; grant number F32CA174319 to J.M.B.] and the California Institute of Regenerative Medicine [grant number RB5-07409 to V.M.W.; grant number TG2-01153 to L.P.]. Deposited in PMC for release after 12 months.
References
- Abaskharoun M., Bellemare M., Lau E. and Margolis R. U. (2010a). Expression of hyaluronan and the hyaluronan-binding proteoglycans neurocan, aggrecan, and versican by neural stem cells and neural cells derived from embryonic stem cells. Brain Res. 1327, 6-15. 10.1016/j.brainres.2010.02.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abaskharoun M., Bellemare M., Lau E. and Margolis R. U. (2010b). Glypican-1, phosphacan/receptor protein-tyrosine phosphatase-zeta/beta and its ligand, tenascin-C, are expressed by neural stem cells and neural cells derived from embryonic stem cells. Asn Neuro 2, e00039 10.1042/AN20100001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acerbi I., Cassereau L., Dean I., Shi Q., Au A., Park C., Chen Y. Y., Liphardt J., Hwang E. S. and Weaver V. M. (2015). Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 7, 1120-1134. 10.1039/C5IB00040H [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti E., Hartmann A., Schutz H.-J. and Schreckenberger F. (1978). The effect of large doses of dexamethasone on the cerebrospinal fluid pressure in patients with supratentorial tumors. J. Neurol. 217, 173-181. 10.1007/BF00312958 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A. and Lim D. A. (2004). For the long run: maintaining germinal niches in the adult brain. Neuron 41, 683-686. 10.1016/S0896-6273(04)00111-4 [DOI] [PubMed] [Google Scholar]
- Back S. A., Tuohy T. M., Chen H., Wallingford N., Craig A., Struve J., Luo N. L., Banine F., Liu Y., Chang A. et al. (2005). Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 11, 966-972. 10.1038/nm1279 [DOI] [PubMed] [Google Scholar]
- Barnes J. M., Nauseef J. T. and Henry M. D. (2012). Resistance to fluid shear stress is a conserved biophysical property of malignant cells. PLoS ONE 7, e50973 10.1371/journal.pone.0050973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani T., Bari V., Marchi A., Tassin S., Dalla Vecchia L., Canesi M., Barbic F., Furlan R. and Porta A. (2014). Model-free causality analysis of cardiovascular variability detects the amelioration of autonomic control in Parkinson's disease patients undergoing mechanical stimulation. Physiol. Meas. 35, 1397-1408. 10.1088/0967-3334/35/7/1397 [DOI] [PubMed] [Google Scholar]
- Beggs H. E., Schahin-Reed D., Zang K., Goebbels S., Nave K.-A., Gorski J., Jones K. R., Sretavan D. and Reichardt L. F. (2003). FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron 40, 501-514. 10.1016/S0896-6273(03)00666-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. (2011). Hyperechogenicity of the substantia nigra: pitfalls in assessment and specificity for Parkinson's disease. J. Neural Transm. 118, 453-461. 10.1007/s00702-010-0469-5 [DOI] [PubMed] [Google Scholar]
- Bilston L. E., Liu Z. and Phan-Thien N. (1997). Linear viscoelastic properties of bovine brain tissue in shear. Biorheology 34, 377-385. 10.1016/S0006-355X(98)00022-5 [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D. and Wiley C. A. (2009). Brain extracellular matrix in neurodegeneration. Brain Pathol. 19, 573-585. 10.1111/j.1750-3639.2008.00195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell V. and Götz M. (2014). Role of radial glial cells in cerebral cortex folding. Curr. Opin. Neurobiol. 27, 39-46. 10.1016/j.conb.2014.02.007 [DOI] [PubMed] [Google Scholar]
- Butcher D. T., Alliston T. and Weaver V. M. (2009). A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108-122. 10.1038/nrc2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos L. S. (2005). Beta1 integrins and neural stem cells: making sense of the extracellular environment. Bioessays 27, 698-707. 10.1002/bies.20256 [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network, Brat D. J., Verhaak R. G., Aldape K. D., Yung W. K., Salama S. R., Cooper L. A., Rheinbay E., Miller C. R., Vitucci M., Morozova O. et al. (2015). Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 372, 2481-2498. 10.1056/NEJMoa1402121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos T. M., Clark R. S., Franicola-Higgins D., Schiding J. K. and Kochanek P. M. (1997). Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J. Leukoc. Biol. 61, 279-285. [DOI] [PubMed] [Google Scholar]
