Pulmonary hypertension (PH) is a complex pulmonary vascular condition with increasing global prevalence and with particularly severe forms, such as pulmonary arterial hypertension (PAH), that are often fatal. In PAH, remodeling in diseased pulmonary arteries places an increasing hemodynamic burden on the right ventricle, leading to right ventricular failure, multiorgan dysfunction, and death. Current PH medications primarily target three major vasodilatory pathways (nitric oxide, endothelin, and prostacyclin signaling) but do not target the elusive upstream molecular origins of PH. Thus, they neither prevent nor reverse disease. Hence, there is a great need to identify the upstream molecular triggers of disease and apply those discoveries to clinical benefit.
However, the pathogenesis of PH is complex and multifactorial. It involves both multiple vascular and nonvascular cell types and depends on several pathogenic events (i.e., genetic susceptibility, hypoxia, inflammation, viral infection, DNA damage, and shear stress, among others) for disease manifestation and progression (1, 2). An inability to fully unravel these molecular complexities has led to multiple clinical challenges in developing both new diagnostics and therapeutics for this disease.
Discoveries have identified the dysregulation of microRNAs (miRNAs) as integral events that shape the development and progression of PH (3). miRNAs are small noncoding RNA molecules that negatively regulate gene expression and perform pervasive regulatory functions in all aspects of biology, both in the animal and plant kingdoms (4). A number of key reports have implicated miRNAs in a wide range of pulmonary vascular processes, spanning pulmonary vascular development, physiology, and disease and leading to speculation on their usefulness in clinical diagnosis and prognosis. Yet, we still are at the inception of this growing field of study, and a number of challenges remain in applying this biology to clinical practice. In this review, we discuss both the fundamental biology of miRNAs in PH and the translational potential and obstacles in their development as novel biomarkers and direct therapeutics.
Fundamental Pathobiology of MicroRNAs in PH
Canonical Functions of miRNAs and In Vivo Detection Strategies
At present, more than 5,500 distinct miRNA species have been predicted to be encoded by the human genome (5), some of which are conserved throughout mammalian evolution and others encoded only in primates and humans. On transcription from their genomic sites, primary and premature miRNA forms are processed in the nucleus and cytoplasm to mature forms (6). These mature, active species are double-stranded RNA molecules 19–24 nt in length. Each active miRNA encodes a 6-nt “seed sequence” (positions 2–7 at the 5′ end of the active strand), which is a crucial determinant for target sequence binding on the complementary mRNA transcript—typically positioned in the 3′ untranslated region (3′ UTR) of an mRNA molecule. This miRNA–mRNA interaction results in the down-regulation of target gene expression, via either translational repression or transcript degradation (Figure 1). Because of the importance of conserved Watson–Crick binding for such complementary RNA binding, several freely available computational algorithms have been developed (i.e., TargetScan 5 [Conserved] [7] and DIANA [8] among many others) to predict the mRNA targets of given miRNAs. Although they suffer from a variable level of false-positive prediction, these algorithms continue to be used extensively to accelerate the discovery of the biological roles of miRNAs based on their predicted target pool.
Figure 1.
MicroRNA (miRNA) biogenesis and canonical actions of post-transcriptional gene regulation. Left: Canonical protein-coding genes embedded throughout the human chromosomal structure are transcribed, spliced, and transported from the nucleus to the cytoplasm as mature mRNAs. miRNAs are encoded as discrete single genes, as gene families, or embedded in introns of protein-coding genes (mirtrons). After transcription, primary miRNAs (pri-miRNAs) undergo nuclear and cytosolic processing steps to premature miRNAs (pre-miRNAs) before nuclear export and further maturation to a biologically active 19- to 24-nt molecule containing a 6-nt “seed” region. Driven primarily by seed region binding to complementary sites of target mRNAs, miRNAs repress gene expression via translational repression or mRNA degradation (bottom). Right: Technological advances have allowed more efficient and more comprehensive strategies for quantifying miRNA levels in human tissue and fluids. miRNA* duplex = antisense strand of the miRNA duplex; PCR = polymerase chain reaction; RISC = RNA-induced silencing complex; UTR = untranslated region.
Unlike the detection of proteins by antibody-based strategies, the quantitation of miRNAs in vascular tissue or bloodstream relies on the ability to extract and detect or amplify RNA sequences (Figure 1). Historically, in research applications, detection of any RNA molecules, including miRNAs, relied on a more cumbersome method of gel electrophoresis and oligonucleotide probe hybridization (Northern blot). Such electrophoretic methods are still used in research settings, but are not feasible in clinical contexts. Methods of quantitative amplification of single and specific miRNA molecules, such as reverse transcription-quantitative polymerase chain reaction (RT-qPCR) or droplet digital PCR (ddPCR), have been used as extremely sensitive and specific methods to quantify accurately as little as one miRNA molecule in samples of human tissue and body fluids. Beyond single miRNA quantification, the era of comprehensive -omics profiling has resulted in the rapid development of technology to assess thousands of miRNAs simultaneously from these samples. Originally, arrayed technology was developed via positioning hundreds of labeled oligonucleotide probes on a chip and hybridizing to miRNA sequences that would allow for quantification of miRNA levels. Arrayed and robotic technology has also been developed to perform single RT-qPCRs relevant to thousands of miRNAs; such a platform has been used effectively to quantify the landscape of miRNAs in the bloodstream of thousands of subjects in the Framingham Heart Study (9). More recently, so-called next-generation RNA sequencing and single-cell RNA sequencing are quickly gaining favor for the comprehensive and accurate assessment of miRNA levels, with applications in a variety of precision medicine initiatives to attain molecular profiles of human health and disease.
MicroRNAs in PH
In PH, accumulating evidence indicates that dysregulation of miRNAs is intimately linked to the hyperproliferative and apoptosis-resistant pathophenotypes of pulmonary vascular cells including pulmonary arterial endothelial cells (PAECs), pulmonary arterial smooth muscle cells (PASMCs), and pulmonary arterial adventitial fibroblasts (PAAFs) (Figure 2A). Because providing an exhaustive list of such molecules is beyond the scope of this Perspective, we instead focus on key miRNAs with well-established, and often complementary and convergent, mechanistic roles in PH pathogenesis (Figures 2A–2C).
Figure 2.
Dysregulated microRNAs (miRNAs) implicated in vascular remodeling and extrapulmonary sites in pulmonary arterial hypertension (PAH). (A) Aberrantly expressed miRNAs are organized by their convergent molecular actions and cellular localization during pulmonary artery remodeling in PAH. (B) Down-regulation of miR-208 in right ventricular (RV) cardiomyocytes and miR-126 in RV endothelial cells induces angiogenic and contractile alterations in RV function, promoting the progression of adaptive hypertrophy to overt RV failure. Numerous other miRNAs are dysregulated in the RV during both adaptive hypertrophy and failure, and their precise mechanistic roles are currently being defined. (C) Microcirculation loss and impaired angiogenesis secondary to miR-126 down-regulation contribute to exercise intolerance in patients with PAH. BMPR2 = bone morphogenetic protein receptor 2; BRD4 = bromodomain containing 4; ET-1 = endothelin-1; FGF = fibroblast growth factor; FGFR1 = fibroblast growth factor receptor 1; IGF-1 = insulin-like growth factor 1; ISCU = iron–sulfur cluster assembly protein; KLFs = Krüppel-like factors; miR = microRNA; NFAT = nuclear factor of activated T cells; PAEC = pulmonary arterial endothelial cells; PARP-1 = poly(ADP-ribose) polymerase 1; PASMC = pulmonary arterial smooth muscle cells; PPARγ = peroxisome proliferator-activated receptor γ; PTPB1 = polypyrimidine tract–binding protein 1; SMURF1 = Smad ubiquitination regulatory factor 1; STAT3 = signal transducer and activator of transcription 3.
