ABSTRACT
Cells dynamically assemble and organize into complex tissues during development, and the resulting three-dimensional (3D) arrangement of cells and their surrounding extracellular matrix in turn feeds back to regulate cell and tissue function. Recent advances in engineered cultures of cells to model 3D tissues or organoids have begun to capture this dynamic reciprocity between form and function. Here, we describe the underlying principles that have advanced the field, focusing in particular on recent progress in using mechanical constraints to recapitulate the structure and function of musculoskeletal tissues.
KEY WORDS: 3D model, Microtissue, Contractility, Extracellular matrix, TFM
Summary: Engineered 3D cultures of cells have mainly focused on epithelial tissues. In this Commentary, advances in engineered microtissue systems to model fibrous tissues under mechanical load are discussed.
Introduction
Cells have been cultured ex vivo for over a century (Carrel and Burrows, 1911; Harrison, 1959), providing a key platform for the elucidation of many key cellular processes, such as mitosis, transcription, translation and migration. Although complex multicellular tissue functions, such as immunity and development, have largely been reserved for studies in animals, recent advances in the culture of cells within 3D contexts has enabled us to begin to model more complex behaviors and tissue functions (Schmeichel and Bissell, 2003).
Recent advances in inducing pluripotency in stem cells – differentiating these cells into tissue-specific progenitors and then allowing these cells to organize in a 3D setting – have resulted in a number of ‘organoid’ models (Clevers, 2016). In many ways, these models emerge through the recapitulation of developmental stages – embryonic development begins with a single totipotent cell that expands to a staggering 5 trillion to 10 trillion cells in a human newborn. Important early tissue-forming events during this process occur in cellular condensations and are strongly dependent on cell–cell interactions (White and Plachta, 2015). The earliest studies of cellular condensation in developing embryos identified the Spemann organizer, a cell aggregate in the amphibian blastula that induces neural differentiation in embryonic cells, formation of the neural plate and defines the anterior–posterior axis of the embryo (De Robertis, 2006). Similarly, mesenchymal progenitor cells cluster into mesenchymal condensations before differentiating into chondrocytes and osteoblasts, and odontoblasts during limb and tooth development (Hall and Miyake, 2000; Mammoto et al., 2011).
It is therefore not surprising that scientists have been able to induce cells to recapitulate many early stages of morphogenesis in a variety of tissues by simply culturing progenitor populations in aggregates. One classic example of such a system is the embryoid body that has been generated by culturing clusters of embryonic stem cells (or induced pluripotent stem cells) and allowed to differentiate into teratoma-like masses (Itskovitz-Eldor et al., 2000; Thomson et al., 1998). Although such systems have been used as models for generating early ‘tissues’, including developing brain structures (Muguruma et al., 2015, 2010), maturation of tissues into their more adult forms and functions is also characterized by the substantial presence of extracellular matrix (ECM).
The ECM constitutes the scaffolding material that cells synthesize, secrete and deposit to form tissues, and is an important component of many tissues as development proceeds (Bonnans et al., 2014; Lu et al., 2011; Rozario and DeSimone, 2010). Once deposited, cells adhere to the ECM and release soluble proteins, such as growth factors and cytokines, that are sequestered within the ECM and affect cell signaling and function. As the embryo continues to develop and grow, more ECM is produced, and thus the amount of information stored in the ECM progressively increases. This bi-directional interaction of cells with the ECM to define ECM composition and tissue architecture on the one hand, and the ECM regulation of cellular behavior through growth factor signaling and cell–matrix interactions on the other hand, coined ‘dynamic reciprocity’, constitutes a central paradigm with regards to the regulation of tissue development, homeostasis and pathology (Bissell et al., 1982; Nelson and Bissell, 2006).
Engineered three-dimensional (3D) cultures incorporating ECM and embedded cells provides a means to model more mature tissues. Early examples of such organotypic models include the use of Matrigel™ with epithelial cells to model mammary acini (Barcellos-Hoff et al., 1989), lung (Schuger et al., 1990), salivary gland (Hoffman et al., 1996) and intestine (Sanderson et al., 1996). More recently, coupling these approaches with advances in induced pluripotent stem cell (iPSC) technology and stem cell biology have resulted in numerous additional organoid models. For example, neuro-epithelial differentiated human embryonic stem cells cultured in small clusters embedded in Matrigel™ spontaneously form stratified retinal-like structures (Nakano et al., 2012), and similarly, neuronal stem cells can stratify to form structures that are reminiscent of the cortex of the brain (Lancaster et al., 2013). Similar organoids, also sometimes referred to as microtissues, have been developed to study morphogenesis and function of lung, kidney, salivary gland, liver, skin and blood vessels (reviewed in Clevers, 2016; Passier et al., 2016). Many of these models have been widely adopted and successfully used to capture the functional behavior of numerous epithelia.
In contrast to epithelial cells, which line surfaces and glands throughout the body, mesenchymal stromal cells play a central role in depositing and crosslinking large amounts of diverse ECM proteins in many tissues, including bone, muscle, tendon and skin. Mechanical cues within the ECM play an unusually central role in the terminal differentiation, maturation and healing responses in these cells. For example, mechanical loading of the provisional cartilage matrix in the growth plate is an essential regulator of the columnar organization and maturation of chondrocytes to direct longitudinal growth of appendicular bones (Carter et al., 1987; Kronenberg, 2003). Similarly, loading forces within collagen-type-I-rich bone matrix promotes terminal differentiation of osteoblasts (Papachroni et al., 2009; Sittichockechaiwut et al., 2009). Indeed, the mechanical load generated by fetal muscle cell activity is thought to be crucial for the maintenance and maturation of all connective tissues. Importantly, this prominent role of forces in the maturation and maintenance of tissues continues throughout life to regulate tissue adaptation to mechanical loading (Kjaer, 2004). Therefore, to model stromal and musculoskeletal tissues, a specialized class of 3D culture models has been developed that introduces forces and physical constraints, which are crucial determinants of connective tissue function.
Given a number of excellent reviews regarding 3D culture models in general (Passier et al., 2016; Shamir and Ewald, 2014), as well as those specifically focused on stem-cell-based organoid cultures (Clevers, 2016) and classic epithelial organotypic culture models (Schmeichel and Bissell, 2003), in this Commentary, we will focus on engineered microtissue systems that have been developed to model tissues under mechanical load. We will first examine the principal mechanisms underlying microtissue formation, and discuss the structural organization and mechanics of assembled microtissues. Then, we will explore the use of engineered microtissues to emulate tissue-specific functions in vitro. Lastly, we will conclude with a perspective on how microtissues could provide a crucial platform to study tissue morphogenesis, homeostasis and disease.
Principles for forming 3D microtissues in vitro
Microtissues, with or without the addition of ECM, are generated by allowing a suspension of cells to aggregate before cells can spontaneously undergo compaction, a process wherein cells adhere to one another and/or to their surrounding ECM, and contract, thereby increasing the density of the microtissue.