- Carulli D., Rhodes K. E., Brown D. J., Bonnert T. P., Pollack S. J., Oliver K., Strata P. and Fawcett J. W. (2006). Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J. Comp. Neurol. 494, 559-577. 10.1002/cne.20822 [DOI] [PubMed] [Google Scholar]
- Chang E. H., Adorjan I., Mundim M. V., Sun B., Dizon M. L. V. and Szele F. G. (2016). Traumatic brain injury activation of the adult subventricular zone neurogenic niche. Front. Neurosci. 10, 332 10.3389/fnins.2016.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet D., Imbault M., Capelle L., Demene C., Mossad M., Karachi C., Boch A. L., Gennisson J. L. and Tanter M. (2015). In vivo measurement of brain tumor elasticity using intraoperative shear wave elastography. Ultraschall. Med. [Epub ahead of print] doi: 10.1055/s-0034-1399152 10.1055/s-0034-1399152 [DOI] [PubMed] [Google Scholar]
- Christensen A. P. and Corey D. P. (2007). TRP channels in mechanosensation: direct or indirect activation? Nat. Rev. Neurosci. 8, 510-521. 10.1038/nrn2149 [DOI] [PubMed] [Google Scholar]
- Cowman M. K., Schmidt T. A., Raghavan P. and Stecco A. (2015). Viscoelastic properties of hyaluronan in physiological conditions. F1000res 4, 622 10.12688/f1000research.6885.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross S. E., Jin Y.-S., Rao J. and Gimzewski J. K. (2007). Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2, 780-783. 10.1038/nnano.2007.388 [DOI] [PubMed] [Google Scholar]
- Day J. M., Olin A. I., Murdoch A. D., Canfield A., Sasaki T., Timpl R., Hardingham T. E. and Aspberg A. (2004). Alternative splicing in the Aggrecan G3 domain influences binding interactions with tenascin-C and other extracellular matrix proteins. J. Biol. Chem. 279, 12511-12518. 10.1074/jbc.M400242200 [DOI] [PubMed] [Google Scholar]
- Doetsch F., Petreanu L., Caille I., Garcia-Verdugo J.-M. and Alvarez-Buylla A. (2002). EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron 36, 1021-1034. 10.1016/S0896-6273(02)01133-9 [DOI] [PubMed] [Google Scholar]
- DuFort C. C., Paszek M. J. and Weaver V. M. (2011). Balancing forces: architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 12, 308-319. 10.1038/nrm3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S. et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature 474, 179-183. 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- Duscher D., Maan Z. N., Wong V. W., Rennert R. C., Januszyk M., Rodrigues M., Hu M., Whitmore A. J., Whittam A. J., Longaker M. T. et al. (2014). Mechanotransduction and fibrosis. J. Biomech. 47, 1997-2005. 10.1016/j.jbiomech.2014.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L. and Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677-689. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Farge E. (2011). Mechanotransduction in development. Curr. Top. Dev. Biol. 95, 243-265. 10.1016/B978-0-12-385065-2.00008-6 [DOI] [PubMed] [Google Scholar]
- Fox J. W., Lamperti E. D., Ekşioğlu Y. Z., Hong S. E., Feng Y., Graham D. A., Scheffer I. E., Dobyns W. B., Hirsch B. A., Radtke R. A. et al. (1998). Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron 21, 1315-1325. 10.1016/S0896-6273(00)80651-0 [DOI] [PubMed] [Google Scholar]
- Franze K., Janmey P. A. and Guck J. (2013). Mechanics in neuronal development and repair. Annu. Rev. Biomed. Eng. 15, 227-251. 10.1146/annurev-bioeng-071811-150045 [DOI] [PubMed] [Google Scholar]
- Garcion E., Faissner A. and Ffrench-Constant C. (2001). Knockout mice reveal a contribution of the extracellular matrix molecule tenascin-C to neural precursor proliferation and migration. Development 128, 2485-2496. [DOI] [PubMed] [Google Scholar]
- Gavara N. (2016). A Beginner's guide to atomic force microscopy probing for cell mechanics. Microsc. Res. Tech. [Epub ahead of print] doi: 10.1002/jemt.22776 10.1002/jemt.22776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges P. C., Miller W. J., Meaney D. F., Sawyer E. S. and Janmey P. A. (2006). Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys. J. 90, 3012-3018. 10.1529/biophysj.105.073114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene N. D. E. and Copp A. J. (2009). Development of the vertebrate central nervous system: formation of the neural tube. Prenat. Diagn. 29, 303-311. 10.1002/pd.2206 [DOI] [PubMed] [Google Scholar]
- Guirao B., Meunier A., Mortaud S., Aguilar A., Corsi J.-M., Strehl L., Hirota Y., Desoeuvre A., Boutin C., Han Y.-G. et al. (2010). Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol. 12, 341-350. 10.1038/ncb2040 [DOI] [PubMed] [Google Scholar]