Metabolism and Proliferation
There exists an increasing appreciation that cancer and diseased vascular cells in PH present numerous molecular similarities (10). In many regards, PH disease progression mirrors tumorigenesis in relying on a metabolic switch from mitochondrial oxidative phosphorylation to cytoplasmic glycolysis (known as the Warburg effect) (2) to provide a survival advantage to highly proliferating cells (11). The hypoxia-dependent miR-210 is one of several miRNAs implicated in this metabolic rewiring (12–14). Among its pleiotropic actions, miR-210 was found to repress directly the iron–sulfur cluster assembly proteins (ISCU1/2) essential for mitochondrial respiration, leading to Fe–S deficiencies and PH development. Accordingly, forced expression of miR-210 was sufficient to induce pulmonary vascular dysfunction in mice, whereas molecular or genetic inhibition of miR-210 prevented and reversed chronic hypoxic PH (14).
Highlighting additional similarities to tumor progression, miR-204 has been found to act as a tumor suppressor with a pivotal role in PAH (15). Reduced expression of miR-204 in PAH-PASMCs secondary to STAT3 (signal transducer and activator of transcription 3) activation was observed to enhance STAT3 (creating a positive feedback loop) and BRD4 (bromodomain containing 4) expression, leading to the overactivation of NFAT (nuclear factor of activated T cells), HIF-1α (hypoxia inducible factor-1α) (16), and RUNX2 (runt-related transcription factor 2) (17), all of which contribute to metabolic dysfunction, exaggerated proliferation, and often calcification. The critical implication of miR-204 dysregulation in PH was further supported by experiments in two animal models of PAH, in which restoration of miR-204 expression reversed remodeling and improved hemodynamics (15, 16). Two additional miRNAs expressed by PAECs, miR-424 and miR-503, were found to be down-regulated in human PAH (18). Such down-regulation was accompanied by an increase in fibroblast growth factor 2 (FGF2) and fibroblast growth factor receptor 1 (FGFR1) expression and hyperproliferation of PAECs and PASMCs, indicating that PAEC-specific alterations in miRNAs compromise the PASMC phenotype through miRNA-dependent paracrine signaling. Similarly, the miR-130/301 family was found to be increased in PAH (19), thus repressing a cohort of target genes and subordinate miRNAs, thus affecting PASMC proliferation (19) and vasoconstriction (20). PAH-PASMCs and PAH models are also characterized by a down-regulation of miR-193. Although its role remains to be clarified, it has been demonstrated that apolipoprotein A-I–mimetic peptides can successfully rescue experimental PAH by inducing the expression of miR-193, most likely via the suppression of the peroxisome proliferator-activated receptor γ (PPARγ)–retinoid X receptor α (RXRα) signaling pathway, which in turn inhibits PASMC proliferation by diminishing insulin growth factor (IGF)-1 receptor expression (21).
Inherently related to the hyperproliferative vascular state of PAH is the central role of bone morphogenetic protein receptor 2 (BMPR2), a gene in which naturally occurring mutations accounts for 70% of all hereditary cases of PH (22, 23). Indeed, there exists a predominance of functional connections of miRNAs to BMP signaling in PH. For instance, the polycistronic miR-17-92 cluster was found to be down-regulated in vascular cells from patients with PAH. Overexpression of this miRNA cluster resulted in direct transcript binding of miR-17-5p and miR-20a (24) and consequent expression of BMPR2. Correspondingly, in vivo inhibition of several members of the cluster, using oligonucleotide inhibitors (i.e., antagomiRs) or targeted genetic deletion of the entire cluster, was shown to improve experimental PH (25–27). These beneficial effects were attributed at least in part to the restoration of BMPR2 expression and the up-regulation of the cyclin-dependent kinase inhibitor p21 (25, 27). In addition to being the target of miRNAs, BMPR2 signaling also represses expression of another critical miRNA, miR-145. Elevated miR-145 expression was observed in primary PASMCs cultured from patients with PAH with loss-of-function BMPR2 mutations as well as in the lungs of BMPR2-deficient mice. Importantly, mice with genetic deletions of miR-145 exhibited protection against PH (28). Conversely, miR-140 has been implicated in the regulation of Smad ubiquitination regulatory factor 1 (SMURF1), an E3-ubiquitin protein ligase 1, that in turn modulates BMPR2 signaling and PAH in vivo (29). Numerous additional miRNAs implicated in PAH have been reported to respond to BMPR2 signaling, such as miR-130/301 (19), and to regulate BMPR2 directly, such as miR-21 (30).
Beyond PAEC and PASMC biology, the cellular pathophenotypes of PAAFs, including proliferation, migration, and differentiation, promote vascular remodeling and are influenced by miRNA activity. In vitro gain- and loss-of-function experiments revealed that decreased miR-124 in PAAFs from patients with PAH led to increased proliferation and migration through modulating the polypyrimidine tract–binding protein 1 (PTPB1) and subsequent inhibition of the cell cycle (28). Separately, the miR-130/301 family has been found to modulate extracellular matrix stiffening in PAAFs (31) via interfacing with the transcriptional coactivators YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif). The connection between YAP/TAZ and this miRNA family has led to additional understanding of the broader links among pulmonary vascular stiffness, glutamine metabolism, and proliferation (32, 33).
DNA Damage
DNA damage predates the onset of clinical PAH and is likely an intrinsic property in cells of individuals at risk for this disease (34). DNA damage is increased in circulating peripheral blood mononuclear cells, PAECs, and PASMCs (35, 36). DNA damage was reported to down-regulate miR-223 in PAH-PASMCs (37). This down-regulation induced poly(ADP-ribose) polymerase (PARP)-1, which mediates the DNA damage response and contributes to DNA repair. Activation of PARP-1 increased cell survival and proliferation through a mechanism that involves miR-204 down-regulation (38).
Vasoconstriction
The miR-130/301 family has been shown to control vasoconstriction in PH by stimulating production of the vasoconstrictor endothelin-1 in PAECs (20). Other miRNAs, including miR-328 and miR-190, influence vascular tone in PH by targeting K+ and Ca2+ channels (39, 40), both of which are critical to the physiological (hypoxic) and pathological response of pulmonary arteries (41).
Estrogen Signaling and Sex-Specific miRNA Biology in PH
The actions of two miRNAs have been implicated in the female predominance of PAH. A sex-specific decrease in miR-96 was found to increase expression of the 5-hydroxytryptamine 1B receptor (42), thus predisposing to alteration of serotonin signaling and proliferative signals in the diseased pulmonary vasculature. Separately, a sex-specific interaction of miR-29 was described with estrogen metabolism (43), suggesting a molecular mechanism correlating female hormone signaling with the predisposition of PAH in women in the general population.
Angiogenesis and MicroRNAs in PH and Extrapulmonary Sites
Impaired angiogenesis and subsequent rarefaction of microvessels is a common feature of pulmonary, right ventricular, and skeletal muscle abnormalities seen in patients with PAH (44). Studies revealed that down-regulation of the myocyte enhancer factor 2 (MEF2C)–miR-208 axis (45) and miR-126 (46) drives the transition from a compensated hypertrophic right ventricle (RV) to a decompensated RV by affecting contractility and angiogenesis, respectively (Figure 2B). Consistently, microcirculation loss and impaired angiogenesis secondary to miR-126 down-regulation was also reported to contribute to exercise intolerance in patients with PAH (Figure 2C) (47). Additional insights regarding the roles of miRNAs in the extrapulmonary manifestations of PH await further study, particularly in the setting of a growing number of miRNAs found to be dysregulated in RV dysfunction but with actions that have yet to be fully clarified (48).
