To the Editor:
We previously identified pediatric septic shock endotypes by computer-assisted image analysis of gene expression mosaics representing the expression patterns of 100 genes (1–4). The endotypes differ with respect to outcome and treatment response. Because the endotype-defining genes reflect adaptive immunity and glucocorticoid receptor signaling, assigning patients to an endotype might enable precision critical care medicine. Using the expression pattern for 100 genes to assign endotype is currently impractical for time-sensitive decision-making concerning critically ill patients with septic shock (5). A strategy that uses a much smaller number of genes would more readily translate into a rapid clinical test amenable to decision-making for this patient population. We aimed to reduce the existing 100 endotype-defining genes into a minimum subset needed to accurately differentiate endotypes.
Methods
Using classification and regression tree methodology (Salford Predictive Modeler, version 7.0; Salford Systems, San Diego, CA), we developed a decision tree to accurately predict assignment to endotype A or endotype B, using the smallest possible subset of genes from among the original 100. We derived the tree using data from the 300 subjects enrolled in the prior study (1) and tested the tree in 43 newly enrolled subjects. RNA was derived from whole blood collected within 24 hours of a septic shock diagnosis, and gene expression was measured with the NanoString nCounter (NanoString Technologies, Seattle, WA) and a custom-made code set as previously detailed (1).
The primary outcome was allocation to either endotype A or endotype B. The modeling procedure considered all 100 genes as candidate predictor variables and used the class probability method. We pruned terminal nodes having less than 5% of the subjects in the root node and terminal nodes that did not improve classification. Weighting of cases and costs for misclassification were not used. Tenfold cross validation was used to estimate model performance. The code and data used to generate the model are available from the authors.
Results and Discussion
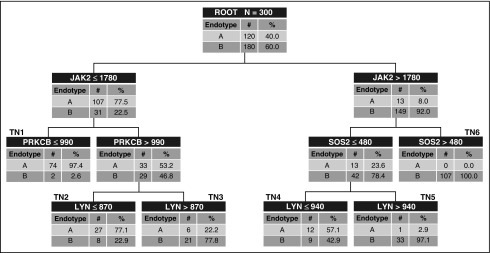
The clinical characteristics and demographics of the 300 derivation subjects are described elsewhere (1). There were 120 endotype A subjects (40%) and 180 endotype B subjects. Figure 1 shows the derived decision tree, consisting of four genes. Using our original endotype classification strategy as the criterion standard, the area under the receiver operating curve (AUROC) of the decision tree was 0.97 (95% confidence interval [CI], 0.95–0.99) for differentiating between endotypes A and B. The tenfold cross-validation procedure yielded an AUROC of 0.90. Subjects allocated to terminal nodes 1, 2, and 4 had a higher probability (57.1–97.4%) of being an endotype A, whereas subjects allocated to terminal nodes 3, 5, and 6 had a lower probability (0.0–22.2%) of being an endotype A. On the basis of allocation to these terminal nodes, 19 subjects allocated to endotype A were originally endotype B, and 7 subjects allocated to endotype B were originally endotype A. This results in the following diagnostic test characteristics for identifying endotype A subjects: sensitivity, 94% (95% CI, 88–97%); specificity, 89% (84–93%); positive predictive value, 86% (78–91%); negative predictive value, 96% (91–98%); positive likelihood ratio, 8.9 (5.8–13.7); and negative likelihood ratio, 0.07 (0.03–0.13).
Figure 1.
The derived decision tree. The decision tree includes Janus kinase 2 (JAK2), protein kinase C, β (PRKCB), SOS Ras/Rho guanine nucleotide exchange factor 2 (SOS2), and LYN proto-oncogene, Src family tyrosine kinase (LYN). The gene expression values are provided in arbitrary units of mRNA counts, as generated by the NanoString nCounter platform and normalized to four housekeeping genes. The root node provides the total number of subjects originally allocated to endotypes A and B, and their respective rates. Each daughter node provides the respective decision rule criterion based on a gene expression level, and the number of endotype A and B subjects, with the respective rates. Terminal nodes (TN) TN1, TN2, and TN4 contained subjects having a higher probability of being an endotype A (57.1–97.4%), whereas TN3, TN5, and TN6 contained subjects having a higher probability of being an endotype B (77.8–100%).
Using the original endotyping strategy, there were 14 endotype A subjects and 29 endotype B subjects in the test cohort. When these subjects were classified according to the derived four-gene tree, the AUROC was 0.97 (95% CI, 0.93–1.00). The sensitivity and specificity for identifying endotype A were 100% (95% CI, 73–100%) and 79% (95% CI, 60–91%), respectively.
