Abstract
It is commonly assumed that creatine kinase (CK) activity in plasma is related to the state of an inflammatory response at 24-48 h, and also it has shown biphasic patterns after a marathon run. No information is available on CK isoenzymes after an ultra-marathon run. The purpose of the present study is to examine the CK isoenzymes after a 200 km ultra-marathon run and during the subsequent recovery. Blood samples were obtained during registration 1 2 h before the 200-km race and during the race at 100 km, 150 km and at the end of 200 km, as well as after a 24 h period of recovery. Thirty-two male ultra-distance runners participated in the study. Serum CPK showed a marked increase throughout the race and 24 h recovery period (p < 0.001). Serum CK during the race occurs mostly in the CK-MM isoform and only minutely in the CK-MB isoform and is unchanged in the CK-BB isoform. High-sensitivity C-reactive protein (hs-CRP), oestradiol, AST and ALT increased significantly from the pre-race value at 100 km and a further increase took place by the end of the 200 km run. The results of our study demonstrate a different release pattern of creatine kinase after an ultra-distance (200 km) run compared to the studies of marathon running and intense eccentric exercise, and changes in several biomarkers, indicative of muscle damage during the race, were much more pronounced during the latter half (100–200 km) of the race. However, the increases in plasma concentration of muscle enzymes may reflect not only structural damage, but also their rate of clearance.
Keywords: aerobic exercise, biomarkers, endurance, monitoring, runners
INTRODUCTION
Ultra-endurance exercises favours a catabolic state by activating protein degradation [1, 2]. Creatine kinase (CK) is, therefore, often used as a biomarker for skeletal muscle damage after exercise and myocardial infarction. Plasma CK concentration differs with respect to contractile characteristics, such as with different types of exercise [3]. Mechanisms of CK efflux are well accepted as being due to the damage of muscle tissue or changes in myocyte membrane permeability. With regard to the cause of increased membrane permeability, there are various hypotheses of ion-distribution change, enzyme insufficiency, and ATP reduction [4–6], and sex hormones seem to modify membrane permeability [7, 8]. However, an increase of CK has been observed in rats after level running and also in in-vitro studies without evidence of histological damage [2, 8], suggesting that increased permeability is not necessarily associated with histological damage [2].
CK is a dimeric protein found as three principle isoenzymes: muscle (MM), heart (MB), and brain (BB). Within skeletal muscle, CK occurs mostly in the MM isoform (approximately 90% of total CK) and only minutely in the MB and BB forms. CK-MM is further distinguished into three isoforms (MM1-3), where its location and the cleavage of amino acid residue from the carboxyl terminus of CK enzyme constitute distinguishing characteristics [6].
Most studies of CK efflux have been examined with different modes of exercise including ultra-marathon run, but there has been no study to examine the CK isoenzymes during a long distance run, especially ultra-distance running. During an ultra-marathon run, muscles contract repeatedly and use energy substrates in the race with a repeated pause of short rests/walking between periods of running (<50% of maximal capacity), compared to other repetitive exercise with a short pause (>50% of maximal capacity).
It is also important that the sex hormone oestradiol affects CK efflux [7, 9]. Previous studies have demonstrated that oestrogen may potentially influence contractile properties of muscle fibre and reduce muscle damage, including the release of creatine kinase into the bloodstream [10, 11]. While previous studies have shown an increase in oestradiol following prolonged running [1], since the point at which oestradiol increases is not confirmed in this race, the present study may confirm the point during the race that oestradiol concentrations begin to increase.
The purpose of the present study is, therefore, to examine the biomarkers including CPK isoenzymes of muscle damage and sex hormone during and after a 200 km ultra-marathon run and the subsequent recovery.
MATERIALS AND METHODS
Subjects
Thirty-two male ultra-distance runners volunteered to participate in the present study. The mean age, body weight and height of the runners were 59 years (range 56–70), 69 kg (range 56–78), and 171 cm (range 165–178), respectively, and all subjects had been training for more than 3 years (range 3–10 years). Subjects were affiliated with Korean marathon clubs or with the Korean Ultra-marathon Federation. All the subjects were volunteers and agreed to the experimental procedures.