- Halfter W., Dong S., Yip Y. P., Willem M. and Mayer U. (2002). A critical function of the pial basement membrane in cortical histogenesis. J. Neurosci. 22, 6029-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg C.-P. and Bellaiche Y. (2013). Forces in tissue morphogenesis and patterning. Cell 153, 948-962. 10.1016/j.cell.2013.05.008 [DOI] [PubMed] [Google Scholar]
- Hetz C. and Mollereau B. (2014). Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat. Rev. Neurosci. 15, 233-249. 10.1038/nrn3689 [DOI] [PubMed] [Google Scholar]
- Hingorani S. R., Harris W. P., Beck J. T., Berdov B. A., Wagner S. A., Pshevlotsky E. M., Tjulandin S. A., Gladkov O. A., Holcombe R. F., Korn R. et al. (2016). Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin. Cancer Res. 22, 2848-2854. 10.1158/1078-0432.CCR-15-2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmuth R. M. (2000). Micropipette aspiration of living cells. J. Biomech. 33, 15-22. 10.1016/s0021-9290(99)00175-x [DOI] [PubMed] [Google Scholar]
- Humphrey J. D., Dufresne E. R. and Schwartz M. A. (2014). Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15, 802-812. 10.1038/nrm3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H., Nakamura H., Ono T., Okumura M. S. and Anraku Y. (1994). MID1, a novel saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell. Biol. 14, 8259-8271. 10.1128/MCB.14.12.8259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita M., Kataoka N., Toida K. and Kosodo Y. (2014). Systematic profiling of spatiotemporal tissue and cellular stiffness in the developing brain. Development 141, 3793-3798. 10.1242/dev.109637 [DOI] [PubMed] [Google Scholar]
- Jacot J. G., Dianis S., Schnall J. and Wong J. Y. (2006). A simple microindentation technique for mapping the microscale compliance of soft hydrated materials and tissues. J. Biomed. Mater. Res. A 79A, 485-494. 10.1002/jbm.a.30812 [DOI] [PubMed] [Google Scholar]
- Jain R. K. (1999). Transport of molecules, particles, and cells in solid tumors. Annu. Rev. Biomed. Eng. 1, 241-263. 10.1146/annurev.bioeng.1.1.241 [DOI] [PubMed] [Google Scholar]
- Johnson C. L., Schwarb H., D J McGarry M., Anderson A. T., Huesmann G. R., Sutton B. P. and Cohen N. J. (2016). Viscoelasticity of subcortical gray matter structures. Hum. Brain Mapp 37, 4221-4233. 10.1002/hbm.23314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumi A., Orr A. W., Tzima E. and Schwartz M. A. (2004). Integrins in mechanotransduction. J. Biol. Chem. 279, 12001-12004. 10.1074/jbc.R300038200 [DOI] [PubMed] [Google Scholar]
- Keung A. J., de Juan-Pardo E. M., Schaffer D. V. and Kumar S. (2011). Rho GTPases mediate the mechanosensitive lineage commitment of neural stem cells. Stem Cells 29, 1886-1897. 10.1002/stem.746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung A. J., Asuri P., Kumar S. and Schaffer D. V. (2012). Soft microenvironments promote the early neurogenic differentiation but not self-renewal of human pluripotent stem cells. Integr. Biol. 4, 1049-1058. 10.1039/c2ib20083j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. and Kumar S. (2014). Cd44-mediated adhesion to hyaluronic acid contributes to mechanosensing and invasive motility. Mol. Cancer Res. 12, 1416-1429. 10.1158/1541-7786.MCR-13-0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C., Hain E. G., Braun J., Riek K., Mueller S., Steiner B. and Sack I. (2014). Enhanced adult neurogenesis increases brain stiffness: in vivo magnetic resonance elastography in a mouse model of dopamine depletion. PLoS ONE 9, e92582 10.1371/journal.pone.0092582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knupfer M. M., Poppenborg H., Hotfilder M., Kuhnel K., Wolff J. E. A. and Domula M. (1999). Cd44 expression and hyaluronic acid binding of malignant glioma cells. Clin. Exp. Metastasis 17, 81-86. 10.1023/A:1026425519497 [DOI] [PubMed] [Google Scholar]
- Koser D. E., Thompson A. J., Foster S. K., Dwivedy A., Pillai E. K., Sheridan G. K., Svoboda H., Viana M., Costa L. D., Guck J. et al. (2016). Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 10.1038/nn.4394 [Epud ahead of print] doi:10.1038/nn.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress B. T., Iliff J. J., Xia M., Wang M., Wei H. S., Zeppenfeld D., Xie L., Kang H., Xu Q., Liew J. A. et al. (2014). Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 76, 845-861. 10.1002/ana.24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullberg G. and West K. A. (1965). Influence of corticosteroids on the ventricular fluid pressure. Acta Neurol. Scand. Suppl. 13, 445-452. 10.1111/j.1600-0404.1965.tb01913.x [DOI] [PubMed] [Google Scholar]