Leveraging Network Biology to Consider the Molecular Complexity of miRNAs in PH
Although it is clear that a number of key miRNAs play powerful roles in the control of pulmonary vascular biology, challenges exist in understanding their integrated and likely overlapping actions. Computational network modeling may be a powerful and emerging tool to glean biological information in the context of complicated molecular interactions (49). Initial attempts to model computationally and interrogate miRNA regulatory networks in the pulmonary vasculature have yielded positive results. Chan and colleagues designed an in silico approach to rank miRNAs with systems-wide effects on known downstream PH-relevant genes and their first degree interactors, based on several independent gene network architectural parameters (19, 30). Not only was the miR-130/301 family identified as the top-ranked miRNA family, but also such network analyses outlined a model of downstream actions of these miRNAs to include two known proliferative pathways, a pathway influencing vasoconstriction, and a novel signaling mechanism controlling extracellular matrix deposition and remodeling. These findings were verified in vivo and in vitro (15, 19, 20, 24, 31), thus demonstrating the power of combining computational bioinformatics with experimental biology and affording distinct advantages as compared with traditional scientific approaches.
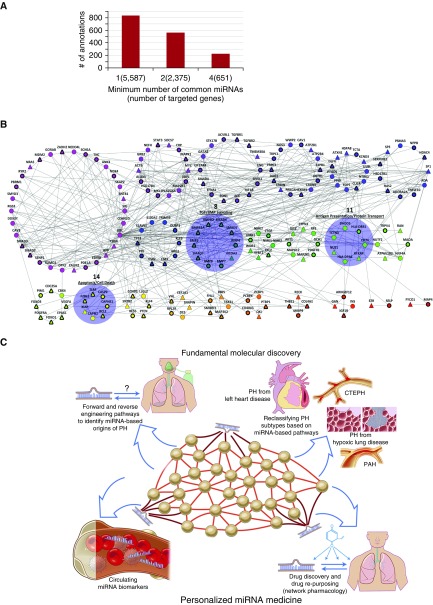
Network gene analyses may be applied to the current challenges of understanding the collective actions of PH-specific miRNAs on overall disease manifestation rather than merely focusing on single actions of an miRNA in isolation. For example, we considered a list of 25 PH-relevant miRNAs (Table 1) and attempted to identify functional pathways that may be targeted coordinately by multiple miRNAs. We compiled a list of more than 5,500 target genes of these miRNAs from miRTarBase (50), a database cataloging validated miRNA–gene interactions, and TargetScan (51), a database predicting mRNA binding sites for miRNAs (Figure 3A). Gene set enrichment analysis (GSEA) is a popular statistical tool often used to identify previously annotated pathways that have a significant representation in a given gene set (52). Here, we enriched target genes via GSEA with four databases of pathway annotations: Reactome (53), KEGG (54), BioCarta (55), and Gene Ontology (56, 57). After correcting for the false discovery rate, we found more than 800 significant annotations. We repeated the enrichment by analyzing only genes targeted by multiple PH-relevant miRNAs. However, even when examining genes targeted by 4 or more miRNAs (651 genes), pathways enriched among these target genes still numbered more than 220—a number outweighing our ability to discern obvious shared actions of these miRNAs and outstripping our ability to validate experimentally these predictions. Thus, a “brute force” analysis of these data demonstrates the immense pleiotropy of miRNAs but alone fails to filter these results in a meaningful way for the purpose of uncovering the interconnected but hidden actions of these molecules.
Table 1.
Pulmonary Hypertension–Relevant MicroRNAs Used for Construction of a Pulmonary Hypertension Gene Subnetwork
| miRNA |
|---|
| miR-124-3p |
| miR-125a-5p |
| miR-126-3p |
| miR-130a-3p |
| miR-130b-3p |
| miR-140-5p |
| miR-145-5p |
| miR-17-5p |
| miR-18a-5p |
| miR-19a-3p |
| miR-19b-1-5p |
| miR-204-5p |
| miR-208a-3p |
| miR-20a-5p |
| miR-210-3p |
| miR-21-5p |
| miR-223-3p |
| miR-29a-3p |
| miR-301a-3p |
| miR-301b-3p |
| miR-424-5p |
| miR-503-5p |
| miR-92a-1-5p |
| miR-92a-3p |
| miR-96-5p |
Definition of abbreviation: miR, miRNA = microRNA.
Figure 3.
Application of network theory to gain insight into microRNA (miRNA) activity in pulmonary arterial hypertension (PAH). (A) Gene set enrichment analysis (GSEA) demonstrates the vast pleiotropy of miRNA activity. The number of significant pathway annotations was gathered after GSEA enrichment on genes targeted by at least 1, 2, or 4 pulmonary hypertension (PH)-relevant miRNAs (among a group of 25 total miRNAs). A large number of statistically significant annotations was observed in all cases, indicating the need for additional statistical filtering techniques to discern the convergent functions of miRNAs. (B) Network architecture analysis improves the resolution of testable hypotheses regarding convergent miRNA function. From genes targeted by four or more PH-relevant miRNAs and overlaid on an extended network of PH genes and their first-degree interactors, a single connected component of 190 genes was partitioned into clusters based on its topology, using the “MAP” algorithm (58). Each node represents a gene, and each gray line denotes an interaction between genes. Rings of same-colored nodes represent clusters. Triangular nodes are those genes targeted by four or more PH-relevant miRNAs, and circular nodes are first-degree interactors necessary to make a single connected component. Nodes outlined in thick black lines are genes already known to play a role in PH, curated from the scientific literature. Of the 22 clusters, we found 3 to be of particular interest (highlighted in blue). Clusters 8, 11, and 14 are involved in transforming growth factor/bone morphogenetic protein (TGF/BMP) signaling, antigen presentation and protein transport, and apoptosis and cell death, respectively. (C) Leveraging computational gene network analysis for discovery of fundamental miRNA biology in PH and application to personalized medicine initiatives in PH. CTEPH = chronic thromboembolic pulmonary hypertension.
To identify more clearly the convergent functions of these miRNAs, we designed an additional in silico algorithm to leverage the interconnected network architecture of those genes targeted by 4 or more PH-specific miRNAs and known to be directly relevant to PH (85 genes). We overlaid the list of 85 genes onto an updated and extended PH network, constructed similarly to a previous description (19) and comprising 747 interconnected genes that represent known downstream PH-relevant genes and their first-degree interactors. In doing so, we created a subgraph of 190 genes including all 85 of the original target genes and several additional genes to create a single connected component (Figure 3B). We then used “MAP” (58), a well-established clustering method, to partition the 190 genes into 22 separate clusters based on the density distribution of interactions (Table 2). Theoretically, these clusters represent interconnected pathways that are particularly integrated in the actions of multiple PH-relevant miRNA, and several testable hypotheses emerged after GSEA enrichment. For example, cluster 14 included a number of known PH genes targeted by multiple PH-relevant miRNAs and were enriched as a group for their involvement in apoptosis and cell death (Table 3). Included in that cluster was the X-linked inhibitor of apoptosis protein (XIAP), a gene that has never been studied in the context of PH yet one with statistically significant connections among other cluster 14 genes and one that is targeted by more than 4 PH-relevant miRNAs. Separately, cluster 8 contained 10 genes, 9 of which were well-known mediators of transforming growth factor/bone morphogenetic protein (TGF/BMP) signaling. The only additional gene was nuclear receptor coactivator 3 (NCOA3), a gene that also has not been studied in the context of PH or BMP signaling, but one that clearly has connections among other cluster 8 genes and one that is also targeted by more than four PH-relevant miRNAs. Finally, cluster 11 (Table 4) carried mostly first-degree interactors of known PH genes, some of which (NUS1, RFXAP) are targeted by multiple PH miRNAs but have never been studied directly in PH. GSEA enrichment revealed a predominant representation of antigen presentation and intracellular protein transport, pathways that have been implicated in PH but vastly understudied (59, 60).
Table 2.
