Abstract
Rationale: Lower respiratory tract illness is a major cause of childhood morbidity and mortality. It is unknown whether infants are predisposed to illness because of impaired lung function or whether respiratory illness reduces lung function.
Objectives: To investigate the impact of early life exposures, including lower respiratory tract illness, on lung function during infancy.
Methods: Infants enrolled in the Drakenstein child health study had lung function at 6 weeks and 1 year. Testing during quiet natural sleep included tidal breathing, exhaled nitric oxide, and multiple breath washout measures. Risk factors for impaired lung health were collected longitudinally. Lower respiratory tract illness surveillance was performed and any episode investigated.
Measurements and Main Results: Lung function was tested in 648 children at 1 year. One hundred and fifty (29%) infants had a lower respiratory tract illness during the first year of life. Lower respiratory tract illness was independently associated with increased respiratory rate (4%; 95% confidence interval [CI], 1.01–1.08; P = 0.02). Repeat episodes further increased respiratory rate (3%; 95% CI, 1.01–1.05; P = 0.004), decreased tidal volume (−1.7 ml; 95% CI, −3.3 to −0.2; P = 0.03), and increased the lung clearance index (0.13 turnovers; 95% CI, 0.04–0.22; P = 0.006) compared with infants without illness. Tobacco smoke exposure, lung function at 6 weeks, infant growth, and prematurity were other independent predictors of lung function at 1 year.
Conclusions: Early life lower respiratory tract illness impairs lung function at 1 year, independent of baseline lung function. Preventing early life lower respiratory tract illness is important to optimize lung function and promote respiratory health in childhood.
Keywords: infant, lung function, epidemiology, LRTI, lung growth and development
At a Glance Commentary
Scientific Knowledge on the Subject
Lower respiratory tract illness is a major cause of childhood morbidity and mortality. It is unknown whether infants are predisposed to illness because of impaired lung function or whether respiratory illness reduces lung function.
What This Study Adds to the Field
Early life lower respiratory tract illness impairs lung function at 1 year, independent of baseline lung function. This effect is stronger if respiratory illness is severe or recurrent.
Respiratory disease is a leading cause of childhood mortality and morbidity globally, particularly in low- and middle-income countries (LMIC) (1, 2). Among these, lower respiratory tract illness (LRTI) is the leading cause of mortality, with the peak incidence in infancy (1). Despite this the impact of LRTI on lung growth and development has not been well studied, especially in LMIC where the burden of LRTI is particularly high. Lung development and maturation is incomplete at birth and continues during the first years of life making lungs particularly vulnerable to damage during this critical time of lung development. Low lung function in early life has been shown to track through childhood and into adulthood (3, 4); furthermore, children born with low lung function have an increased risk of LRTI or wheezing illnesses in childhood (5–10). LRTI in infancy has been associated with reduced lung function in later childhood and adulthood (11–14). However, the sparse data from longitudinal studies of lung function in high-income settings suggest that the effect of respiratory tract illness on later lung function may be mediated through reduced lung function before the disease episode (9, 10). Understanding the importance of LRTI and other risk factors on lung development and subsequent risk of respiratory illness, particularly in LMIC where disease burden is concentrated (15), may inform strategies for optimizing lung health and preventing chronic respiratory disease.
We aimed to investigate lung function longitudinally from 6 weeks to 1 year of age and the impact of early life exposures, including LRTI during infancy in an African birth cohort study, the Drakenstein Child Health Study (DCHS). Some of the results of this study have been previously reported in the form of an abstract (16).
Methods
Study Design and Participants
Infants enrolled in the DCHS, were followed from birth until 1 year of age, during which time lung function testing was done and any LRTI episode was investigated. The DCHS is a birth cohort study situated in a periurban, low socioeconomic area outside Cape Town in South Africa (17). Mothers were enrolled antenatally and followed through pregnancy at one of two primary care clinics; mother-child pairs were followed from birth. Infants attended scheduled study visits at 6, 10, and 14 weeks and 6, 9, and 12 months of age, with lung function assessed at 6 weeks and 1 year.
Household tobacco smoke exposure or maternal smoking information was collected by study questionnaire at each study visit. Maternal alcohol intake during pregnancy was assessed using the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) self-reported questionnaires completed at 28–32 weeks gestation (18, 19). Infants were classified as being alcohol-exposed in utero if ASSIST scored heavy exposure with high risk for alcohol-related problems. All study variables and outcomes are defined in Table E1 in the online supplement.
A strong LRTI surveillance system was established as part of the DCHS and detailed investigation was done at any episode of LRTI (20). The World Health Organization clinical case definitions were used to define an episode of LRTI (defined as cough or difficulty breathing and increased respiratory rate or lower chest wall in-drawing in a child older than 2 mo old). Severe LRTI was defined as increased respiratory rate or lower chest wall in-drawing, in a child younger than 2 months of age or any general danger sign in a child of any age (21). Each suspected case of LRTI was examined by study staff to confirm the presence of LRTI. All children with an LRTI had a nasopharyngeal swab for respiratory organisms (22). LRTI surveillance was established through community health workers, use of cell phones, a dedicated study contact person available 24 hours, and a network of study staff at community-based sites (20).
The study was approved by the Faculty of Health Sciences, Human Research Ethics Committee, University of Cape Town (401/2009; 423/2012) and by the Western Cape Provincial Health Research Committee. Mothers gave informed, written consent in their first language for their infants to participate.