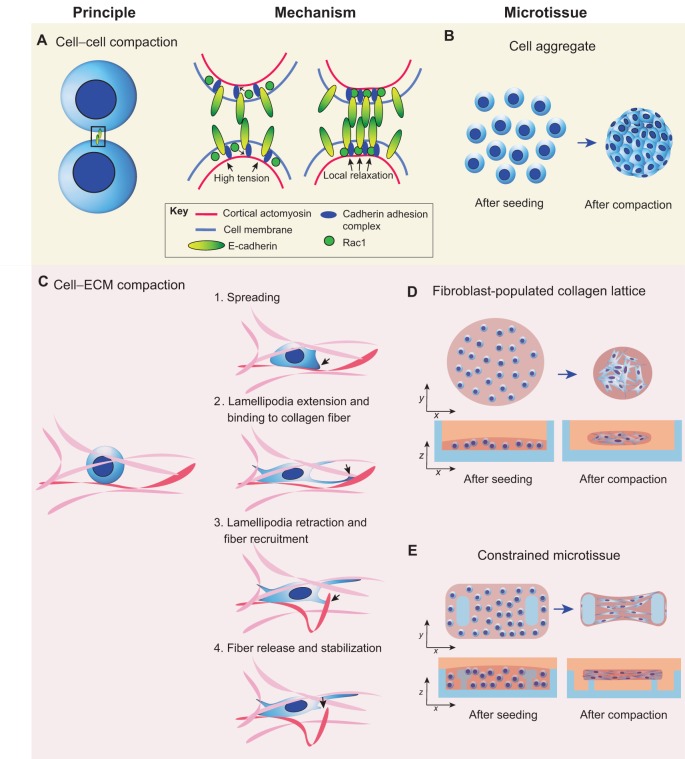
In cellular aggregates, epithelial cells mainly interact through cadherins, a family of transmembranous cell–cell adhesion proteins that, upon engagement, form clusters and physically link neighboring cells. In suspension, cells are spherical owing to the cortical tension of the submembranous cytoskeleton that drives the surface tension of the lipid cell membrane (Cavey and Lecuit, 2009; Manning et al., 2010). When cadherins engage, for instance by touching other cells, the GTPase Rac1 becomes activated and reduces the cortical tension around the cadherin complexes (Maitre et al., 2012; Yamada and Nelson, 2007). The reduction in cortical tension flattens the cell membrane locally, thus increasing the adhesive surface area; this engages more cadherins and strengthens the adhesion between the cells (Fig. 1A). Thus, the reduction in cortical tension is essential for the attachment of cells over extended contact areas and determines the cell shape in aggregates (Fig. 1B) and tissues (Winklbauer and Parent, 2016).
Fig. 1.
Mechanical basis of microtissue formation coupled with high-level microtissue architecture. (A) Schematic overview of cell aggregate formation. Epithelial cell–cell contact is initiated by engagement of E-cadherin adhesion proteins. Upon binding of the extracellular domain of E-cadherin to other cadherins, the intracellular cadherin adhesion complex connects the intracellular domain of E-cadherin to cortical actin. At the same time, activated Rac1 suppresses cortical actomyosin activity, which locally decreases the cortical tension at the junction. This decrease in cortical tension flattens the cell membrane, thus increasing the adhesive surface between two cells, and more juxtapositional cadherins are activated, reinforcing cell–cell contact. When multiple cells are involved, this mechanism drives the formation of aggregates (B). (C) Schematic overview of cell–ECM interactions during microtissue formation. Fibroblasts seeded in a fibrous collagen matrix (fibers are pink, red fiber is fiber of interest) spread in the matrix. After extension of lamellipodia (black arrow) and binding to a collagen fiber (red), the lamellipodia retracts and the fiber is recruited and released once it reaches the cell body, where it is crosslinked with the surrounding matrix. This mechanism drives the compaction of (D) unconstrained fibroblast-populated collagen lattices (blue, culture dish) and (E) constrained microtissues (blue, culture device with pillars).
Intriguingly, differences in the strength of adhesions between cells of the same type (homotypic) or different populations (heterotypic) drive cellular rearrangements in aggregates composed of mixed cell populations, a process known as cell sorting (Steinberg, 1963). Typically, strongly self-cohesive cells will cluster in the core of the aggregate and weakly cohesive cells arrange at the surface (Steinberg, 1963, 1970; Steinberg and Takeichi, 1994). Although cell sorting driven by differential adhesion has been proposed to control germ layer separation during gastrulation (Krieg et al., 2008) and mammary gland assembly (Chanson et al., 2011), reconstituting these tissues in vitro results in a spatial organization of the cells opposite to what is found in vivo (Cerchiari et al., 2015; Krieg et al., 2008), suggesting that other factors regulate cell sorting. Indeed, when encapsulating a heterotypic cell aggregate with an ECM that anchors the most self-cohesive cell type, therefore preventing movement of this cell type to the core of the aggregate, the spatial organization is restored (Cerchiari et al., 2015). Thus, in the presence of ECM, cell–ECM adhesion can be dominant over cell–cell cohesion, which determines the final tissue organization after cell sorting. Harnessing this principle of self-assembly could provide a strategy to engineer layered microtissues in vitro.
Unlike cell aggregates, which are assembled through cell–cell adhesions, stromal cells that have been seeded in collagen matrices form microtissues through cell–matrix adhesion molecules. After seeding fibroblasts in a collagen matrix, α2β1 integrins at the surface of the cells engage, anchor the cell to the matrix and initiate spreading. During cell spreading, fibroblast lamellipodia extend along the polymerized collagen fibers and, upon binding, retract, thereby transporting the fiber towards the center of the cell where it is released (Fig. 1C). This dynamic transport of collagen fibers in 3D is directly controlled by myosin IIB activity (Meshel et al., 2005). After release, the fibers are stabilized by the cells, and as such the contracted collagen matrix maintains its shape even when cells are removed (Guidry and Grinnell, 1987). Such a remodeling mechanism is essential for preserving tissue architecture and allows the migration of cells without any recoil of the fibrous matrix into its original loose state.
In free-floating aggregates and hydrogels, adhesive and contractile forces shape the tissue into isotropic spherical structures (Fig. 1D). Although such systems can provide a 3D environment that induces some cell types to behave in a more similar manner to that of their in vivo counterparts, many tissues in the body are under a considerable mechanical load that alters matrix structure and cell function. Albert Harris was perhaps the first to systematically model this loading by anchoring fibroblast-laden collagen gels with pins in the culture dish (Stopak and Harris, 1982). With two pins, compaction of the gels results in alignment of the ECM and cells along the axis of the mechanical stress between pins, mimicking structures such as the tendon; with four pins or more, compaction results in a planar structure, mimicking flat tissues such as skin. Given that needles can be most simply placed in a collagen gel before the onset of compaction, this method is only practical when the dimensions of the tissues are in the centimeter scale. Numerous methods have since been developed to scale the approach down to sub-millimeter scale microtissues. By using microfabrication techniques, our laboratory has engineered a culture vessel that comprises hundreds of microwells, each containing two or more flexible elastomer pillars (Legant et al., 2009). Through successive centrifugation steps, cells in a soluble collagen matrix are spun into the microwells and form microtissues after the collagen has been polymerized (Fig. 1E) (Legant et al., 2009; Sakar et al., 2016). Using this method, tissue shape can be controlled by changing the spacing and angle between the pillars in the mold design of the culture vessel.
