Abstract
We sought to confirm the prognostic importance of simple clinically available biomarkers of C-reactive protein, serum albumin, and ferritin prior to allogeneic hematopoietic cell transplantation. The study population consisted of 784 adults with acute myeloid leukemia in remission or myelodysplastic syndromes undergoing unrelated donor transplant reported to the Center for International Blood and Marrow Transplant Research. C-reactive protein and ferritin were centrally quantified by ELISA from cryopreserved plasma whereas each center provided pre-transplant albumin. In multivariate analysis, transplant-related mortality was associated with the pre-specified thresholds of C-reactive protein more than 10 mg/L (P=0.008) and albumin less than 3.5 g/dL (P=0.01) but not ferritin more than 2500 ng/mL. Only low albumin independently influenced overall mortality. Optimal thresholds affecting transplant-related mortality were defined as: C-reactive protein more than 3.67 mg/L, log(ferritin), and albumin less than 3.4 g/dL. A 3-level biomarker risk group based on these values separated risks of transplant-related mortality: low risk (reference), intermediate (HR=1.66, P=0.015), and high risk (HR=2.7, P<0.001). One-year survival was 74%, 67% and 56% for low-, intermediate- and high-risk groups. Routinely available pre-transplant biomarkers independently risk-stratify for transplant-related mortality and survival.
Introduction
Successful outcomes following allogeneic hematopoietic cell transplant (HCT) may be offset by risks of transplant-related morbidity and mortality.1 Traditional factors used to gauge transplant risks include conditioning regimen intensity,2 immunosuppression and HLA matching,3 along with hematopoietic cell source, and graft cell yield.4,5 The importance of patient factors on morbidity and transplant-related mortality (TRM) has gained greater appreciation, particularly in the era of HCT for older and/or more fragile patients.6 Comorbidity determined by the hematopoietic cell transplantation-comorbidity index (HCT-CI),7 predicts for worse survival, generally through higher TRM. Impaired performance status interferes with transplant success.7–9 Detailed inventories of patient health through Geriatric Assessment (GA) enhance risk-stratification10 but are time consuming and mostly apply to older adults.
Simple, objective, available, and validated prognostic markers are required to more precisely estimate TRM and ideally inform targeted interventions to mitigate adverse risks. Serum biomarkers hold promise in this regard. Prior single institutional studies have suggested elevated C-reactive protein (CRP),11,12 ferritin13,14 and lower albumin15 prior to allogeneic HCT are associated with greater TRM and worse overall survival (OS). Although inflammation affects all three measures, each biomarker has separate and distinct underlying pathways and clinical uses. CRP is an acute phase reactant clinically applied to quantify inflammation, but is also prognostic for long-term function and mortality outside the HCT setting.16 Serum ferritin estimates iron stores.13 Albumin reflects protein synthesis and levels decline in relation to protein restriction and inflammation.17 Hypoalbuminemia represents a risk factor for mortality in older patients in general.18
Prior studies of these biomarkers in HCT have shown inconsistent results on transplant outcomes. We sought to validate the independent prognostic value of pre-conditioning serum CRP, ferritin, and albumin in a large, homogenous, sample derived from transplant registry data.
Methods
Data source
The Center for International Blood and Transplant Research (CIBMTR) is a voluntary working group of more than 450 transplant centers worldwide who contribute data on consecutive allogeneic HCT to a statistical center housed both at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program (NMDP) in Minneapolis. Patients are followed longitudinally with yearly follow up. Computerized checks for errors and on-site audits of participating centers ensure data quality. Physician review of data and additional requested information from reporting centers are included. Observational studies conducted by the CIBMTR are performed with a waiver of informed consent and in compliance with HIPAA regulations, as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
Patient selection
Patients aged 18 years or over, receiving either an 8/8 or 7/8 human leukocyte antigen matched (i.e. HLA-A, B, C, DRB1) unrelated donor allograft for acute myeloid leukemia (AML) in remission or myelodysplastic syndromes (MDS) with less than 5% blasts at time of transplant and available, cryopreserved, plasma samples collected before HCT conditioning were considered. The study period covered transplant from 2008 to 2010 to allow for comorbidity adjustment as routine capture of the HCT-CI began in 2008. Recipients of T-cell depleted allografts and prior autologous or allogeneic transplant were excluded.