- Kung C., Martinac B. and Sukharev S. (2010). Mechanosensitive channels in microbes. Annu. Rev. Microbiol. 64, 313-329. 10.1146/annurev.micro.112408.134106 [DOI] [PubMed] [Google Scholar]
- Kwok J. C. F., Dick G., Wang D. and Fawcett J. W. (2011). Extracellular matrix and perineuronal nets in CNS repair. Dev. Neurobiol. 71, 1073-1089. 10.1002/dneu.20974 [DOI] [PubMed] [Google Scholar]
- Lau L. W., Cua R., Keough M. B., Haylock-Jacobs S. and Yong V. W. (2013). Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat. Rev. Neurosci. 14, 722-729. 10.1038/nrn3550 [DOI] [PubMed] [Google Scholar]
- Laywell E. D., Dorries U., Bartsch U., Faissner A., Schachner M. and Steindler D. A. (1992). Enhanced expression of the developmentally regulated extracellular matrix molecule tenascin following adult brain injury. Proc. Natl. Acad. Sci. USA 89, 2634-2638. 10.1073/pnas.89.7.2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipzig N. D. and Shoichet M. S. (2009). The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 30, 6867-6878. 10.1016/j.biomaterials.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Levental K. R., Yu H., Kass L., Lakins J. N., Egeblad M., Erler J. T., Fong S. F. T., Csiszar K., Giaccia A., Weninger W. et al. (2009). Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891-906. 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew R. R., Abbas Z., Anderca M. I. and Free S. J. (2008). Phenotype of a mechanosensitive channel mutant, mid-1, in a filamentous fungus, Neurospora crassa. Eukaryot. Cell 7, 647-655. 10.1128/EC.00411-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Deng J., Zhou J. and Li X. (2016). Elastic and viscoelastic mechanical properties of brain tissues on the implanting trajectory of sub-thalamic nucleus stimulation. J. Mater. Sci. Mater. Med. 27, 163 10.1007/s10856-016-5775-5 [DOI] [PubMed] [Google Scholar]
- Lo E. H., Wang X. and Cuzner M. L. (2002). Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J. Neurosci. Res. 69, 1-9. 10.1002/jnr.10270 [DOI] [PubMed] [Google Scholar]
- Long K., Moss L., Laursen L., Boulter L. and Ffrench-Constant C. (2016). Integrin signalling regulates the expansion of neuroepithelial progenitors and neurogenesis via Wnt7a and Decorin. Nat. Commun. 7, 10354 10.1038/ncomms10354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis D. N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W. K., Ohgaki H., Wiestler O. D., Kleihues P. and Ellison D. W. (2016). The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131, 803-820. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- Lu Y.-B., Franze K., Seifert G., Steinhauser C., Kirchhoff F., Wolburg H., Guck J., Janmey P., Wei E.-Q., Kas J. et al. (2006). Viscoelastic properties of individual glial cells and neurons in the CNS. Proc. Natl. Acad. Sci. USA 103, 17759-17764. 10.1073/pnas.0606150103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K. V., Chang J. P., Parachoniak C. A., Pandika M. M., Aghi M. K., Meyronet D., Isachenko N., Fouse S. D., Phillips J. J., Cheresh D. A. et al. (2012). VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 22, 21-35. 10.1016/j.ccr.2012.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmanus D. B., Pierrat B., Murphy J. G. and Gilchrist M. D. (2016). A viscoelastic analysis of the p56 mouse brain under large-deformation dynamic indentation. Acta Biomater. [Epud ahead of print] doi: 10.1016/j.actbio.2016.10.029 10.1016/j.actbio.2016.10.029 [DOI] [PubMed] [Google Scholar]
- Majkut S., Idema T., Swift J., Krieger C., Liu A. and Discher D. E. (2013). Heart-specific stiffening in early embryos parallels matrix and myosin expression to optimize beating. Curr. Biol. 23, 2434-2439. 10.1016/j.cub.2013.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan Y. K., Glaser K. J. and Ehman R. L. (2010). Magnetic resonance elastography: a review. Clin. Anat. 23, 497-511. 10.1002/ca.21006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinac B. and Kloda A. (2003). Evolutionary origins of mechanosensitive ion channels. Prog. Biophys. Mol. Biol. 82, 11-24. 10.1016/S0079-6107(03)00002-6 [DOI] [PubMed] [Google Scholar]