In addition to geometrical spacing of the pillars, microtissue shape can also be determined by the pillar profile. As cells compact the matrix around the pillars, the tissue tension increases and causes the tissue to move upwards along the pillar until it slips off. By manufacturing T-shaped pillars, the tissue is prevented from slipping off and thus the tissue boundaries are outlined by the spacing of the pillars (Kalman et al., 2016; Legant et al., 2009). However, by introducing conical shaped pillars, one can control where and when the tissue is released from a pillar, and thus change the shape of the microtissue over time (Svoronos et al., 2014). Although pillar spacing and profiles are two variables that control tissue geometry, other factors, such as microwell size and shape, still need to be characterized in order to establish the design rules for controlling the assembly and maintenance of stromal microtissues (Svoronos et al., 2014).
Tissue architecture in stromal microtissues
In contrast to free-floating collagen gels, anchoring tissues to pillars prevents isotropic contraction of the tissue. As a consequence, the cells and the matrix align according to the direction of the principal stresses, which are dictated by the geometry of the tissue (Schell et al., 2016). In other words, cells and matrix are highly uniaxially aligned in tissues anchored to two pillars and randomly organized in equibiaxial hexagonal tissues (Obbink-Huizer et al., 2014; Schell et al., 2016). Given that fibroblasts can bind and apply forces to different ECM molecules, fiber alignment is not restricted to the exogenous collagen matrix alone, but also applies to other ECM proteins, such as tenascin-C, collagen type II and fibronectin (Legant et al., 2012; Schell et al., 2016).
In vivo, cells employ fibronectin in order to assemble a provisional template that directs the assembly of more permanent collagen fibers (Kadler et al., 2008; Sottile et al., 2007). Unlike during de novo tissue morphogenesis, in vitro microtissues are compacted with collagen that has been already introduced into the system. During tissue compaction, soluble fibronectin from medium is quickly absorbed and is stabilized by the collagen fibers. Nonetheless, stromal cells eventually assemble fibrillar fibronectin – a combination of plasma and cellular fibronectin – as they remodel the tissue. Initially, this activity is concentrated predominantly at the periphery of the tissue (Legant et al., 2012).
Intriguingly, full-length fibrillar fibronectin that is concentrated at the periphery drives migration of mouse embryonic fibroblasts (MEFs) to the periphery of the microtissue, resulting in an accumulation of cells at the surface of the tissue (Foolen et al., 2016). Although the mechanism is unclear, it is likely that cells at the periphery of the tissue engage α5β1 integrins to interact with the full-length fibronectin; this activates downstream RhoA–Rock–myosin-II signaling, thus increasing contractility as well as adhesion maturation and stability (da Rocha-Azevedo et al., 2013; Schiller et al., 2013). We speculate that localization of cells to the periphery could be due to multiple factors – haptotactic sensing of the higher peripheral levels of fibronectin, a preference for the strongly aligned ECM fibers at the periphery versus less-aligned ECM in the tissue core – perhaps due to tensional remodeling (Abhilash et al., 2014) – or even a durotactic preference for increased ECM stiffness at the periphery due to changes in ECM alignment, composition or crosslinking (Baker et al., 2015; Pelham and Wang, 1997; Plotnikov et al., 2012). Thus, the use of a microtissue system could provide insights into how gradients in provisional matrix assembly lead to changes in cell and matrix distribution.
Interestingly, in contrast to collagen gels, fibroblasts remain in the bulk matrix when embedded in fibrin (Midwood and Schwarzbauer, 2002). Conversely, chondrocytes don't segregate in collagen type-I gels (Galois et al., 2006). Thus, in analogy to cell–cell sorting (Steinberg, 1970), not all combinations of cells and ECM lead to spatial segregation of cells. We propose that a systematic screening of cells in hydrogels of different ECM composition in constrained microtissues could serve as a model system to investigate the molecular mechanisms underpinning how cells organize as a function of their surrounding matrix.
Tissue mechanics of stromal microtissues
As discussed above, cell-generated contractile forces drive tissue compaction and cell–ECM alignment, two mechanisms that modulate tissue architecture. Conversely, the physical properties of the ECM, such as geometry (Théry et al., 2006), rigidity (Califano and Reinhart-King, 2010; Saez et al., 2007) and topography (Ghibaudo et al., 2009; Schvartzman et al., 2011), feed back to modulate cell contractility, which in turn regulates cell shape (Murrell et al., 2015), migration (Doyle et al., 2015) and differentiation (Engler et al., 2006; Kilian et al., 2010; McBeath et al., 2004). Hence, to investigate this mechanical reciprocity between cells and ECM during tissue morphogenesis, one needs to be able to measure the contractile forces of the cells within their 3D environment.
Although cell-generated contractile forces cannot be directly measured, they can be calculated from the deformations they impose on compliant structures. Indeed, contracting microtissues bend the free end of the anchoring pillars or needles towards the center of the tissue (John et al., 2010; Legant et al., 2009; Zimmermann et al., 2000). Provided that the pillar stiffness is well characterized and the displacement of the top of the pillar is measured, one can calculate the tissue contractile force using beam theory (Legant et al., 2009; Polacheck and Chen, 2016). In contrast to estimating forces exerted by single cells using conventional 3D traction force microscopy (TFM) – a technique measuring displacement of fluorescent markers surrounding cells embedded in an elastic matrix (Legant et al., 2010) – deflection of the pillars is the result of the sum of all cell-generated forces within the microtissue (Polacheck and Chen, 2016). Hence, to estimate the contractility of a single cell in microtissues, the total contractility is divided by the number of cells present in the microtissue. Assuming that forces are distributed homogenously throughout the tissue, this straightforward method yields a meaningful approximation of cellular contractility in two-pillar uniaxial microtissues given that most cells are aligned parallel to the long axis of the tissue (John et al., 2010).
Using this TFM method, cellular contractility has been assessed in collagen matrices for a variety of cells, including NIH/3T3 fibroblasts (Legant et al., 2009), human foreskin fibroblasts (Delvoye et al., 1991), lung fibroblasts (Chen et al., 2016b), cardiomyocytes (Boudou et al., 2012; Zimmermann et al., 2000) and valvular interstitial cells (Kural and Billiar, 2014). Depending on the culture conditions, cell type and method of force measurement, the obtained values for force generation per cell ranges from approximately 0.1 to 450 nN/cell (John et al., 2010). Interestingly, despite this large variation in reported contractile forces, all cell types exhibit higher contractility when anchored to stiff pillars compared to more compliant pillars. Cells thus actively sense the boundary stiffness of the tissue and respond by adapting their contractility. Furthermore, temporary ‘stiffening’ of the pillar through attachment of a magnetic bead and placement within a magnetic field, thereby preventing bending, increases contractility of valvular interstitial cells. Surprisingly, this increased contractility persists even after ‘softening’ the pillar by removing the magnet (Kural and Billiar, 2016). Although not well understood, such mechanisms that involve persistence of cell contraction after reduction of the mechanical load could be important drivers in the progression of matrix-related diseases, including fibrosis (Wells, 2013) and cancer (Schedin and Keely, 2011).