Study end points and definitions
For validation of TRM, pre-specified, literature-derived, biomarker thresholds were defined as follows: CRP more than 10 mg/L,11 ferritin more than 2500 ng/mL,14 and albumin less than 3.5 g/dL.15 Time to death without evidence of disease relapse defined TRM. Relapse is the competing risk, and patients surviving in continuous complete remission were censored at last follow up. Secondary end points included cumulative incidence of relapse, progression-free survival (PFS), overall survival (OS), acute graft-versus-host disease (GvHD) grade II–IV and grade III–IV. A secondary analysis was planned to explore the optimal biomarker cut-off points and construct an overall biomarker model for future testing.
Sample collection and processing
Peripheral blood plasma samples were obtained prior to conditioning and cryopreserved at −80°C in the CIBMTR biorepository. The University of Chicago later analyzed the samples by enzyme-linked immunosorbent assay (ELISA) for ferritin and high-sensitivity CRP (kits from MP Biomedicals). Each center provided pre-transplant patient albumin values as available from the medical record before conditioning started. The limited sample volume precluded automated chemistry machine analysis for biomarkers.
Correlation of biomarkers by automated methods and ELISA
To correlate automated chemistry derived CRP and ferritin (as routinely performed in clinical laboratories) to ELISA, we obtained de-identified serum samples analyzed on the Roche Cobas© 8000 (Roche Diagnostics, USA) covering the entire biomarker range after institutional review board approval. CRP encompassed four ranges in mg/L: < 3, 3–5, 6–10, and >10. Likewise, ferritin spanned four groups as follows: < 500, 500–1000, >1000–1500, > 1500 (ng/mL). These samples were cryopreserved, thawed and then simultaneously analyzed on the Roche Cobas© 8000 and by ELISA as above for correlation analysis by each technique.
Statistical analysis
Univariate probabilities of PFS and OS were calculated using the Kaplan-Meier estimator with variance estimated by Greenwood’s formula. Probabilities of acute GvHD, TRM and relapse were calculated using cumulative incidence curves to accommodate competing risks. Ninety-five percent confidence intervals (95%CI) for all probabilities and P-values of pairwise comparisons were derived from pointwise estimates and calculated using standard techniques. Pearson correlation coefficients were generated and compared for each biomarker and standard clinical characteristics. Multivariate Cox models were fit for each outcome of interest after adjusting for clinical characteristics by stepwise regression, omitting the biomarkers. We modeled biomarkers in several ways. First, the analysis used protocol specified biomarker cut-off points, entering each biomarker individually and generating hazard ratios, 95% confidence intervals (CI) and probability values for each. Second, the functional form of the relationship between each biomarker and TRM was determined using splines by examining Martingale residual plots, and considering potential interactions, leading to models with an optimal threshold. Finally, we constructed a combined model using optimal biomarker levels in their final functional form entered in the multivariate model simultaneously. Risk factors with P<0.05 were included in the model. All computations were performed using the statistical package SAS v.9.1. Linear regression was used to correlate CRP and ferritin values by ELISA and chemistry procedures. Due to variability at very high values, prior to regression, biomarkers underwent log transformation.
Results
Patients’ characteristics
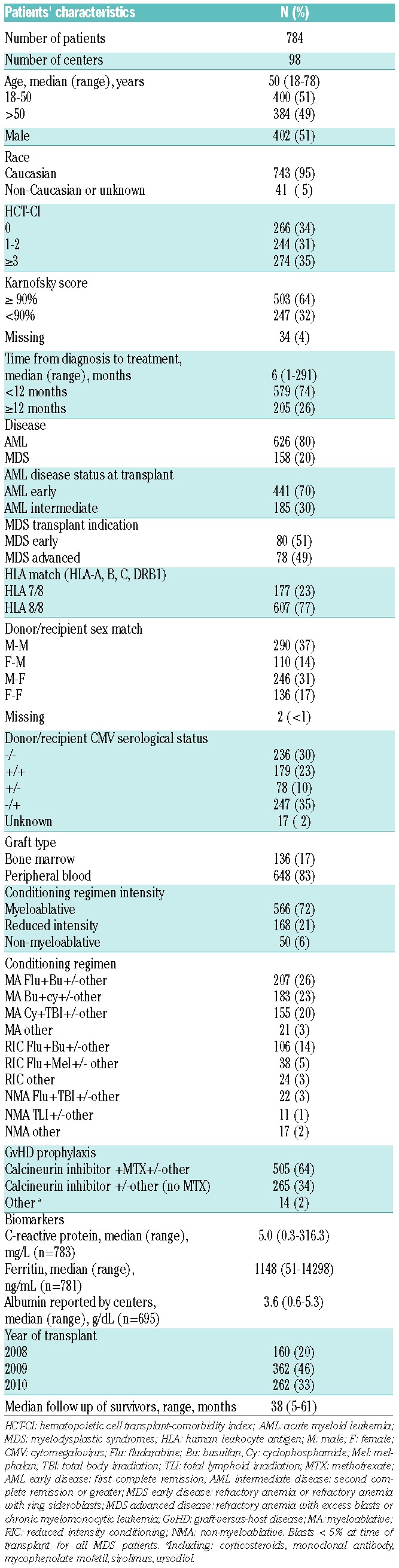
Table 1 summarizes baseline characteristics of the study cohort. In brief, the median age was 50 years (range 18–78 years) among the 784 patients across 98 centers. Most subjects were Caucasian (95%), and received myeloablative regimens (72%). The primary disease indication was AML (80%), and the major graft source was peripheral blood (83%). Median follow up was 38 months.
