Abstract
Objective
To generate recombinant Bacillus subtilis (B. subtilis) engineered for expression of porcine β-defensin-2 (pBD-2) and cecropin P1 (CP1) fusion antimicrobial peptide and investigate their anti-bacterial activity in vitro and their growth-promoting and disease resisting activity in vivo.
Methods
The pBD-2 and CP1 fused gene was synthesized using the main codons of B. subtilis and inserted into plasmid pMK4 vector to construct their expression vector. The fusion peptide-expressing B. subtilis was constructed by transformation with the vector. The expressed fusion peptide was detected with Western blot. The antimicrobial activity of the expressed fusion peptide and the recovered pBD-2 and CP1 by enterokinase digestion in vitro was analyzed by the bacterial growth-inhibitory activity assay. To analyze the engineered B. subtilis on growth promotion and disease resistance, the weaned piglets were fed with basic diet supplemented with the recombinant B. subtilis. Then the piglets were challenged by enteropathogenic Escherichia coli (E. coli). The weight gain and diarrhea incidence of piglets were measured after challenge.
Results
The recombinant B. subtilis engineered for expression of pBD-2/CP1 fusion peptide was successfully constructed using the main codons of the B. subtilis. Both expressed pBD-2/CP1 fusion peptide and their individual peptides recovered from parental fusion peptide by enterokinase digestion possessed the antimicrobial activities to a variety of the bacteria, including gram-negative bacteria (E. coli, Salmonella typhimurium, and Haemophilus parasuis) and gram-positive bacteria (Staphylococcus aureus). Supplementing the engineered B. subtilis to the pig feed could significantly promote the piglet growth and reduced diarrhea incidence of the piglets.
Conclusion
The generated B. subtilis strain can efficiently express pBD-2/CP1 fusion antimicrobial peptide, the recovered pBD-2 and CP1 peptides possess potent antimicrobial activities to a variety of bacterial species in vitro. Supplementation of the engineered B. subtilis in pig feed obviously promote piglet growth and resistance to the colibacillosis.
Keywords: Porcine β-defensin-2, Cecropin P1, Antimicrobial Activity, Engineered Bacillus subtilis, Piglets
INTRODUCTION
Antibiotics have been widely used as an antimicrobial feed additive in food animal production to enhance feed efficiency, promote growth, and prevent animal diseases [1]. Undoubtedly, the antibiotics once played an important role in animal production especially under poor feeding conditions. However, the misuse of antibiotics has given rise to antibiotics residues in animal-based food (such as milk, eggs, and meat) and the development of drug resistant bacteria [2,3]. Residual antibiotics in the food could be transmitted via food chain to humans and threaten human health [4]. Therefore, the safety of animal-based food was of significant concern since the 1980s, Sweden first prohibited the use of some antibiotics in animal feeds in 1986 [5]. However, the antimicrobial agents were inevitably added to animal feed under the current animal feeding conditions in many countries, especially in developing countries [6]. Therefore, development of alternative antimicrobial agents that would not produce drug residues and drug-resistant bacteria is imperative.
Due to their many advantages, including no toxicity, no residues, no tolerance and low cost probiotics have become one of the ideal alternatives for antibiotics widely used in animal production, especially in swine production [7]. Bacillus subtilis (B. subtilis), a gram positive bacterum, was identified as an important strain of probiotics found in animal gastrointestinal tracts [8]. Its ability to form a tough, protective endospore contributes to its tolerance to extreme environmental conditions. Furthermore, its high production of digestive enzymes, lack of pathogenicity and freedom from lipopolysaccharides (endotoxins) make it one of the favorite probiotics to be selected for animal production [9].
Antimicrobial peptides (AMPs), as another alternative for antibiotics, are extensively investigated and applied in animal production [10]. One of the major AMP subclasses is the β-defensin family, which are mainly expressed in epithelial cells in animal skin, gastrointestinal and respiratory tracts [11]. The β-defensin-2 (pBD-2) is crucial AMP for gut protection owing to their antimicrobial and immunomodulatory activities [12]. Cecropin peptide family is another subclass of AMPs, mainly derived from roundworms in animal intestinal tracts [13,14]. Cecropin P1 has been proved to have a broad spectrum antimicrobial activity against gram-negative bacteria, it has an especially strong inhibitory and killing effect on Escherichia coli (E. coli) [15].
Although the AMPs as potent antimicrobial substances were considered as a potential alternative for antibiotics, their preparations, either by extraction from natural biological materials or by expression through genetic engineering, are costly, which severely affect their application in animal production. It has been known that the function of probiotics mainly involves the regulation of flora balance and restriction of pathogenic bacteria, but lacking direct antimicrobial activity. Since antimicrobial activity in animal gastrointestinal tract is essential for animal growth and production especially under poor husbandry conditions [16,17], the maintenance of the flora balance and antimicrobial activity are equally important for animal growth and production. To meet the requirement in swine production, we used B. subtilis to construct a derived strain engineered to express AMPs of pBD-2 and cecropin P1, and then analyzed its antimicrobial activity and growth-promoting effects in vitro and in vivo.