200-km ultra-marathon race description
All subjects completed the 200 km race within a cut-off time of 36 h (individual records ranged from 29:18 to 33:59). The race was held on the first weekend of April on Cheju Island in South Korea (at sea level), where most of the race course is roughly flat. The temperature during the race ranged from 5.1 to 11.7°C (mean 8.5°C) with a relative humidity of 44.6% and wind speed of 3.2 m · s−1. All runners were provided with food and drink ad libitum during the race.
Blood sampling and analysis
A blood sample was obtained during registration 1–2 h before the 200-km race from a forearm vein. Further samples were also obtained at 100 km, 150 km and at the end of 200 km, as well as after a 24 h period of recovery. Three subjects failed to complete all of the experiment in the 200-km race. On each occasion, blood was divided into tubes containing EDTA (1 mg · mL−1), or dry tubes for separating serum. Samples were stored at −80°C after centrifugation (3,000 rpm for 15 min). Plasma levels of creatine phosphokinase (CPK), high-sensitivity C-reactive protein (hs-CRP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured using an auto-analyzer (ADVIA 1650, Bayer, USA). CK isoenzymes were measured using a semi-automated gel electrophoresis system (Spife3000, HELENA) and oestradiol was measured using a Gamma-10 analyser by radioimmunoassay (Estradiol MAIA Kit). All measurements were performed in duplicate.
Statistics
Each variable is expressed as a mean and standard deviation (SD). All statistical computations were performed using SPSS 11 software (SPSS Inc. Champaign, IL). One-way analysis of variance (ANOVA) with repeated measures and Pearson correlation test were used to analyze variables. Where a significant F test was found, a contrast test was used for comparison of the changes from the pre-race level. The level of significance was accepted at a value of P < 0.05.
RESULTS
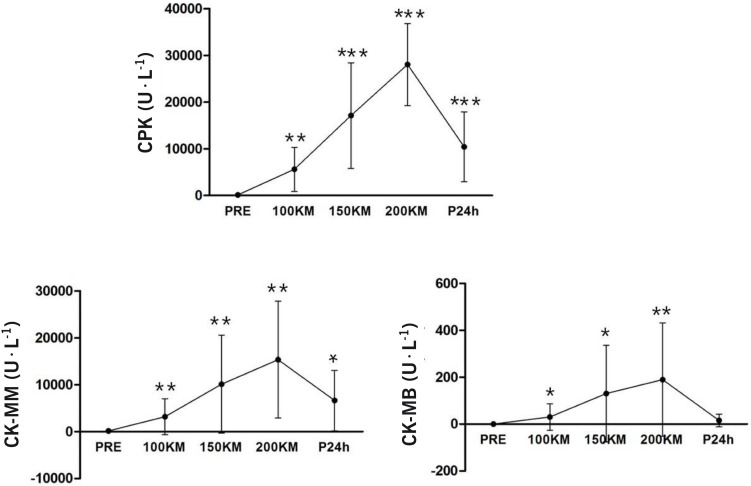
Total serum CPK activity during the ultra-marathon run and after 24h of recovery.
The total CPK increased gradually and significantly at a given distance during the 200 km race from the pre-race value by 22-, 71- and 98-fold, at 100 km, 150 km and 200 km, respectively, and then decreased by 44-fold after 24 h of recovery (Figure 1).
FIG. 1.
Changes in serum CPK, CPK-MM and CPK-MB activity at different distances and during 24 h of recovery.
Note: Values are means ± SD. Significantly different from pre-value and between groups at given distances and recovery at P < 0.01.