Lung Function Measures
Lung function testing was undertaken at 6 (5–11) weeks of age corrected for prematurity (<37 wk) and then at 1 year (11–13 mo). All testing was done in unsedated, behaviorally assessed quiet sleep as previously described (23, 24). Testing included measures of tidal breathing (tidal volume, respiratory rate, inspiratory and expiratory flow ratios); exhaled nitric oxide (eNO), a measure of eosinophilic airway inflammation; sulfur-hexafluoride multiple breath washout (MBW), measuring FRC; and lung clearance index (LCI), a measure of ventilation distribution. Tidal breathing and eNO measures were collected using the Exhalyser D with ultrasonic flow meter (Ecomedics AG, Duernton, Switzerland) and analyzed using analysis software (Wbreath version 3.28.0; Ndd Medizintechnik, AG, Zurich, Switzerland) and the MBW measures were performed using 4% sulfur-hexafluoride as a tracer gas and ultrasonic flow meter (Spiroson; Ecomedics, Duernten, Switzerland) with acquisition and analysis software (Wbreath version 3.28.0; Ndd Medizintechnik), as previously described (23, 24). Low lung function at 6 weeks was defined as lung function outcome below the lower limit of normal using locally derived reference equations (24).
Statistical Analysis
Lung function outcome measurements were modeled using multiple linear regression to assess the impact of different early life exposures on lung function attained at 1 year. The base model was constructed for lung function outcomes using directed acyclic graphs, which graphically illustrate assumptions made regarding the causal relationships between variables of interest with the goal of minimizing bias for the effect estimates in our chosen models. Initial relationships between clinically relevant variables were entered into a directed acyclic graphs based on previous literature and univariate associations in this data set. It was implemented in a graphical interface software DAGitty (www.dagitty.net version 2.2, 2014) (25). A minimal set of confounders was selected by automated approach in DAGitty described here (26). As a result, for each lung function outcome the base model consisted of matched lung function at 6 weeks, sex, body mass index for age z score, ethnicity, socioeconomic status quartile, and gestation length.
For each lung function outcome separately, early life exposures of interest were then added to the base model one at a time and assessed individually. The only exception to this was infants’ lung function measurements at baseline (6 wk) because it explained a large percentage of the variability of lung function at 1 year. The z scores of body mass index, height, and weight for age were not included in any models simultaneously because of high correlation between them. Similarly length of gestation (continuous variable), preterm and very preterm (binary variables), were not included simultaneously.
Interactions were explored among all the variables in the final set of confounders using a Wald test with significance level of 0.05. Interactions between weight, height, and age were avoided by including body mass index for age z score variable. The only nonlinear term retained in the final model was age at visit squared.
Estimated coefficients, 95% confidence intervals (CIs), and P values were recorded for each early life exposure of interest (see Tables E2–E7).
Infants were stratified into quartile by overall model’s predicted lung function at 1 year, excluding early life exposure of interest. Odds ratios (ORs) were calculated for each early life exposure based on the direction of effect of each lung function outcome: either the upper quartile was compared with the lower quartile (for one-sided effects; Vt, LCI, eNO) or upper and lower quartiles were compared with the middle quartile separately (for two-sided effects; respiratory rate, FRC, time to peak tidal expiratory flow over total expiratory time [tPTEF/tE]). Estimated ORs, 95% CIs, and P values were recorded for each early life exposure in the online supplement.
Statistical analyses were performed using STATA 13 for windows (STATS Corporation, College Station, TX). Weight (WAZ) and height for age z scores were calculated using the World Health Organization Child Growth Standards “I grow up” STATA package (27).
Results
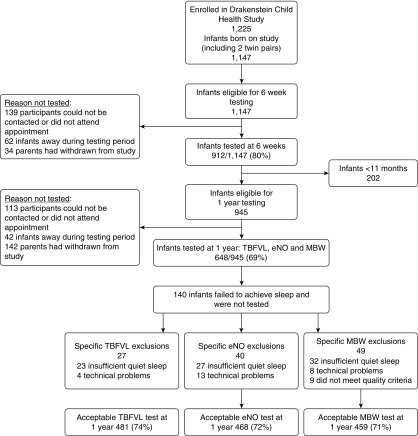
Of 1,147 infants born in the DCHS between July 2012 and February 2016, a total of 912 (80%) had lung function testing at 6 weeks (mean [SD] age, 1.9 [0.4] mo) and 648 (69%) at 1 year of age (mean [SD] age 12.0 [0.7] mo) Figure 1. Paired unsedated 6-week and 1-year lung function was completed in 434 children, of which 404 (93%) tidal breathing measures, 380 (88%) eNO, and 361 (83%) MBW tests were successful with good quality results. Reasons for exclusions or failed tests are shown in Figure 1.
Figure 1.
Cohort description before exclusions (n = 1,225). eNO = exhaled nitric oxide; MBW = sulfur-hexafluoride multiple breath washout; TBFVL = tidal breathing measures.
The cohort demographics and exposures are shown in Table 1. Four hundred and one (79%) infants were exposed to household tobacco, with 169 (34.4%) infants having a mother who smoked during pregnancy. Twenty-one (4.5%) infants had mothers who drank alcohol heavily during pregnancy. One hundred and nine (21.4%) mothers were HIV-infected; however, because of the well-functioning prevention of mother-to-child transmission program only one infant was HIV infected. There were 234 episodes of LRTI in 150 (29.5%) infants during the first year. Fifty-one (10%) infants had more than one episode and 32 infants (6.3%) had severe LRTI. Of the 234 LRTI episodes, 61 (26%) required hospitalization and 27 (11.5%) required supplemental oxygen.