Using the 100 gene mosaics, we previously showed that endotype A subjects had worse outcomes compared with endotype B subjects, and corticosteroid prescription was associated with increased mortality risk among endotype A subjects (1). To determine whether reclassification modified these observations, we combined the derivation and test cohorts (n = 343), and compared the clinical characteristics of the endotype A and B subjects, as defined by the four-gene decision tree. Table 1 shows that endotype A patients had a higher mortality rate and a higher rate of complicated course compared with endotype B subjects. Using logistic regression to adjust for age and illness severity (Pediatric Risk of Mortality score), we found that allocation to endotype A was associated with increased odds of mortality (odds ratio [OR], 2.3; 95% CI, 1.1–4.9; P = 0.022) and complicated course (OR, 2.1; 95% CI, 1.3–3.6; P = 0.004). Among endotype A subjects, corticosteroid prescription was associated with increased odds of mortality (OR, 3.7; 95% CI, 1.4–9.8; P = 0.008).
Table 1.
Clinical and Demographic Data for Combined Derivation and Test Cohort Subjects Allocated to Endotypes A and B, Using the Four-Gene Decision Tree
| Variable | Endotype A | Endotype B | P Value |
|---|---|---|---|
| n | 152 | 191 | — |
| Males, n (%) | 77 (58) | 96 (57) | 0.929 |
| Age, yr, median (IQR) | 1.6 (0.6–4.7) | 3.2 (1.4–6.6) | <0.001 |
| PRISM score, median (IQR) | 13 (8–20) | 11 (8–17) | 0.163 |
| Mortality, n (%) | 27 (18) | 15 (8) | 0.009 |
| Complicated course,* n (%) | 60 (39) | 41 (21) | <0.001 |
Definition of abbreviations: IQR = interquartile range; PRISM = Pediatric Risk of Mortality.
Defined as persistence of two or more organ failures on Day 7 of septic shock or 28-day mortality (1).
These data suggest that we successfully reduced our septic shock endotyping strategy to a decision tree consisting of just four genes. The decision tree has excellent test characteristics for distinguishing endotype A from endotype B in both the derivation and test cohorts, although there were some reclassifications of subjects relative to the original, reference criterion endotyping strategy. Despite these reclassifications, allocation to endotype A remained independently associated with increased odds of poor outcome, and corticosteroid prescription remained independently associated with increased odds of mortality among endotype A subjects.
After decades of study, the role of adjunctive corticosteroids in septic shock remains controversial (6, 7). It is relatively unclear which patients stand to gain the most benefit from adjunctive corticosteroids (8, 9). The septic shock endotypes we report might provide an opportunity to estimate corticosteroid responsiveness and therefore inform clinical trial design and perhaps clinical care. In another recent post hoc analysis, we demonstrated that by combining mortality risk stratification with endotype assignment, it might be possible to identify a subgroup of patients most likely to benefit from corticosteroids (10).
In summary, we have simplified our septic shock endotyping strategy to a four-gene decision tree. The simplified strategy is amenable to translation to the bedside of critically ill patients and therefore warrants further evaluation.
Footnotes
Supported by National Institutes of Health grants R01GM099773 and R01GM108025.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Wong HR, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT, Weiss SL, Fitzgerald J, Checchia PA, Meyer K, et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med. 2015;191:309–315. doi: 10.1164/rccm.201410-1864OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong HR, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Willson DF, Freishtat RJ, Anas N, Meyer K, Checchia PA, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009;7:34. doi: 10.1186/1741-7015-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong HR, Cvijanovich NZ, Allen GL, Thomas NJ, Freishtat RJ, Anas N, Meyer K, Checchia PA, Lin R, Shanley TP, et al. Validation of a gene expression-based subclassification strategy for pediatric septic shock. Crit Care Med. 2011;39:2511–2517. doi: 10.1097/CCM.0b013e3182257675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong HR, Wheeler DS, Tegtmeyer K, Poynter SE, Kaplan JM, Chima RS, Stalets E, Basu RK, Doughty LA. Toward a clinically feasible gene expression-based subclassification strategy for septic shock: proof of concept. Crit Care Med. 2010;38:1955–1961. doi: 10.1097/CCM.0b013e3181eb924f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maslove DM, Wong HR. Gene expression profiling in sepsis: timing, tissue, and translational considerations. Trends Mol Med. 2014;20:204–213. doi: 10.1016/j.molmed.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troché G, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 7.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, et al. CORTICUS Study Group. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson SJ, Cvijanovich NZ, Thomas NJ, Allen GL, Anas N, Bigham MT, Hall M, Freishtat RJ, Sen A, Meyer K, et al. Corticosteroids and pediatric septic shock outcomes: a risk stratified analysis. PLoS One. 2014;9:e112702. doi: 10.1371/journal.pone.0112702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson SJ, Wong HR. Identifying critically ill patients who may benefit from adjunctive corticosteroids: not as easy as we thought. Pediatr Crit Care Med. 2014;15:769–771. doi: 10.1097/PCC.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong HR, Atkinson SJ, Cvijanovich NZ, Anas N, Allen GL, Thomas NJ, Bigham MT, Weiss SL, Fitzgerald JC, Checchia PA, et al. Combining prognostic and predictive enrichment strategies to identify children with septic shock responsive to corticosteroids Crit Care Med 201644e1000–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]