- Mashayekhi F., Draper C. E., Bannister C. M., Pourghasem M., Owen-Lynch P. J. and Miyan J. A. (2002). Deficient cortical development in the hydrocephalic Texas (H-Tx) rat: a role for CSF. Brain 125, 1859-1874. 10.1093/brain/awf182 [DOI] [PubMed] [Google Scholar]
- Mayor R. and Theveneau E. (2013). The neural crest. Development 140, 2247-2251. 10.1242/dev.091751 [DOI] [PubMed] [Google Scholar]
- McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K. and Chen C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483-495. 10.1016/S1534-5807(04)00075-9 [DOI] [PubMed] [Google Scholar]
- McClenahan F. K., Sharma H., Shan X., Eyermann C. and Colognato H. (2016). Dystroglycan suppresses notch to regulate stem cell niche structure and function in the developing postnatal subventricular zone. Dev. Cell 38, 548-566. 10.1016/j.devcel.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F. (2016). Fractones: extracellular matrix niche controlling stem cell fate and growth factor activity in the brain in health and disease. Cell. Mol. Life Sci. 73, 4661-4674. 10.1007/s00018-016-2314-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroshnikova Y. A., Mouw J. K., Barnes J. M., Pickup M. W., Lakins J. N., Kim Y., Lobo K., Persson A. I., Reis G. F., Mcknight T. R. et al. (2016). Tissue mechanics promote IDH1-dependent Hif1alpha-tenascin C feedback to regulate glioblastoma aggression. Nat. Cell Biol. [Epud ahead of print] doi:10.1038/ncb3429. 10.1038/ncb3429 [DOI] [PubMed] [Google Scholar]
- Mohan H., Krumbholz M., Sharma R., Eisele S., Junker A., Sixt M., Newcombe J., Wekerle H., Hohlfeld R., Lassmann H. et al. (2010). Extracellular matrix in multiple sclerosis lesions: fibrillar collagens, biglycan and decorin are upregulated and associated with infiltrating immune cells. Brain Pathol. 20, 966-975. 10.1111/j.1750-3639.2010.00399.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. A. and Lemischka I. R. (2006). Stem cells and their niches. Science 311, 1880-1885. 10.1126/science.1110542 [DOI] [PubMed] [Google Scholar]
- Moore S. A., Saito F., Chen J., Michele D. E., Henry M. D., Messing A., Cohn R. D., Ross-Barta S. E., Westra S., Williamson R. A. et al. (2002). Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 418, 422-425. 10.1038/nature00838 [DOI] [PubMed] [Google Scholar]
- Moustakas A. and Heldin P. (2014). TGFbeta and matrix-regulated epithelial to mesenchymal transition. Biochim. Biophys. Acta 1840, 2621-2634. 10.1016/j.bbagen.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Mouw J. K., Ou G. and Weaver V. M. (2014). Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 15, 771-785. 10.1038/nrm3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J. M. and Shieh A. C. (2014). Interstitial fluid flow in cancer: implications for disease progression and treatment. Cancer Manag. Res. 6, 317-328. 10.2147/CMAR.S65444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J. M., Bellamkonda R. V. and Swartz M. A. (2013). Interstitial flow in a 3D microenvironment increases glioma invasion by a CXCR4-dependent mechanism. Cancer Res. 73, 1536-1546. 10.1158/0008-5472.CAN-12-2838 [DOI] [PubMed] [Google Scholar]
- Murphy M. C., Huston J. III, Jack C. R. Jr, Glaser K. J., Manduca A., Felmlee J. P. and Ehman R. L. (2011). Decreased brain stiffness in Alzheimer's disease determined by magnetic resonance elastography. J. Magn. Reson. Imaging 34, 494-498. 10.1002/jmri.22707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. C., Jones D. T., Jack C. R. Jr., Glaser K. J., Senjem M. L., Manduca A., Felmlee J. P., Carter R. E., Ehman R. L. and Huston J. III (2016). Regional brain stiffness changes across the Alzheimer's disease spectrum. Neuroimage Clin. 10, 283-290. 10.1016/j.nicl.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan R. K., Kishore P. R. S., Becker D. P., Ward J. D., Enas G. G., Greenberg R. P., Da Silva A. D., Lipper M. H., Choi S. C., Mayhall C. G. et al. (1982). Intracranial pressure: to monitor or not to monitor? A review of our experience with severe head injury. J. Neurosurg. 56, 650-659. 10.3171/jns.1982.56.5.0650 [DOI] [PubMed] [Google Scholar]
- Netti P. A., Berk D. A., Swartz M. A., Grodzinsky A. J. and Jain R. K. (2000). Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 60, 2497-2503. [PubMed] [Google Scholar]
- Nicholson C. and Sykova E. (1998). Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21, 207-215. 10.1016/S0166-2236(98)01261-2 [DOI] [PubMed] [Google Scholar]