Table 1.
Demographics of Infants Tested at 1 Year of Age (n = 508)
| n (%) | |
|---|---|
| Male sex | 259 (50.9) |
| Black African ethnicity | 246 (51.6) |
| Socioeconomic status* | |
| Highest quartile | 115 (22.6) |
| Moderate-high quartile | 115 (22.6) |
| Low-moderate quartile | 152 (29.9) |
| Lowest quartile | 126 (24.8) |
| Preterm | |
| <37 wk gestation | 80 (15.8) |
| <32 wk gestation | 12 (2.4) |
| LRTI | |
| Infants with LRTI | 150 (29.5) |
| >1 LRTI episode | 51 (10.0) |
| Severe LRTI† | 32 (6.3) |
| LRTI total episodes | 234 |
| Hospitalized LRTI | 61/234 (26.0) |
| LRTI requiring oxygen | 27/234 (11.5) |
| Household tobacco smoke exposure, excluding mother (n = 508) | 401 (78.8) |
| Maternal antenatal smoking (n = 491) | 169 (34.4) |
| Maternal alcohol use in pregnancy, high risk (n = 465) | 21 (4.5) |
| Maternal HIV infection‡ | 109 (21.4) |
Definition of abbreviation: LRTI = lower respiratory tract illness.
Quartiles are internal comparisons within a low socioeconomic cohort.
Severe LRTI was diagnosed in children younger than 2 months with tachypnea (>60 breaths/min) or lower chest wall in-drawing, or in children of any age if the child had a general danger sign.
No infants were HIV infected.
Unsedated lung function testing was successful in more than 75% of 1-year infants (Figure 1). The anthropometry and results of lung function at 6 weeks and 1 year, with average within-subject interval change, is shown in Table 2. Between the 6-week and 1-year test the respiratory rate decreased by an average of 20 breaths per minute, Vt increased by an average of 58.5 ml, a 2.7-fold increase and the FRC by 121 ml, a 2.6-fold increase. The tPTEF/tE decreased 9% and the average LCI reduced by 0.4 turnovers.
Table 2.
Infant Anthropometry and Lung Function Outcomes at 6 Weeks and 1 Year and Within-Subject Interval Change
| 6-Week Test [Mean (SD)] | 1-Year Test [Mean (SD)] | Within-Subject Interval Change [Mean (SE)] (n = 434) | |
|---|---|---|---|
| Age, mo | 1.85 (0.38) | 12.58 (1.00) | 10.73 (0.04) |
| Weight, kg | 4.91 (0.77) | 9.47 (1.45) | 4.56 (0.06) |
| Weight-for-age z score | −0.52 (1.27) | −0.07 (1.32) | 0.46 (0.07) |
| Height, cm | 55.05 (3.20) | 73.81 (3.18) | 18.75 (0.18) |
| Height-for-age z score | −1.09 (1.66) | −0.71 (1.33) | 0.38 (0.09) |
| Tidal breathing measures (n = 404) | |||
| Respiratory rate, n/min | 48.77 (11.42) | 29.13 (4.86) | −19.64 (0.55) |
| Tidal volume, ml | 34.78 (6.40) | 93.29 (13.72) | 58.51 (0.55) |
| tPTEF/tE, % | 38.21 (12.14) | 29.24 (9.93) | −8.97 (0.65) |
| tI/tTOT, % | 45.14 (4.71) | 43.54 (4.22) | −1.60 (0.26) |
| Exhaled nitric oxide, ppb (n = 380) | 10.43 (7.68) | 12.22 (10.58) | −1.80 (0.51) |
| Multiple breath washout measures (n = 361) | |||
| Functional residual capacity, ml | 77.61 (16.01) | 198.73 (44.27) | 121.12 (1.73) |
| Lung clearance index, n turnovers | 7.16 (0.44) | 6.76 (0.65) | −0.40 (0.03) |
Definition of abbreviations: tPTEF/tE = time to peak tidal expiratory flow over total expiratory time; tI/tTOT = inspiratory time over total breath time.
Lung Function between 6 Weeks and 1 Year
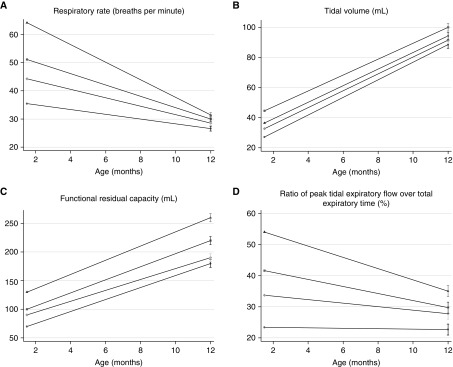
Figure 2 illustrates the tracking of lung function from 6 weeks to 1 year. Infants with low lung function at 6 weeks had 8% higher respiratory rate (95% CI, 1.05–1.12; P < 0.001), 8.0 ml lower Vt (95% CI, −10.4 to −5.6; P < 0.001), 23% lower tPTEF/tE (95% CI, 0.69–0.84; P < 0.001), and 19.6 ml lower FRC (95% CI, −29.4 to −9.8; P < 0.001) at 1 year compared with infants who did not have low baseline function.
Figure 2.