List of Pulmonary Hypertension Subnetwork Genes in Their Respective Clusters
| Cluster | Genes |
|---|---|
| 1 | ANK3, APP, CALM2, CAV3, CDK6, CDKN2D, DDX3Y, DPF2, ESRRB, GORAB, MDM2, NEDD4L, NOX4, NRAS, PBRM1, PDE1A, RGS3, RYR2, SCN5A, SENP1, SKAP2, SMAD2, SMAD3, SMYD3, SNTB1, SRI, SUMO2, TNC, UBC, and ZADH2 |
| 2 | ACTB, ALOX5, CRP, GRB2, HSD17B4, MAP2K5, MAPK1, MKL1, MYC, NEFH, PLA2G4A, SOCS7, STAT3, and XBP1 |
| 3 | ACE, ACVRL1, EDN1, EFNA1, EIF2AK4, ENG, GATA2, HIF1A, SMAD4, SPHK1, STK17B, TGFBR1, TGFBR2, and TMEM30A |
| 4 | AGTR1, ATP2B1, ATP2B4, CALM1, CAV1, DAG1, ERBIN, HEXIM1, NOS3, PRKCA, S1PR1, TRPC1, TRPC3, and WWP2 |
| 5 | ATXN1, CLIC4, EGFR, FDPS, HSPA8, ILVBL, KCND3, NTRK1, PPID, PTGDS, and TCTA |
| 6 | ADA, ADORA2A, HAS2, HDAC4, HSD17B1, NPPB, PSMA3, SERPINE1, SP1, SP3, and TNFSF10 |
| 7 | ARG1, DUSP1, ELAVL1, ESR1, ESR2, KLF5, PRL, S100A7, TP53, and TRIM59 |
| 8 | BMP2, BMP4, BMP6, BMP7, BMPR1A, BMPR2, NCOA3, SMAD5, SMAD6, and SMAD7 |
| 9 | CFL2, CTGF, HPRT1, LIMK1, NME1-NME2, NME2, and WDR1 |
| 10 | AKT1, GCH1, GIT2, MAP2K1, MAPK12, PDGFRL, PTPN3, and RPE |
| 11 | CD74, DHDDS, HLA-DPA1, HLA-DRB1, NUS1, RFXAP, and TCTN3 |
| 12 | ATP6V1B2, HNF4A, MAOA, NUTF2, RAN, and TRPV4 |
| 13 | CBX4, CDC25A, EPAS1, FOXO1, FOXO3, PDGFRA, PIM1, and VEGFA |
| 14 | BCL2, CAPN2, CAPNS1, CASP9, F2RL1, TLR4, and XIAP |
| 15 | CCND1, HES5, KLF4, LLGL2, PTEN, and SNTB2 |
| 16 | DES, EEF1A1, RPL29, SNAPIN, and VHL |
| 17 | FHL1, MAP3K2, RBPJ, SMURF1, and TBX21 |
| 18 | CD40LG, PCBP1, PCBP4, PTBP1, and QKI |
| 19 | COL4A1, MMP9, RECK, and THBS1 |
| 20 | ARHGEF12, GAN, IGF1R, and INS |
| 21 | EZR and SELP |
| 22 | FYCO1 and MAP4 |
Table 3.
Significant Results from Gene Set Enrichment Analysis of Cluster 14
| Database | Annotation | P Value | Corrected P Value |
|---|---|---|---|
| Reactome | SMAC-mediated apoptotic response | 0.000001 | 0.018322 |
| Reactome | Apoptosis | 0 | 0.005601 |
| BioCarta | Mitochondria pathway | 0 | 0.002736 |
| KEGG | Apoptosis | 0 | 0.001818 |
| Reactome | SMAC-mediated dissociation of IAP:caspase complexes | 0.000001 | 0.01649 |
| BioCarta | HIVNEF pathway | 0.000001 | 0.018034 |
| Reactome | SMAC binds to IAPs | 0.000001 | 0.01649 |
| BioCarta | Death pathway | 0 | 0.004703 |
| Reactome | Intrinsic pathway for apoptosis | 0 | 0.007846 |
| KEGG | Small cell lung cancer | 0.000002 | 0.032358 |
| GO | IL-1β secretion | 0.000003 | 0.0355 |
| Reactome | Programmed cell death | 0 | 0.004703 |
| Reactome | Apoptotic factor–mediated response | 0.000002 | 0.031471 |
Definition of abbreviations: GO = Gene Ontology; HIVNEF = HIV negative effector of Fas and TNF; IAP = inhibitor of apoptosis; KEGG = Kyoto Encyclopedia of Genes and Genomes; SMAC = second mitochondria-derived activator of caspase.
Enrichment was performed by Fisher exact test. P values were corrected for false discovery rate, using the Benjamini-Hochberg method.
Table 4.
Significant Results from Gene Set Enrichment Analysis of Cluster 11
| Database | Annotation | P Value | Corrected P Value |
|---|---|---|---|
| KEGG | Antigen processing and presentation | 0 | 0.001905 |
| GO | Transport vesicle membrane | 0 | 0.010629 |
| GO | Integral component of lumenal side of endoplasmic reticulum membrane | 0 | 0.007507 |
| GO | Trans-Golgi network membrane | 0.000002 | 0.035833 |
| Reactome | Synthesis of dolichyl phosphate | 0.000002 | 0.030914 |
| GO | ER to Golgi transport vesicle membrane | 0.000001 | 0.018099 |
| GO | Dolichyl diphosphate biosynthetic process | 0.000002 | 0.030914 |
| GO | Clathrin-coated endocytic vesicle membrane | 0 | 0.010931 |
| GO | Endocytic vesicle membrane | 0.000001 | 0.031164 |
Definition of abbreviations: ER = endoplasmic reticulum; GO = Gene Ontology; KEGG = Kyoto Encyclopedia of Genes and Genomes.
Enrichment was performed by Fisher exact test. P values were corrected for false discovery rate, using the Benjamini-Hochberg method.
Although such computational analyses are useful, our techniques are still developing in assessing miRNA–target gene network architecture and kinetics in relation to PH or any other disease (Figure 3C). Endeavors have commenced in discerning specific molecular pathways important in PH via wide-scale genomic analysis (61, 62). Studies could be expanded to concentrate on the importance of genetic associations relevant to miRNA biogenesis genes, miRNA genes themselves, and target sequences recognized by those miRNAs. At the same time, the vast information emerging regarding mammalian metabolic networks and metabolomics could lead to profound systems-based discoveries regarding miRNA-based control of the pervasive metabolic reprogramming central to PH pathogenesis (63–66). New technologies to extract diseased lung tissue from living patients with PH (67) should also greatly aid our construction of high-throughput miRNA–target gene data sets in this disease. Together, these opportunities could serve as a key transition to an era of personalized miRNA medicine—one that is highly anticipated but not yet realized.
Development of Novel miRNA-based Diagnostic Platforms in PH: An Advent in Biomarker Discovery?
Concomitant with our advancing insights into the gene regulatory biology of intracellular miRNAs, the dynamic alterations of cell-free miRNAs circulating in plasma and the extracellular space have been reported in PH. In general, such released miRNAs may be associated with the Argonaute 2 (AGO2) protein in microvesicles (68), as free RNA–protein complexes (69), or both (70). The functions of circulating miRNAs as endocrine or paracrine messengers to allow for intercellular communication in PH are being described (70, 71). Correspondingly, their usefulness as prognostic and diagnostic biomarkers in PH has been increasingly investigated. For example, circulating levels of miR-150 (72), miR-26a (73), miR-23a (74), and miR-125a (75) have been reported as reduced in patients with PAH. Dysregulation of entire sets of circulating miRNAs in PAH has also been described (74, 76). Other miRNAs with known causative actions in PAH, such as the miR-130/301 family (20) and miR-210 (14), have been found to be elevated in the pulmonary circulation of patients with PH. Moreover, differential expression of plasma miRNAs was also reported in acute pulmonary embolism (77) and chronic thromboembolic PH (78). Direct evidence has been presented regarding the transport and signaling of miR-143 among PAECs and PASMCs in PAH (71).
