Abstract
Context:
Pro-inflammatory markers play a key role in the pathogenesis of various Flavivirus infection.
Aim:
In this study, we evaluated the role of these markers in neurological manifestations of dengue.
Settings and Designs:
Consecutive dengue cases with different neurological manifestations who presented between August 2012 and July 2014 were studied in hospital-based case–control study.
Materials and Methods:
Interleukin (IL-6) and IL-8 level were measured in serum and cerebrospinal fluid (CSF) of dengue cases with different neurological manifestations and also in age- and sex-matched controls. Level was analyzed with various parameters and outcomes.
Statistical Analysis:
Statistical analysis was done using SPSS version 16.0 by applying appropriate statistical methods. P < 0.05 considered statistically significant.
Results:
Out of the 40 enrolled cases of dengue with neurological manifestations, 29 had central nervous system and 11 had peripheral nervous system (CNS/PNS) manifestations. In CNS group, both IL-6 and IL-8 (CSF and serum) were significantly elevated (P < 0.001), whereas CSF IL-6 (P = 0.008), serum IL-6 (P = 0.001), and serum IL-8 (P = 0.005) were significantly elevated in PNS group. CSF IL-6, serum IL-6, and IL-8 were significantly elevated in poor outcome patients in CNS group (P < 0.05). CSF IL-6 and IL-8 were significantly elevated in CSF dengue positive cases as compared to CSF negative patients (P < 0.05). Cytokine level was not significantly correlated with neuroimaging abnormality in CNS group. Nine patients died and the remainder recovered.
Conclusion:
Elevated level of IL-6 and IL-8 is associated with different neurological manifestations and poor outcome, but whether they are contributing to neuropathogenesis or simply a correlate of severe disease remains to be determined.
KEY WORDS: Dengue, encephalitis, Flavivirus, interleukin-6, interleukin-8, nervous system
Introduction
Dengue infection, transmitted by Aedes mosquitoes, is the most important tropical arboviral disease in the world with millions of symptomatic infections annually, caused by one of the 4 serotypes (DEN 1–4) of dengue virus (DENV), family Flaviviridae, genus Flavivirus.[1] Various neurological manifestations such as encephalitis, myelitis, Guillain-Barré syndrome (GBS), hypokalemic paralysis, and myositis are well reported in dengue, but pathogenesis is still controversial. The pathophysiology of neurological involvement has been attributed to three factors; (a) secondary to systemic complications; (b) direct neurotrophic effect of virus; and (c) parainfectious immune mediated.[2] Pro-inflammatory markers are the integral part of the immune response, and the earliest host responses to viral infections are nonspecific and induce cytokines, resulting in facilitation of the both arms of immunity.[3,4] The production and upregulation of cytokines in response to DENV infection are responsible for the development of manifestations such as cerebral edema.[5] Interleukin (IL-6), IL-8 and regulated upon activation normal T-cell expressed and secreted (RANTES) concentration is reported to be higher in cerebrospinal fluid (CSF) of patients with viral encephalitis including dengue.[6]
Literature on the correlation between pro-inflammatory markers and different neurological manifestations of dengue is limited. The present study aimed at evaluating levels of serum and CSF cytokine IL-6 and IL-8 in two clinical groups (central nervous system/peripheral nervous system [CNS and PNS]) of dengue patients with neurological manifestations and compare them with serum and CSF samples of matched healthy controls.
Materials and Methods
This case-control study was carried out in Department of Neurology from August 2012 to July 2014 with Institutional Ethics Committee approval and written informed consent from all participants before the conduct of any study-related procedure.
Selection of cases and controls
All patients of DENV infection with fever and various neurological manifestations during the study period were included in the study. Diagnosis of dengue was based on positive immunoglobulin M antibody (IgM) assayed by IgM capture enzyme-linked immunosorbent assay (ELISA) or nonstructural protein 1 (NS1) antigen detection or positive reverse transcriptase polymerase chain reaction (PCR) for DENV in serum/CSF. Cases with encephalopathy attributable to systemic complication of dengue and patients who were positive for peripheral blood smear for malaria, CSF Gram stain, acid-fast and India ink staining, laboratory testing of CSF and serum samples for HIV-1 and 2, human T-cell lymphotropic virus I, herpes simplex, cytomegalovirus, and Japanese encephalitis (JE) virus were excluded from the study.
