Abstract
Up to 30% of adults with acute myeloid leukemia fail to achieve a complete remission after induction chemotherapy - termed primary refractory acute myeloid leukemia. There is no universally agreed definition of primary refractory disease, nor have the optimal treatment modalities been defined. We studied 8907 patients with newly diagnosed acute myeloid leukemia, and examined outcomes in patients with refractory disease defined using differing criteria which have previously been proposed. These included failure to achieve complete remission after one cycle of induction chemotherapy (RES), less than a 50% reduction in blast numbers with >15% residual blasts after one cycle of induction chemotherapy (REF1) and failure to achieve complete remission after two courses of induction chemotherapy (REF2). 5-year overall survival was decreased in patients fulfilling any criteria for refractory disease, compared with patients achieving a complete remission after one cycle of induction chemotherapy: 9% and 8% in patients with REF1 and REF2 versus 40% (P<0.0001). Allogeneic stem cell transplantation improved survival in the REF1 (HR 0.58 (0.46–0.74), P=0.00001) and REF2 (HR 0.55 (0.41–0.74), P=0.0001) cohorts. The utilization of REF1 criteria permits the early identification of patients whose outcome after one course of induction chemotherapy is very poor, and informs a novel definition of primary refractory acute myeloid leukemia. Furthermore, these data demonstrate that allogeneic stem cell transplantation represents an effective therapeutic modality in selected patients with primary refractory acute myeloid leukemia.
Introduction
Up to 30% of adults with newly diagnosed acute myeloid leukemia (AML) fail to achieve a morphological complete remission (CR) after one or two courses of induction chemotherapy (IC).1 Although the outcome of AML refractory to IC is known to be poor, the optimal management of this important cause of treatment failure remains undetermined.
Whilst a number of recent registry studies have demonstrated long-term survival after allogeneic stem cell transplantation (SCT) in patients with primary refractory AML (PREF AML), interpretation of these data are complicated by multiple factors, including limited cohort size, selection bias and lead time reporting errors.2–7 Nonetheless, the advent of reduced intensity conditioning (RIC) regimens, coupled with increased numbers of alternative stem cell donors, has resulted in allogeneic SCT becoming an increasingly deliverable treatment option in PREF AML, emphasizing the importance of defining its curative potential in this setting.
At the same time there remains considerable debate concerning the definition of primary refractory disease.8 The International Working Group (IWG) and the European LeukemiaNet (ELN) both define resistant disease after induction therapy as persistent leukemic blasts in either the peripheral blood or the bone marrow in a patient alive seven days or more following treatment.9,10 However, most studies investigating the impact of allogeneic SCT in AML refractory to induction therapy have defined refractoriness as a failure to achieve CR following two courses of chemotherapy.2,3,5,7,11,12 A number of reports have demonstrated that failure to achieve CR after one course of IC is an adverse prognostic indicator; however, this has not been universally reported.13 The UK Medical Research Council (MRC) data have previously demonstrated that patients who had between 5–15% residual leukemic blasts following their first cycle of IC had similar relapse rates to those who achieved a CR, although they demonstrated a reduced overall survival (OS).14 Schlenk et al. analyzed 223 patients enrolled on the HD93 trial and defined those with a <50% reduction in bone marrow blasts following one course of IC as having refractory disease. In this relatively small study, patients with refractory disease defined using this criterion demonstrated a lower OS than patients in CR.15 Previous studies which have defined refractory disease as failure to achieve a CR after two courses of IC, have consistently demonstrated an extremely poor survival rate in this sizeable proportion of newly diagnosed patients.5,16,17 Importantly, to our knowledge, there have been no systematic comparisons of outcome according to different definitions of putative refractoriness in a large cohort of patients, nor has the impact of allogeneic SCT been systematically evaluated.
We have therefore analyzed the outcome of patients with AML resistant to induction therapy, utilizing different definitions of PREF AML, in order to generate diagnostic criteria and examine whether patients genuinely refractory to IC can be identified earlier in their treatment pathway. This has allowed us to study the role of allogeneic SCT in the management of PREF AML - an important but largely ignored disease entity.