Challenges exist in developing these plasma miRNAs further as clinical biomarkers in PH. As most of these miRNAs are expressed throughout multiple tissues, the definitive source of these plasma miRNAs is typically unknown. Furthermore, because the relative importance of the type of molecular packaging of circulating miRNAs (within vs. outside of microvesicles) has yet to be defined, it has been challenging to determine a definitive diagnostic strategy in PH of quantifying total or subpopulations of circulating miRNAs. Moreover, stemming from their low expression, some circulating miRNAs are difficult to quantify accurately, even with the most sensitive techniques. When considering the possibility of contamination from blood cells or extraneous tissues, circulating miRNA measurements can be fraught with error (79). Endogenous controls for normalization have not been standardized, and there exist no reference values for normal expression. In fact, interindividual and intraindividual variations (e.g., with exercise, sleep/wake cycles, sex, etc.) can influence final results, making clinical development of these biomarkers difficult, particularly for single miRNAs. Finally, it is not obvious which type of assay system for miRNA measurements would be ideal for clinical use, ranging from next-generation sequencing to various types of PCR (Figure 1). Although enthusiasm remains high to develop these plasma miRNAs as biomarkers, obstacles influencing accuracy, cost, and speed of detection will certainly influence the future implementation of these methodologies in clinical practice.
Therapeutic Potential of miRNA in Pulmonary Vascular Disease
Whereas our knowledge of miRNA biology in pulmonary vascular disease continues to expand, strategies to therapeutically deliver either miRNA mimics or inhibitors to the lungs remain in their infancy. Many of the strategies used in preclinical studies primarily focus on pulmonary vascular specific outcome variables, such as improvements in right ventricular systolic pressure and the Fulton index [RV/(left ventricle [LV] + septum) weight ratio], but most fall short of addressing key elements that will be critical for this therapeutic strategy to move forward to clinical trials. This section details the advancements and challenges in miRNA delivery to the pulmonary vasculature, as we continue our efforts to refine translation of miRNA-based therapies for PAH (Figure 4).
Figure 4.
Schematic of strategies to optimize delivery of oligonucleotide therapies to the pulmonary vasculature in humans. Potential delivery routes include intravenous, subcutaneous, and via the airway. The most effective therapeutic strategies will preferentially target the pulmonary vasculature while minimizing targeting of other organs. miRNA = microRNA.
Oligonucleotide therapies, including miRNA and short interfering RNA (siRNA), have entered the clinical stage for a number of diseases beyond PAH. These include mipomersen, an siRNA targeting apolipoprotein B and approved by the U.S. Food and Drug Administration for the treatment of homozygous familial hypercholesterolemia (80), as well as a number of other siRNAs and miRNAs for conditions including amyloidosis (81), hepatitis C (82), hemophilia, tissue fibrosis, and hematological malignancies, which are at various stages of clinical trials. Although no miRNA candidate has reached the stage of clinical trial in PAH, Table 5 summarizes the strategies used thus far to achieve oligonucleotide delivery to the lungs. Multiple routes of delivery have been used in rodents, including intravenous, inhalational, subcutaneous, and intraperitoneal. The modalities of delivery of miRNA mimic or inhibitor candidates have included (1) delivery of naked oligonucleotide, (2) delivery via viral vectors, and (3) delivery via packaging with vasculature-targeting nanoparticles. What is remarkable is that each of these delivery routes has demonstrated efficacy in (1) reaching the lungs, as determined by changes in the miRNA and respective target expression levels, and (2) altering the severity of PH.
Table 5.
Strategies for Oligonucleotide Delivery to Target MicroRNAs in the Pulmonary Vasculature of Animal Models of Pulmonary Arterial Hypertension
| miRNA | Ref. | Mimic/Inhibitor | Formulation | Delivery | Validation | Effect |
|---|---|---|---|---|---|---|
| miR-17 | 25 | Inhibitor | Naked RNA | IV | Lung qPCR | Decreased RVSP, RVH, and PA muscularization |
| miR-130/301 | 19 | Inhibitor | Liposomal | Airway | Lung qPCR | Decreased RVSP, RVH, and PA muscularization |
| miR-143/145 | 71, 84 | Inhibitor | Naked, liposomal | SQ, IV | Lung qPCR | Decreased RVSP, RVH, and PA muscularization |
| miR-20a | 27 | Inhibitor | Naked RNA | IP | Lung qPCR | Decreased PA muscularization and RVH |
| miR-210 | 14 | Inhibitor | Liposomal | IV | Lung qPCR | Decreased RVSP and PA muscularization |
| miR-204 | 15 | Mimic | Liposomal | Airway | Lung qPCR | Decreased mean PAP and PA muscularization |
| miR-424/503 | 18 | Mimic | Lentiviral | Airway | Lung qPCR | Decreased RVSP, RVH, and PA muscularization |
| miR-96 | 42 | Mimic | Liposomal | IV | PA qPCR | Decreased RVSP, RVH, and PA muscularization |
Definition of abbreviations: IP = intraperitoneal; IV = intravenous; miR, miRNA = microRNA; PA = pulmonary artery; PAP = pulmonary arterial pressure; qPCR = quantitative polymerase chain reaction; RVH = right ventricular hypertrophy; RVSP = right ventricular systolic pressure; SQ = subcutaneous.
Although the signaling axes implicated in miRNA biology have been robustly validated in many cases, replication of the direct therapeutic efficacy of miRNA mimetics or inhibitors in vivo has often been varied or has been attempted only by the discovering laboratory alone. For example, inhibition of miR-145 has been validated by independent groups using different delivery strategies (83, 84), but others have observed mixed results (H. Chun, personal communication). Thus, because of the limitations of financial resources in general in PAH research, the choice of which of several miRNAs to develop as therapeutic targets is not a straightforward decision. There exist numerous variables to consider regarding the inherent biology of any given miRNA, including its breadth (narrow vs. broad) of target gene engagement, efficacy of repression of any single target gene, and landscape of activity across single or different tissue/cell types. Tailoring each variable may offer advantages and compromises in terms of potency, pleiotropy, and selectivity/side effect profile, but it is currently not clear whether a systematic method to address or prioritize such biological activity may facilitate more efficient miRNA-based drug discovery.
Route of administration may also provide potential selective advantages, but also a number of potential disadvantages. Intravenous delivery may provide the most direct route to reach the pulmonary vasculature; however, it is limited by the challenges of gaining venous access for each administration, achieving preferential targeting of the pulmonary vasculature (over other vascular beds), and having to cross the endothelial layer for miRNAs that may be the most active in other cells involved in PH, such as PASMCs and PAAFs. Inhalational delivery provides the potential advantage of limiting systemic effects while enhancing lung-specific effects; however, such a strategy would have to ensure that the delivered oligonucleotide can cross the lung epithelium in ultimately reaching the relevant pulmonary vascular cells. Moreover, any potential inflammatory reaction in the airway, due to the delivery complex, would need to be addressed. Other routes of administration, such as subcutaneous and intraperitoneal delivery, provide for ease of administration, but the relative efficacy of the delivered oligonucleotide in actually reaching the pulmonary vasculature remains to be fully validated.
Although most of these delivery techniques demonstrate efficacy in ameliorating the severity of PH in the experimental models, a number of key limitations remain inadequately addressed. One of the major obstacles remains characterization of the potential off-target effects. Although most of the published studies have confirmed delivery to the lungs, by quantitative PCR for either the miRNA itself or the proposed targets, only a limited number of these studies have evaluated the off-target effects, including effects on the liver, which is widely known to take up oligonucleotides from the circulation (85). Moreover, because current studies are based on short-term therapeutic interventions (less than 1 mo), long-term toxicities and putative rebound effects remain to be established. The stability of the oligonucleotide-based therapies also remains to be optimized, especially for therapies using nonviral delivery strategies. Dosage and frequency of delivery have also not been fully optimized, and converting effective doses in rodents to humans remains a major hurdle. Last, the financial costs of translating such findings to humans represent a key challenge, especially for a disease such as PAH, for which life-long therapies are warranted.