Table 1.
Baseline characteristics for AML and MDS patients undergoing unrelated donor hematopoietic cell transplantation.
Biomarker levels and correlations
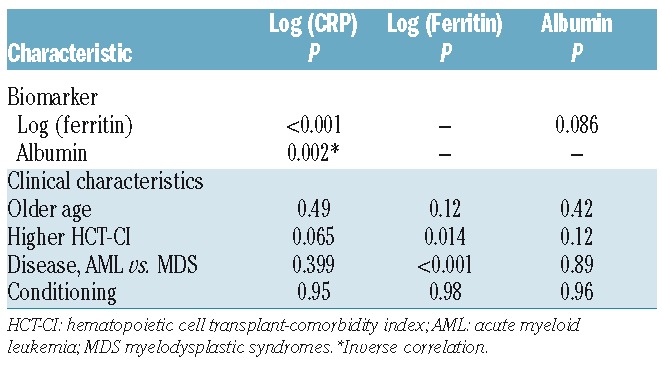
Almost all patients were evaluable for CRP (99.9%) and ferritin (99.6%) by ELISA. The transplant centers supplied albumin results in 88.6% of patients. The median values were 5.0 mg/L for CRP, 1148 ng/dL for ferritin and 3.6 g/dL for albumin (Table 1). Table 2 shows the correlation of biomarkers and clinical factors. A modest correlation was found between CRP and ferritin (r=0.35, P<0.001) and inverse association with albumin (r=−0.12, P=0.002). In addition, CRP and albumin were not associated with patient age, HCT-CI, disease or conditioning intensity. Higher log ferritin was significantly associated with greater comorbidity by HCT-CI (P=0.014) and AML relative to MDS (P<0.001).
Table 2.
Association of biomarkers with clinical characteristics.
Overall outcomes
The cumulative incidence of acute GvHD grades II–IV was 43% (95%CI: 39%–46%) and grade III–IV was 15% (95%CI: 13%–18%) at 100 days. Transplant-related mortality was 18% (95%CI: 16%–21%) at one year and 25% (95%CI: 22%–28%) at three years. Progression-free survival ranged from 57% (95%CI: 54%–61%) at one year to 45% (95%CI: 43%–49%) at three years, whereas 1-year survival was 65% (95%CI: 62%–68%) and 3-year survival was 49% (95%CI: 45%–52%).
Multivariate analysis of outcomes
Table 3 summarizes the multivariate analysis adjusted for clinical factors for TRM and OS for the biomarkers. We validated the association of greater TRM for CRP above 10 mg/L (P=0.008) and albumin less than 3.5 g/L (P=0.01) but not for ferritin above 2500 ng/mL (P=0.27), seen in only a small fraction of patients (12%), ferritin levels exceeded 2500 ng/mL. Only hypoalbuminemia was associated with worse OS as CRP was of borderline significance (P=0.07). The optimally defined biomarker thresholds to predict TRM in unadjusted models specified CRP more than 3.67 mg/dL (P=0.01), linear ferritin (P=0.07) after log transformation and albumin less than 3.4 g/dL (P=0.004).
Table 3.