In addition to cell contractility, matrix stiffness is an equally important mechanical variable that regulates cell behavior (Discher et al., 2005). Using a magnetic actuation platform and biochemical treatments to perturb cellular contractility, Zhao and colleagues have decoupled the contribution of cells and collagen to the overall stiffness of the microtissue in response to mechanical load (Zhao et al., 2013). Similar to changing the pillar stiffness, they found that under static load, the collagen matrix is the primary determinant of the tissue stiffness and that the cells adjust their own stiffness to match that of the matrix. In contrast, under short-term cyclic loading, changes in cell contractility predominantly contribute to the stiffness increase of the microtissues. These findings highlight that in response to mechanical loading, cells not only increase their contractility, but also remodel the structure of their matrix irreversibly. This reciprocal regulation between cell contractility and matrix remodeling in response to mechanical load is a fundamental mechanism of mechanically regulated tissue maturation, wherein the remodeled ECM echoes the mechanical history of the cells.
Despite the quintessential role of contractile forces in tissue formation and remodeling, the same forces can cause necking and failure of a microtissue (Wang et al., 2013). Indeed, maintaining microtissues on the pillars for periods of more than four days is a major challenge. In analogy to pulling a rubber band, tissue failure occurs when the mechanical stresses that are generated by cellular forces and the boundary conditions are higher than the ultimate tensile strength of the ECM. Owing to the reciprocal regulation of cell contractility and the mechanical properties of the ECM, any approach to alter cellular contractility (e.g. by using cytoskeletal inhibitors) or ECM remodeling (with matrix metalloproteinase inhibitors or crosslinking enzymes) is likely to maintain any mechanical imbalances and lead to morphological instability and failure (West et al., 2013). Instead, tissue stability can be improved by changing the ratio between the cells and ECM, such as seeding supporting cells in the matrix (West et al., 2013) or varying the amount of ECM, or by tuning the stiffness of the constraining pillars (Wang et al., 2013). Given the large spread of cellular contractility across cell types (0.1 to 450 nN/cell), microtissue cultures thus need to be optimized for each cell type in order to maintain the tissue stability that is necessary to enable study of tissue morphogenesis over longer time scales.
Implementation of mechanically constrained microtissues within tissue-specific contexts
In the human body, most, if not all tissues experience mechanical loading during physical activity; at the same time, the cells within these tissues pull, push, bend and twist their surrounding ECM. As discussed above, constrained microtissues have comparable mechanical dynamics and might therefore be a universal model system in which to study the behavior of any matrix-adherent cell type. However, as with most in vitro models, inspiring scientific advancements only emerge when a model system captures organ function on a systems level. Therefore, as examples, we will discuss recent advances in wound healing and cardiac morphogenesis, two fields in which constrained microtissues have enabled a deeper exploration of organ level function in vitro.
After injury, open adult skin wounds undergo dramatic morphological changes involving formation of granulation tissue, tissue contraction and re-epithelialization to close the wound and restore tissue architecture (Martin, 1997). To parse out the myriad of mechanisms driving dermal wound healing, in vitro models have been developed to emulate particular stages of the wound healing process. For example, the biology seen in the widely adopted 2D scratch wound assay (Liang et al., 2007), a model involving ‘scratching’ of a monolayer of cells with a pipette tip and measuring the time needed for the cells to invade and cover the ‘scratch’, is most closely related to the re-epithelialization of wound beds in vivo. In a different model, namely fibroblast-populated collagen lattices (FPCLs), dermal fibroblasts embedded in a collagen gel spread and contract the gel, mimicking the role of granulation tissue in wound contraction and scarring (Bell et al., 1979; Carlson and Longaker, 2004). Both models have substantially advanced our knowledge of the tissue mechanics during wound healing; however, they lack essential features of the physical process of wound closure. Scratch wound assays display ‘closure’ but don't capture the deformation of the entire tissue, which brings the wound margins together and is associated with gap closure. Conversely, FPCLs exhibit entire tissue deformations but no closure.
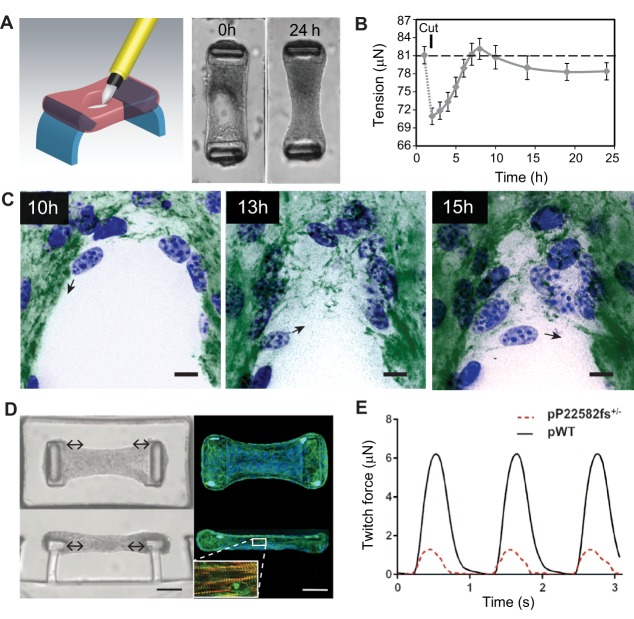
Using constrained microtissues, we have recently developed an in vitro wound healing model that captures multiple stages of wound closure, including tissue contraction and gap closure (Sakar et al., 2016). In this model, suspended microtissues that comprised NIH/3T3 fibroblasts and collagen were damaged with a microdissection knife, leaving an open hole in the tissue (Fig. 2A). Analogous to opening of a skin cut after injury, the hole in the microtissue widens, thereby lowering the tissue stress of the matrix (Fig. 2B). Subsequently, fibroblasts in the collagen matrix start to contract and so bring the margins of the wound closer, before migrating cells enter into the gap area and assemble a provisional fibrous fibronectin template bridging the gap (Fig. 2C). The fibronectin template enables more cells to move in and to restore the multi-layered cellular organization of the microtissue. Despite its fundamental role in gap closure, it is yet unclear to what extent the fibronectin template mirrors granulation tissue in vivo. Given that other matrix molecules that are typically found in granulation tissue, such as tenascin-C and collagen type-I, are deposited on the original rat tail collagen in microtissues (Legant et al., 2009), we hypothesize that the fibronectin template continues to remodel into a ‘mature’ granulation tissue, which, if true, would further validate the use of this model for studying the early stages of dermal tissue repair.
Fig. 2.
Application of constrained microtissues in wound healing and cardiac morphogenesis studies. Microtissues under tension exhibit entire tissue contractions and matrix remodeling during wound closure. (A) Using a microsurgical knife, full-thickness wounds are made in microtissues that are anchored to two flexible pillars (blue). Within 24 h, NIH/3T3 fibroblasts close the open gaps and restore tissue integrity. (B) Quantification of tissue contractility by measuring the deflection of the pillars shows that wound closure in microtissues is a staged process comprising relaxation after damage that is followed by tissue contraction and a steady-state sub-baseline tension stage. Error bars, s.e.m.; dashed line represents baseline tension before microtissue damage. (C) During the latter stage, fibroblasts (blue, nuclei) tow fibronectin (green) from the microtissue and assemble a provisional fibronectin template to bridge the gap, which serves as a substrate on which cells migrate further into the gap area (black arrows indicate migration direction of the cell). Scale bars: 25 µm. Panels A–C are adapted from Sakar et al., 2016 under CC BY 4.0 licence. In addition to wound healing, constrained microtissues have been employed to model cardiac function of human iPSC-differentiated cardiomyocytes. (D) Microscopy images [brightfield (left) and fluorescence (right; blue, nuclei; green, phalloidin)] of iPSC-cardiac microtissue (iPSC-CMT) suspended between two flexible pillars from top-down (upper) and side (lower) views. Upon maturation, iPSC-CMT forms aligned sarcomere structures (inset D; red, F-actin; green, α-actinin A) that beat synchronously and dynamically pull the caps of the pillars to the center of the tissue (double-headed arrows). Scale bars: 50 µm. (E) By measuring the deflection of the pillars, twitch force, a metric for cardiac function, can be assessed in iPSC-CMTs constituted from wild-type human iPSC-cardiomyocytes (pWT) or from individuals with mutations in titin, a structural sarcomere protein (pP22582fs+/−). Panels D and E are adapted from Hinson et al., 2015 with permission from AAAS.