Control CSF was obtained from 40 age- and sex-matched otherwise healthy persons undergoing spinal anesthesia in various surgical departments of our institution.
Patient evaluation
Demographic and clinical data were collected from all enrolled cases with detailed medical history and physical examination. Baseline investigations including prothrombin time, activated partial thromboplastin time, chest radiograph, and electrocardiogram were done in all enrolled patients. Serum creatine phosphokinase levels, thyroid function test, ultrasound abdomen, electromyography, nerve conduction study, and neuroimaging (computed tomography [CT]/magnetic resonance imaging [MRI] brain/MRI spine) were carried out as indicated. CSF cell count, protein, and glucose level were analyzed in all patients.
Measurement of IL-6, IL-8 in serum and CSF samples of both patients and controls was performed by sandwich ELISA as per manufacturer's instructions using Legend Max Human ELISA Kits (BioLegend Inc., San Diego, California, USA).
Follow-up
All cases were followed up at 3 months after hospital discharge, and outcome analyzed using the modified Rankin Scale (mRS) in CNS group and the GBS disability score in PNS group.[7] The outcome was defined as good (scores ≤3) or poor (scores >3 or death).[8]
Statistical analysis
Statistical analysis was performed using SPSS version 16.0 for windows (Chicago, IL, USA). Categorical variables were expressed as percentages. Continuous variables were expressed as mean ± standard deviation if the data were normally distributed; they were expressed as median if the data were not normally distributed. Shapiro–Wilk test was used to check whether a variable was normally distributed or not. Chi-square test was used to compare categorical variables. Independent sample t-test was used to compare means. Mann–Whitney U-test was used for comparison of continuous variables between two groups if they were not normally distributed. All P < 0.05 were considered statistically significant.
Results
A total of 66 consecutive cases of dengue viral infection with neurological manifestations were enrolled in the study period. Twenty-six cases were excluded with encephalopathy attributable to systemic complication of dengue such as perfusion abnormalities, electrolyte imbalances, or bleeding complication secondary to deranged hemostasis.
Baseline characteristics
Forty laboratory-confirmed dengue infection patients with neurological manifestations were evaluated whose mean age was 22.85 ± 17.308 years (range 1–60 years) with majority of cases (52.5%) ≤20 years age. Apart from serum positivity in all patients, dengue IgM antibody was also positive in CSF in 17 (42.5%) patients. Dengue NS1 antigen was positive in serum and CSF in five and two patients, respectively, whereas DENV PCR was positive in only serum of two patients.
Clinical, laboratory, and neuroimaging characteristics of the study groups:
Of 40 patients with neurologic manifestations, 29 had CNS involvement and 11 had PNS involvement. In CNS group, 27 cases had encephalitis and two had myelitis, whereas in PNS group, five had GBS, four had hypokalemic paralysis, and two had myositis. Clinical and laboratory characteristics of patients in both groups are shown in Table 1.
Table 1.