Multivariate analysis of transplant-related mortality and overall survival for biomarkers and Biomarker Panel Risk Score.*
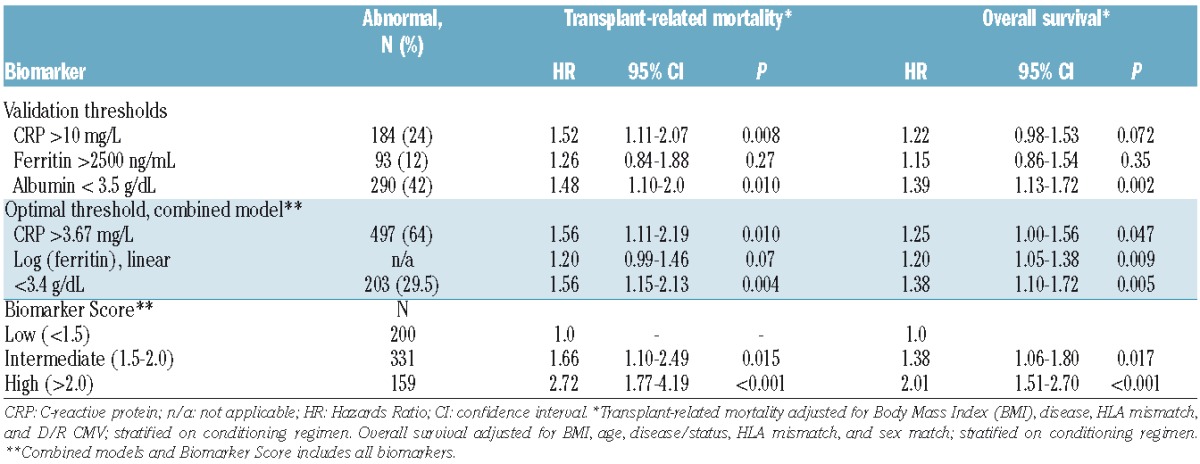
A combined model incorporated all three biomarkers at these optimal cut-off points and adjusted for significant clinical factors. Higher comorbidity (HCT-CI of 3 or more vs. HCT-CI of 0) was of borderline significance for OS (HR=1.27, 95%CI: 0.99–1.6, P=0.06) without a significant effect on TRM (HR=1.14, 95%CI: 0.79–1.6, P=0.48). Inclusion of HCT-CI did not materially influence the biomarkers effects in this model. The optimal biomarker thresholds for TRM also adversely influenced OS (Table 3). None of the biomarkers, either at pre-specified or optimal thresholds, were associated with relapse or incidence of acute GvHD, either grade II–IV or grade III–IV (data not shown).
Biomarker Risk Score
To improve clinical use for prognostication and create a simpler tool for future studies, we next generated a 3-level risk group for TRM based on a biomarker panel alone applying the following formula: Panel =0.44633*I(CRP>3.67) + 0.18330*ln(ferritin) + 0.44730*I(albumin<3.4), where I() is an indicator function which has the value 1 if the expression in parentheses is true, and 0 otherwise. The ROC characteristics of this 3-level panel on day 180 TRM of low (panel<=1.5, n=200), intermediate (1.5<panel<=2.0, n=331), and high (panel>2.0, n=159) biomarker risk panel group were similar to those employing a continuous biomarker panel (data not shown). The area under the ROC curve was 0.61, 0.65, and 0.68 for models using only the biomarker panel, only clinical characteristics, and both, respectively (Figure 1). The high-risk biomarker panel group exacted a similar or greater toll on TRM (HR=2.72, P<0.001) among known factors (Online Supplementary Table S1). Clinical characteristics and biomarkers thus may provide added utility for predicting TRM. The multivariate analyses of this biomarker panel are provided in Table 3 for TRM and OS. Figure 2 depicts TRM (Figure 2A) and overall survival (Figure 2B) by each group showing significantly different survival when grouped according to biomarker alone. Clinically, 1-year survival was 74%, 67% and 56% by low, intermediate and high biomarker scores, respectively. Figure 3 shows the significant association of this biomarker panel on OS (Figure 3A and B) and TRM (Figure 3C and D) for both myeloablative and reduced intensity/nonmyeloablative regimens.
Figure 1.