Early in vitro models simulating cardiac morphogenesis, such as the hanging drop culture and spheroid techniques, have been successfully used to study molecular mechanisms during cardiomyocyte differentiation of embryonic stem cells (ESCs) (Sargent et al., 2009) and, more recently, iPSCs (Beauchamp et al., 2015). A hallmark of differentiated cardiac cells is the formation of sarcomeres, the fundamental contractile unit of cardiomyocytes, and ensuing spontaneous beating. Unlike heart muscle tissue, cellular and ECM organization in hanging drop cultures and spheroids is random, therefore hampering efficient mechanical output, which is crucial for cardiac function in vivo. In contrast, in uniaxial microtissues, cardiomyocytes within the collagen matrix uniformly align along the long axis of the tissue, resulting in aligned sarcomeres and synchronous beating of the cardiac cells (Boudou et al., 2012; Thavandiran et al., 2013; van Spreeuwel et al., 2014). Hence, this is a good example of how ECM alignment dictates cellular architecture and function.
In addition to providing aligned cardiac structures, constrained microtissues also make it possible to quantify the contractile forces generated by the cardiomyocytes. In clinical settings, the contractility of the heart is related to the cardiac output, a diagnostic metric to assess heart disease. Thus, in combination with iPSC technology, constrained microtissues could be a powerful model to investigate cardiac pathologies in human cells. Indeed, using constrained microtissues, Hinson and colleagues have demonstrated that human iPSCs with certain missense mutations in titin, a structural sarcomere protein, result in impaired contractility (Fig. 2D,E) and responses to β-adrenergic stress that are similar to those in individuals that develop dilated cardiomyopathy owing to truncated titin variants (Hinson et al., 2015). Moreover, highly aligned microtissues with human pluripotent-stem-cell-derived cardiomyocytes have enabled the development of a tachycardic model of arrhythmogenesis, an aspect of cardiophysiology that has not been previously recapitulated in other microtissue models (Thavandiran et al., 2013). Given these examples, constrained microtissues could one day serve as a platform for the screening and testing of small compounds for heart-regeneration therapies and to assess off-target effects of non-cardiac drugs.
In addition to skin and heart, the use of constrained microtissues is currently being explored to model several other tissues. In particular, progress has been made in developing a model for skeletal muscle (Juhas et al., 2014; Sakar et al., 2012; Uzel et al., 2014), which could be useful for understanding and modeling dystrophic muscle diseases. Constrained microtissues also have been applied to model airway smooth muscle (West et al., 2013), aortic valve tissue (Kural and Billiar, 2016) and lung (Chen et al., 2016b). Looking forward, it is anticipated that this platform could provide a common platform for recapitulating the biology of numerous connective tissues.
Conclusions and perspectives
Despite their broad potential for application in cell biology, constrained microtissues have not yet been widely adopted by standard biology laboratories, in part because fabrication and access to the devices required to constrain 3D cultures involves substantial expertise and specialized equipment to generate them (Polacheck and Chen, 2016). However, new protocols to facilitate the fabrication process of silicon masters are being developed (Kalman et al., 2016). Furthermore, evolving 3D printing techniques are approaching the resolution needed to accurately print the molds of microtissue devices and could thus replace current microfabrication methods in the near future. Therefore, we anticipate that it will only be a matter of time before microtissue culture becomes a widely accessible culture model for biology laboratories.
Although efforts are being made to make microtissue culture more accessible, there is also a need to engineer heterotypic microtissues to better emulate in vivo cellular microenvironments (Schmeichel and Bissell, 2003). On the one hand, a deeper understanding of how cell types organize with respect to each other would enable better control of how they organize in engineered microtissues; on the other, engineering technologies continue to advance to allow specification of the initial arrangements of cells in a 3D environment. For example, a recent set of studies has used DNA-complementarity to synthetically enforce adhesion between distinct cell populations, or between cells and material, in order to organize populations into unique arrangements (Chen et al., 2016a; Gartner and Bertozzi, 2009; Todhunter et al., 2015). Ultimately, the ability to organize multiple cell types would allow for the incorporation of stromal populations in the context of a parenchymal tissue, for example to model fibrosis in heart muscle, liver or lung, or during cancer progression. Overall, it is now clear that providing culture environments that can recapitulate aspects of in vivo 3D architecture and mechanics will lead to a crucial platform for deeper understanding of how tissues form, maintain homeostatic function and spiral into pathologic states.
Acknowledgements
We'd like to thank William Polacheck for critical reading of this manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported in part by grants from the National Institutes of Health (HL080494), National Institute of Biomedical Imaging and Bioengineering (EB017103), and the National Science Foundation (CMMI-1462710). Deposited in PMC for release after 12 months.