MATERIALS AND METHODS
Plasmids, bacteria and reagents
Plasmid pMK4 as an expression vector used in this B. subtilis expression system was purchased from Addgene (Cambrige, Cambrige, MA, USA). B. subtilis 168 as host cells for recombinant peptide expression was from American Type Culture Collection (ATCC, Manassas, VA, USA). E. coli DH5α and enteropathogenic E. coli 2134P (F18ac) were from our laboratory. Salmonella typhimurium (S. typhimurium), Haemophilus parasuis (H. parasuis), and Staphylococcus aureus (S. aureus) were presented by the Laboratory of Microbiology in College of Food Science and technology.
Restriction enzymes, T 4 DNA ligase and PureYield Plasmid Miniprep were from Promega (Madison, WI, USA). Enterokinase was from Sigma (St. Louis, MO, USA). DL-2000 DNA markers were from Takara Biotechnology (Dalian, China). DNA Purification Kit, Protein Markers were from Transgen Biotechnology (Bejing, China). Mouse anti-His monoclonal antibody and goat anti-mouse IgG-AP antibody were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Ni-NTA-agarose beads were from Qiagen (Hilden, Germany).
Construction of codon-optimized pBD-2 and cecropin P1 expression vector
To construct the expression vector of codon-optimized pBD-2 and cecropin P1 fusion gene, the pBD-2 (GenBank: AY506573.1) and cecropin P1 (GenBank: AB186032.1) genes were codon-optimized using B. subtilis main codons based on Codon Usage Database (Kazusa DNA Research Institute, Japan; Website: http://www.kazusa.or.jp/codon), To reduce the potential toxic effects of expressed AMPs on B. subtilis host cells, we adopted the fusion expression strategy, i.e the pBD-2 and cecropin P1 genes were fused by Ala-Ser-Ala-Ser-Ala linker followed by Asp-Asp-Asp-Asp-Lys (DDDDK) enterokinase site, the former for maintaining the peptide stability, while the latter for recovering the two individual AMP by enterokinase digestion. In addition, the fused gene was His-tagged at the C-terminus for the fusion protein purification, and fused with signal peptide of bacterial alkaline protease at the N-terminus for pBD-2/cecropin P1 secretion into culture medium. The codon-optimized pBD-2 and cecropin P1 genes were synthesized by Sangon (Shanghai, China) and cloned into pMK4 vector by BamH I and EcoR I sites to construct pBD-2/cecropin P1 expression vector pMK4-BD/CP/His, in which the pBD-2 and cecropin P1 genes were fused by linker and tagged by 6×His, and controlled by Lac promoter.
Transformation and expression
B. subtilis transformation was performed based on a previously described technique [18]. For pBD-2/cecropin P1 fusion peptide expression, B. subtilis bearing the pMK4-BD/CP/His or pMK4 empty plasmid (as a negative control) were cultured in 5 mL Luria-Bertani (LB) medium containing 100 μg/mL ampicillin at 37°C for overnight. The culture was then inoculated into a fresh 50 mL LB culture in a 250 mL flask and allowed to continue to grow at 37°C with shaking 200 rpm for 24 h. Then the culture medium was harvested by centrifugation.
For large-scale preparation for animal expreiment, overnight cultured 5 mL-bacterial seed liquid was centrifuged to remove the supernatant containing ampicillin. After washing with LB medium twice, the bacteria were inocubated into 50 mL LB medium without ampicillin and continued to culture at 37°C with shaking 200 rpm until the the optical density (OD600) value of the culture reached 0.6 to 0.8, and then bacteria were inocubated into 500 mL LB ampicillin-free medium in a 3,000 mL-flask and continued to culture under the same conditions as above until culture OD600 value reached 2.5 to 3.0. Then the bacteria were harvested by centrifugation.
Fusion peptide purification and pBD-2 and cecropin P1 recovery
The pBD-2/cecropin P1 fusion peptide in the culture medium was purified by Ni-NTA agarose affinity chromatography as described previously [19]. For pBD-2 and cecropin P1 recovery, the purified pBD-2/cecropin P1 fusion peptides were digested with enterokinase (5 units/μg of fusion peptide) to cleave the fusion protein to release the pBD-2 and cecropin P1. The cecropin P1 in the digestion mixture was further isolated by Ni-NTA agarose affinity chromatography.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western-blot
The fusion peptides, pBD-2 and cecropin P1 were detected by Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot. The samples were run on 15% SDS-PAGE or 18% tricine SDS-PAGE gels. The separated peptides on the gels were either strained with Coomassie Brilliant Blue or electro-blotted onto a nitrocellulose membrane by using semi-dry electro-bloting apparatus (Bio-Rad, Hercules, CA, USA). Blotted peptides on the membrane were detected by Western blot using mouse anti-His monoclonal antibody followed by goat anti-mouse IgG conjugated with alkaline phosphatase. The blots were developed using BCIP/NBT substrate (Bio-Rad, USA).