(A–D) Lung function outcomes in quartiles at 6 weeks and 1 year.
Determinants and Independent Predictors of Lung Function at 1 Year
The results of the linear regression model of associations with lung function at 1 year and the results of early life exposures and risk of having low lung function at 1 year expressed as OR, 95% CI, and P values are summarized in Table 3, with full analyses shown in Tables E2–E12.
Table 3.
Associations of Lung Function and Predictors of Lowest Quartile Lung Function Outcomes at 1 Year of Age
| Overall Model Coefficient, Crude Lung Function (95% CI) | Lowest Predicted Lung Function Quartile [Odds Ratio (95% CI)] | |
|---|---|---|
| Baseline low lung function | ||
| Respiratory rate, % change* | 1.08 (1.05 to 1.12) | 1.63 (1.0 to 2.68) |
| Tidal volume, ml | −8.02 (−10.43 to −5.62) | 9.73 (4.94 to 19.17) |
| tPTEF/tE, % change* | 0.77 (0.69 to 0.84) | N/A |
| Functional residual capacity, ml | −19.58 (−29.37 to −9.78) | 0.29 (0.12 to 0.67) |
| Lung clearance index, n turnovers | 0.10 (−0.06 to 0.27) | 2.28 (1.04 to 4.99) |
| Lower respiratory tract illness | ||
| Respiratory rate, % change* | 1.04 (1.01 to 1.08) | 1.74 (1.11 to 2.45) |
| Tidal volume, ml | −2.61 (−5.28 to 0.05) | 1.34 (0.79 to 2.30) |
| tPTEF/tE, % change* | 1.04 (0.97 to 1.11) | 1.41 (0.88 to 2.37) |
| Functional residual capacity, ml | −4.70 (−13.29 to 13.38) | 0.83 (0.54 to 1.30) |
| Lung clearance index, n turnovers | 0.12 (−0.04 to 0.28) | 1.55 (0.87 to 2.77) |
| Infant growth, change in WAZ | ||
| Respiratory rate, % change* | 1.00 (0.98 to 1.02) | 0.89 (0.71 to 1.11) |
| Tidal volume, ml | 3.26 (1.97 to 4.56) | 0.64 (0.49 to 0.84) |
| tPTEF/tE, % change* | 0.96 (0.92 to 1.00) | 1.31 (1.06 to 1.63) |
| Functional residual capacity, ml | 11.67 (6.32 to 17.01) | 1.03 (0.83 to 1.28) |
| Lung clearance index, n turnovers | −0.09 (−0.17 to 0.00) | 0.89 (0.69 to 1.15) |
| Household tobacco smoke | ||
| Respiratory rate, % change* | 1.02 (0.98 to 1.06) | 0.86 (0.49 to 1.49) |
| Tidal volume, ml | 0.46 (−2.64 to 3.57) | 1.10 (0.60 to 2.00) |
| tPTEF/tE, % change* | 0.99 (0.92 to 1.07) | 1.34 (0.76 to 2.38) |
| Functional residual capacity, ml | −5.26 (−16.73 to 2.45) | 1.03 (0.59 to 1.80) |
| Lung clearance index, n turnovers | 0.24 (0.06 to 0.43) | 1.61 (0.87 to 2.99) |
| Maternal smoking in pregnancy | ||
| Respiratory rate, % change* | 1.03 (0.99 to 1.06) | 1.14 (0.71 to 1.80) |
| Tidal volume, ml | −2.87 (−5.62 to −0.11) | 2.83 (1.60 to 5.02) |
| tPTEF/tE, % change* | 1.01 (0.94 to 1.08) | 0.95 (0.06 to 1.52) |
| Functional residual capacity, ml | −6.05 (−14.98 to 2.88) | 0.89 (0.54 to 1.46) |
| Lung clearance index, n turnovers | 0.05 (−0.12 to 0.21) | 1.24 (0.70 to 2.18) |
| Prematurity (<37 wk) | ||
| Respiratory rate, % change* | 0.99 (0.94 to 1.04) | 1.00 (0.54 to 1.86) |
| Tidal volume, ml | −0.06 (−5.10 to 5.22) | 1.99 (0.98 to 4.05) |
| tPTEF/tE, % change* | 1.02 (0.93 to 1.10) | 1.61 (0.89 to 2.90) |
| Functional residual capacity, ml | −16.79 (−27.81 to −5.76) | 1.54 (0.92 to 2.60) |
| Lung clearance index, n turnovers | 0.07 (−0.24 to 0.38) | 1.35 (0.64 to 2.87) |
| Maternal HIV | ||
| Respiratory rate, % change* | 1.04 (0.99 to 1.08) | 1.27 (0.76 to 2.12) |
| Tidal volume, ml | 0.70 (−3.41 to 4.82) | 0.95 (0.51 to 1.78) |
| tPTEF/tE, % change* | 1.05 (0.95 to 1.15) | 0.76 (0.42 to 1.36) |
| Functional residual capacity, ml | 0.29 (−12.10 to 12.67) | 0.16 (0.71 to 1.92) |
| Lung clearance index, n turnovers | 0.18 (−0.06 to 0.41) | 1.07 (0.56 to 2.05) |
| Maternal regular alcohol use | ||
| Respiratory rate, % change* | 0.99 (0.96 to 1.03) | 0.45 (0.13 to 1.66) |
| Tidal volume, ml | −1.04 (−8.40 to 6.33) | 1.09 (0.27 to 4.47) |
| tPTEF/tE, % change* | 1.02 (0.81 to 1.22) | 2.02 (0.74 to 5.53) |
| Functional residual capacity, ml | −2.68 (−28.71 to 23.36) | 1.46 (0.60 to 3.53) |
| Lung clearance index, n turnovers | 0.07 (−0.38 to 0.52) | 7.14 (0.86 to 59.11) |
Definition of abbreviations: CI = confidence interval; N/A = not applicable; tPTEF/tE = time to peak tidal expiratory flow over total expiratory time; WAZ = weight-for-age z score.