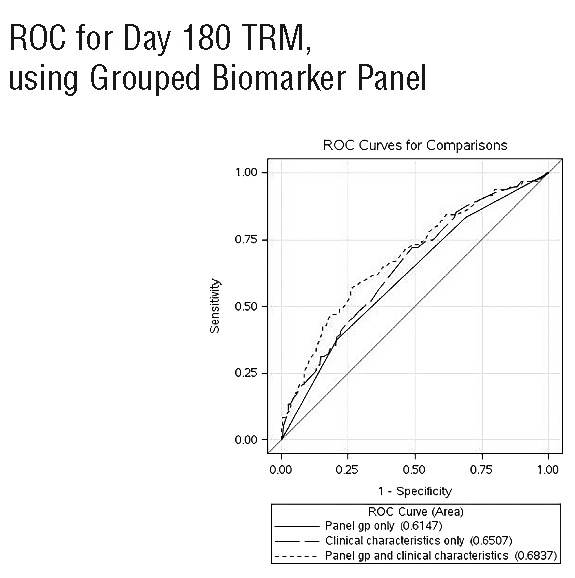
Receiver operating characteristic (ROC) curves for day 180 transplant-related mortality for three groups: biomarker panel group alone (panel gp only), clinical characteristics alone, or both [panel group (gp) and clinical characteristics].
Figure 2.
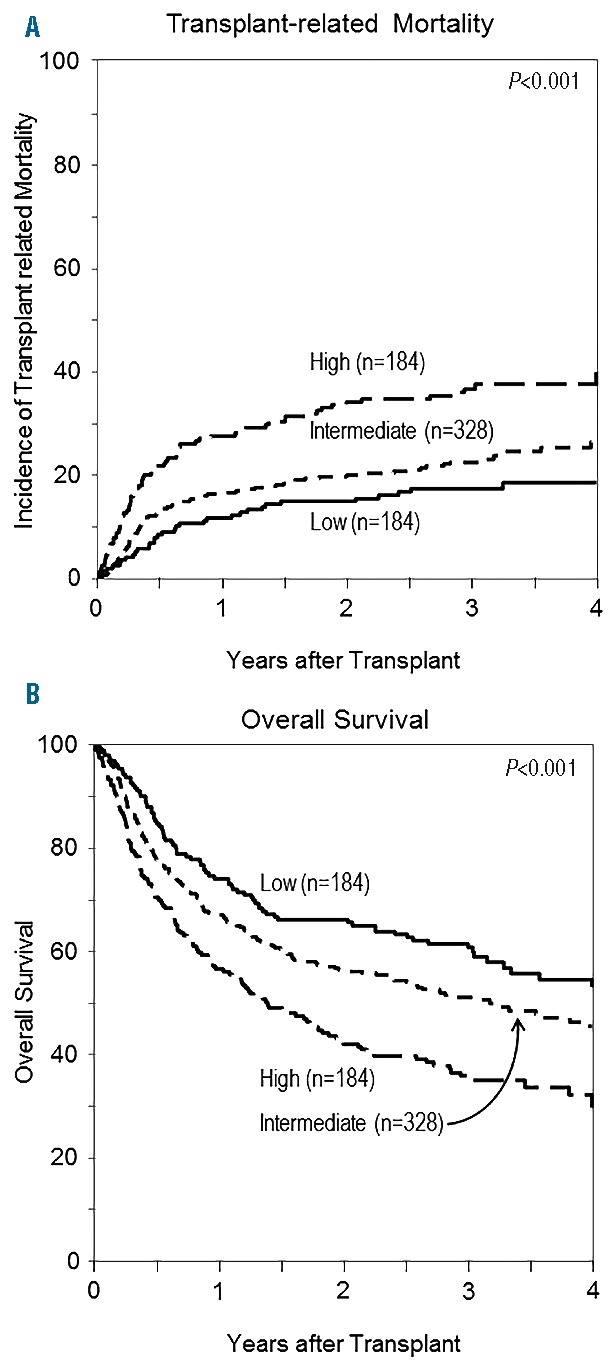
Outcomes after unrelated donor allogeneic transplant in each biomarker risk score group. (A) Transplant related mortality for low, intermediate and high biomarker risk panel group. (B) Overall survival for low, intermediate and high biomarker risk panel group.
Figure 3.