Fortunately, published studies and endeavors of industry partners have begun to address some of these key limitations, as we pursue advancement of miRNA modifiers to the clinical stage. Preferential targeting of the pulmonary endothelium rather than the systemic endothelium has been demonstrated and promoted using different nanoparticle-based oligonucleotide delivery partners (86). Multiple strategies for proprietary modifications of the nucleotide backbone provide resistance to degradation in vivo, and provide the means to improve stability and efficacy. Such stabilization strategies also provide for the ability to extend the time between doses and deliver lower concentrations of oligonucleotide-based therapies. Moreover, development of small-molecule inhibitors of specific miRNAs has shown reasonable promise and could provide an alternative and possibly less expensive strategy for targeting specific miRNAs (87, 88). Therefore, although miRNA-based therapy for PH has yet to reach the clinical realm, advancements in our knowledge of the role and function of these oligonucleotide-based therapies provide the key foundation of knowledge that deserves further exploration.
Conclusions
In summary, scientific endeavors have been accelerating, identifying the fundamental and robust actions of miRNAs in controlling PH initiation and development. Attendant with those discoveries, the potential has been growing for clinical application in the development of new RNA-based biomarkers of disease and in the advent of a new generation of medications based on RNA-specific biology (82). Yet, in spite of these promising results, challenges still remain regarding the decipherment of the expansive complexity of miRNA-target gene networks in PH, demonstrating the feasibility and usefulness of quantifying plasma miRNAs for diagnosis or prognosis, and proving the efficacy and specificity of miRNA-based therapies in the pulmonary vasculature. Given the extent of these obstacles, key collaborative efforts among federal, academic, and industry partners will be necessary to advance these technologies in the years ahead.
Acknowledgments
Acknowledgment
The authors thank Diane Margaria for expert administrative assistance, Adam Handen for computational and biostatistical analyses, and Birck Cox for art composition.
Footnotes
Supported by a Canadian Institutes of Health Research Tier-2 Canadian Research Chair (S.B.); the Heart and Stroke Foundation of Canada (S.B.); a Canadian Institutes of Health Research Operating Grant Program (S.B.); National Institutes of Health grants HL096834 (S.Y.C.), HL124021 (S.Y.C.), and HL113005 (H.J.C.); and the American Heart Association (H.J.C. and S.Y.C.).
Author Contributions: All authors have contributed equally.
Originally Published in Press as DOI: 10.1164/rccm.201604-0886PP on September 20, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulin R, Courboulin A, Barrier M, Bonnet S. From oncoproteins/tumor suppressors to microRNAs, the newest therapeutic targets for pulmonary arterial hypertension. J Mol Med. 2011;89:1089–1101. doi: 10.1007/s00109-011-0788-5. [DOI] [PubMed] [Google Scholar]
- 3.Boucherat O, Potus F, Bonnet S. microRNA and pulmonary hypertension. Adv Exp Med Biol. 2015;888:237–252. doi: 10.1007/978-3-319-22671-2_12. [DOI] [PubMed] [Google Scholar]
- 4.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 5.Londin E, Loher P, Telonis AG, Quann K, Clark P, Jing Y, Hatzimichael E, Kirino Y, Honda S, Lally M, et al. Analysis of 13 cell types reveals evidence for the expression of numerous novel primate- and tissue-specific microRNAs. Proc Natl Acad Sci USA. 2015;112:E1106–E1115. doi: 10.1073/pnas.1420955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White K, Loscalzo J, Chan SY. Holding our breath: the emerging and anticipated roles of microRNA in pulmonary hypertension. Pulm Circ. 2012;2:278–290. doi: 10.4103/2045-8932.101395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlachos IS, Hatzigeorgiou AG. Functional analysis of miRNAs using the DIANA Tools online suite. Methods Mol Biol. 2017;1517:25–50. doi: 10.1007/978-1-4939-6563-2_2. [DOI] [PubMed] [Google Scholar]
- 9.Freedman JE, Gerstein M, Mick E, Rozowsky J, Levy D, Kitchen R, Das S, Shah R, Danielson K, Beaulieu L, et al. Diverse human extracellular RNAs are widely detected in human plasma. Nat Commun. 2016;7:11106. doi: 10.1038/ncomms11106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guignabert C, Tu L, Le Hiress M, Ricard N, Sattler C, Seferian A, Huertas A, Humbert M, Montani D. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. Eur Respir Rev. 2013;22:543–551. doi: 10.1183/09059180.00007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cottrill KA, Chan SY. Metabolic dysfunction in pulmonary hypertension: the expanding relevance of the Warburg effect. Eur J Clin Invest. 2013;43:855–865. doi: 10.1111/eci.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gou D, Ramchandran R, Peng X, Yao L, Kang K, Sarkar J, Wang Z, Zhou G, Raj JU. miR-210 has an antiapoptotic effect in pulmonary artery smooth muscle cells during hypoxia. Am J Physiol Lung Cell Mol Physiol. 2012;303:L682–L691. doi: 10.1152/ajplung.00344.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron–sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White K, Lu Y, Annis S, Hale AE, Chau BN, Dahlman JE, Hemann C, Opotowsky AR, Vargas SO, Rosas I, et al. Genetic and hypoxic alterations of the microRNA-210–ISCU1/2 axis promote iron–sulfur deficiency and pulmonary hypertension. EMBO Mol Med. 2015;7:695–713. doi: 10.15252/emmm.201404511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courboulin A, Paulin R, Giguère NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Côté J, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meloche J, Potus F, Vaillancourt M, Bourgeois A, Johnson I, Deschamps L, Chabot S, Ruffenach G, Henry S, Breuils-Bonnet S, et al. Bromodomain-containing protein 4: the epigenetic origin of pulmonary arterial hypertension. Circ Res. 2015;117:525–535. doi: 10.1161/CIRCRESAHA.115.307004. [DOI] [PubMed] [Google Scholar]
- 17.Ruffenach G, Chabot S, Tanguay VF, Courboulin A, Boucherat O, Potus F, Meloche J, Pflieger A, Breuils-Bonnet S, Nadeau V, et al. Role for RUNX2 in proliferative and calcified vascular lesions in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194:1273–1285. doi: 10.1164/rccm.201512-2380OC. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, McLean DL, Park H, Comhair SA, Greif DM, et al. An endothelial apelin–FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 2013;19:74–82. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, Saggar R, Wallace WD, Ross DJ, Vargas SO, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest. 2014;124:3514–3528. doi: 10.1172/JCI74773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertero T, Cottrill K, Krauszman A, Lu Y, Annis S, Hale A, Bhat B, Waxman AB, Chau BN, Kuebler WM, et al. The microRNA-130/301 family controls vasoconstriction in pulmonary hypertension. J Biol Chem. 2015;290:2069–2085. doi: 10.1074/jbc.M114.617845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma S, Umar S, Potus F, Iorga A, Wong G, Meriwether D, Breuils-Bonnet S, Mai D, Navab K, Ross D, et al. Apolipoprotein A-I mimetic peptide 4F rescues pulmonary hypertension by inducing microRNA-193-3p. Circulation. 2014;130:776–785. doi: 10.1161/CIRCULATIONAHA.114.007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newman JH, Wheeler L, Lane KB, Loyd E, Gaddipati R, Phillips JA, III, Loyd JE. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N Engl J Med. 2001;345:319–324. doi: 10.1056/NEJM200108023450502. [Published errata appear in N Engl J Med 2001;345:1506; 2002;346:1258.] [DOI] [PubMed] [Google Scholar]