Antimicrobial activity assay in vitro
The antimicrobial activity assay for the AMPs was performed by measuring bacterial growth-inhibitory activity using E. coli, S. typhimurium, H. parasuis, and S. aureus. The bacteria were cultured in LB (H. parasuis was cultured in tryptone soya agar medium) at 37°C overnight, then inoculated to the fresh LB medium and cultured until the OD600 value reached 0.5. The fusion peptide, pBD-2, and cecropin P1 were separately added to the different bacterial cultures at the final concentrations of 20 mM, the bacteria were continuously cultured at the same conditions for 12 h. The ampicillin (100 μg/mL) and phosphate-buffered saline (PBS) were separately added to the bacterial cultures as positive and negative controls, respectively. The OD600 value was measured using Bio-Tek microplate reader (Winooski, VT, USA) every two hours. The growth of bacteria was determined by measuring OD600 values. The experiment was performed in triplicates.
Animal housing and management
The animal study was performed according to the Guide for the Care and Use of Animals of the Agricultural University of Hebei. The protocol was approved by the Ethical Committee for Animal Research of the Agricultural University of Hebei. The tested weaned piglets were selected from a commercial pig farm and housed in a constant temperature (24°C±1°C) and humidity (65%±5%) room with clean air-conditioning system. The piglets were fed with non-contaminated basal diet reported previously [23], the autoclaved water was available ad libitum.
Antimicrobial activity assay in vivo
The forty-eight piglets (Landrace×Large white, initial body weight about 6.5 kg, 30±2 d of age) were divided into four groups (n = 12): untreated group (negative control), chlortetracycline-treated group (positive control), wild-type B. subtilis-treated group and engineered B. subtilis-treated group. The piglets in the control and chlortetracycline-treated groups were fed with the basal diet and the basal diet supplemented with chlortetracycline antibiotics (100 mg/kg of feed) for 30 d, respectively. The piglets in wild-type and engineered B. subtilis-treated groups were fed with the basal diet supplemented with wide-type B. subtilis and engineered B. subtilis (5×109 colony-forming units [CFU]/kg of feed) for 30 d, respectively. The supplementing levels of the B. subtilis preparations were selected based on the results of earlier several experiments, with which the maximal weight gain in piglets were achieved (data not shown). During the experiment period, the piglets were challenged using enteropathogenic E. coli 2134P (F18ac) at 2×109 CFU in 50 mL milk replacer at 15 d after treatments. Piglets were closely observed after the challenges at least three times daily to check for diarrheal pigs based on faecal properties. The diarrhea incidence was calculated with the formula: Diarrhea incidence (%) = total number of pigs with diarrhea observed each day/(number of pig×total experimental day)×100. Feed intake and body weight were measured at 15 d and 30 d after treatment to determine average daily gain, average daily feed intake, and feed/weight gain ratio.
Statistics
The data were compared among the different treatment groups by the Duncan’s multiple range test following analysis of variance using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). The level of statistical significance was preset at p<0.05.
RESULTS
Codon optimization of pBD-2 and cecropin P1 genes and their fusion expression vector construction
To efficiently express porcine pBD-2 and cecropin P1 in B. subtilis, we optimized these two genes with B. subtilis prefered codons (Figure 1A). To construct its prokaryotic expression vector, the synthesized pBD-2/cecropin P1 fusion gene was cloned into pMK4 plasmid, to generate pBD-2/cecropin P1 fusion gene expression vector (pMK4-pBD/CP/His) (Figure 1B). Results showed that the constructed vector was correct by restriction analysis (Figure 1C).
Figure 1.