Clinical and laboratory features of patients in Group A and B
| Parameters | CNS group (n=29) | PNS group (n=11) | P |
|---|---|---|---|
| Age (years) | 19.10±16.398 | 32.73±16.353 | 0.024* |
| Sex (%) | |||
| Male | 22 (75.9) | 9 (81.8) | 0.687 |
| Female | 7 (36) | 2 (18.2) | 0.687 |
| Mean duration of fever | 7.97±3.168 | 4.73±1.794 | <0.001* |
| Mean temperature of fever (F°) | 102.56±1.45 | 100.87±1.69 | 0.003* |
| Headache (%) | 25 (86.2) | 2 (18.2) | <0.001* |
| Rash (%) | 04 (13.8) | 03 (27.3) | 0.316 |
| Bleeding (%) | 03 (10.3) | 01 (9.1) | 0.906 |
| Nausea - vomiting (%) | 15 (51.7) | 00 (0) | 0.003* |
| Diarrhea (%) | 4 (13.8) | 1 (9.1) | 0.688 |
| Abdominal pain (%) | 5 (17.2) | 00 (0) | 0.141 |
| Myalgia (%) | 6 (20.7) | 2 (18.2) | 0.859 |
| Arthralgia (%) | 6 (20.7) | 2 (18.2) | 0.859 |
| TLC (cells/μl) | 8494±3206 | 6931±3078 | 0.172 |
| Hemoglobin (gm/dl) | 11.4±2.54 | 11.72±2.33 | 0.723 |
| Hematocrit (%) | 36.1379±4.01 | 35.4818±4.38 | 0.655 |
| Platelet count (106 cells/μl) | 1.3±0.87 | 1.15±1.13 | 0.657 |
| Serum creatinine (mg/dl) | 1.42±0.397 | 1.46±0.42 | 0.746 |
| Serum urea (mg/dl) | 40.55±17.95 | 38.95±10.22 | 0.783 |
| AST (IU/L) | 82.76±47.38 | 75.64±78.26 | 0.727 |
| ALT (IU/L) | 71.1±41.77 | 73±45.4 | 0.901 |
| Total bilirubin (mg/dl) | 1.35±0.63 | 1.47±0.48 | 0.557 |
| Serum sodium (mmol/L) | 140.48±4.24 | 140.09±3.59 | 0.788 |
| Serum potassium (mmol/L) | 3.69±0.29 | 3.68±0.76 | 0.974 |
| CSF sugar (mg/dl) | 62.97±23.6 | 59.91±8.61 | 0.552 |
| CSF protein (mg/dl) | 66±32.6 | 61.09±31.48 | 0.670 |
| CSF TLC (cells/μl) | 45.69±46.34 | 9.09±7.36 | <0.001* |
*Significant P value (P<0.05). AST: Aspartate transaminase, ALT: Alanine transaminase, TLC: Total leukocyte count, CNS: Central nervous system, PNS: Peripheral nervous system, CSF: Cerebrospinal fluid
Of total 28 patients in CNS group who underwent neuroimaging (5 MRI brain, 2 MRI spine, and 21 contrast-enhanced CT brain), eighteen patients (62.07%) had abnormal imaging with sixteen suggestive of encephalitis and two of myelitis. Imaging could not be done in one patient due to rapid deterioration to death.
Clinical outcome
In CNS group, at presentation, 6 (20.69%) patients had mRS scores ≤3, 23 (79.31%) had mRS scores between 4 and 5, and nine patients died during the hospital stay. In remaining twenty patients at 3-months, 19 (95%) patients recovered (mRS ≤3) and one (5%) patient had poor recovery (mRS 4). In PNS group, 3 (27.27%) patients had GBS disability scores ≤3 and 8 (72.73%) had scores between 4 and 5 at presentation. By 3-month, all patients recovered (GBS ≤3) with no mortality in this group.
Pro-inflammatory cytokine levels
Serum IL-6, CSF IL-6, and serum IL-8 were significantly elevated compared to controls in both CNS and PNS groups, whereas CSF IL-8 was significant only in CNS group [Table 2 and Figure 1a and b]. Serum IL-6, CSF IL-6, and CSF IL-8 were significantly higher in poor outcome patients of CNS group as compared to good outcome; however, there was no significant difference in serum concentration of IL-8 [Figure 1c]. No significant difference in any serum or CSF cytokine concentration was noted between good and poor baseline GBS disability score of PNS group. By Spearman's rank order test, serum IL-6 (P = 0.042, r = −0.379) and CSF IL-6 (P = 0.047, r = −0.371) correlated significantly with mean duration of fever in this group. The serum/CSF IL-6 and IL-8 levels did not correlate with radiological abnormalities in CNS patients (P > 0.05).
Table 2.