- 23.Trembath RC, Thomson JR, Machado RD, Morgan NV, Atkinson C, Winship I, Simonneau G, Galie N, Loyd JE, Humbert M, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med. 2001;345:325–334. doi: 10.1056/NEJM200108023450503. [DOI] [PubMed] [Google Scholar]
- 24.Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, Ulrich S, Speich R, Huber LC. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3–microRNA cluster 17/92 pathway. Circ Res. 2009;104:1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 25.Pullamsetti SS, Doebele C, Fischer A, Savai R, Kojonazarov B, Dahal BK, Ghofrani HA, Weissmann N, Grimminger F, Bonauer A, et al. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2012;185:409–419. doi: 10.1164/rccm.201106-1093OC. [DOI] [PubMed] [Google Scholar]
- 26.Chen T, Zhou G, Zhou Q, Tang H, Ibe JC, Cheng H, Gou D, Chen J, Yuan JX, Raj JU. Loss of microRNA-17∼92 in smooth muscle cells attenuates experimental pulmonary hypertension via induction of PDZ and LIM domain 5. Am J Respir Crit Care Med. 2015;191:678–692. doi: 10.1164/rccm.201405-0941OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brock M, Samillan VJ, Trenkmann M, Schwarzwald C, Ulrich S, Gay RE, Gassmann M, Ostergaard L, Gay S, Speich R, et al. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J. 2014;35:3203–3211. doi: 10.1093/eurheartj/ehs060. [DOI] [PubMed] [Google Scholar]
- 28.Wang D, Zhang H, Li M, Frid MG, Flockton AR, McKeon BA, Yeager ME, Fini MA, Morrell NW, Pullamsetti SS, et al. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res. 2014;114:67–78. doi: 10.1161/CIRCRESAHA.114.301633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothman AM, Arnold ND, Pickworth JA, Iremonger J, Ciuclan L, Allen RM, Guth-Gundel S, Southwood M, Morrell NW, Thomas M, et al. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest. 2016;126:2495–2508. doi: 10.1172/JCI83361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, Cottrill KA, Shaik RS, Waxman AB, Zhang YY, et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012;125:1520–1532. doi: 10.1161/CIRCULATIONAHA.111.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, Hale A, Bhat B, Kaimal V, Zhang YY, et al. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Rep. 2015;13:1016–1032. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest. 2016;126:3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kudryashova TV, Goncharov DA, Pena A, Kelly N, Vanderpool R, Baust J, Kobir A, Shufesky W, Mora AL, Morelli AE, et al. HIPPO–integrin linked kinase crosstalk controls self-sustaining proliferation and survival in pulmonary hypertension. Am J Respir Crit Care Med. 2016;194:866–877. doi: 10.1164/rccm.201510-2003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Federici C, Drake KM, Rigelsky CM, McNelly LN, Meade SL, Comhair SA, Erzurum SC, Aldred MA. Increased mutagen sensitivity and DNA damage in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:219–228. doi: 10.1164/rccm.201411-2128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranchoux B, Meloche J, Paulin R, Boucherat O, Provencher S, Bonnet S. DNA damage and pulmonary hypertension. Int J Mol Sci. 2016;17:17. doi: 10.3390/ijms17060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Courboulin A, Ranchoux B, Cohen-Kaminsky S, Perros F, Bonnet S. MicroRNA networks in pulmonary arterial hypertension: share mechanisms with cancer? Curr Opin Oncol. 2016;28:72–82. doi: 10.1097/CCO.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 37.Meloche J, Le Guen M, Potus F, Vinck J, Ranchoux B, Johnson I, Antigny F, Tremblay E, Breuils-Bonnet S, Perros F, et al. miR-223 reverses experimental pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2015;309:C363–C372. doi: 10.1152/ajpcell.00149.2015. [DOI] [PubMed] [Google Scholar]
- 38.Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, Graydon C, Courboulin A, Breuils-Bonnet S, Tremblay E, et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129:786–797. doi: 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- 39.Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, Tian H, Jiang C, Zhu D. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-α1C. Hypertension. 2012;59:1006–1013. doi: 10.1161/HYPERTENSIONAHA.111.185413. [DOI] [PubMed] [Google Scholar]
- 40.Li C, Li X, Gao X, Zhang R, Zhang Y, Liang H, Xu C, Du W, Zhang Y, Liu X, et al. MicroRNA-328 as a regulator of cardiac hypertrophy. Int J Cardiol. 2014;173:268–276. doi: 10.1016/j.ijcard.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 41.Boucherat O, Chabot S, Antigny F, Perros F, Provencher S, Bonnet S. Potassium channels in pulmonary arterial hypertension. Eur Respir J. 2015;46:1167–1177. doi: 10.1183/13993003.00798-2015. [DOI] [PubMed] [Google Scholar]
- 42.Wallace E, Morrell NW, Yang XD, Long L, Stevens H, Nilsen M, Loughlin L, Mair KM, Baker AH, MacLean MR. A sex-specific microRNA-96/5-hydroxytryptamine 1B axis influences development of pulmonary hypertension. Am J Respir Crit Care Med. 2015;191:1432–1442. doi: 10.1164/rccm.201412-2148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, Talati M, Fessel JP, Hemnes AR, Gladson S, French J, Shay S, Trammell A, Phillips JA, Hamid R, et al. Estrogen metabolite 16a-hydroxyestrone exacerbates bone morphogenetic protein receptor type II-associated pulmonary arterial hypertension through microRNA-29-mediated modulation of cellular metabolism. Circulation. 2016;133:82. doi: 10.1161/CIRCULATIONAHA.115.016133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potus F, Graydon C, Provencher S, Bonnet S. Vascular remodeling process in pulmonary arterial hypertension, with focus on miR-204 and miR-126 (2013 Grover Conference series) Pulm Circ. 2014;4:175–184. doi: 10.1086/675980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paulin R, Sutendra G, Gurtu V, Dromparis P, Haromy A, Provencher S, Bonnet S, Michelakis ED. A miR-208–Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res. 2015;116:56–69. doi: 10.1161/CIRCRESAHA.115.303910. [DOI] [PubMed] [Google Scholar]
- 46.Potus F, Ruffenach G, Dahou A, Thebault C, Breuils-Bonnet S, Tremblay È, Nadeau V, Paradis R, Graydon C, Wong R, et al. Downregulation of microRNA-126 contributes to the failing right ventricle in pulmonary arterial hypertension. Circulation. 2015;132:932–943. doi: 10.1161/CIRCULATIONAHA.115.016382. [DOI] [PubMed] [Google Scholar]
- 47.Potus F, Malenfant S, Graydon C, Mainguy V, Tremblay È, Breuils-Bonnet S, Ribeiro F, Porlier A, Maltais F, Bonnet S, et al. Impaired angiogenesis and peripheral muscle microcirculation loss contribute to exercise intolerance in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190:318–328. doi: 10.1164/rccm.201402-0383OC. [DOI] [PubMed] [Google Scholar]
- 48.Thum T, Batkai S. MicroRNAs in right ventricular (dys)function (2013 Grover Conference series) Pulm Circ. 2014;4:185–190. doi: 10.1086/675981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan SY, Loscalzo J. The emerging paradigm of network medicine in the study of human disease. Circ Res. 2012;111:359–374. doi: 10.1161/CIRCRESAHA.111.258541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chou CH, Chang NW, Shrestha S, Hsu SD, Lin YL, Lee WH, Yang CD, Hong HC, Wei TY, Tu SJ, et al. miRTarBase 2016: updates to the experimentally validated miRNA–target interactions database. Nucleic Acids Res. 2016;44:D239–D247. doi: 10.1093/nar/gkv1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:4. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fabregat A, Sidiropoulos K, Garapati P, Gillespie M, Hausmann K, Haw R, Jassal B, Jupe S, Korninger F, McKay S, et al. The Reactome pathway Knowledgebase. Nucleic Acids Res. 2016;44:D481–D487. doi: 10.1093/nar/gkv1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 2010;38:D355–D360. doi: 10.1093/nar/gkp896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nishimura D. A view from the web: BioCarta. Biotech Software Internet Rep. 2004;2:117–120. [Google Scholar]