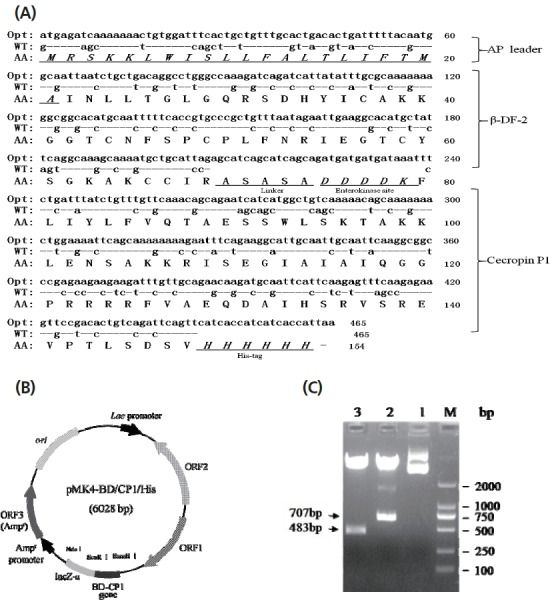
Codon optimization and expression vector construction of pBD-2/cecropin P1 fusion gene. (A) Sequence comparison between wild-type and codon optimized pBD-2/cecropin P1 fusion gene. Opt, codon-optimized; WT, wild-type; AA, animo acids; AP leader, signal peptide of alkaline protease; ASASA, a linker; DDDDK, enterokinase site; His-tag, 6-histidine tag. (B) Prokaryotic expression vector of pBD-2/cecropin P1 fusion gene. The fusion gene (465 bp) was controlled by Lac promoter. BD-CP1, pBD-2/cecropin P1 fusion gene. (C) Restriction analysis of the pMK4-BD/CP1/His plasmid. M, DL 2000DNA Marker; Lane 1, the pMK4-BD/CP/His plasmid; Lane 2, digested fragments of the pMK4-BD/CP/His plasmid by Nde I and BamH I; Lane 3, digested fragments by BamH I and EcoR I.
Engineered Bacillus subtilis can efficiently express pBD-2/cecropin P1 fusion peptide
To generate B. subtilis strain engineered for pBD-2/cecropin P1 expression, pMK4-pBD/CP/His plasmids were transformed into the B. subtilis 168 strain with Spizizen method [18]. The engineered B. subtilis was selected by ampicillin resistance and fusion peptide expression. The fusion peptide in the culture medium was purified with Ni-NTA-agarose affinity chromatography and detected by SDS–PAGE and Western blot (Figure 2). As shown in Figure 2A and 2B, the fusion peptides with the size of 14 kDa were detected, indicating that the expressed pBD-2/cecropin P1 fusion peptides, mediated by signal peptide of bacterial alkaline protease, could be secreted into the medium. The yield of the fusion peptide expression was about 45 mg/L culture medium after purification. To further prove the purified peptides were pBD-2/cecropin P1 fusion peptides, Western blot was performed using anti-His tag antibodies and a 14 kDa-positive bloting band was detected. To recover the individual peptides of pBD-2 and cecropin P1, the fusion peptide was digested with enterokinase and pBD-2 and cecropin P1 were recovered (Figure 2D). The cecropin P1 (8 kDa) and pBD-2 (6 kDa) were further separated by Ni-NTA-agarose affinity chromatography (Figure 2D).
Figure 2.
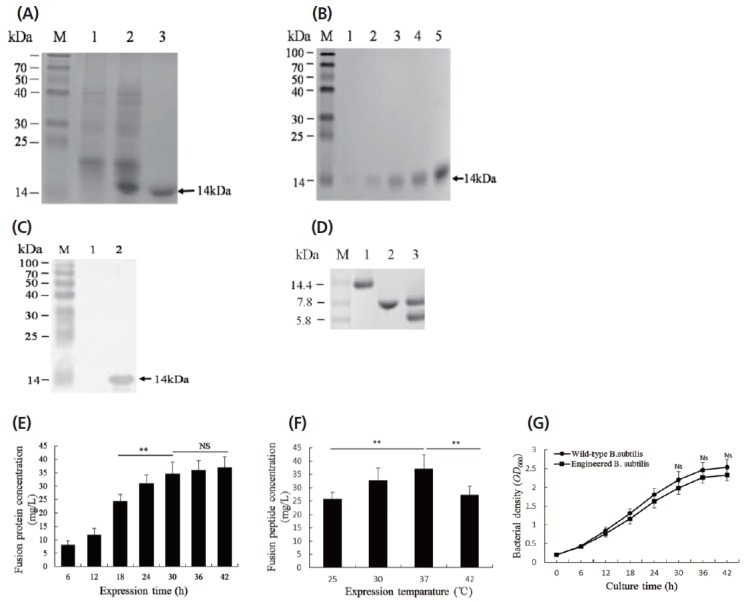
pBD-2/cecropin P1 fusion peptide expression and some factors affecting the expression. (A) The fusion peptides in the culture media detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. M, protein markers; Lane 1, pMK4 empty vector-transformed sample; Lane 2, pMK4-pBD/CP/His vector-tramsformed sample; Lane 3, pMK4-pBD/ CP/His vector-tramsformed sample after purification with Ni-NTA-agarose beads. (B) The different fractions of the fusion peptides eluted from Ni-NTA-agarose beads. M, protein markers; Lane 1–4, elute 4-1. (C) The purified fusion peptide detected by Western blot with mouse anti-His-tag antibody and goat anti-mouse IgG-AP. M, prestained protein markers; Lane 1, sample from the culture medium of pMK-4 empty vector-transformed Bacillus subtilis (B. subtilis); Lane 2, sample from the culture medium of pMK4-pBD/CP/His vector-transformed B. subtilis. (D) The fusion peptide and its cleaved forms by enterokinase. M, protein markers; Lane 1, pBD-2/cecropin P1 fusion peptide; Lane 2, pBD-2 (6 kDa) and cecropin P1 (8 kDa) peptides generated from the fusion peptide by enterokinase digestion. Lane 3, cecropin P1 (8 kDa) isolated from pBD-2 and cecropin P1 mixture. (E) and (F), Effects of expression time and temperature on fusion peptide expression. (G) Comparison of bacterial growth between wide-type and engineered B. subtilis at 37°C for 48 h. The data are shown as means±standard deviation, and representative of three independent experiments. * p<0.05; ** p<0.01. NS, no significance.