- Nieto M. A. (2013). Epithelial plasticity: a common theme in embryonic and cancer cells. Science 342, 1234850 10.1126/science.1234850 [DOI] [PubMed] [Google Scholar]
- Ohata S. and Alvarez-Buylla A. (2016). Planar organization of multiciliated ependymal (E1) cells in the brain ventricular epithelium. Trends Neurosci. 39, 543-551. 10.1016/j.tins.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr A. W., Helmke B. P., Blackman B. R. and Schwartz M. A. (2006). Mechanisms of mechanotransduction. Dev. Cell 10, 11-20. 10.1016/j.devcel.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Padera T. P., Stoll B. R., Tooredman J. B., Capen D., Di Tomaso E. and Jain R. K. (2004). Pathology: cancer cells compress intratumour vessels. Nature 427, 695 10.1038/427695a [DOI] [PubMed] [Google Scholar]
- Paszek M. J., Dufort C. C., Rossier O., Bainer R. O., Mouw J. K., Godula K., Hudak J. E., Lakins J. N., Wijekoon A. C., Cassereau L. et al. (2014). The cancer cell glycocalyx mechanically primes integrin dependent growth and survival. Nature 511, 319-325. 10.1038/nature13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak M. M., Nourse J. L., Tran T., Hwe J., Arulmoli J., Le D. T. T., Bernardis E., Flanagan L. A. and Tombola F. (2014). Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 111, 16148-16153. 10.1073/pnas.1409802111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips H. S., Kharbanda S., Chen R., Forrest W. F., Soriano R. H., Wu T. D., Misra A., Nigro J. M., Colman H., Soroceanu L. et al. (2006). Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9, 157-173. 10.1016/j.ccr.2006.02.019 [DOI] [PubMed] [Google Scholar]
- Pickup M. W., Mouw J. K. and Weaver V. M. (2014). The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 15, 1243-1253. 10.15252/embr.201439246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope W. B., Kim H. J., Huo J., Alger J., Brown M. S., Gjertson D., Sai V., Young J. R., Tekchandani L., Cloughesy T. et al. (2009). Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology 252, 182-189. 10.1148/radiol.2521081534 [DOI] [PubMed] [Google Scholar]
- Pope W. B., Mirsadraei L., Lai A., Eskin A., Qiao J., Kim H. J., Ellingson B., Nghiemphu P. L., Kharbanda S., Soriano R. H. et al. (2012). Differential gene expression in glioblastoma defined by ADC histogram analysis: relationship to extracellular matrix molecules and survival. Am. J. Neuroradiol. 33, 1059-1064. 10.3174/ajnr.A2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsch H., Bernert B., Mehić M., Theocharis A. D., Heldin C.-H. and Heldin P. (2013). Efficient TGFbeta-induced epithelial-mesenchymal transition depends on hyaluronan synthase HAS2. Oncogene 32, 4355-4365. 10.1038/onc.2012.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla L., Lakins J. N. and Weaver V. M. (2016a). Tissue mechanics orchestrate WNT-dependent human embryonic stem cell differentiation. Cell Stem Cell 19, 462-475. 10.1016/j.stem.2016.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla L., Muncie J. M. and Weaver V. M. (2016b). Mechanical control of epithelial-to-mesenchymal transitions in development and cancer. Annu. Rev. Cell Dev. Biol. 32, 527-554. 10.1146/annurev-cellbio-111315-125150 [DOI] [PubMed] [Google Scholar]
- Radner S., Banos C., Bachay G., Li Y. N., Hunter D. D., Brunken W. J. and Yee K. T. (2013). Beta2 and Gamma3 laminins are critical cortical basement membrane components: ablation of Lamb2 and Lamc3 genes disrupts cortical lamination and produces dysplasia. Dev. Neurobiol. 73, 209-229. 10.1002/dneu.22057 [DOI] [PubMed] [Google Scholar]
- Rakic P. (2003). Elusive radial glial cells: historical and evolutionary perspective. Glia 43, 19-32. 10.1002/glia.10244 [DOI] [PubMed] [Google Scholar]
- Ranade S. S., Syeda R. and Patapoutian A. (2015). Mechanically activated ion channels. Neuron 87, 1162-1179. 10.1016/j.neuron.2015.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A. C., Guimond S., Krufka A. and Olwin B. B. (1994). Regulation by heparan sulfate in fibroblast growth factor signaling. Methods Enzymol. 245, 219-240. 10.1016/0076-6879(94)45013-7 [DOI] [PubMed] [Google Scholar]
- Reinhard J., Brosicke N., Theocharidis U. and Faissner A. (2016). The extracellular matrix niche microenvironment of neural and cancer stem cells in the brain. Int. J. Biochem. Cell Biol. [Epub ahead of print] doi:10.1016/j.biocel.2016.05.002. 10.1016/j.biocel.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Reiss-Zimmermann M., Streitberger K.-J., Sack I., Braun J., Arlt F., Fritzsch D. and Hoffmann K.-T. (2015). High resolution imaging of viscoelastic properties of intracranial tumours by multi-frequency magnetic resonance elastography. Clin. Neuroradiol. 25, 371-378. 10.1007/s00062-014-0311-9 [DOI] [PubMed] [Google Scholar]