Bold indicates statistically significant changes at the 0.05 level of significance.
Variables log transformed.
After controlling for low baseline lung function, growth, and other confounding factors, infants who had an LRTI episode had higher respiratory rates at 1 year compared with infants who did not have a LRTI during the first year of life, with an average 4% higher respiratory rate (95% CI, 1.01–1.08; P = 0.02). Timing of an LRTI was not associated with lung function at 1 year, but infants with more than one episode of LRTI had a further 3% higher respiratory (95% CI, 1.01–1.05; P = 0.004), a 2 ml lower Vt (95% CI, −3.27 to −0.21; P = 0.03), and a higher LCI (0.13 turnover higher; 95% CI, 0.00–0.26; P = 0.05), compared with infants who did not have an LRTI. The effect on 1-year lung function was greater if LRTI was severe with an average 5.5 ml lower Vt (95% CI, −10.93 to −0.12; P = 0.045) and if LRTI required hospitalization with an increased LCI (average 0.36 turnovers higher; 95% CI, 0.09–0.63; P = 0.01). Low baseline lung function was not associated with risk of LRTI during the first year of life, but low baseline lung function and LRTI had an additive effect on lung function outcome at 1 year. eNO was only associated with severe LRTI, being 5 ppb lower compared with infants who had no LRTI (95% CI, −9.52 to −0.51; P = 0.03).
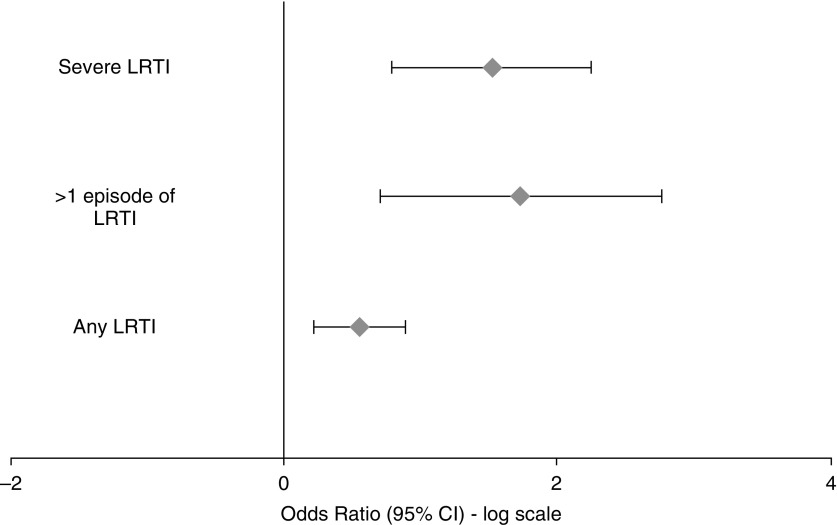
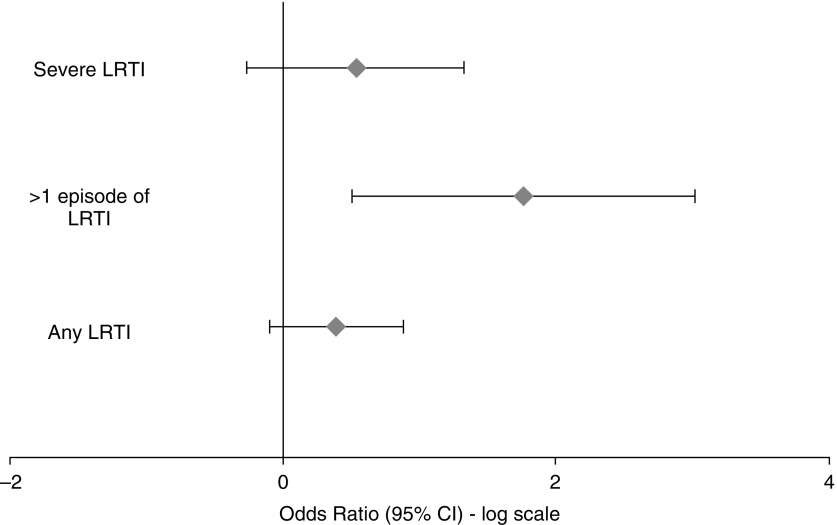
LRTI in the first year of life increased a child’s risk of having a respiratory rate in the highest quartile at 1 year by 74% (OR, 1.74; 95% CI, 1.1–2.5; P = 0.03) (Figure 3). Having a severe LRTI in the first year of life trebled a child’s risk of highest quartile respiratory rate (OR, 3.1; 95% CI, 1.3–7.3; P = 0.008). Every additional LRTI increased a child’s risk of having an increased respiratory rate at 1 year by 50% (OR, 1.5; 95% CI, 1.2–1.9; P = 0.002), of having a tPTEF/tE in the lowest quartile by 30% (OR, 1.3; 95% CI, 1.02–1.59; P = 0.035) and highest quartile LCI by 40% (OR, 1.4; 95% CI, 1.05–1.96; P = 0.02) (Figure 4).