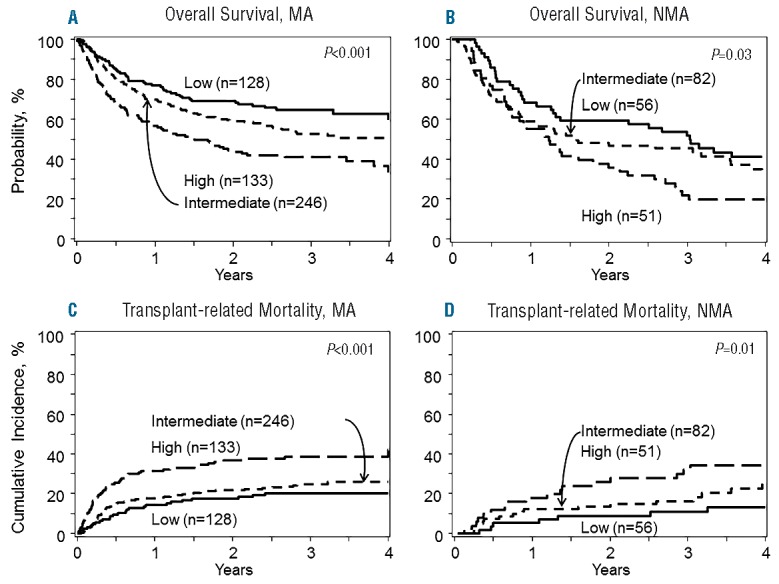
Overall survival for low, intermediate and high biomarker risk panel groups in myeloablative (MA) conditioning cohort (A) and reduced intensity/non-myeloablative regimen cohorts (NMA) (B). Transplant-related mortality for low, intermediate and high biomarker risk panel groups in MA conditioning cohort (C) and reduced intensity/NMA regimen cohorts (D).
Finally, we summarized causes of death for each biomarker category (Online Supplementary Table S2). Most causes of non-relapse death appeared relatively similar including GvHD, except perhaps a greater fraction of “organ failure” for high biomarker panel risk patients.
Correlation of biomarkers by automated methods and ELISA
We performed a pairwise analysis of 80 samples for CRP and 73 samples for ferritin unrelated to these study samples by ELISA and automated chemistry. The R squared for CRP and ferritin were both 95% and Online Supplementary Figures S1 and S2 depict scatterplots for each. CRP by ELISA of 10 mg/L (protocol-defined) and 3.67 mg/L (optimal threshold) corresponding to a calculated chemistry value of 8.45 mg/L and 3.54 mg/L, respectively. Applying the corresponding chemistry values for ELISA-derived CRP and ferritin would give a slightly modified biomarker risk panel as follows: Panel =0.44633*I(CRP_Chemistry>3.54) + 0.07184+0.19646 *ln(ferritin_Chemistry) + 0.44730*I(albumin<3.4), where I() is an indicator function which has the value 1 if the expression in parentheses is true, and 0 otherwise. CRP converts to more than 3.0 g/dL assuming the values are reported as an integer in mg/L. Thus, for future studies or clinical use for biomarkers analyzed by routine automated chemistry methods, this formula may be preferred.
Discussion
We report the first validation study regarding the impact of simple, clinically available, serum biomarkers of CRP, ferritin and albumin obtained prior to allogeneic transplant conditioning transplant on subsequent outcomes. We confirmed that the protocol-specified thresholds of CRP more than 10 mg/L and albumin less than 3.5 g/dL each predicted for approximately 50% greater risks of TRM independent of clinical factors. Hypoalbuminemia showed a stronger association with inferior survival (P=0.002) whereas the significance for CRP more than 10 mg/L was borderline (P=0.072). Ferritin in excess of 2500 ng/mL did not heighten risks of TRM or impair survival. None of the biomarkers significantly affected relapse or acute GvHD suggesting abnormal biomarkers represent a non-specific vulnerability to transplant toxicity.
Variability of biomarker thresholds and lack of validation have hampered adoption of biomarkers as prognostic tools in practice. For validation, we initially specified thresholds based on the published literature. The lack of an association of ferritin and outcome contradicts prior reports and underscores the critical importance of confirmatory findings. Ferritin thresholds in prior reports covered a range of 500 ng/mL to above 2600 ng/mL;14,19–21 we elected to approximate the inflection point originally reported by Armand et al. in similar patients. In the study by Vaughn et al. and a meta-analysis, ferritin of 1000 ng/mL best stratified for outcome.22,23 Still, even at a threshold of 1000 ng/mL, we found no association with OS (HR=1.02, P=0.92), arguing against the prognostic value of a discrete ferritin cut-off point in myeloid malignancy patients undergoing allogeneic HCT. Prior studies likely have been biased as testing may have been performed as clinically indicated, enriching for patients at risk of iron overload and/or complications. For example, in the original Armand report, ferritin was available in 64% and a recent study by Vaughn et al. revealed serum ferritin only evaluable in 50%.23 Furthermore, most studies select a cutoff point best fitting the available data which also tends to overestimate effects.
We next explored optimal biomarker thresholds and established CRP more than 3.67 mg/L, linear ferritin after log transformation and albumin less than 3.4 g/dL best predicted for TRM. These optimal biomarker thresholds for TRM generally held for an adverse effect on overall survival. Patients with high-risk biomarker scores fared worse after myeloablative or reduced intensity/non-myeloablative regimens, although the effects may have been more pronounced after myeloablative rgimens.