Comparison of cytokines between cases and control group, outcomes in central nervous system patients, baseline disabilities in peripheral nervous system group and cerebrospinal fluid dengue positivity
| Cytokines (pg/ml) | Median (range) | P | Z | |
|---|---|---|---|---|
| CNS group | ||||
| Cases (n=29) | Controls (n=29) | |||
| Serum IL-6 | 57.43 (4.55-427) | 5.94 (5.04-12.1) | <0.001* | −5.545 |
| CSF IL-6 | 48.09 (3-388) | 5.86 (0-11) | <0.001* | −4.660 |
| Serum IL-8 | 237.2 (0-1328.55) | 9.12 (0-33.72) | <0.001* | −5.953 |
| CSF IL-8 | 114.05 (0-1305.6) | 0 (0-23.86) | <0.001* | −4.026 |
| Good outcome (n=19) | Poor outcome (n=10) | |||
| Serum IL-6 | 107.33 (4.55-374.03) | 176.69 (25.77-427.12) | 0.022* | −2.294 |
| CSF IL-6 | 16.84 (3.33-332.53) | 279.73 (3.73-388.13) | 0.005* | −2.776 |
| Serum IL-8 | 235.53 (16.59-1328.55) | 274.31 (0-1317.64) | 0.521 | −0.642 |
| CSF IL-8 | 80.49 (0-388.56) | 852.96 (0-1305.61) | 0.009* | −2.596 |
| PNS group | ||||
| Cases (n=11) | Controls (n=11) | |||
| Serum IL-6 | 32 (5.04-410.84) | 6.77 (0-11.38) | 0.001* | −3.189 |
| CSF IL-6 | 19.51 (0-149) | 5.28 (0-8) | 0.008* | −2.664 |
| Serum IL-8 | 570.38 (0-1328.55) | 15.57 (0-25.41) | 0.005* | −2.820 |
| CSF IL-8 | 0 (0-592.63) | 0 (0-25.33) | 0.918 | −0.103 |
| Good outcome (n=3) | Poor outcome (n=8) | |||
| Serum IL-6 | 19.60 (5.04-345.44) | 74.39 (8.69-410.84) | 0.540 | −0.612 |
| CSF IL-6 | 9.27 (0-143) | 37.12 (4-149.00) | 0.540 | −0.612 |
| Serum IL-8 | 133.81 (0-1064.22) | 764.15 (0-1328.55) | 0.357 | −0.921 |
| CSF IL-8 | 71.44 (0-370.04) | 00 (0-592.63) | 0.316 | −1.002 |
| CSF dengue | ||||
| Positive (n=17) | Negative (n=23) | |||
| Serum IL-6 | 60.14 (7.60-427.12) | 32.0 (4.55-410.84) | 0.436 | −0.780 |
| CSF IL-6 | 142.39 (3.73-388.13) | 19.17 (0-332.53) | 0.038* | −2.079 |
| Serum IL-8 | 347.43 (16.59-1328.55) | 190.09 (0-1328.55) | 0.147 | −1.450 |
| CSF IL-8 | 231.88 (0-1305.61) | 5.80 (0-592.63) | 0.008* | −2.656 |
*Significant P value (P<0.05). IL: Interleukin, CNS: Central nervous system, PNS: Peripheral nervous system, CSF: Cerebrospinal fluid
Figure 1.
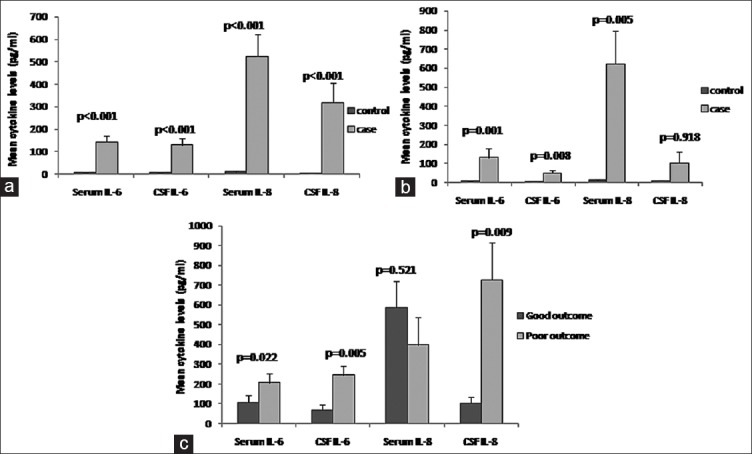
Serum and cerebrospinal fluid cytokine level in dengue patients with central nervous system and peripheral nervous system manifestations. (a) Cytokine level in central nervous system manifesting patients and control (b) cytokine level in peripheral nervous system manifesting patients and control (c) cytokine level in outcome groups of central nervous system manifesting patients
CSF IL-6 and CSF IL-8 were significantly higher in CSF dengue positive patients compared to CSF dengue-negative patients; however, no difference was noticed in serum IL-6 and serum IL-8 [Table 2].