Figure 3.
Odds ratio of belonging to the upper quartile of respiratory rate at 1 year after lower respiratory tract illness. CI = confidence interval; LRTI = lower respiratory tract illness.
Figure 4.
Odds ratio of belonging to the upper quartile of lung clearance index at 1 year after lower respiratory tract illness. CI = confidence interval; LRTI = lower respiratory tract illness.
Infant growth was a strong predictor of lung function at 1 year. For every WAZ increase infants had a 4.7-ml higher Vt (95% CI, 3.0–6.4; P < 0.001), 12.9 ml higher FRC (95% CI, 9.9–15.9; P < 0.001), and 0.1 turnover lower LCI (95% CI, −0.16 to −0.05; P < 0.001). Improved nutrition during the first year of life as measured by a change in WAZ between 6 weeks and 1 year was also associated with increased Vt and FRC and predicted better Vt at 1 year. Every unit increase in WAZ reduced the chance of having a low Vt at 1 year by 36% (OR, 0.64; 95% CI, 0.5–0.8; P = 0.001). However, an increased WAZ increased the chance of a tPTEF/tE in the lowest quartile (OR, 1.31; 95% CI, 1.06–1.63; P = 0.01).
Infants whose mothers smoked during pregnancy had a lower Vt at 1 year compared with infants whose mother’s did not smoke (average 3 ml lower; 95% CI, −5.6 to −0.1; P = 0.04). Having a mother who smoked during pregnancy trebled an infant’s risk of having Vt in the lowest quartile at 1 year (OR, 2.8; 95% CI, 1.6–5.0; P < 0.001).
Exposure to household tobacco smoke during the first year of life was associated with a higher LCI (0.24 turnovers; 95% CI, 0.06–0.43; P = 0.01) compared with infants who were unexposed.
Being born premature was associated with a lower FRC at 1 year compared with term infants (average 16.8 ml lower; 95% CI, −27.8 and −5.8; P = 0.003). Very preterm infants (<32 wk gestation) had four times the risk of having a tPTEF/tE in the lowest quartile (OR, 4.2; 95% CI, 1.03–17.0; P = 0.05) compared with infants born at term.
Maternal HIV exposure was not associated with lung function outcomes at 1 year. Similarly, high-risk maternal alcohol intake during pregnancy was not associated with lung function change at 1 year after adjusting for confounding factors.
Discussion
This is the first study in an LMIC to describe changes in lung function and the impact of early life LRTI on child lung health. Lung function during this critical period of lung development tracked from early life, with low lung function soon after birth a strong predictor of lung function at 1 year. LRTI was identified as a key factor associated with reduced lung function at 1 year, with recurrent illnesses and more severe disease associated with further reductions in lung function. Other factors identified that impacted normal lung growth included infant nutrition, prematurity, and antenatal or postnatal exposure to tobacco smoke.
Previous reports of LRTI in early life and later lung function suggested that the effect of LRTI on lung function was mediated by preexisting low lung function (10, 28). In the current study low lung function at 6 weeks, with the measurements used, was not associated with increased risk of LRTI during the first year. The independent effect of LRTI and episode severity on lung function outcomes at 1 year is an important finding in view of the fact that lung function is known to track to later life and increase risk of later respiratory disease (3, 4). In addition, the increased risk of low lung function with recurrent events (Figures 2 and 3) highlights the potential negative impact of recurrent ambulatory LRTI on lung function outcomes. Longitudinal follow-up of these infants is currently underway.
The tracking of lung function during the first year of life is in keeping with described patterns of early lung development (29, 30). The increase in Vt and FRC during the first year reflects the rapid and nonlinear association of lung and somatic growth (31) and the decrease of tPTEF/tE, the dysynaptic growth of lung parenchyma, and airways during this period of development (32).
Although postnatal growth has been associated with increased lung volumes, large postnatal weight gain has been associated with reduced expiratory flows (33, 34). We found infants who increased their WAZ between 6 weeks and 1 year had an increased risk of having lower expiratory flow ratios (tPTEF/tE) and increased lung volumes. Hence optimizing nutrition is important in promoting respiratory health in infancy.
We have already described the negative impact of maternal smoking during pregnancy on lung function at 6 weeks in this cohort (35, 36). The current study extends this by showing that the negative effect of maternal smoking persists to 1 year, an effect independent of infant growth and baseline lung function. Subsequent household exposure to tobacco smoke had the additional effect of increased LCI. This may reflect airway inflammation and/or damage rather than impaired growth. However, in this study assessing the effects of antenatal and postnatal tobacco smoke exposure independently is not possible, because the same infants whose mothers smoked during pregnancy had postnatal exposure.
Prematurity was independently associated with reduced FRC at 1 year, and being born very premature was a strong predictor of having an expiratory flow ratio tPTEF/tE in the lowest quartile. This suggests that decreased lung volumes in preterm infants are mediated by reduced somatic size (37).
Strengths of this study are the large sample size of matched lung function collected by the same investigators, using the same testing techniques. Lung function was undertaken in a community-based cohort with robust LRTI surveillance and simultaneous measurement of comprehensive risk factors for impaired lung growth. Our symptom-based definition of LRTI allowed us to assess the impact on lung function at a population level of community-acquired LRTI. This definition aligns with relevant epidemiologic studies investigating LRTI in high-burden settings (38). A further strength is measurement of lung function before an illness episode, enabling accurate assessment of lung health before illness. Limitations of the study are the potential lack of generalizability to other settings with different exposures; however, many of these exposures are common in LMIC settings.