To obtain a high-level expression of pBD-2/cecropin P1 fusion peptide, we analyzed the effects of expression time and temperature on fusion peptide expression. Results showed that the high expression levels should be obtained with LB medium at 37°C for 24 h (Figure 2E, 2F).
To investigate whether expressed fusion peptides can interfere with the growth of the host bacteria, we compared the growth rate between the wild-type and engineered B. subtilizes. As shown in Figure 2G, the expressed fusion peptides have an effect on the growth of the engineered B. subtilis, but it was of no significance since during 48 h-cultivation, the engineered B. subtilis still maintained more than 90% of the growth rate of wild-type B. subtilis.
Both pBD-2/cecropin P1 fusion peptide and their individual peptides possess antimicrobial activities to the bacteria
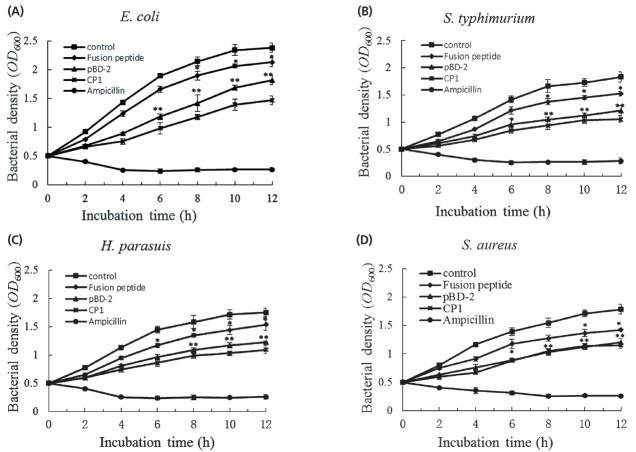
The antimicrobial activity of the fusion AMP or peptide was evaluated by measuring reduction of bacterial density. Results showed that both fusion peptides and their individual peptides possessed antimicrobial activity against all species of bacteria tested in this study (Figure 3A to 3D). However, the different antibacterial peptides showed different antimicrobial activity against certain species of bacteria. Under the same molar concentrations, the antimicrobial activity of the cecropin P1 to bacteria was higher than that of pBD-2 and the fusion peptide, especially to the gram-negative bacteria (Figure 3A to 3C). However, there was no difference in antimicrobial activity against the gram-positive S. aureus. Both cecropin P1 and pBD-2 showed the higher antibacterial activity compared with their parental fusion peptide, which was as we expected since exogenous AMP expressed in the host tended to kill it (B. subtilis). By adopting a fusion strategy, the damage of the antibacterial peptide to the host bacteria was reduced to an acceptable level. After proteolytic processing in vitro, the antibacterial peptides with high antimicrobial activity were recovered from their parental fusion peptide and then allowed to display their antibacterial activity.
Figure 3.
Antimicrobial activities of different antibacterial peptides to the different bacteria. The different species of bacteria, including Escherichia coli (A), Salmonella typhimurium (B), Haemophilus parasuis (C) and Staphylococcus aureus (D), were used to test the antimicrobial activities of different antibacterial peptides. The optical density (OD600) values of the bacterial cultures reflect the antimicrobial activities of the antimicrobial peptides. The data are shown as means±standard deviation, and representative of three independent experiments. * p<0.05; ** p<0.01.
Supplementing the engineered Bacillus subtilis to the feed promotes weaned piglet growth and resistance to experimental colibacillosis
To test growth-promoting effects of the engineered B. subtilis in vivo, the weaned piglets were fed with basal diets supplemented with wild-type B. subtilis, engineered B. subtilis or chlortetracycline as a positive control (untreated piglets as a negative control) for 15 d. The piglet weight gain and feed/weight gain ratio were measured. Results showed both wild-type and engineered B. subtilis significantly increased the piglet weight gains and feed/weight gain ratio compared with the untreated control groups (Table 1), indicating that B. subtilis, either wild-type or engineered, significantly promoted weight gains and feed/weight gain ratio of the weaned piglets.