Discussion
Studies on cytokines in DENV-specific central as well as PNS manifestations are lacking in the literature. Our sample included forty confirmed dengue patients with different neurologic manifestations, and the majority of those had CNS infection (72.5%) with predominantly encephalitis.
Serum and CSF IL-6 and IL-8 were higher in CNS group as compared to controls. IL-6 increases blood-brain barrier permeability and CNS viral infection may induce proliferation of microglia and astrocytes resulting in liberation of IL-8.[9] It regulates selective chemotaxis, degranulation, and activation of neutrophils.[9] Experimentally induced dengue encephalitis in newborn mice showed that expression of mRNA encoding for IL-6 and other cytokines were elevated,[10] suggesting that dengue encephalitis could be associated with IL-6 production. Increased levels of various pro-inflammatory mediators such as CSF IL-6, IL-8, and RANTES in viral encephalitis were observed in a recent study. However, in contrast to our study, those levels were not associated with poor outcome.[6] This may be due to small sample size with heterogeneous etiology (8 JE, 1 dengue, and 5 nonspecific encephalitis) and less number of severe cases (only 2 patients died) in that study.[6] In a study done in JE patients, higher levels of CSF IL-6 and IL-8 were associated with increased mortality.[9] A markedly elevated IL-6 level in the spinal fluid of myelitis patients was also highly predictive of disability irrespective to etiology. Marked upregulation of IL-6 correlates with increased nitric oxide production and this elevation is related to tissue injury causing clinical disability in acute transverse myelitis.[11] Similar mechanism may occur in dengue myelitis, but further studies are required with large sample size to explore the pathogenesis.
Serum IL-6, CSF IL-6, and serum IL-8 were significantly elevated in PNS group compared to control. The level of IL-6 and IL-8 was found to be higher in CSF and serum samples of GBS patients irrespective of the cause according to some earlier studies.[12,13,14] Besides, the pro-inflammatory role of IL-6 in experimental autoimmune neuritis and GBS, it is shown that it may have additional neuroprotective effects by reducing TNF, IL-1, and promoting SC proliferation and remyelination.[15] IL-8 along with IL-1ra (receptor alpha) mediates the recruitment and activation of lymphocytes and monocytes expressed in the CSF of GBS patients.[12] Both IL-6 and IL-8 along with other cytokines released by tubular epithelia in response to reduced total or regional blood flow to the kidney enhances inflammation and cell injury.[16,17] This supports the observation of raised serum level of both cytokines in our study. Myositis secondary to myotoxic cytokines particularly TNF-α has also been shown in patients of dengue.[18] Potential mediators of skeletal muscle damage in meningococcal disease are TNF-α and IL-8. IL-8 is an important regulator of neutrophil activation and migration, and TNF-α activates neutrophils to induce the production of other cytokines.[19] Raised levels of IL-6 and IL-8 in our study suggest some role of these markers in dengue myositis also.