Methods
We performed a retrospective analysis of patient data on 8907 patients with non-promyelocytic AML treated with intensive chemotherapy regimens on the MRC/NCRI AML 10, 11, 12, 14, 15 and 16 trials. The AML 11, 14 and 16 trials were predominantly for older AML patients (>60 years), and their treatment intensity was reduced compared with trials for younger AML patients. The trial chemotherapy regimens used have been previously outlined,1,18–21 and are summarized in the Online Supplementary Figure S1. Trials were conducted in accordance with the declaration of Helsinki, were approved by the Wales multi-center research ethics committee and participating institutions ethical review committees, and patients provided written informed consent for their inclusion in each trial and for the use of their clinical data in the outcome analysis. Karyotype risk stratification was designated according to Grimwade et al.22 Bone marrow blasts were analyzed for FLT3 internal tandem duplications (ITD) and NPM1 mutations as previously described.1
Response to IC was assessed by bone marrow evaluation performed 14–21 days after completion of chemotherapy. Complete response was defined as the presence of less than 5% blasts in the bone marrow. In patients who failed to achieve a CR after their first course (C1) of IC response assessment was repeated after a second course of IC (C2). CR after a second course of IC was defined as CR occurring within 42 days of commencing C2, or 75 days after trial entry, if the date of administration of C2 was not available. Patients failing to achieve a CR after two courses of IC were typically treated off study. Failure to respond to either the first or second course of IC was defined according to four definitions of refractoriness, namely: RES: resistant disease with failure to achieve CR after C1, PR: those deemed to have had a partial response to IC with failure to achieve CR after C1 and fewer than 15% blasts or a greater than 50% proportional reduction in blast percentage, REF1: those deemed to have had a minor or no response to IC with more than 15% blasts and a less than 50% proportional reduction in blast percentage after C1, and REF2: failure to achieve CR after two courses of IC (Figure 1). 371 patients were deemed to be refractory but their blast percentage was not available, and for the purpose of this analysis these patients were classified as PR. Separately, patients fulfilling either REF1 or REF2 criteria were combined and analyzed separately in a compendious cohort, REF1/2.
Figure 1.
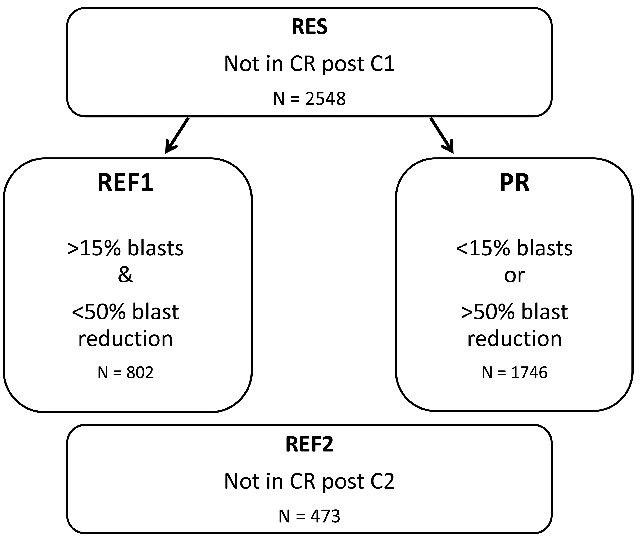
Diagram illustrating the four definitions of refractory AML (acute myeloid leukemia) used in this study. C1: course 1; C2: course 2; CR: complete remission; RES: resistant disease with failure to achieve a complete remission after C1; REF1: those deemed to have had a minor or no response to IC with more than 15% blasts and a less than 50% proportional reduction in blast percentage after C1; REF2: failure to achieve a complete remission after two courses of IC; PR: those deemed to have had a partial response after C1 with fewer than 15% blasts or a greater than 50% proportional reduction in blast percentage.
Survival in patients fulfilling the different criteria of refractoriness was measured from the time at which refractoriness was ascertained according to the defined criteria. Survival percentages are measured using the method of Kaplan-Meier, or that of Mantel-Byar for the analyses of transplant versus not. In comparing allograft with no transplant, patients receiving other types of transplant were censored on the date of transplant. The outcomes of patients allografted after the year 2000 were analyzed according to age (greater or less than 50 years), to take into account the introduction of reduced intensity conditioning (RIC) regimens in older patients from this date. Models for risk of refractoriness or prognosis after being defined as refractory were built using Cox proportional hazards regression with forward selection; because molecular data were not uniformly available, this was performed in 2 stages – first using clinical variables, and then adding the presence of mutations in the FLT3 or NPM1 genes to the model.