Serum CPK isozymes
Serum CPK isoenzymes during the race occur mostly in the CK-MM isoform (consisting of 99% of total CK) and only minutely in the CK-MB isoform (Fig. 1). Serum CK-BB levels were undetectable. Serum CK-MM activity already began to increase significantly at 100 km, and there was a further increase at 200 km, but it decreased during recovery (p < 0.01). CK-MB showed the same trend as CK-MM (p < 0.05) at the 150 km and the 200 km race, and rapidly decreased compared to CK-MM during the 24 h period of recovery (p < 0.05).
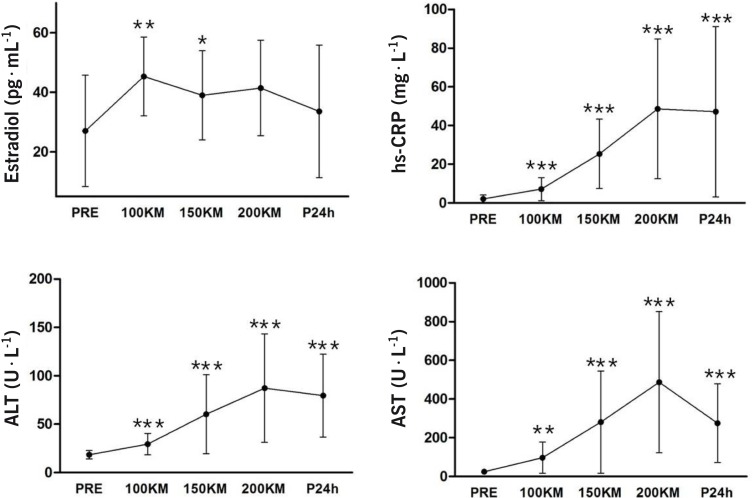
Serum hs-CRP, oestradiol, AST, and ALT levels
Serum hs-CRP increased by 23-fold at 200 km [(p < 0.001), 3.3- fold at 100 km] from the pre-race value (Fig. 2), but remained the same at 24 h of recovery from the 200 km race. Oestradiol concentration increased significantly at 100 km and 150 km from the pre-race values (p < 0.05) by 1.7-fold and 1.4-fold, respectively and remained the same during the rest of the race and the 24 h period of recovery (Figure 2). Both AST and ALT activities increased gradually and significantly during the 200 km race from the pre-race value by 3.9- vs 1.6-, 11.5- vs 3.3-, and 20.3- vs 4.7-fold, respectively at 100 km, 150 km, 200 km (Figure 2), but ALT was unchanged at 24 h of recovery from the 200 km race.
FIG. 2.
Changes in serum hs-CRP, oestradiol, AST and ALT activity at different distances and during 24 h of recovery.
Note: Values are means ± SD. Significantly different from pre-value at P < 0.05.
DISCUSSION
The increases in CPK activity seen in the present study are in general agreement with the proposal made by Noakes [12] that the release of CK is principally related to the duration of the exercise, at least up to 200 km (25-35 hrs; Kim et al. [13], Kim et al. [14]), but not in strenuous daily running (1600 km for 11 days), in which it decreased from day 4 (∼400 km; Fallon et al. [4]).
Mostly the plasma CK-MM isoform was increased (>98% of total CK), with minutely increased CK-MB (∼1%) and unaffected CK-BB forms during and after the 200 km run.
For the first time we have demonstrated that CPK isoenzyme release after a ultra-distance run is a different response to those of a marathon run or an intense eccentric exercise. CK-MB is expressed in the brain, in smooth muscle, and in cardiac and developing muscle, and is released into the circulation when myocardial infarction occurs. Our data agree well with the previous investigations on long distance exercise [15, 16], which reported that CK-MB levels increased after endurance exercise due to myocardial lesions and damaged skeletal muscle. However, it is unclear whether the increased CK-MB level was solely from heart muscle after a 200 km run.
The CK-MM isoenzyme activities are an important marker of muscle cell disruption or damage. In the present study, the CK-MM levels were variable: 51-42 876 IU · L−1 at 150 km and 111141726 U · L− at 200 km. In addition, the CK-MB levels are also unstable: 34-875 U · L−1 at 150 km and 22-921 U · L−1 at 200 km. In a previous study, Totsuka et al. [17] suggested that the serum CK activities are associated with distinctive individual muscle properties in non athletes during and after endurance exercise.