- Reitman Z. J., Olby N. J., Mariani C. L., Thomas R., Breen M., Bigner D. D., McLendon R. E. and Yan H. (2010). IDH1 and IDH2 hotspot mutations are not found in canine glioma. Int. J. Cancer 127, 245-246. 10.1002/ijc.25017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B., Beggs H. E., Schahin-Reed D., Kimes N., Schmidt A. and Reichardt L. F. (2004). Control of axonal branching and synapse formation by focal adhesion kinase. Nat. Neurosci. 7, 1059-1069. 10.1038/nn1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy L. D., Sahraei M., Subramani D. B., Besmer D., Nath S., Tinder T. L., Bajaj E., Shanmugam K., Lee Y. Y., Hwang S. I. L. et al. (2011). MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 30, 1449-1459. 10.1038/onc.2010.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. (1996). Brain extracellular matrix. Glycobiology 6, 489-492. 10.1093/glycob/6.5.489 [DOI] [PubMed] [Google Scholar]
- Saha K., Keung A. J., Irwin E. F., Li Y., Little L., Schaffer D. V. and Healy K. E. (2008). Substrate modulus directs neural stem cell behavior. Biophys. J. 95, 4426-4438. 10.1529/biophysj.108.132217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M. S., Lopez J. I., McGhee E. J., Croft D. R., Strachan D., Timpson P., Munro J., Schröder E., Zhou J., Brunton V. G. et al. (2011). Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 19, 776-791. 10.1016/j.ccr.2011.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K., Wichterle H., Gonzalez-Perez O., Cholfin J. A., Yamada M., Spassky N., Murcia N. S., Garcia-Verdugo J. M., Marin O., Rubenstein J. L. et al. (2006). New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 311, 629-632. 10.1126/science.1119133 [DOI] [PubMed] [Google Scholar]
- Scholz M., Noack V., Pechlivanis I., Engelhardt M., Fricke B., Linstedt U., Brendel B., Ing D., Schmieder K., Ermert H. et al. (2005). Vibrography during tumor neurosurgery. J. Ultrasound Med. 24, 985-992. [DOI] [PubMed] [Google Scholar]
- Scholz M., Lorenz A., Pesavento A., Brendel B., Khaled W., Engelhardt M., Pechlivanis I., Noack V., Harders A. and Schmieder K. (2007). Current status of intraoperative real-time vibrography in neurosurgery. Ultraschall Med. 28, 493-497. 10.1055/s-2006-927359 [DOI] [PubMed] [Google Scholar]
- Serrels A., Lund T., Serrels B., Byron A., McPherson R. C., von Kriegsheim A., Gómez-Cuadrado L., Canel M., Muir M., Ring J. E. et al. (2015). Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell 163, 160-173. 10.1016/j.cell.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. J. and Iliff J. J. (2016). Regulation of cerebrospinal fluid (Csf) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim. Biophys. Acta 1862, 442-451. 10.1016/j.bbadis.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singha N. C., Nekoroski T., Zhao C., Symons R., Jiang P., Frost G. I., Huang Z. and Shepard H. M. (2015). Tumor-associated hyaluronan limits efficacy of monoclonal antibody therapy. Mol. Cancer Ther. 14, 523-532. 10.1158/1535-7163.MCT-14-0580 [DOI] [PubMed] [Google Scholar]
- Smith D. H., Johnson V. E. and Stewart W. (2013). Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol. 9, 211-221. 10.1038/nrneurol.2013.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleman S., Filippov M. A., Dityatev A. and Fawcett J. W. (2013). Targeting the neural extracellular matrix in neurological disorders. Neuroscience 253, 194-213. 10.1016/j.neuroscience.2013.08.050 [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L. and Sepich D. S. (2012). Gastrulation: making and shaping germ layers. Annu. Rev. Cell Dev. Biol. 28, 687-717. 10.1146/annurev-cellbio-092910-154043 [DOI] [PubMed] [Google Scholar]
- Spicer A. P., Joo A. and Bowling R. A. Jr (2003). A Hyaluronan binding link protein gene family whose members are physically linked adjacent to chrondroitin sulfate proteoglycan core protein genes: the missing links. J. Biol. Chem. 278, 21083-21091. 10.1074/jbc.M213100200 [DOI] [PubMed] [Google Scholar]
- Stocchetti N. and Maas A. I. R. (2014). Traumatic intracranial hypertension. N. Engl. J. Med. 370, 2121-2130. 10.1056/nejmra1208708 [DOI] [PubMed] [Google Scholar]