Results
Characterization of induction failure cohorts
8907 patients were treated with intensive chemotherapy and form the subject of this study (Figure 2). 5480 patients achieved a CR following C1 and there were 879 induction deaths. A total of 2548 patients did not achieve remission with C1 (RES) of whom 802 fulfilled the criteria for refractoriness according to definition REF1. Of those not in CR post C1, 1059 patients achieved a CR after C2, with 100 patients dying during C2. 473 patients fulfilled the criteria for REF2. Of 802 patients fulfilling the criteria for REF1, 204 achieved remission after C2. The total number of patients who received an allogeneic SCT was 498. Of these, 351 underwent a myeloablative conditioning regimen whilst 147 received a RIC regimen. The demographics of the patients with refractory disease as defined by these criteria are outlined in the Online Supplementary Table S1.
Figure 2.
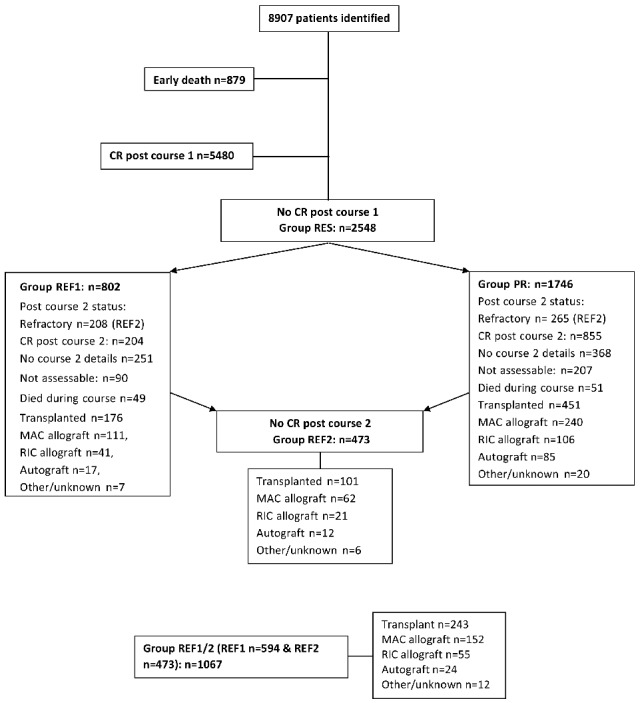
Consort diagram of patient cohorts and treatment outcomes. CR: Complete remission; MAC: myeloablative conditioning; RIC: reduced intensity conditioning; RES: resistant disease with failure to achieve a complete remission after C1; REF1: those deemed to have had a minor or no response to IC with more than 15% blasts and a less than 50% proportional reduction in blast percentage after C1; PR: those deemed to have had a partial response after C1 with fewer than 15% blasts or a greater than 50% proportional reduction in blast percentage; REF2: failure to achieve a complete remission after two courses of IC; REF 1/2; all patients in groups REF1 and REF2.