Table 1.
Effects of engineered Bacillus subtilis on weaned piglet weight gain and feed/weight gain ratio at 15 d post Bacillus subtilis treatments
| Groups | Untreated control | Wild-type Bacillus subtilis | Engineered Bacillus subtilis | Chlortetracycline |
|---|---|---|---|---|
| Average initial weight (kg) | 6.59±0.14 | 6.62±0.15 | 6.65±0.15 | 6.55±0.10 |
| Average final weight (kg) | 9.10±0.27a | 9.64±0.32b | 10.49±0.28c | 10.71±0.19c |
| Average daily gain (kg) | 0.17±0.024a | 0.20±0.024b | 0.26±0.020c | 0.28±0.010d |
| Average daily feed intake (kg/d) | 0.316a | 0.348a | 0.396b | 0.453c |
| Feed/weight gain ratio | 1.89a | 1.73b | 1.55c | 1.63bc |
Mean values in the same row with different superscripts differ significantly (p<0.05).
To test disease-resisting activity of the engineered B. subtilis in vivo, the piglets in above four groups were challenged with enteropathogenic E. coli at 15 d after B. subtilis treatment. The diarrhea incidence, weight gain and feed/weight gain ratio were measured at 15 d after E. coli challenge. Results showed that upon enteropathogenic E. coli challenge, the piglet weight gains in engineered B. subtilis-treated group were significantly higher than that in wild-type B. subtilis-treated and control groups (Table 2). Importantly, the diarrhea incidence of engineered B. subtilis-treated enteropathogenic E. coli-challenged piglets were significantly lower than that of negative control groups, and similar to the chlortetracycline-treated group, indicating that the engineered B. subtilis possessed a potent ability to protect the weaned piglets from colibacillosis (Table 3).
Table 2.
Effects of engineered Bacillus subtilis on weight gain of weaned piglets at 15 d after challenge with enteropathogenic Escherichia coli
| Groups | Untreated control | Wild-type Bacillus subtilis | Engineered Bacillus subtilis | Chlortetracycline |
|---|---|---|---|---|
| Average initial weight (kg) | 9.10±0.27a | 9.64±0.32b | 10.49±0.28c | 10.71±0.19c |
| Average final weight (kg) | 14.48±0.24a | 15.45±0.35b | 16.57±0.15c | 16.84±0.13d |
| Average daily gain (kg) | 0.358±0.024a | 0.386±0.034b | 0.405±0.021bc | 0.410±0.015c |
| Average daily feed intake (kg/d) | 0.652a | 0.665a | 0.667a | 0.747b |
| Feed/weight gain ratio | 1.82b | 1.69a | 1. 64a | 1.83b |
Mean values in the same row with different superscripts differ significantly (p<0.05).
Table 3.
Effects of engineered Bacillus subtilis on diarrhea incidence of weaned piglets at 15 d after challenge with enteropathogenic Escherichia coli
| Phase | Diarrhea incidence1) (%) | |||
|---|---|---|---|---|
|
| ||||
| Untreated control | Wild-type Bacillus subtilis | Engineered Bacillus subtilis | Chlortetracycline | |
| Day 1–15 | 31.7c | 26.1b | 20a | 18.3a |
| Day 16–30 | 38.9c | 33.3b | 28.3a | 27.2a |
Diarrhea incidence = Days of diarrhea piglets/12 (pigs)×15 d.
Mean values in the same row with different superscripts differ significantly (p<0.05).
DISCUSSION
In this study, we used probiotics B. subtilis to construct a derived strain engineered for expression of the two porcine AMPs, pBD-2, and cecropin P1, in a fusion form using their codon-optimized genes. By adopting codon optimization and fusion expression strategies, the pBD-2/cecropin P1 fused peptide was efficiently expressed in the B. subtilis in a secretory manner. After protease-catalyzed hydrolysis by the specific enterokinase, the pBD-2 and cecropin P1 were successfully released and their antimicrobial activities were recovered from their parental fusion peptide. Results showed that both pBD-2 and cecropin P1 possessed potent antimicrobial activity against Gram-positive and Gram-negative bacteria in vitro, significantly higher than that of their parental fusion peptide. By adopting the fusion expression strategy, the antimicrobial activity of pBD-2/cecropin P1 fusion peptide against the engineered B. subtilis was significantly decreased, which would benefit host cell growth and fusion peptide expression. Our results also showed that supplementation of the engineered B. subtilis to the piglet feed promoted weight gain and reduced feed/weight gain ratio. More importantly, the engineered B. subtilis significantly reduced the incidence of diarrhea in experimental colibacillosis in piglets. All of the results above indicated that the engineered B. subtilis might be a potential feed additive as a probiotics for application in swine breeding, especially in weaned piglet rearing. This study laid the foundation for further investigation on its regulation in porcine intestinal flora balance and restriction for pathogenic bacteria, as well as for its application in swine production.