The HCT-CI is perhaps the best established factor7 predicting transplant-related mortality, and the report by Vaughn et al. showing ferritin, platelet count and albumin augment HCT-CI reinforces our findings.23 Although the HCT-CI was only of borderline significance for OS, this was attributable to the less robust sample size as the hazard ratio of 1.27 for higher comorbidity mirrored recent registry studies with larger samples.24 Additional tools to estimate TRM, such as Geriatric Assessment (GA), likely further refine prognostication in older adults but CRP and albumin retain prognostic significance when considering GA.10,25 Biomarkers will likely supplement rather than replace the HCT-CI. Therefore, a wealth of date now support biomarkers as independently prognostic for TRM and survival in the allogeneic HCT setting independent of all clinical factors studied.
A biomarker-based panel for risk stratification would be a clinically valuable tool and thus confirmatory studies are critically important. Objective and accurate estimates of low rates of early TRM would reassure both patients and providers in considering allogeneic HCT especially in older patients where transplant rates are very low.26 Conversely, patients at high risk of TRM should proceed with caution, contemplate avoiding myeloablative regimens, pursue more thorough evaluation by Geriatric Assessment, or escalate post-transplant monitoring. The ready clinical availability of these laboratory tests position biomarkers well to facilitate comparison of patient populations in prospective studies or even retrospectively when data and/or samples are available.
This study has several limitations. Although the biomarker panel demonstrated a profound effect on TRM beyond all clinical factors (HR=2.7), this must be validated, especially with different diseases and conditioning regimens. We opted to include ferritin in our final biomarker panel even though there was only a borderline association with TRM (P=0.07) as the association with survival was strong (P=0.009). Future studies should examine the utility of measuring biomarkers earlier, such as at the time of transplant referral. The analysis and cut-off points utilized ELISA-generated values from cryopreserved samples. As data comparing these methods were limited, we initiated a sub-study using entirely different samples and found a very strong correlation of ELISA and automated chemistry, as suggested in prior studies.27,28 As centers begin to perform and use these biomarkers routinely in the clinical laboratory, these data provide reassurance that CRP and ferritin likely approximate the values in this report.
The biological drivers for abnormal biomarkers including inflammation, iron burden, nutritional status, infection and genetic polymorphisms require further exploration and are critically important in moving beyond patient selection and toward mitigating adverse outcomes in those at heightened risk. Inflammation may be a central theme, yet the modest correlations of biomarkers to each other and prognostically independent effects of each biomarker support the role of non-inflammatory pathways as well. Even then, inflammation can be influenced by divergent pathways of infection, hepatic sources, the primary disease, or genetic origins. The notion of high ferritin as merely a measure of high iron stores appears too simplistic, as high levels of iron stores evaluated according to liver iron content do not solely contribute to adverse outcomes.22,29 Albumin crudely estimates nutrition and more broadly reflects the equilibrium between protein synthesis and catabolism. Poor nutrition itself can result from a multitude of factors and organ dysfunctions. Finally, low albumin increases bioavailability of drugs and could increase toxicity. Future studies elucidating the pathophysiology of biomarkers before transplant may guide biomarker-driven interventional studies to ameliorate the increased risks of TRM. For example, statins have been tested to dampen inflammation in those with elevated CRP.30
In summary, pre-transplant CRP and albumin independently predict for greater TRM but not high ferritin. The association of a new biomarker risk score based on optimal thresholds for ferritin, CRP and albumin on higher risks of TRM and inferior survival should be validated in independent populations. Future research should investigate the biology underlying biomarker abnormalities to design studies to mitigate the high risks of TRM.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/11/1426
Funding
The work was partially supported from CTSA Grant UL1 RR024999 and the National Center for Advancing Translational Sciences of the National Institutes of Health UL1 TR000430 (AA)
CIBMTR Support List
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Be the Match Foundation; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Mesoblast; *Millennium: The Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; Oxford Immunotec; Perkin Elmer, Inc.; Pharmacyclics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Telomere Diagnostics, Inc.; TerumoBCT; Therakos, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Corporate Members
References