In summary, this study describes the tracking of lung function in the first year of life in a cohort of infants living in a high respiratory disease burden setting. It identifies LRTI as an independent risk factor for reduced lung function at 1 year, highlighting the importance of development of strategies to prevent LRTI in early life. Other risk factors include infant nutrition, prematurity, or tobacco smoke exposure, factors amenable to public health interventions. Given that infant lung function tracks into later life and plays a role in chronic respiratory disease, preventing LRTI in young children, reducing exposure to tobacco smoke, and optimizing nutrition are further key priorities to strengthen child respiratory health.
Acknowledgments
Acknowledgment
The authors thank the study and clinical staff at Paarl Hospital, Mbekweni and Newman clinics, as well as the CEO of Paarl Hospital, Dr. Kruger, and the Western Cape Health Department for their support of the study. The authors thank the families and children who participated in this study.
Footnotes
Supported by the Wellcome Trust (#098479/z/12/z), Bill and Melinda Gates Foundation (OPP1017641), Thrasher Foundation (#9207), MRC South Africa, Worldwide University Network Research Mobility Award, and University of Cape Town equipment grant.
Author Contributions: D.M.G. conceived the study, worked on study design, was involved in data collection, recording lung function data, analysis of data, and was primary author of the manuscript. G.L.H. and H.J.Z. conceived the study and contributed to study design. L.T. contributed to planning statistical methods and data analysis. A. Vanker, D.J.S., and P.D.S. contributed to study design and commented on manuscript draft. A. Visagie, L.W., and A. Vanker were involved in recruiting patients and collecting data. All authors have seen and approved the submitted manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201601-0188OC on August 10, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2.Zar HJ, Ferkol TW. The global burden of respiratory disease-impact on child health. Pediatr Pulmonol. 2014;49:430–434. doi: 10.1002/ppul.23030. [DOI] [PubMed] [Google Scholar]
- 3.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner SW, Palmer LJ, Rye PJ, Gibson NA, Judge PK, Young S, Landau LI, Le Souëf PN. Infants with flow limitation at 4 weeks: outcome at 6 and 11 years. Am J Respir Crit Care Med. 2002;165:1294–1298. doi: 10.1164/rccm.200110-018OC. [DOI] [PubMed] [Google Scholar]
- 5.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319:1112–1117. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 6.Dezateux C, Stocks J, Dundas I, Fletcher ME. Impaired airway function and wheezing in infancy: the influence of maternal smoking and a genetic predisposition to asthma. Am J Respir Crit Care Med. 1999;159:403–410. doi: 10.1164/ajrccm.159.2.9712029. [DOI] [PubMed] [Google Scholar]
- 7.Bisgaard H, Jensen SM, Bønnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185:1183–1189. doi: 10.1164/rccm.201110-1922OC. [DOI] [PubMed] [Google Scholar]
- 8.van der Gugten AC, Uiterwaal CS, van Putte-Katier N, Koopman M, Verheij TJ, van der Ent CK. Reduced neonatal lung function and wheezing illnesses during the first 5 years of life. Eur Respir J. 2013;42:107–115. doi: 10.1183/09031936.00214711. [DOI] [PubMed] [Google Scholar]
- 9.Håland G, Carlsen KH, Devulapalli CS, Pettersen M, Mowinckel P, Lødrup Carlsen KC. Lung function development in the first 2 yr of life is independent of allergic diseases by 2 yr. Pediatr Allergy Immunol. 2007;18:528–534. doi: 10.1111/j.1399-3038.2007.00555.x. [DOI] [PubMed] [Google Scholar]
- 10.Castro-Rodríguez JA, Holberg CJ, Wright AL, Halonen M, Taussig LM, Morgan WJ, Martinez FD. Association of radiologically ascertained pneumonia before age 3 yr with asthmalike symptoms and pulmonary function during childhood: a prospective study. Am J Respir Crit Care Med. 1999;159:1891–1897. doi: 10.1164/ajrccm.159.6.9811035. [DOI] [PubMed] [Google Scholar]
- 11.Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015;135:607–616. doi: 10.1542/peds.2014-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold DR, Tager IB, Weiss ST, Tosteson TD, Speizer FE. Acute lower respiratory illness in childhood as a predictor of lung function and chronic respiratory symptoms. Am Rev Respir Dis. 1989;140:877–884. doi: 10.1164/ajrccm/140.4.877. [DOI] [PubMed] [Google Scholar]
- 14.Johnston ID, Strachan DP, Anderson HR. Effect of pneumonia and whooping cough in childhood on adult lung function. N Engl J Med. 1998;338:581–587. doi: 10.1056/NEJM199802263380904. [DOI] [PubMed] [Google Scholar]
- 15.Zar HJ, Barnett W, Stadler A, Gardner-Lubbe S, Myer L, Nicol MP. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. Lancet Respir Med. 2016;4:463–472. doi: 10.1016/S2213-2600(16)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray D, Turkovic L, Willemse L, Alberts A, Vanker A, Stein DJ, Sly P, Hall GL, Zar HJ. Lung function in the first year of life in African infants: effect of early life pneumonia. Cape Town, South Africa: South African Thoracic Society; 2015. [Google Scholar]