Factors predicting resistance to induction chemotherapy
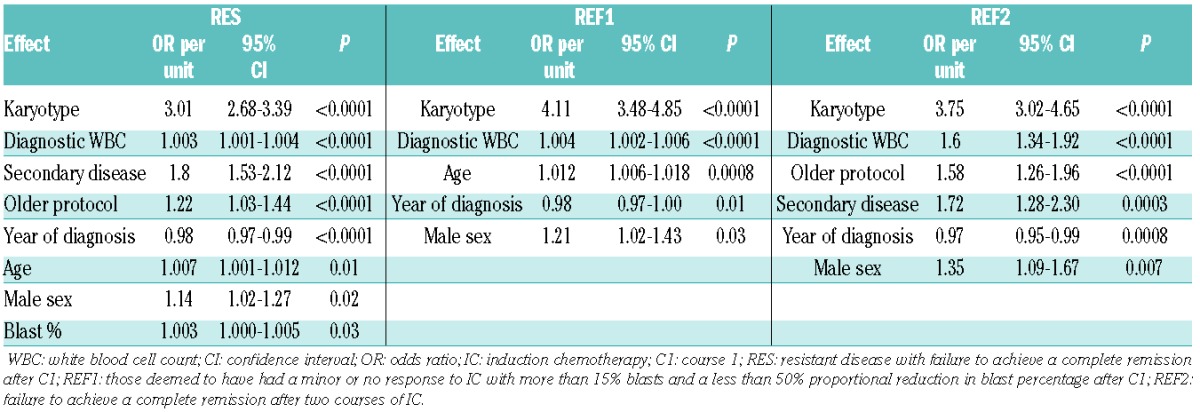
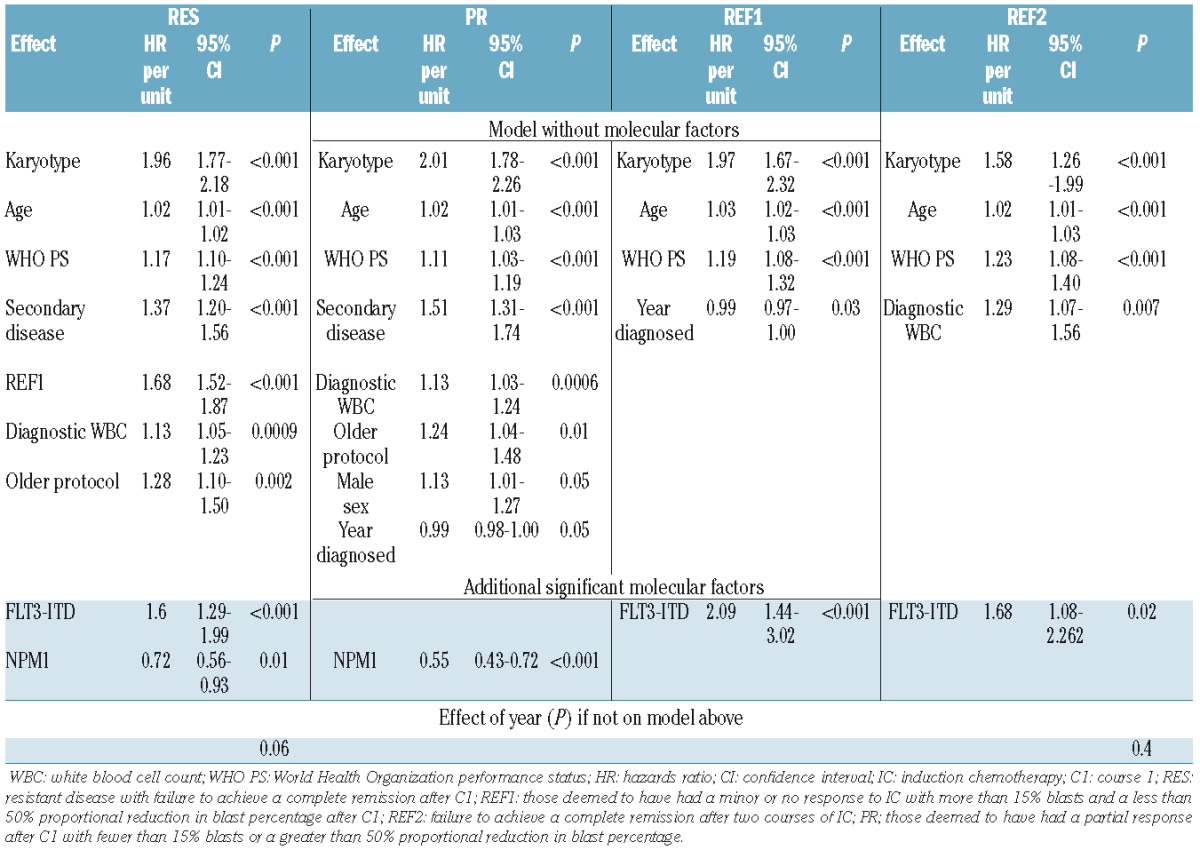
The factors determining the presence of refractory disease after IC, according to the studied definitions, are summarized in Table 1. Factors common to patients fulfilling REF1, PR and REF2 criteria included the year of diagnosis, presentation of white blood cell count (WBC) and karyotype.
Table 1.
Factors predicting the presence of refractory disease in RES, REF1 and REF 2 cohorts.
Patient outcomes according to category of refractory disease
The 5-year OS for patients in RES, REF1, PR, REF2 and REF1/2 cohorts was 17%, 9%, 21%, 8% and 9%, respectively, compared with 40% for patients achieving a CR after one course of IC (P<0.0001). REF1 criteria identify a distinct sub-population of patients who fail to achieve CR after course 1, with significantly worse 5-year OS compared with PR patients (P<0.0001) (Figure 3A). The 5-year OS for REF1 patients (9%) was equivalent to REF2 patients (8%). The 5-year OS for the minority (204) of REF1 patients who achieved CR with C2 was markedly reduced compared with patients achieving CR with their first course of IC (HR 1.39 (1.15–1.69) P=0.0008) (Figure 3B).