Conclusion
Ours is a preliminary study which has evaluated the cytokine responses in patients of dengue with different neurological manifestation. IL-6 and IL-8 were higher in both CNS and PNS groups compared to controls. Higher levels of CSF IL-6 and IL-8 in CNS manifestation infection were poor prognostic markers but whether they are contributing to neuropathogenesis or simply a correlate of severe disease remains to be determined. Further studies with more pro-inflammatory markers are needed in various individual groups of neurological manifestation to elucidate a mechanism of immunopathogenesis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Murray NE, Quam MB, Wilder-Smith A. Epidemiology of dengue: Past, present and future prospects. Clin Epidemiol. 2013;5:299–309. doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma R, Sahu R, Holla V. Neurological manifestations of dengue infection: A review. J Neurol Sci. 2014;346:26–34. doi: 10.1016/j.jns.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 3.Biron C, Sen G. Interferons and other cytokines. In: Knipe DM, Howley PM, editors. Fields Virology. 4th ed. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 321–52. [Google Scholar]
- 4.Goodbourn S, Didcock L, Randall RE. Interferons: Cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol. 2000;81(Pt 10):2341–64. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi UC, Agarwal R, Elbishbishi EA, Mustafa AS. Cytokine cascade in dengue hemorrhagic fever: Implications for pathogenesis. FEMS Immunol Med Microbiol. 2000;28:183–8. doi: 10.1111/j.1574-695X.2000.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalita J, Srivastava R, Mishra MK, Basu A, Misra UK. Cytokines and chemokines in viral encephalitis: A clinicoradiological correlation. Neurosci Lett. 2010;473:48–51. doi: 10.1016/j.neulet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial prednisolone in acute polyneuropathy. Lancet. 1978;2:750–3. doi: 10.1016/s0140-6736(78)92644-2. [DOI] [PubMed] [Google Scholar]
- 8.Sahu R, Verma R, Jain A, Garg RK, Singh MK, Malhotra HS, et al. Neurologic complications in dengue virus infection: A prospective cohort study. Neurology. 2014;83:1601–9. doi: 10.1212/WNL.0000000000000935. [DOI] [PubMed] [Google Scholar]
- 9.Winter PM, Dung NM, Loan HT, Kneen R, Wills B, Thu le T, et al. Proinflammatory cytokines and chemokines in humans with Japanese encephalitis. J Infect Dis. 2004;190:1618–26. doi: 10.1086/423328. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Burgos G, Hernández-Pando R, Campbell IL, Ramos-Castañeda J, Ramos C. Cytokine production in brain of mice experimentally infected with dengue virus. Neuroreport. 2004;15:37–42. doi: 10.1097/00001756-200401190-00009. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan C, Kaplin AI, Deshpande DM, Pardo CA, Kerr DA. Transverse myelitis: Pathogenesis, diagnosis and treatment. Front Biosci. 2004;9:1483–99. doi: 10.2741/1351. [DOI] [PubMed] [Google Scholar]
- 12.Sainaghi PP, Collimedaglia L, Alciato F, Leone MA, Naldi P, Molinari R, et al. The expression pattern of inflammatory mediators in cerebrospinal fluid differentiates Guillain-Barré syndrome from chronic inflammatory demyelinating polyneuropathy. Cytokine. 2010;51:138–43. doi: 10.1016/j.cyto.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Sivieri S, Ferrarini AM, Lolli F, Matà S, Pinto F, Tavolato B, et al. Cytokine pattern in the cerebrospinal fluid from patients with GBS and CIDP. J Neurol Sci. 1997;147:93–5. doi: 10.1016/s0022-510x(96)00319-x. [DOI] [PubMed] [Google Scholar]
- 14.Maimone D, Annunziata P, Simone IL, Livrea P, Guazzi GC. Interleukin-6 levels in the cerebrospinal fluid and serum of patients with Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Neuroimmunol. 1993;47:55–61. doi: 10.1016/0165-5728(93)90284-6. [DOI] [PubMed] [Google Scholar]
- 15.Lu MO, Zhu J. The role of cytokines in Guillain-Barré syndrome. J Neurol. 2011;258:533–48. doi: 10.1007/s00415-010-5836-5. [DOI] [PubMed] [Google Scholar]
- 16.Bonventre JV, Zuk A. Ischemic acute renal failure: An inflammatory disease? Kidney Int. 2004;66:480–5. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 17.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–37. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 18.Acharya S, Shukla S, Mahajan SN, Diwan SK. Acute dengue myositis with rhabdomyolysis and acute renal failure. Ann Indian Acad Neurol. 2010;13:221–2. doi: 10.4103/0972-2327.70882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrol ED, Thomson AP, Mobbs KJ, Fraser WD, Sills JA, Hart CA. Myositis in children with meningococcal disease: A role for tumour necrosis factor-alpha and interleukin-8? J Infect. 2002;44:17–21. doi: 10.1053/jinf.2001.0923. [DOI] [PubMed] [Google Scholar]