Serum CK activities can also reflect both the rate of loss of CK from muscle and the rate of clearance of CK from blood. An increase of CK has been observed in rats after level running and also in in-vitro studies without evidence of histological damage [8, 18]. This suggests that increased permeability is not necessarily associated with histological damage [2]. Thus, circulating CK activities following exercise may not necessarily reflect the degree of muscle damage alone and could also be influenced by the CK removal rate [19]. Sex hormones seem to modify membrane permeability, i.e., androgens enhance CK efflux, whereas estrogens inhibit CK efflux [7, 8]. However, it is a matter of speculation whether any role of the reticuloendothelial system, which plays an important role in the clearance of enzymes from the muscle [9], may be less functional, especially after an ultra-marathon race. The oestrogen activity in plasma was increased by 1.7-fold after 100 km and remained the same up to the end of 200 km and after the 24 h period of recovery. We also found no correlation between a muscle damage marker and oestradiol. By the virtue of oestrogen antioxidant and/or membrane stabilizing abilities, oestrogen can diminish exercise-induced damage to muscle sarcoplasmic structures during recovery. Jones et al. [2] demonstrated that in male animals the disruption of muscular microarchitecture as well as histopathological changes associated with loss of microstructures of fibre and fibre swelling occurred to a significantly greater extent and in a much earlier time course than in female animals, and evidenced in humans as well [20].
In our recent study, the increase in muscle-specific E3 ligases, muscle-specific ring finger-1 and muscle atrophy F box, and in ubiquitin specific proteasome-28 mRNAs in the vastus lateralis muscle after a 200 km run suggests that the ubiquitination/deubiquitination cycle could be enhanced in muscle cells during ultra-endurance exercise [13], and also suggests the presence of an inhibitory mechanism which down-regulates the proteasomal degradation during ultra-endurance exercise, although this mechanism of regulation is unknown. Therefore, the use of plasma enzyme activities for estimating the amount of muscle damage may be carefully interpreted.
Hs-CRP increased 22-fold at the end of the 200-km race and maintained this elevated level during the 24 h recovery period. Hs- CRP release from hepatocytes is induced by IL-6 [16] and plays a role both in the induction of anti-inflammatory cytokines from circulating monocytes and in suppression of the synthesis of pro-inflammatory cytokines from tissue macrophages [21]. In our study, changes in plasma hs-CRP occurred after 100 km and a further increase took place by the end of the race. Therefore, the increase in hs-CRP in the latter half of the race may represent the synergistic effect of accelerated muscle or liver damage. Hepatic marker enzymes, such as AST and ALT in plasma, increased at 100 km, with a further increase in the latter half of the race, but it is also unclear whether hepatic damage occurs during the ultra-marathon race, as hepatic marker enzymes are found in both liver and muscle cells [11].
Another interesting finding in the present study was that there was no further increase in CK release after a 200-km run, as was found after 24 h of recovery after a marathon or intensive eccentric exercise, but rather it decreased after 24 h of recovery, even though the increase in CK release at the end of the 200-km race was proportionately much greater than that of 100 km (assuming a linear relationship to distance). In our previous studies [13, 14], plasma CK concentrations were not with commonly accepted CK release which has shown biphasic patterns after a marathon run and intense eccentric exercise, in which it is assumed that the peak immediately following exercise is associated with an acute and transient increased sarcolemmal permeability due to a decline in the pool of energy-rich phosphates [2, 18], A second peak is assumed to be related to an inflammatory response [22], which is released in the blood stream during 24-48 hours of recovery. However, plasma CK concentration after a 200 km ultra-marathon run already declined after 24 h of recovery [13, 14]. There was no second peak, in spite of a very high CK concentration after the 200 km run. Fallon et al. [11] also reported an early peak on day 4 (∼438 km) and subsequent decrease on day 11 (∼1090 km) and after the race (1600 km) in CK, despite strenuous daily running. As an explanation for the different response in CK release between immediately after an ultra-marathon and a marathon run or a short intense exercise, it is possible that this reflects a progressive decline in pace at the end of the 200-km race, which will often result in a greater degree of walking with less eccentric contractions in the last quarter of the ultra-marathon race. Another possibility is that the peak in CK release in the 200-km race occurred earlier in the race, at ∼24 hours or 150 km, or even earlier (4-6h), as suggested by Noakes [12], and therefore plasma CK was already falling by the end of the race, and certainly after 24 h of recovery. However, this was not the case. We found that there was no evidence of any early peak before 200 km, even though we measured it once at around 24 h (150 km) after the start of the race. Instead, differences in the rate of clearance of plasma CK are likely to be an explanation.