Selecting endogenous AMPs as an animal feed additive should be superior to exogenous AMPs in maintaining animal intestinal flora balance and restricting pathogenic bacteria invasion since the intestinal flora balance mainly depends on the interaction and regulation between endogenous probiotics and endogenous AMPs [20]. The mutual adaption of the endogenous AMPs and probiotics in the animal gastrointestinal tract should have been established in the long process of animal evolution with the endogenous probiotics tolerating the co-existing endogenous AMPs [21]. In contrast, the exogenous AMPs might interfere with the growth of probiotics when they are killing the pathogenic bacteria. In addition, the host immune response to the exogenous AMPs might attenuate their antimicrobial activity. Therefore, in this study, we selected porcine endogenous AMPs, pBD-2, and cecropin P1 as candidate AMPs to construct the engineered B. subtilis strain.
Compared with recombinant AMPs prepared from E. coli expression system in vitro [22–24], the AMP-producing probiotics showed a superiority in animal production as feed anti-microbial additives. Firstly, the expressed AMPs in probiotics were directly secreted into the intestinal tract. Secondly, the toxic substance derived from E. coli could be prevented from invading the animal’s gastrointestinal tract. Finally, the AMP-producing probiotics not only act as host bacteria for expressing AMPs, but also an important microorganism on regulating microbial ecological balance and immune functions in an animal’s intestinal tract [25,26]. B. subtilis is one of the important probiotics in human and animal gastrointestinal tracts [27], therefore in this study it was selected as a candidate probiotic to generate a derived strain engineered for expressing AMPs.
The fusion expression strategy is commonly used to express recombinant toxic proteins or peptides including AMPs expressed in prokaryotic or eukaryotic expression systems. The expressed toxic proteins severely interfere with the survival of host bacterial cells leading to bacteria death or causing significantly defects in bacteria growth, which dramatically decrease host’s expression capacity [28]. By fusing protein tags, such as thioredoxin and glutathione S-transferase or maltose-binding protein tag, the toxicity of recombinant proteins or peptides to the bacterial host is obviously reduced and the protein or peptide expressions were not significantly affected [29]. To reduce the toxicity of pBD-2 or cecropin P1 to the host B. subtilis, we tried the two-AMP-fusion strategy to attenuate the peptide toxicity and showed that the expressed pBD-2/cecropin P1 fusion peptide did not seriously interfere with B. subtilis growth and the peptide expression. Before that, when we had tried to use B. subtilis to express pBD-2 without fusing any protein tag, we could not express pBD-2 peptide efficiently using B. subtilis (Data not shown). How the pBD-2/cecropin P1 fusion peptide showed the low toxicity to the B. subtilis remains to be elucidated. We speculate that the natural structure of pBD-2 and cecropin P1 in their fused form might have been changed and consequently their antimicrobial activities have been reduced.
ACKNOWLEDGMENTS
This study was supported by the Hebei Province Science and Technology Support Program (14226605D-3).
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Cheng G, Hao H, Xie S, et al. Antibiotic alternatives: the substitution of antibiotics in animal husbandry? Front Microbiol. 2014;5:217. doi: 10.3389/fmicb.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jukes TH. Antibiotics in meat production. JAMA. 1975;232:292–3. [PubMed] [Google Scholar]
- 3.Stanton TB. A call for antibiotic alternatives research. Trends Microbiol. 2013;21:111–3. doi: 10.1016/j.tim.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Marshall B, Levy SB. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev. 2011;24:718–33. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castanon JI. History of the use of antibiotic as growth promoters in european poultry feeds. Poult Sci. 2007;86:2466–71. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- 6.Wang N, Guo X, Xu J, et al. Pollution characteristics and environmental risk assessment of typical veterinary antibiotics in livestock farms in Southeastern China. J Environ Sci Health B. 2014;49:468–79. doi: 10.1080/03601234.2014.896660. [DOI] [PubMed] [Google Scholar]
- 7.Collins MD, Gibson GR. Probiotics, prebiotics and symbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999;69:1052–7. doi: 10.1093/ajcn/69.5.1052s. [DOI] [PubMed] [Google Scholar]