Figure 3.
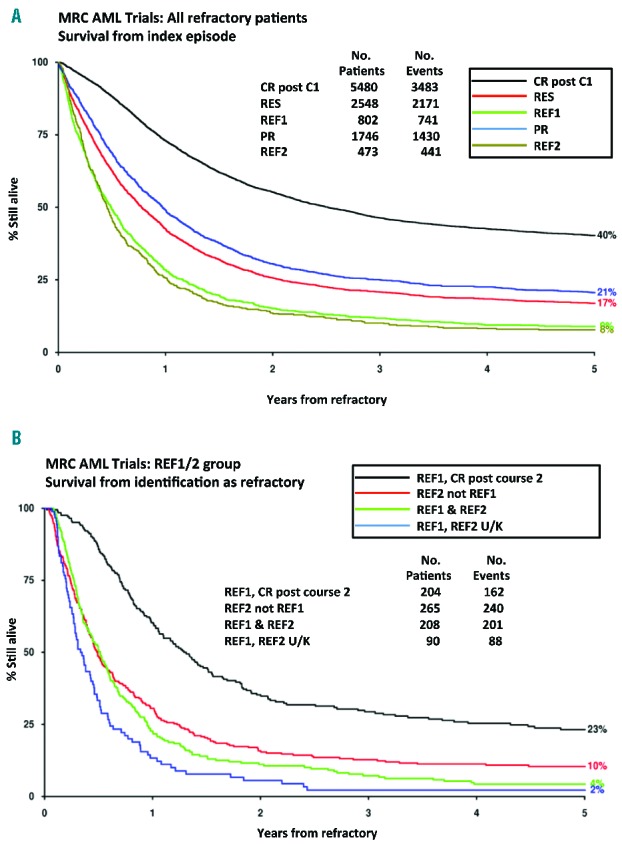
Survival from first being identified as refractory according to the definitions studied or entering complete remission (CR) after one course (C1) of induction chemotherapy a) CR post C1, RES, REF1, PR, REF 2; b) comparison of REF1 patients who achieve CR after course two of IC with REF 2 patients not identified in the REF1 cohort (REF2 not REF1), REF1 patients included in the REF 2 cohort (REF1 & REF2) and REF1 patients whose REF 2 status is unknown (REF1, REF2 U/K). RES: resistant disease with failure to achieve a complete remission after C1; REF1: those deemed to have had a minor or no response to IC with more than 15% blasts and a less than 50% proportional reduction in blast percentage after C1; PR: those deemed to have had a partial response after C1 with fewer than 15% blasts or a greater than 50% proportional reduction in blast percentage; REF2: failure to achieve a complete remission after two courses of IC; REF 1/2; all patients in groups REF1 and REF2.
Factors predicting long term survival in refractory disease
The prognostic factors associated with survival for each of the defined populations with refractory disease are outlined in Table 2, and are broadly similar to those which predicted the presence of refractory disease. Karyotype, age and performance status were predictive of survival across all cohorts. When we included the mutational status for FLT3 ITD and NPM1, we found that NPM1 mutations predicted for survival in the REF1 cohort with FLT3 ITD being predictive for survival in the REF1, REF2 and REF1/2 cohorts.
Table 2.
Prognostic factors for survival of the defined cohorts of patients studied.