The present study is descriptive. That is its main limitation. Neither the exercise intensity nor the food intake was controlled, and there was no group with unexercised subjects. Nevertheless, our field observations provide novel results in which biphasic patterns of CPK release, previously demonstrated in studies of marathon running and intense eccentric exercise, were not seen after ultra-marathon running.
CONCLUSIONS
In summary, the results of our study demonstrate a different release pattern of creatine kinase after an ultra-distance (200 km) run compared to the studies of marathon running and intense eccentric exercise, and changes in several biomarkers, indicative of muscle damage during the race, were much more pronounced during the latter half (100–200 km) of the race. Mostly the plasma CK-MM isoform was increased (>98% of total CK), with minutely increased CK-MB (∼1%) and unaffected CK-BB forms during and after the 200 km run. Hs- CRP increased 22-fold at the end of the 200-km race and maintained this elevated level during the 24 h recovery period. The increase in hs-CRP in the latter half of the race may represent the synergistic effect of accelerated muscle. However, the increases in plasma concentration of muscle enzymes may not only reflect structural damage, but also its rate of clearance.
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2010- 413-G00007) and grant from Korea National Sport University.
Conflict of interests
The authors declared no conflict of interests regarding the publication of this manuscript.
REFERENCES
- 1.Dohm GL, Tapscott EB, Kasperek GJ. Protein degradation during endurance exercise and recovery. Med Sci Sports Exerc. 1987;19(5):166–71. [PubMed] [Google Scholar]
- 2.Jones DA, Jackson MJ, Edwards RHT. Release intercellular enzymes from an isolated mammalian skeletal muscle preparation. Clin Sci Lond. 1983;65:193–201. doi: 10.1042/cs0650193. [DOI] [PubMed] [Google Scholar]
- 3.Nosaka K, Clarkson PM. Relationship between post-exercise plasma CK elevation and muscle mass involved in the exercise. Int J Sports Med. 1992;13(6):471–5. doi: 10.1055/s-2007-1021300. [DOI] [PubMed] [Google Scholar]
- 4.Highman B, Altland PD. Serum enzyme raise after hypoxia and effect of autonomic blockade. Am J Physiol. 1960;199:981–986. doi: 10.1152/ajplegacy.1960.199.6.981. [DOI] [PubMed] [Google Scholar]
- 5.Olerud JE, Homer LD, Carroll HW. Incidence of acute exertional rhabdomyolysis. Serum myoglobin and enzyme levels as indicators of muscle injury. Arch Intern Med. 1976;136:692–697. doi: 10.1001/archinte.136.6.692. [DOI] [PubMed] [Google Scholar]
- 6.Yamashita K, Yoshioka T. Profiles of creatine kinase isoenzyme compositions in single muscle fibres of different types. J Muscle Res Cell Motil. 1991;12(1):37–44. doi: 10.1007/BF01781172. [DOI] [PubMed] [Google Scholar]
- 7.Amelink GJ, Bär PR. Exercise-induced muscle protein leakage in the rat. Effects of hormonal manipulation. J Neurol Sci. 1986;76(1):61–8. doi: 10.1016/0022-510x(86)90142-5. [DOI] [PubMed] [Google Scholar]