- 17.Zar HJ, Barnett W, Myer L, Stein DJ, Nicol MP. Investigating the early-life determinants of illness in Africa: the Drakenstein Child Health Study. Thorax. 2015;70:592–594. doi: 10.1136/thoraxjnl-2014-206242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, de Lacerda RB, Ling W, Marsden J, Monteiro M, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction. 2008;103:1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 19.Stein DJ, Koen N, Donald KA, Adnams CM, Koopowitz S, Lund C, Marais A, Myers B, Roos A, Sorsdahl K, et al. Investigating the psychosocial determinants of child health in Africa: the Drakenstein Child Health Study. J Neurosci Methods. 2015;252:27–35. doi: 10.1016/j.jneumeth.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.le Roux DM, Myer L, Nicol MP, Zar HJ. Incidence of childhood pneumonia: facility-based surveillance estimate compared to measured incidence in a South African birth cohort study. BMJ Open. 2015;5:e009111. doi: 10.1136/bmjopen-2015-009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO. Integrated management of childhood illness: distance learning course. Geneva: WHO; 2014. [Google Scholar]
- 22.Zar HJ, Barnett W, Stadler A, Gardner-Lubbe S, Myer L, Nicol MP. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. Lancet Respir Med. 2016;4:463–472. doi: 10.1016/S2213-2600(16)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray DM, Willemse L, Alberts A, Simpson S, Sly PD, Hall GL, Zar HJ. Lung function in African infants: a pilot study. Pediatr Pulmonol. 2015;50:49–54. doi: 10.1002/ppul.22965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray D, Willemse L, Visagie A, Smith E, Czövek D, Sly PD, Hantos Z, Hall GL, Zar HJ. Lung function and exhaled nitric oxide in healthy unsedated African infants. Respirology. 2015;20:1108–1114. doi: 10.1111/resp.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22:745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 26.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization World Child Growth Standards 2006. Available from: http://www.who.int/childgrowth/standards/en/
- 28.Håland G, Lødrup Carlsen KC, Mowinckel P, Munthe-Kaas MC, Devulapalli CS, Berntsen S, Carlsen KH. Lung function at 10 yr is not impaired by early childhood lower respiratory tract infections. Pediatr Allergy Immunol. 2009;20:254–260. doi: 10.1111/j.1399-3038.2008.00781.x. [DOI] [PubMed] [Google Scholar]
- 29.Young S, Sherrill DL, Arnott J, Diepeveen D, LeSouëf PN, Landau LI. Parental factors affecting respiratory function during the first year of life. Pediatr Pulmonol. 2000;29:331–340. doi: 10.1002/(sici)1099-0496(200005)29:5<331::aid-ppul1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 30.Hoo AF, Dezateux C, Henschen M, Costeloe K, Stocks J. Development of airway function in infancy after preterm delivery. J Pediatr. 2002;141:652–658. doi: 10.1067/mpd.2002.128114. [DOI] [PubMed] [Google Scholar]
- 31.Stocks J, Quanjer PH Official Statement of The European Respiratory Society. Reference values for residual volume, functional residual capacity and total lung capacity: ATS Workshop on Lung Volume Measurements. Eur Respir J. 1995;8:492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 32.Horsfield K, Gordon WI, Kemp W, Phillips S. Growth of the bronchial tree in man. Thorax. 1987;42:383–388. doi: 10.1136/thx.42.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucas JS, Inskip HM, Godfrey KM, Foreman CT, Warner JO, Gregson RK, Clough JB. Small size at birth and greater postnatal weight gain: relationships to diminished infant lung function. Am J Respir Crit Care Med. 2004;170:534–540. doi: 10.1164/rccm.200311-1583OC. [DOI] [PubMed] [Google Scholar]
- 34.Turner S, Zhang G, Young S, Cox M, Goldblatt J, Landau L, Le Souëf P. Associations between postnatal weight gain, change in postnatal pulmonary function, formula feeding and early asthma. Thorax. 2008;63:234–239. doi: 10.1136/thx.2006.064642. [DOI] [PubMed] [Google Scholar]
- 35.Gray D, Czövek D, Smith E, Willemse L, Alberts A, Gingl Z, Hall GL, Zar HJ, Sly PD, Hantos Z. Respiratory impedance in healthy unsedated South African infants: effects of maternal smoking. Respirology. 2015;20:467–473. doi: 10.1111/resp.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray D, Willemse L, Visagie A, Czovek D, Nduru P, Vanker A, Stein DJ, Koen N, Sly PD, Hantos Z, et al. Thorax. Determinants of early-life lung function in African infants. [online ahead of print] 17 Nov 2016. DOI: 10.1136/thoraxjnl-2015-207401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merth IT, de Winter JP, Borsboom GJ, Quanjer PH. Pulmonary function during the first year of life in healthy infants born prematurely. Eur Respir J. 1995;8:1141–1147. doi: 10.1183/09031936.95.08071141. [DOI] [PubMed] [Google Scholar]
- 38.Levine OS, O’Brien KL, Deloria-Knoll M, Murdoch DR, Feikin DR, DeLuca AN, Driscoll AJ, Baggett HC, Brooks WA, Howie SR, et al. The Pneumonia Etiology Research for Child Health Project: a 21st century childhood pneumonia etiology study. Clin Infect Dis. 2012;54:S93–S101. doi: 10.1093/cid/cir1052. [DOI] [PMC free article] [PubMed] [Google Scholar]