- 56.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene Ontology Consortium. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gene Ontology Consortium. Gene Ontology Consortium: going forward. Nucleic Acids Res. 2015;43:D1049–D1056. doi: 10.1093/nar/gku1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosvall M, Axelsson D, Bergstrom CT. The map equation. Eur Phys J Spec Top. 2009;178:13–23. [Google Scholar]
- 59.Sehgal PB, Mukhopadhyay S, Xu F, Patel K, Shah M. Dysfunction of Golgi tethers, SNAREs, and SNAPs in monocrotaline-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1526–L1542. doi: 10.1152/ajplung.00463.2006. [DOI] [PubMed] [Google Scholar]
- 60.Sutendra G, Dromparis P, Wright P, Bonnet S, Haromy A, Hao Z, McMurtry MS, Michalak M, Vance JE, Sessa WC, et al. The role of Nogo and the mitochondria–endoplasmic reticulum unit in pulmonary hypertension. Sci Transl Med. 2011;3:88ra55. doi: 10.1126/scitranslmed.3002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benza RL, Gomberg-Maitland M, Demarco T, Frost AE, Torbicki A, Langleben D, Pulido T, Correa-Jaque P, Passineau MJ, Wiener HW, et al. Endothelin-1 pathway polymorphisms and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:1345–1354. doi: 10.1164/rccm.201501-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hemnes AR, Zhao M, West J, Newman JH, Rich S, Archer SL, Robbins IM, Blackwell TS, Cogan J, Loyd JE, et al. Critical genomic networks and vasoreactive variants in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;194:464–475. doi: 10.1164/rccm.201508-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fessel JP, Hamid R, Wittmann BM, Robinson LJ, Blackwell T, Tada Y, Tanabe N, Tatsumi K, Hemnes AR, West JD. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm Circ. 2012;2:201–213. doi: 10.4103/2045-8932.97606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Y, Peng J, Lu C, Hsin M, Mura M, Wu L, Chu L, Zamel R, Machuca T, Waddell T, et al. Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS One. 2014;9:e88727. doi: 10.1371/journal.pone.0088727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014;19:558–573. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Malenfant S, Potus F, Fournier F, Breuils-Bonnet S, Pflieger A, Bourassa S, Tremblay È, Nehmé B, Droit A, Bonnet S, et al. Skeletal muscle proteomic signature and metabolic impairment in pulmonary hypertension. J Mol Med. 2015;93:573–584. doi: 10.1007/s00109-014-1244-0. [DOI] [PubMed] [Google Scholar]
- 67.Pollett JB, Benza RL, Murali S, Shields KJ, Passineau MJ. Harvest of pulmonary artery endothelial cells from patients undergoing right heart catheterization. J Heart Lung Transplant. 2013;32:746–749. doi: 10.1016/j.healun.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 68.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 69.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hale A, Lee C, Annis S, Min PK, Pande R, Creager MA, Julian CG, Moore LG, Mitsialis SA, Hwang SJ, et al. An Argonaute 2 switch regulates circulating miR-210 to coordinate hypoxic adaptation across cells. Biochim Biophys Acta. 2014;1843:2528–2542. doi: 10.1016/j.bbamcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deng L, Blanco FJ, Stevens H, Lu R, Caudrillier A, McBride M, McClure JD, Grant J, Thomas M, Frid M, et al. MicroRNA-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res. 2015;117:870–883. doi: 10.1161/CIRCRESAHA.115.306806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rhodes CJ, Wharton J, Boon RA, Roexe T, Tsang H, Wojciak-Stothard B, Chakrabarti A, Howard LS, Gibbs JS, Lawrie A, et al. Reduced microRNA-150 is associated with poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;187:294–302. doi: 10.1164/rccm.201205-0839OC. [DOI] [PubMed] [Google Scholar]
- 73.Schlosser K, White RJ, Stewart DJ. miR-26a linked to pulmonary hypertension by global assessment of circulating extracellular microRNAs. Am J Respir Crit Care Med. 2013;188:1472–1475. doi: 10.1164/rccm.201308-1403LE. [DOI] [PubMed] [Google Scholar]
- 74.Sarrion I, Milian L, Juan G, Ramon M, Furest I, Carda C, Cortijo Gimeno J, Mata Roig M. Role of circulating miRNAs as biomarkers in idiopathic pulmonary arterial hypertension: possible relevance of miR-23a. Oxid Med Cell Longev. 2015;2015:792846. doi: 10.1155/2015/792846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huber LC, Ulrich S, Leuenberger C, Gassmann M, Vogel J, von Blotzheim LG, Speich R, Kohler M, Brock M. microRNA-125a in pulmonary hypertension: regulator of a proliferative phenotype of endothelial cells. Exp Biol Med (Maywood) 2015;240:1580–1589. doi: 10.1177/1535370215579018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei C, Henderson H, Spradley C, Li L, Kim IK, Kumar S, Hong N, Arroliga AC, Gupta S. Circulating miRNAs as potential marker for pulmonary hypertension. PLoS One. 2013;8:e64396. doi: 10.1371/journal.pone.0064396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao J, Jing ZC, Ellinor PT, Liang D, Zhang H, Liu Y, Chen X, Pan L, Lyon R, Liu Y, et al. MicroRNA-134 as a potential plasma biomarker for the diagnosis of acute pulmonary embolism. J Transl Med. 2011;9:159. doi: 10.1186/1479-5876-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo L, Yang Y, Liu J, Wang L, Li J, Wang Y, Liu Y, Gu S, Gan H, Cai J, et al. Differentially expressed plasma microRNAs and the potential regulatory function of Let-7b in chronic thromboembolic pulmonary hypertension. PLoS One. 2014;9:e101055. doi: 10.1371/journal.pone.0101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng HH, Yi HS, Kim Y, Kroh EM, Chien JW, Eaton KD, Goodman MT, Tait JF, Tewari M, Pritchard CC. Plasma processing conditions substantially influence circulating microRNA biomarker levels. PLoS One. 2013;8:e64795. doi: 10.1371/journal.pone.0064795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raal FJ, Santos RD, Blom DJ, Marais AD, Charng MJ, Cromwell WC, Lachmann RH, Gaudet D, Tan JL, Chasan-Taber S, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 81.Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 82.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 83.Caruso P, Dempsie Y, Stevens HC, McDonald RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, et al. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res. 2012;111:290–300. doi: 10.1161/CIRCRESAHA.112.267591. [DOI] [PubMed] [Google Scholar]
- 84.McLendon JM, Joshi SR, Sparks J, Matar M, Fewell JG, Abe K, Oka M, McMurtry IF, Gerthoffer WT. Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension. J Control Release. 2015;210:67–75. doi: 10.1016/j.jconrel.2015.05.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lorenzer C, Dirin M, Winkler AM, Baumann V, Winkler J. Going beyond the liver: progress and challenges of targeted delivery of siRNA therapeutics. J Control Release. 2015;203:1–15. doi: 10.1016/j.jconrel.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 86.Dahlman JE, Barnes C, Khan OF, Thiriot A, Jhunjunwala S, Shaw TE, Xing Y, Sager HB, Sahay G, Speciner L, et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014;9:648–655. doi: 10.1038/nnano.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naro Y, Thomas M, Stephens MD, Connelly CM, Deiters A. Aryl amide small-molecule inhibitors of microRNA miR-21 function. Bioorg Med Chem Lett. 2015;25:4793–4796. doi: 10.1016/j.bmcl.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 88.Gumireddy K, Young DD, Xiong X, Hogenesch JB, Huang Q, Deiters A. Small-molecule inhibitors of microRNA miR-21 function. Angew Chem Int Ed Engl. 2008;47:7482–7484. doi: 10.1002/anie.200801555. [DOI] [PMC free article] [PubMed] [Google Scholar]