- 8.Oggioni MR, Pozzi G, Valensin PE, Galieni P, Bigazzi C. Recurrent Septicemia in an Immunocompromised Patient Due to Probiotic Strains of Bacillus subtilis. J Clin Microbiol. 1998;36:325–6. doi: 10.1128/jcm.36.1.325-326.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker AA, Davis E, Spencer JD, Moser R, Rehberger T. The effect of a -based direct-fed microbial supplemented to sows on the gastrointestinal microbiota of their neonatal piglets. J Anim Sci. 2013;91:3390–9. doi: 10.2527/jas.2012-5821. [DOI] [PubMed] [Google Scholar]
- 10.Sang Y, Blecha F. Antimicrobial peptides and bacteriocins: alternatives to traditional antibiotics. Anim Health Res Rev. 2008;9:227–35. doi: 10.1017/S1466252308001497. [DOI] [PubMed] [Google Scholar]
- 11.Fellermann K, Stange EF. Defensinsinnate—immunity at the epithelial frontier. Eur J Gastroenterol Hepatol. 2001;13:771–6. doi: 10.1097/00042737-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Han F, Zhang H, Xia X, et al. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J Immunol. 2015;194:1882–93. doi: 10.4049/jimmunol.1402300. [DOI] [PubMed] [Google Scholar]
- 13.Wei L, Zhong F, Xiu L, et al. Expression of Ascaris suum cecropin P1 gene in Pichia pastoris and its antimicrobial activity. Chin J Vet Sci. 2011 [Google Scholar]
- 14.Arcidiacono S, Soares JW, Meehan A, Marek P, Kirby R. Membrane permeability and antimicrobial kinetics of cecropin P1 against Escherichia coli. J Pept Sci. 2009;15:398–403. doi: 10.1002/psc.1125. [DOI] [PubMed] [Google Scholar]
- 15.Gregory K, Mello CM. Immobilization of Escherichia coli cells by use of the antimicrobial peptide cecropin P1. Appl Environ Microbiol. 2005;71:1130–4. doi: 10.1128/AEM.71.3.1130-1134.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee S, Hooper LV. Antimicrobial defense of the intestine. Immunity. 2015;42:28–39. doi: 10.1016/j.immuni.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Ostaff MJ, Stange EF, Wehkamp J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med. 2013;5:1465–83. doi: 10.1002/emmm.201201773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spizizen J. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonuclease. Proc Natl Acad Sci USA. 1958;44:1072–8. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Li X, Zhang Z, Shen X, Zhong F. Codon optimization enhances secretory expression of Pseudomonas aeruginosa exotoxin A in E. coli. Protein Expr Purif. 2010;72:101–6. doi: 10.1016/j.pep.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Yang W, Feng J, Xiang F, et al. Endogenous animal toxin-like human β-defensin 2 inhibits own K+ channels through interaction with channel extracellular pore region. Cell Mol Life Sci. 2014;72:845–53. doi: 10.1007/s00018-014-1715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harwig SS, Eisenhauer PB, Chen NP, Lehrer RI. Cryptdins: endogenous antibiotic peptides of small intestinal paneth cells. Adv Exp Med Biol. 1995:251–5. doi: 10.1007/978-1-4615-1941-6_53. [DOI] [PubMed] [Google Scholar]
- 22.Vargues T, Morrison G, Seo ES, et al. Efficient production of human β-Defensin 2 (HBD2) in Escherichia coli. Protein Pept Lett. 2009;16:668–76. doi: 10.2174/092986609788490122. [DOI] [PubMed] [Google Scholar]
- 23.Wu S, Zhang F, Huang Z, et al. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides. 2012;35:225–30. doi: 10.1016/j.peptides.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Zhao Y, Song X, Huang X, Zhao W. Molecular cloning, expression and characterization of the porcine beta defensin 2 in E. coli. Protein Pept Lett. 2013;20:715–23. doi: 10.2174/0929866511320060010. [DOI] [PubMed] [Google Scholar]
- 25.Bonavita R, Isticato R, Maurano F, Ricca E, Rossi M. Mucosal immunity induced by gliadin-presenting spores of Bacillus subtilis in HLA-DQ8-transgenic mice. Immunol Lett. 2015;165:84–9. doi: 10.1016/j.imlet.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Zokaeifar H, Balcazar JL, Saad CR, et al. Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2012;33:683–9. doi: 10.1016/j.fsi.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Sorokulova I, Pinchuk IV, Denayrolles M, et al. The safety of two bacillus probiotic strains for human use. Dig Dis Sci. 2008;53:954–63. doi: 10.1007/s10620-007-9959-1. [DOI] [PubMed] [Google Scholar]
- 28.Saida F, Uzan M, Odaert B, Bontems F. Expression of highly toxic genes in E. coli: special strategies and genetic tools. Curr Protein Pept Sci. 2006;7:47–56. doi: 10.2174/138920306775474095. [DOI] [PubMed] [Google Scholar]
- 29.Tatsuda D, Arimura H, Tokunaga H, et al. Expression and purification of cytokine receptor homology domain of human granulocyte-colony-stimulating factor receptor fusion protein in Escherichia coli. Protein Expr Purif. 2001;21:87–91. doi: 10.1006/prep.2000.1343. [DOI] [PubMed] [Google Scholar]