References
- Abhilash A. S., Baker B. M., Trappmann B., Chen C. S. and Shenoy V. B. (2014). Remodeling of fibrous extracellular matrices by contractile cells: predictions from discrete fiber network simulations. Biophys. J. 107, 1829-1840. 10.1016/j.bpj.2014.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. M., Trappmann B., Wang W. Y., Sakar M. S., Kim I. L., Shenoy V. B., Burdick J. A. and Chen C. S. (2015). Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 14, 1262-1268. 10.1038/nmat4444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff M. H., Aggeler J., Ram T. G. and Bissell M. J. (1989). Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105, 223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp P., Moritz W., Kelm J. M., Ullrich N. D., Agarkova I., Anson B. D., Suter T. M. and Zuppinger C. (2015). Development and characterization of a scaffold-free 3D spheroid model of induced pluripotent stem cell-derived human cardiomyocytes. Tissue Eng. Part C Methods 21, 852-861. 10.1089/ten.tec.2014.0376 [DOI] [PubMed] [Google Scholar]
- Bell E., Ivarsson B. and Merrill C. (1979). Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA 76, 1274-1278. 10.1073/pnas.76.3.1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G. and Parry G. (1982). How does the extracellular matrix direct gene expression. J. Theor. Biol. 99, 31-68. 10.1016/0022-5193(82)90388-5 [DOI] [PubMed] [Google Scholar]
- Bonnans C., Chou J. and Werb Z. (2014). Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786-801. 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudou T., Legant W. R., Mu A., Borochin M. A., Thavandiran N., Radisic M., Zandstra P. W., Epstein J. A., Margulies K. B. and Chen C. S. (2012). A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. Part A 18, 910-919. 10.1089/ten.tea.2011.0341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Califano J. P. and Reinhart-King C. A. (2010). Substrate stiffness and cell area predict cellular traction stresses in single cells and cells in contact. Cell. Mol. Bioeng. 3, 68-75. 10.1007/s12195-010-0102-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M. A. and Longaker M. T. (2004). The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Repair Regen. 12, 134-147. 10.1111/j.1067-1927.2004.012208.x [DOI] [PubMed] [Google Scholar]
- Carrel A. and Burrows M. T. (1911). Cultivation of tissues in vitro and its technique. J. Exp. Med. 13, 387-396. 10.1084/jem.13.3.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D. R., Orr T. E., Fyhrie D. P. and Schurman D. J. (1987). Influences of mechanical stress on prenatal and postnatal skeletal development. Clin. Orthop. Relat. Res. 219, 237-250. 10.1097/00003086-198706000-00034 [DOI] [PubMed] [Google Scholar]
- Cavey M. and Lecuit T. (2009). Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harb. Perspect. Biol. 1, a002998 10.1101/cshperspect.a002998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerchiari A. E., Garbe J. C., Jee N. Y., Todhunter M. E., Broaders K. E., Peehl D. M., Desai T. A., LaBarge M. A., Thomson M. and Gartner Z. J. (2015). A strategy for tissue self-organization that is robust to cellular heterogeneity and plasticity. Proc. Natl. Acad. Sci. USA 112, 2287-2292. 10.1073/pnas.1410776112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson L., Brownfield D., Garbe J. C., Kuhn I., Stampfer M. R., Bissell M. J. and LaBarge M. A. (2011). Self-organization is a dynamic and lineage-intrinsic property of mammary epithelial cells. Proc. Natl. Acad. Sci. USA 108, 3264-3269. 10.1073/pnas.1019556108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Bremer A. W., Scheideler O. J., Na Y. S., Todhunter M. E., Hsiao S., Bomdica P. R., Maharbiz M. M., Gartner Z. J. and Schaffer D. V. (2016a). Interrogating cellular fate decisions with high-throughput arrays of multiplexed cellular communities. Nat. Commun. 7, 10309 10.1038/ncomms10309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Wang Q., Asmani M., Li Y., Liu C., Li C., Lippmann J. M., Wu Y. and Zhao R. (2016b). Lung microtissue array to screen the fibrogenic potential of carbon nanotubes. Sci. Rep. 6, 31304 10.1038/srep31304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. (2016). Modeling development and disease with organoids. Cell 165, 1586-1597. 10.1016/j.cell.2016.05.082 [DOI] [PubMed] [Google Scholar]
- da Rocha-Azevedo B., Ho C.-H. and Grinnell F. (2013). Fibroblast cluster formation on 3D collagen matrices requires cell contraction dependent fibronectin matrix organization. Exp. Cell Res. 319, 546-555. 10.1016/j.yexcr.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. M. (2006). Spemann's organizer and self-regulation in amphibian embryos. Nat. Rev. Mol. Cell Biol. 7, 296-302. 10.1038/nrm1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvoye P., Wiliquet P., Levêque J.-L., Nusgens B. V. and Lapieèe C. M. (1991). Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. J. Invest. Dermatol. 97, 898-902. 10.1111/1523-1747.ep12491651 [DOI] [PubMed] [Google Scholar]
- Discher D. E., Janmey P. and Wang Y.-L. (2005). Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139-1143. 10.1126/science.1116995 [DOI] [PubMed] [Google Scholar]
- Doyle A. D., Carvajal N., Jin A., Matsumoto K. and Yamada K. M. (2015). Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat. Commun. 6, 8720 10.1038/ncomms9720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L. and Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677-689. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Foolen J., Shiu J.-Y., Mitsi M., Zhang Y., Chen C. S. and Vogel V. (2016). Full-length fibronectin drives fibroblast accumulation at the surface of collagen microtissues during cell-induced tissue morphogenesis. PLoS ONE 11, e0160369 10.1371/journal.pone.0160369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galois L., Hutasse S., Cortial D., Rousseau C. F., Grossin L., Ronziere M.-C., Herbage D. and Freyria A. M. (2006). Bovine chondrocyte behaviour in three-dimensional type I collagen gel in terms of gel contraction, proliferation and gene expression. Biomaterials 27, 79-90. 10.1016/j.biomaterials.2005.05.098 [DOI] [PubMed] [Google Scholar]
- Gartner Z. J. and Bertozzi C. R. (2009). Programmed assembly of 3-dimensional microtissues with defined cellular connectivity. Proc. Natl. Acad. Sci. USA 106, 4606-4610. 10.1073/pnas.0900717106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghibaudo M., Trichet L., Le Digabel J., Richert A., Hersen P. and Ladoux B. (2009). Substrate topography induces a crossover from 2D to 3D behavior in fibroblast migration. Biophys. J. 97, 357-368. 10.1016/j.bpj.2009.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry C. and Grinnell F. (1987). Contraction of hydrated collagen gels by fibroblasts: evidence for two mechanisms by which collagen fibrils are stabilized. Coll. Relat. Res. 6, 515-529. 10.1016/S0174-173X(87)80050-X [DOI] [PubMed] [Google Scholar]
- Hall B. K. and Miyake T. (2000). All for one and one for all: condensations and the initiation of skeletal development. Bioessays 22, 138-147. [DOI] [PubMed] [Google Scholar]
- Harrison R. G. (1959). The outgrowth of the nerve fiber as a mode of protoplasmic movement. J. Exp. Zool. 142, 5-73. 10.1002/jez.1401420103 [DOI] [PubMed] [Google Scholar]
- Hinson J. T., Chopra A., Nafissi N., Polacheck W. J., Benson C. C., Swist S., Gorham J., Yang L., Schafer S., Sheng C. C. et al. (2015). Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 349, 982-986. 10.1126/science.aaa5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman M. P., Kibbey M. C., Letterio J. J. and Kleinman H. K. (1996). Role of laminin-1 and TGF-beta 3 in acinar differentiation of a human submandibular gland cell line (HSG). J. Cell Sci. 109, 2013-2021. [DOI] [PubMed] [Google Scholar]
- Itskovitz-Eldor J., Schuldiner M., Karsenti D., Eden A., Yanuka O., Amit M., Soreq H. and Benvenisty N. (2000). Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 6, 88-95. [PMC free article] [PubMed] [Google Scholar]