- Streitberger K.-J., Reiss-Zimmermann M., Freimann F. B., Bayerl S., Guo J., Arlt F., Wuerfel J., Braun J., Hoffmann K.-T. and Sack I. (2014). High-resolution mechanical imaging of glioblastoma by multifrequency magnetic resonance elastography. PLoS ONE 9, e110588 10.1371/journal.pone.0110588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Yong K. M. A., Villa-Diaz L. G., Zhang X., Chen W., Philson R., Weng S., Xu H., Krebsbach P. H. and Fu J. (2014). Hippo/Yap-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat. Mater. 13, 599-604. 10.1038/nmat3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram H., Voigts B., Beer K. and Meland M. (2010). Comparison of the rheological properties of viscosity and elasticity in two categories of soft tissue fillers: calcium hydroxylapatite and hyaluronic acid. Dermatol. Surg. 36 Suppl. 3:1859-1865. 10.1111/j.1524-4725.2010.01743.x [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kanamaru K., Shiba M., Fujimoto M., Kawakita F., Imanaka-Yoshida K., Yoshida T. and Taki W. (2015). Tenascin-C is a possible mediator between initial brain injury and vasospasm-related and -unrelated delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Acta Neurochir. Suppl. 120, 117-121. 10.1007/978-3-319-04981-6_20 [DOI] [PubMed] [Google Scholar]
- Tavazoie M., Van Der Veken L., Silva-Vargas V., Louissaint M., Colonna L., Zaidi B., Garcia-Verdugo J. M. and Doetsch F. (2008). A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3, 279-288. 10.1016/j.stem.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Shepard H. M., O'Connor P. M., Kadhim S., Jiang P., Osgood R. J., Bookbinder L. H., Li X., Sugarman B. J., Connor R. J. et al. (2010). Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol. Cancer Ther. 9, 3052-3064. 10.1158/1535-7163.MCT-10-0470 [DOI] [PubMed] [Google Scholar]
- Traber P. G., Chou H., Zomer E., Hong F., Klyosov A., Fiel M.-I. and Friedman S. L. (2013). Regression of fibrosis and reversal of cirrhosis in rats by Galectin inhibitors in thioacetamide-induced liver disease. PLoS ONE 8, e75361 10.1371/journal.pone.0075361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler W. J. (2012). The mechanobiology of brain function. Nat. Rev. Neurosci. 13, 867-878. 10.1038/nrn3383 [DOI] [PubMed] [Google Scholar]
- Tzima E., Del Pozo M. A., Shattil S. J., Chien S. and Schwartz M. A. (2001). Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 20, 4639-4647. 10.1093/emboj/20.17.4639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E., Del Pozo M. A., Kiosses W. B., Mohamed S. A., Li S., Chien S. and Schwartz M. A. (2002). Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 21, 6791-6800. 10.1093/emboj/cdf688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich T. A., de Juan Pardo E. M. and Kumar S. (2009). The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 69, 4167-4174. 10.1158/0008-5472.CAN-08-4859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaak R. G., Hoadley K. A., Purdom E., Wang V., Qi Y., Wilkerson M. D., Miller C. R., Ding L., Golub T., Mesirov J. P. et al. ; Cancer Genome Atlas Research Network. (2010). Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98-110. 10.1016/j.ccr.2009.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J. A., Stys P. K., Lusardi T., Meaney D. and Smith D. H. (2001). Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J. Neurosci. 21, 1923-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Lin Y., Han J. C., Xi Z. N., Shen H. and Gao P. Y. (2007). Magnetic resonance elastography of brain tumors: preliminary results. Acta Radiol. 48, 327-330. 10.1080/02841850701199967 [DOI] [PubMed] [Google Scholar]
- Yang C., Liu Y., He Y., Du Y., Wang W., Shi X. and Gao F. (2013). The use of HA oligosaccharide-loaded nanoparticles to breach the endogenous hyaluronan glycocalyx for breast cancer therapy. Biomaterials 34, 6829-6838. 10.1016/j.biomaterials.2013.05.036 [DOI] [PubMed] [Google Scholar]
- Yiu G. and He Z. (2006). Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 7, 617-627. 10.1038/nrn1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Kim H. Y. and Davidson L. A. (2009). Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development 136, 677-688. 10.1242/dev.026211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann D. R. and Dours-Zimmermann M. T. (2008). Extracellular matrix of the central nervous system: from neglect to challenge. Histochem. Cell Biol. 130, 635-653. 10.1007/s00418-008-0485-9 [DOI] [PubMed] [Google Scholar]