- 8.Bar PR, Rodenburg AJB, Koot RW, Amelink GJ. Exercise-induced muscle damage: Recent developments. Basic Appl Myo. 1994;4:5–16. [Google Scholar]
- 9.Bijsterbosch MK, Duursma AM, Smit MJ, Bos OJM, Bouma JMW, Gruber M. Several dehydrogenases and kinases compete for endocytosis from plasma by rat tissues. Biochem J. 1985;229:409–417. doi: 10.1042/bj2290409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendall B, Eston R. Exercise-induced muscle damage and the potential protective role of estrogen. Sports Med. 2002;32(2):103–23. doi: 10.2165/00007256-200232020-00003. [DOI] [PubMed] [Google Scholar]
- 11.Fallon KE, Sivyer G, Sivyer K, Dare A. The biochemistry of runners in a 1600 km ultramarathon. Br J Sports Med. 1999;33(4):264–9. doi: 10.1136/bjsm.33.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noakes TD. Effect of exercise on serum activities in humans. Sports Med. 1987;4:245–267. doi: 10.2165/00007256-198704040-00003. [DOI] [PubMed] [Google Scholar]
- 13.Kim HJ, Lee YH, Kim CK. Biomarkers of muscle and cartilage damage and inflammation during a 200 km run. Eur J Appl Physiol. 2007;99(4):443–7. doi: 10.1007/s00421-006-0362-y. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Lee YH, Kim CK. Changes in serum cartilage oligomeric matrix protein (COMP), plasma CPK and plasma hs-CRP in relation to running distance in a marathon (42.195 km) and an ultra-marathon (200 km) race. Eur J Appl Physiol. 2009;105(5):765–70. doi: 10.1007/s00421-008-0961-x. [DOI] [PubMed] [Google Scholar]
- 15.Hansen KN, Bjerre-Knudsen J, Brodthagen U, Jordal R, Paulev PE. Muscle cell leakage due to long distance training. Eur J Appl Physiol. 1982;48:177–188. doi: 10.1007/BF00422979. [DOI] [PubMed] [Google Scholar]
- 16.Majid A, Evalynne V, Braun A, Olusegun F. Elevated CK-MB levels in marathon runners. JAMA. 1982;247:2368–2369. [PubMed] [Google Scholar]
- 17.Totsuka M, Nakaji S, Suzuki K, Sugawara K, Sato K. Break point of serum creatine kinase release after endurance exercise. J Appl Physiol. 2002;93(4):1280–6. doi: 10.1152/japplphysiol.01270.2001. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong RB, Ogilvie RW, Schwane JA. Ecccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- 19.Van der Meulen JH, Kuiper H, Drukker J. Relationship between exercise-induced muscle damage and enzyme release in rats. J Appl Physiol. 1991;71:999–1004. doi: 10.1152/jappl.1991.71.3.999. [DOI] [PubMed] [Google Scholar]
- 20.Fanò G, Mecocci P, Vecchiet J, Belia S, Fulle S, Polidori MC, Felzani G, Senin U, Vecchiet L, Beal MF. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil. 2001;22(4):345–51. doi: 10.1023/a:1013122805060. [DOI] [PubMed] [Google Scholar]
- 21.Pue CA, Mortensen RF, Marsh CB, Pope HA, Wewers MD. Acute phase levels of C-reactive protein enhance IL-1 beta and IL-1ra production by human blood monocytes but inhibit IL-1 beta and IL-1ra production by alveolar macrophages. J Immunol. 1996;156(4):1594–600. 15. [PubMed] [Google Scholar]
- 22.Clarkson PM, Ebbeling C. Investigation of serum creatine kinase variability after muscle-damaging exercise. Clin Sci(Lond) 1988;75(3):257–61. doi: 10.1042/cs0750257. [DOI] [PubMed] [Google Scholar]