- John J., Quinlan A. T., Silvestri C. and Billiar K. (2010). Boundary stiffness regulates fibroblast behavior in collagen gels. Ann. Biomed. Eng. 38, 658-673. 10.1007/s10439-009-9856-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhas M., Engelmayr G. C. Jr, Fontanella A. N., Palmer G. M. and Bursac N. (2014). Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo. Proc. Natl. Acad. Sci. USA 111, 5508-5513. 10.1073/pnas.1402723111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadler K. E., Hill A. and Canty-Laird E. G. (2008). Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 20, 495-501. 10.1016/j.ceb.2008.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman B., Picart C. and Boudou T. (2016). Quick and easy microfabrication of T-shaped cantilevers to generate arrays of microtissues. Biomed. Microdevices 18, 43 10.1007/s10544-016-0067-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian K. A., Bugarija B., Lahn B. T. and Mrksich M. (2010). Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 107, 4872-4877. 10.1073/pnas.0903269107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer M. (2004). Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 84, 649-698. 10.1152/physrev.00031.2003 [DOI] [PubMed] [Google Scholar]
- Krieg M., Arboleda-Estudillo Y., Puech P.-H., Käfer J., Graner F., Müller D. J. and Heisenberg C.-P. (2008). Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 10, 429-436. 10.1038/ncb1705 [DOI] [PubMed] [Google Scholar]
- Kronenberg H. M. (2003). Developmental regulation of the growth plate. Nature 423, 332-336. 10.1038/nature01657 [DOI] [PubMed] [Google Scholar]
- Kural M. H. and Billiar K. L. (2014). Mechanoregulation of valvular interstitial cell phenotype in the third dimension. Biomaterials 35, 1128-1137. 10.1016/j.biomaterials.2013.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kural M. H. and Billiar K. L. (2016). Myofibroblast persistence with real-time changes in boundary stiffness. Acta Biomater. 32, 223-230. 10.1016/j.actbio.2015.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M. A., Renner M., Martin C.-A., Wenzel D., Bicknell L. S., Hurles M. E., Homfray T., Penninger J. M., Jackson A. P. and Knoblich J. A. (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373-379. 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legant W. R., Pathak A., Yang M. T., Deshpande V. S., McMeeking R. M. and Chen C. S. (2009). Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proc. Natl. Acad. Sci. USA 106, 10097-10102. 10.1073/pnas.0900174106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legant W. R., Miller J. S., Blakely B. L., Cohen D. M., Genin G. M. and Chen C. S. (2010). Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nat. Methods 7, 969-971. 10.1038/nmeth.1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legant W. R., Chen C. S. and Vogel V. (2012). Force-induced fibronectin assembly and matrix remodeling in a 3D microtissue model of tissue morphogenesis. Integr. Biol. 4, 1164-1174. 10.1039/c2ib20059g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C.-C., Park A. Y. and Guan J.-L. (2007). In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2, 329-333. 10.1038/nprot.2007.30 [DOI] [PubMed] [Google Scholar]
- Lu P., Takai K., Weaver V. M. and Werb Z. (2011). Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 3, a005058 10.1101/cshperspect.a005058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre J.-L., Berthoumieux H., Krens S. F. G., Salbreux G., Julicher F., Paluch E. and Heisenberg C.-P. (2012). Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science 338, 253-256. 10.1126/science.1225399 [DOI] [PubMed] [Google Scholar]
- Mammoto T., Mammoto A., Torisawa Y.-S., Tat T., Gibbs A., Derda R., Mannix R., de Bruijn M., Yung C. W., Huh D. et al. (2011). Mechanochemical control of mesenchymal condensation and embryonic tooth organ formation. Dev. Cell 21, 758-769. 10.1016/j.devcel.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning M. L., Foty R. A., Steinberg M. S. and Schoetz E.-M. (2010). Coaction of intercellular adhesion and cortical tension specifies tissue surface tension. Proc. Natl. Acad. Sci. USA 107, 12517-12522. 10.1073/pnas.1003743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. (1997). Wound healing–aiming for perfect skin regeneration. Science 276, 75-81. 10.1126/science.276.5309.75 [DOI] [PubMed] [Google Scholar]
- McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K. and Chen C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483-495. 10.1016/S1534-5807(04)00075-9 [DOI] [PubMed] [Google Scholar]
- Meshel A. S., Wei Q., Adelstein R. S. and Sheetz M. P. (2005). Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat. Cell Biol. 7, 157-164. 10.1038/ncb1216 [DOI] [PubMed] [Google Scholar]
- Midwood K. S. and Schwarzbauer J. E. (2002). Tenascin-C modulates matrix contraction via focal adhesion kinase- and Rho-mediated signaling pathways. Mol. Biol. Cell 13, 3601-3613. 10.1091/mbc.E02-05-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruma K., Nishiyama A., Ono Y., Miyawaki H., Mizuhara E., Hori S., Kakizuka A., Obata K., Yanagawa Y., Hirano T. et al. (2010). Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nat. Neurosci. 13, 1171-1180. 10.1038/nn.2638 [DOI] [PubMed] [Google Scholar]
- Muguruma K., Nishiyama A., Kawakami H., Hashimoto K. and Sasai Y. (2015). Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 10, 537-550. 10.1016/j.celrep.2014.12.051 [DOI] [PubMed] [Google Scholar]
- Murrell M., Oakes P. W., Lenz M. and Gardel M. L. (2015). Forcing cells into shape: the mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 16, 486-498. 10.1038/nrm4012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M. and Sasai Y. (2012). Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771-785. 10.1016/j.stem.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Nelson C. M. and Bissell M. J. (2006). Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 22, 287-309. 10.1146/annurev.cellbio.22.010305.104315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obbink-Huizer C., Foolen J., Oomens C. W. J., Borochin M., Chen C. S., Bouten C. V. C. and Baaijens F. P. T. (2014). Computational and experimental investigation of local stress fiber orientation in uniaxially and biaxially constrained microtissues. Biomech. Model Mechanobiol. 13, 1053-1063. 10.1007/s10237-014-0554-z [DOI] [PubMed] [Google Scholar]
- Papachroni K. K., Karatzas D. N., Papavassiliou K. A., Basdra E. K. and Papavassiliou A. G. (2009). Mechanotransduction in osteoblast regulation and bone disease. Trends Mol. Med. 15, 208-216. 10.1016/j.molmed.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Passier R., Orlova V. and Mummery C. (2016). Complex tissue and disease modeling using hiPSCs. Cell Stem Cell 18, 309-321. 10.1016/j.stem.2016.02.011 [DOI] [PubMed] [Google Scholar]