Identification of treatment factors determining long term survival
We next studied the impact of allogeneic transplantation on outcome in the defined groups of primary refractory disease using a Mantel-Byar approach. Analyses are presented as Forest plots stratified by age (Figure 4). Mantel-Byar analysis demonstrated that OS in allografted patients was significantly improved compared with non-transplant patients in REF1, REF2 and REF1/2 cohorts, with roughly equivalent estimates of the hazard ratio for the benefit of transplantation: REF1 (HR 0.58 (0.46–0.74), P=0.00001), REF2 (HR 0.55 (0.41–0.74), P=0.0001), and REF1/2 (HR 0.58 (0.49–0.69), P<0.00001). In the RES cohort patients over 50 years of age (HR 0.75 (0.62–0.91), P=0.003), allogeneic transplantation improved survival, although there was no difference in RES patients under the age of 50 (HR 1.06 (0.87–1.28), P=0.6; test for interaction P=0.01). When analysis was restricted to PR patients, there was no benefit for transplantation in either age group. In the minority of patients in REF1 who achieved a CR (204/802) with further courses of chemotherapy, there was a trend towards improved OS after allografting, but this did not achieve statistical significance (HR 0.77 (0.57–1.05) P=0.09). In patients with REF2 disease survival after allogeneic transplant was improved in patients who had achieved a CR with subsequent courses of chemotherapy (n=49), compared with those transplanted with active disease (n=37) (38% vs. 17%), although numbers were small. In analyses of the REF1/2 group censored at stem cell transplant, there was no evidence of improvement in survival over time (P=0.3), implying that improved survival is likely to be related to the use of transplantation.
Figure 4.
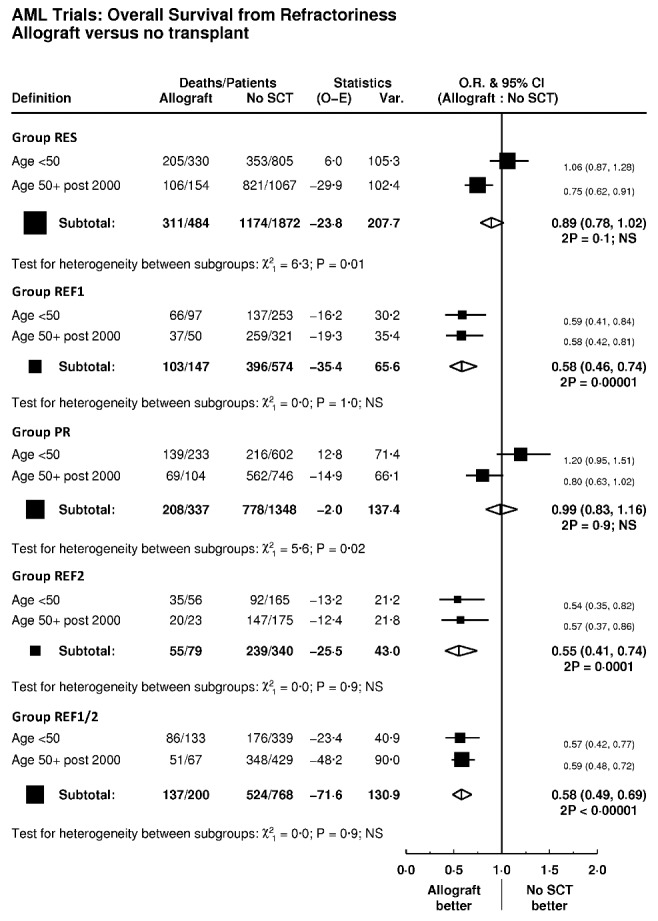
Mantel-Byar analysis of impact of allogeneic transplant on survival according to different definitions of PREF AML (primary refractory acute myeloid leukemia). SCT: stem cell transplant; O.R.: odds ratio; CI: confidence intervals; Var.: variance; 2P: 2-sided P value; NS: non-significant; RES: resistant disease with failure to achieve a complete remission after C1; PR: those deemed to have had a partial response after C1 with fewer than 15% blasts or a greater than 50% proportional reduction in blast percentage; REF1: those deemed to have had a minor or no response to IC with more than 15% blasts and a less than 50% proportional reduction in blast percentage after C1; REF2: failure to achieve a complete remission after two courses of IC; REF 1/2; all patients in groups REF1 and REF2.