- 1.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made¿ J Clin Oncol. 2011;29(7):805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong R, Giralt SA, Martin T, et al. Reduced-intensity conditioning for unrelated donor hematopoietic stem cell transplantation as treatment for myeloid malignancies in patients older than 55 years. Blood. 2003;102(8):3052–3059. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110(13):4576–4583. [DOI] [PubMed] [Google Scholar]
- 4.Pulsipher MA, Boucher KM, Wall D, et al. Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood. 2009;114(7):1429–1436. [DOI] [PubMed] [Google Scholar]
- 5.Gratwohl A, Hermans J, Goldman JM, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998; 352(9134):1087–1092. [DOI] [PubMed] [Google Scholar]
- 6.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306(17):1874–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artz AS, Pollyea DA, Kocherginsky M, et al. Performance status and comorbidity predict transplant-related mortality after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12(9):954–964. [DOI] [PubMed] [Google Scholar]
- 9.Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32(29):3249–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muffly LS, Kocherginsky M, Stock W, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99(8):1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlu J, Kew AK, Taylor-Roberts B, et al. Optimizing patient selection for myeloablative allogeneic hematopoietic cell transplantation in chronic myeloid leukemia in chronic phase. Blood. 2010;115(20):4018–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Artz AS, Wickrema A, Dinner S, et al. Pretreatment C-reactive protein is a predictor for outcomes after reduced-intensity allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(11):1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konijn AM, Hershko C. Ferritin synthesis in inflammation. I. Pathogenesis of impaired iron release. Br J Haematol. 1977;37(1):7–16. [PubMed] [Google Scholar]
- 14.Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109(10):4586–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong R, Shahjahan M, Wang X, et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11(2):108–114. [DOI] [PubMed] [Google Scholar]
- 16.Reuben DB, Cheh AI, Harris TB, et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50(4):638–644. [DOI] [PubMed] [Google Scholar]
- 17.Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC, Yap SH. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest. 1987;79(6):1635–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corti MC, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA. 1994;272(13):1036–1042. [PubMed] [Google Scholar]
- 19.Sucak GT, Yegin ZA, Ozkurt ZN, Aki SZ, Yagci M. Iron overload: predictor of adverse outcome in hematopoietic stem cell transplantation. Transplant Proc. 42(5):1841–1848. [DOI] [PubMed] [Google Scholar]
- 20.Maradei SC, Maiolino A, de Azevedo AM, Colares M, Bouzas LF, Nucci M. Serum ferritin as risk factor for sinusoidal obstruction syndrome of the liver in patients undergoing hematopoietic stem cell transplantation. Blood. 2009;114(6):1270–1275. [DOI] [PubMed] [Google Scholar]
- 21.Lim ZY, Fiaccadori V, Gandhi S, et al. Impact of pre-transplant serum ferritin on outcomes of patients with myelodysplastic syndromes or secondary acute myeloid leukaemia receiving reduced intensity conditioning allogeneic haematopoietic stem cell transplantation. Leuk Res. 2010; 34(6):723–727. [DOI] [PubMed] [Google Scholar]
- 22.Armand P, Kim HT, Virtanen JM, et al. Iron overload in allogeneic hematopoietic cell transplantation outcome: a meta-analysis. Biol Blood Marrow Transplant. 2014;20(8):1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaughn JE, Storer B, Armand P, et al. Design and Validation of an Augmented Hematopoietic Cell Transplantation-Comorbidity Index Comprising Pretransplant Ferritin, Albumin, and Platelet Count for Prediction of Outcomes after Allogeneic Transplantation. Biol Blood Marrow Transplant. 2015;21(8):1418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muffly LS, Boulukos M, Swanson K, et al. Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol Blood Marrow Transplant. 2013;19(3):429–434. [DOI] [PubMed] [Google Scholar]
- 26.Yao S, Hahn T, Zhang Y, et al. Unrelated donor allogeneic hematopoietic cell transplantation is underused as a curative therapy in eligible patients from the United States. Biol Blood Marrow Transplant. 2013;19(10):1459–1464. [DOI] [PubMed] [Google Scholar]
- 27.Simo JM, Joven J, Cliville X, Sans T. Automated latex agglutination immunoassay of serum ferritin with a centrifugal analyzer. Clin Chem. 1994;40(4):625–629. [PubMed] [Google Scholar]
- 28.Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem. 1999;45(12):2136–2141. [PubMed] [Google Scholar]
- 29.Trottier BJ, Burns LJ, DeFor TE, Cooley S, Majhail NS. Association of iron overload with allogeneic hematopoietic cell transplantation outcomes: a prospective cohort study using R2-MRI-measured liver iron content. Blood. 2013;122(9):1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286(1):64–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