- Pelham R. J. Jr and Wang Y.-L. (1997). Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. USA 94, 13661-13665. 10.1073/pnas.94.25.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov S. V., Pasapera A. M., Sabass B. and Waterman C. M. (2012). Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151, 1513-1527. 10.1016/j.cell.2012.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck W. J. and Chen C. S. (2016). Measuring cell-generated forces: a guide to the available tools. Nat. Methods 13, 415-423. 10.1038/nmeth.3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozario T. and DeSimone D. W. (2010). The extracellular matrix in development and morphogenesis: a dynamic view. Dev. Biol. 341, 126-140. 10.1016/j.ydbio.2009.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez A., Ghibaudo M., Buguin A., Silberzan P. and Ladoux B. (2007). Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc. Natl. Acad. Sci. USA 104, 8281-8286. 10.1073/pnas.0702259104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakar M. S., Neal D., Boudou T., Borochin M. A., Li Y., Weiss R., Kamm R. D., Chen C. S. and Asada H. H. (2012). Formation and optogenetic control of engineered 3D skeletal muscle bioactuators. Lab. Chip 12, 4976-4985. 10.1039/c2lc40338b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakar M. S., Eyckmans J., Pieters R., Eberli D., Nelson B. J. and Chen C. S. (2016). Cellular forces and matrix assembly coordinate fibrous tissue repair. Nat. Commun. 7, 11036 10.1038/ncomms11036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson I. R., Ezzell R. M., Kedinger M., Erlanger M., Xu Z. X., Pringault E., Leon-Robine S., Louvard D. and Walker W. A. (1996). Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc. Natl. Acad. Sci. USA 93, 7717-7722. 10.1073/pnas.93.15.7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent C. Y., Berguig G. Y. and McDevitt T. C. (2009). Cardiomyogenic differentiation of embryoid bodies is promoted by rotary orbital suspension culture. Tissue Eng. Part A 15, 331-342. 10.1089/ten.tea.2008.0145 [DOI] [PubMed] [Google Scholar]
- Schedin P. and Keely P. J. (2011). Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb. Perspect. Biol. 3, a003228 10.1101/cshperspect.a003228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell J. Y., Wilks B. T., Patel M., Franck C., Chalivendra V., Cao X., Shenoy V. B. and Morgan J. R. (2016). Harnessing cellular-derived forces in self-assembled microtissues to control the synthesis and alignment of ECM. Biomaterials 77, 120-129. 10.1016/j.biomaterials.2015.10.080 [DOI] [PubMed] [Google Scholar]
- Schiller H. B., Hermann M.-R., Polleux J., Vignaud T., Zanivan S., Friedel C. C., Sun Z., Raducanu A., Gottschalk K.-E., Théry M. et al. (2013). beta1- and alphav-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 15, 625-636. 10.1038/ncb2747 [DOI] [PubMed] [Google Scholar]
- Schmeichel K. L. and Bissell M. J. (2003). Modeling tissue-specific signaling and organ function in three dimensions. J. Cell Sci. 116, 2377-2388. 10.1242/jcs.00503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuger L., O'Shea K. S., Nelson B. B. and Varani J. (1990). Organotypic arrangement of mouse embryonic lung cells on a basement membrane extract: involvement of laminin. Development 110, 1091-1099. [DOI] [PubMed] [Google Scholar]
- Schvartzman M., Palma M., Sable J., Abramson J., Hu X., Sheetz M. P. and Wind S. J. (2011). Nanolithographic control of the spatial organization of cellular adhesion receptors at the single-molecule level. Nano Lett. 11, 1306-1312. 10.1021/nl104378f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamir E. R. and Ewald A. J. (2014). Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 15, 647-664. 10.1038/nrm3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittichockechaiwut A., Scutt A. M., Ryan A. J., Bonewald L. F. and Reilly G. C. (2009). Use of rapidly mineralising osteoblasts and short periods of mechanical loading to accelerate matrix maturation in 3D scaffolds. Bone 44, 822-829. 10.1016/j.bone.2008.12.027 [DOI] [PubMed] [Google Scholar]
- Sottile J., Shi F., Rublyevska I., Chiang H.-Y., Lust J. and Chandler J. (2007). Fibronectin-dependent collagen I deposition modulates the cell response to fibronectin. Am. J. Physiol. Cell Physiol. 293, C1934-C1946. 10.1152/ajpcell.00130.2007 [DOI] [PubMed] [Google Scholar]
- Steinberg M. S. (1963). Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science 141, 401-408. 10.1126/science.141.3579.401 [DOI] [PubMed] [Google Scholar]
- Steinberg M. S. (1970). Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J. Exp. Zool. 173, 395-433. 10.1002/jez.1401730406 [DOI] [PubMed] [Google Scholar]
- Steinberg M. S. and Takeichi M. (1994). Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc. Natl. Acad. Sci. USA 91, 206-209. 10.1073/pnas.91.1.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopak D. and Harris A. K. (1982). Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev. Biol. 90, 383-398. 10.1016/0012-1606(82)90388-8 [DOI] [PubMed] [Google Scholar]
- Svoronos A. A., Tejavibulya N., Schell J. Y., Shenoy V. B. and Morgan J. R. (2014). Micro-mold design controls the 3D morphological evolution of self-assembling multicellular microtissues. Tissue Eng. Part A 20, 1134-1144. 10.1089/ten.tea.2013.0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thavandiran N., Dubois N., Mikryukov A., Masse S., Beca B., Simmons C. A., Deshpande V. S., McGarry J. P., Chen C. S., Nanthakumar K. et al. (2013). Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc. Natl. Acad. Sci. USA 110, E4698-E4707. 10.1073/pnas.1311120110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry M., Pépin A., Dressaire E., Chen Y. and Bornens M. (2006). Cell distribution of stress fibres in response to the geometry of the adhesive environment. Cell Motil. Cytoskeleton 63, 341-355. 10.1002/cm.20126 [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Itskovitz-Eldor J., Shapiro S. S., Waknitz M. A., Swiergiel J. J., Marshall V. S. and Jones J. M. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145-1147. 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- Todhunter M. E., Jee N. Y., Hughes A. J., Coyle M. C., Cerchiari A., Farlow J., Garbe J. C., LaBarge M. A., Desai T. A. and Gartner Z. J. (2015). Programmed synthesis of three-dimensional tissues. Nat. Methods 12, 975-981. 10.1038/nmeth.3553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzel S. G. M., Pavesi A. and Kamm R. D. (2014). Microfabrication and microfluidics for muscle tissue models. Prog. Biophys. Mol. Biol. 115, 279-293. 10.1016/j.pbiomolbio.2014.08.013 [DOI] [PubMed] [Google Scholar]
- van Spreeuwel A. C. C., Bax N. A. M., Bastiaens A. J., Foolen J., Loerakker S., Borochin M., van der Schaft D. W. J., Chen C. S., Baaijens F. P. T. and Bouten C. V. C. (2014). The influence of matrix (an)isotropy on cardiomyocyte contraction in engineered cardiac microtissues. Integr. Biol. 6, 422-429. 10.1039/C3IB40219C [DOI] [PubMed] [Google Scholar]
- Wang H., Svoronos A. A., Boudou T., Sakar M. S., Schell J. Y., Morgan J. R., Chen C. S. and Shenoy V. B. (2013). Necking and failure of constrained 3D microtissues induced by cellular tension. Proc. Natl. Acad. Sci. USA 110, 20923-20928. 10.1073/pnas.1313662110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. G. (2013). Tissue mechanics and fibrosis. Biochim. Biophys. Acta 1832, 884-890. 10.1016/j.bbadis.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West A. R., Zaman N., Cole D. J., Walker M. J., Legant W. R., Boudou T., Chen C. S., Favreau J. T., Gaudette G. R., Cowley E. A. et al. (2013). Development and characterization of a 3D multicell microtissue culture model of airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 304, L4-L16. 10.1152/ajplung.00168.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. D. and Plachta N. (2015). How adhesion forms the early mammalian embryo. Curr. Top. Dev. Biol. 112, 1-17. 10.1016/bs.ctdb.2014.11.022 [DOI] [PubMed] [Google Scholar]
- Winklbauer R. and Parent S. E. (2016). Forces driving cell sorting in the amphibian embryo. Mech. Dev. (in press) doi: 10.1016/j.mod.2016.09.003 10.1016/j.mod.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Yamada S. and Nelson W. J. (2007). Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J. Cell Biol. 178, 517-527. 10.1083/jcb.200701058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Boudou T., Wang W.-G., Chen C. S. and Reich D. H. (2013). Decoupling cell and matrix mechanics in engineered microtissues using magnetically actuated microcantilevers. Adv. Mater. 25, 1699-1705. 10.1002/adma.201203585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W. H., Fink C., Kralisch D., Remmers U., Weil J. and Eschenhagen T. (2000). Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol. Bioeng. 68, 106-114. [DOI] [PubMed] [Google Scholar]