Discussion
This analysis, performed in a large and coherently treated population of adults, confirms previous reports that failure to achieve CR after one course of IC is associated with decreased survival. Furthermore, the presence of more than 15% blasts and a less than 50% reduction in blast percentage after the first course of IC identifies a population of patients whose survival is significantly worse than those who achieve a CR after course one, and equivalent to patients who fail to achieve CR after two courses of IC. Reasoning that the definition of refractoriness is failure to achieve long-term survival if treated with chemotherapy alone, our data support a novel operational definition of PREF AML based either on a minimal response to the first course of IC, defined as a less than 50% proportional reduction in blasts and the presence of more than 15% blasts, or a failure to achieve CR after two courses of IC. In other words, the outcomes for patients fulfilling either REF1 or REF2 criteria, if treated with further intensive chemotherapy, is very poor, consistent with chemorefractoriness. Importantly, our data do not support the continued use of the RES or PR criteria to define PREF AML. Our analysis has identified a number of factors including karyotype, age, sex and diagnostic white cell count as predicting refractoriness, consistent with previous studies of high-risk AML. Interestingly, the use of the MRC risk score designed for risk stratification of younger patients with AML in conjunction with REF1 criteria identifies more than 90% of patients within the REF1/2 group.14
Whilst it has been reported that allogeneic SCT may represent an important treatment modality in patients with PREF AML, the absence of a consensus concerning the definition of refractory disease and the selection bias inherent in registry studies has led to skepticism and therapeutic uncertainty. By applying different definitions of primary refractoriness it has been possible, for the first time, to examine the impact of allogeneic transplant in four different clinical settings. These data demonstrate that allografting confers a marked survival advantage in patients fulfilling REF1 and REF2 criteria. There are a number of limitations in the interpretation of our data. Firstly, it is not possible to quantify the degree to which selection bias contributed to the observed improved outcome in the population of patients who proceeded to transplant. Equally, the impact of an allogeneic transplant may have been underestimated because patients often proceeded to transplant after multiple courses of IC, which has previously been shown to compromise the outcome of patients allografted for PREF AML.11,12,23 It is perhaps of no surprise that the outcome of patients fulfilling REF2 criteria who subsequently achieved a CR prior to transplant appeared to be improved compared with those who never achieved CR, but nonetheless our data demonstrate that allografting represents the only curative option for a proportion of REF2 patients; although the degree to which this benefit is restricted to those who achieve a CR with further chemotherapy will require further study. Since time to transplant is an important predictor of outcome in refractory AML, the use of REF1 criteria to identify patients with refractory disease represents an opportunity to improve transplant outcomes by shortening the time from diagnosis to transplant. More prosaically, these data also underline the importance of tissue typing newly diagnosed adult patients and the commencement of an urgent donor search as a cornerstone of the management of adult AML.
An important potential determinant of chemorefractoriness in AML is the intensity of induction chemotherapy. In this study, it was observed that older patients, for whom lower intensity therapy was felt more appropriate, were at a higher risk of having refractory disease after two courses of IC. This underlines the importance of the development of either more effective, but well tolerated, novel chemotherapeutic agents, or improved delivery strategies such as the use of liposomal preparations.24 This is particularly pertinent given the higher incidence of PREF AML in older patients.25 A weakness of this study is that we have analyzed the outcome in patients treated with standard doses of induction chemotherapy only, and it will be important to repeat this analysis in patients receiving high dose cytosine arabinoside regimens. Our data does, however, support the further exploration of sequential conditioning regimens which incorporate a cycle of intensive chemotherapy as an integral component of the preparative regimen, such as those developed by Kolb and Schmid.12,26 In this context, it is of interest to note the particularly encouraging results reported by these authors using the sequential FLAMSA regimen in patients with PREF AML.
Taken together our data support a clarification of the criteria used to define refractoriness to IC in adult AML. Furthermore, we demonstrate the ability of allogeneic transplantation to improve long-term survival in selected patients with PREF AML. Adoption of the proposed criteria will assist in the early identification of patients with PREF AML who have the potential to benefit from allogeneic transplantation. Such an approach has the potential to reduce transplant toxicity and prevent potential selection of chemotherapy resistant sub-clones.27
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/11/1351
Funding
This study was supported (in part) by research funding from Bloodwise UK, the Medical Research Council and Cancer Research UK to CC, RH, NR.
PV acknowledges funding from The Haematology and Stem Cell Theme of the Oxford Biomedical Research Centre (BRC).
References
- 1.Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30(32):3924–3931. [DOI] [PubMed] [Google Scholar]
- 2.Oyekunle AA, Kroger N, Zabelina T, et al. Allogeneic stem-cell transplantation in patients with refractory acute leukemia: a long-term follow-up. Bone Marrow Transplant. 2006;37(1):45–50. [DOI] [PubMed] [Google Scholar]
- 3.Forman SJ, Schmidt GM, Nademanee AP, et al. Allogeneic bone marrow transplantation as therapy for primary induction failure for patients with acute leukemia. J Clin Oncol. 1991;9(9):1570–1574. [DOI] [PubMed] [Google Scholar]
- 4.Mehta J, Powles R, Horton C, et al. Bone marrow transplantation for primary refractory acute leukaemia. Bone Marrow Transplant. 1994;14(3):415–418. [PubMed] [Google Scholar]
- 5.Othus M, Appelbaum FR, Petersdorf SH, et al. Fate of patients with newly diagnosed acute myeloid leukemia who fail primary induction therapy. Biol Blood Marrow Transplant. 2015;21(3):559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabbour E, Daver N, Champlin R, et al. Allogeneic stem cell transplantation as initial salvage for patients with acute myeloid leukemia refractory to high-dose cytarabine-based induction chemotherapy. Am J Hematol. 2014;89(4):395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung HC, Stein A, Slovak M, et al. A long-term follow-up report on allogeneic stem cell transplantation for patients with primary refractory acute myelogenous leukemia: impact of cytogenetic characteristics on transplantation outcome. Biol Blood Marrow Transplant. 2003;9(12):766–771. [DOI] [PubMed] [Google Scholar]
- 8.Ravandi F. Primary refractory acute myeloid leukaemia - in search of better definitions and therapies. Br J Haematol. 2011;155(4):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–4649. [DOI] [PubMed] [Google Scholar]
- 10.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. [DOI] [PubMed] [Google Scholar]
- 11.Craddock C, Labopin M, Pillai S, et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia. 2011;25(5):808–813. [DOI] [PubMed] [Google Scholar]
- 12.Schmid C, Schleuning M, Schwerdtfeger R, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108(3):1092–1099. [DOI] [PubMed] [Google Scholar]
- 13.Rowe JM, Kim HT, Cassileth PA, et al. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer. 2010;116(21):5012–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheatley K, Burnett AK, Goldstone AH, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council’s Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999;107(1):69–79. [DOI] [PubMed] [Google Scholar]
- 15.Schlenk RF, Benner A, Hartmann F, et al. Risk-adapted postremission therapy in acute myeloid leukemia: results of the German multicenter AML HD93 treatment trial. Leukemia. 2003;17(8):1521–1528. [DOI] [PubMed] [Google Scholar]
- 16.Revesz D, Chelghoum Y, Le QH, Elhamri M, Michallet M, Thomas X. Salvage by timed sequential chemotherapy in primary resistant acute myeloid leukemia: analysis of prognostic factors. Ann Hematol. 2003;82(11):684–690. [DOI] [PubMed] [Google Scholar]
- 17.Ravandi F, Cortes J, Faderl S, et al. Characteristics and outcome of patients with acute myeloid leukemia refractory to 1 cycle of high-dose cytarabine-based induction chemotherapy. Blood. 2010;116(26):5818–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hann IM, Stevens RF, Goldstone AH, et al. Randomized comparison of DAT versus ADE as induction chemotherapy in children and younger adults with acute myeloid leukemia. Results of the Medical Research Council’s 10th AML trial (MRC AML10). Adult and Childhood Leukaemia Working Parties of the Medical Research Council. Blood. 1997;89(7):2311–2318. [PubMed] [Google Scholar]
- 19.Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1302–1311. [DOI] [PubMed] [Google Scholar]
- 20.Burnett AK, Hills RK, Milligan DW, et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol. 2010;28(4):586–595. [DOI] [PubMed] [Google Scholar]
- 21.Burnett AK, Milligan D, Goldstone A, et al. The impact of dose escalation and resistance modulation in older patients with acute myeloid leukaemia and high risk myelodysplastic syndrome: the results of the LRF AML14 trial. Br J Haematol. 2009;145(3):318–332. [DOI] [PubMed] [Google Scholar]
- 22.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. [DOI] [PubMed] [Google Scholar]
- 23.Biggs JC, Horowitz MM, Gale RP, et al. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood. 1992;80(4):1090–1093. [PubMed] [Google Scholar]
- 24.Cortes JE, Goldberg SL, Feldman EJ, et al. Phase II, multicenter, randomized trial of CPX-351 (cytarabine:daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer. 2015;121(2):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29(4):369–377. [DOI] [PubMed] [Google Scholar]
- 26.Pfeiffer T, Schleuning M, Mayer J, et al. Influence of molecular subgroups on outcome of acute myeloid leukemia with normal karyotype in 141 patients undergoing salvage allogeneic stem cell transplantation in primary induction failure or beyond first relapse. Haematologica. 2013;98(4):518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


