Abstract
We compared two dosing schedules for subcutaneous injections of a low-dose humanized anti-CD20 antibody, veltuzumab, in immune thrombocytopenia. Fifty adults with primary immune thrombocytopenia, in whom one or more lines of standard therapy had failed and who had a platelet count <30×109/L but no major bleeding, initially received escalating 80, 160, or 320 mg doses of subcutaneous veltuzumab administered twice, 2 weeks apart; the last group received once-weekly doses of 320 mg for 4 weeks. In all dose groups, injection reactions were transient and mild to moderate; there were no other safety issues. Forty-seven response-evaluable patients had 23 (49%) objective responses (platelet counts ≥30×109/L and ≥2 × baseline) including 15 (32%) complete responses (platelets ≥100×109/L). Responses (including complete responses) and bleeding reduction occurred in all dose groups and were not dose-dependent. In contrast, response duration increased progressively with total dose, reaching a median of 2.7 years with the four once-weekly 320-mg doses. Among nine responders retreated at relapse, three at higher dose levels responded again, including one patient who was retreated four times. In all dose groups, B-cell depletion occurred after the first dose until recovery starting 12 to 16 weeks after treatment. Veltuzumab serum levels increased with dose group according to total dose administered, but terminal half-life and clearance were comparable. Human anti-veltuzumab antibody titers developed without apparent dose dependence in nine patients, of whom six responded including five who had complete responses. Subcutaneous veltuzumab was convenient, well-tolerated, and active, without causing significant safety concerns. Platelet responses and bleeding reduction occurred in all dose groups, and response durability appeared to improve with higher doses. Clinicaltrials.gov identifier: NCT00547066
Introduction
Veltuzumab is a second-generation, humanized, anti-CD20 antibody with structural and functional differences from rituximab.1 Clinical studies in B-cell malignancies found that relatively low doses of this antibody were effective when administered by intravenous infusion2 or subcutaneous (SC) injection.3,4 Case reports in systemic lupus erythematosus5 and pemphigus vulgaris6 indicated that veltuzumab might also be effective in autoimmune disease. We therefore undertook a clinical study in immune thrombocytopenia (ITP), previously reporting that low doses of intravenous or SC veltuzumab could improve platelet counts when administered twice 2 weeks apart,7 a dosing schedule used with rituximab in rheumatoid arthritis and other autoimmune diseases. With veltuzumab formulated at 80 mg/mL, patients received their SC injections at one of three planned increasing dose levels of 80, 160 or 320 mg (delivered by 1 mL, 2 mL, or two 2-mL injections given separately several minutes apart, respectively). No dose-limiting toxicity was encountered during the initial dose escalation and this portion of the study was completed by adding several other patients to provide additional experience. We subsequently amended this study to also evaluate SC veltuzumab administered once-weekly for 4 consecutive weeks, a dosing schedule often used with rituximab in hematologic malignancies and in ITP as well, and for this part of the study only the highest dose level of 320 mg was evaluated. With platelet follow-up data now mature, we report the final results for all ITP patients treated with SC veltuzumab, comparing platelet count improvements, bleeding reduction, and other metrics across both dosing schedules.
Methods
This was an open-label, multi-center, phase I study of patients with relapsed ITP who received either 80, 160, or 320 mg doses of SC veltuzumab administered twice, 2 weeks apart, or else once-weekly 320 mg doses for 4 consecutive weeks. Eligible patients were ≥18 years old with primary ITP according to American Society of Hematology guidelines8 in whom one or more standard ITP therapies had failed and who had platelet counts <30×109/L on two occasions at least 1 week apart. Patients could be recruited irrespectively of whether they had or had not undergone splenectomy and in all stages of their disease (newly-diagnosed, <3 months; persistent, 3–12 months; or chronic, >1 year).9 Using the ITP Bleeding Scale (IBLS) with bleeding at any anatomic site graded as 0 (none), 1 (mild), or marked (2),10 patients with marked bleeding were excluded, as were patients with other significant cytopenias (patients with Evans syndrome, etc.). Patients had to be off ITP medications, except for prednisone ≤20 mg/day and danazol, which were allowed if the patients were continuing on stable doses. Patients who had previously been treated with rituximab could enter the trial only if they had achieved at least a partial response for ≥6 months and were either 1 year beyond rituximab therapy or had evidence of B-cell recovery.
The treatment response to SC veltuzumab was based upon a patient’s best platelet count in the absence of major bleeding or rescue interventions. An objective response was defined as platelet counts ≥30×109/L twice, 1 week apart, and at least doubled from baseline. Objective responses were categorized as a complete response if the platelet counts were ≥100×109/L twice, 1 week apart, and otherwise as a partial response.9 Time to response was measured from the first injection to onset of the objective response. Responders were followed up to 5 years, with time to relapse measured from first injection to first occurrence of platelet counts <30×109/L on at least two separate occasions at least 1 day apart. Adverse events and safety with regards to laboratory findings were classified by NCI CTC v3 toxicity grades and bleeding by IBLS grades. Blood B-cell levels (CD19) were used to determine the pharmacodynamics of the drug. For pharmacokinetics and immunogenicity, enzyme-linked immunosorbent assays performed by the sponsor measured veltuzumab serum levels (lower level of quantitation, 0.5 μg/mL) and titers of any human anti-veltuzumab antibody (HAHA) (lower level of quantitation, 50 ng/mL), respectively. Pharmacokinetic parameters following the last injection were determined by WinNonLin 2.1 (Pharsight Corporation, Mountain View, CA, USA) using a non-compartmental model. Time to relapse was analyzed by Kaplan-Meier methods. Other study results are summarized using descriptive statistics. At each participating institution, the governing ethics committee approved the study, and written informed consent was obtained from all patients.
Results
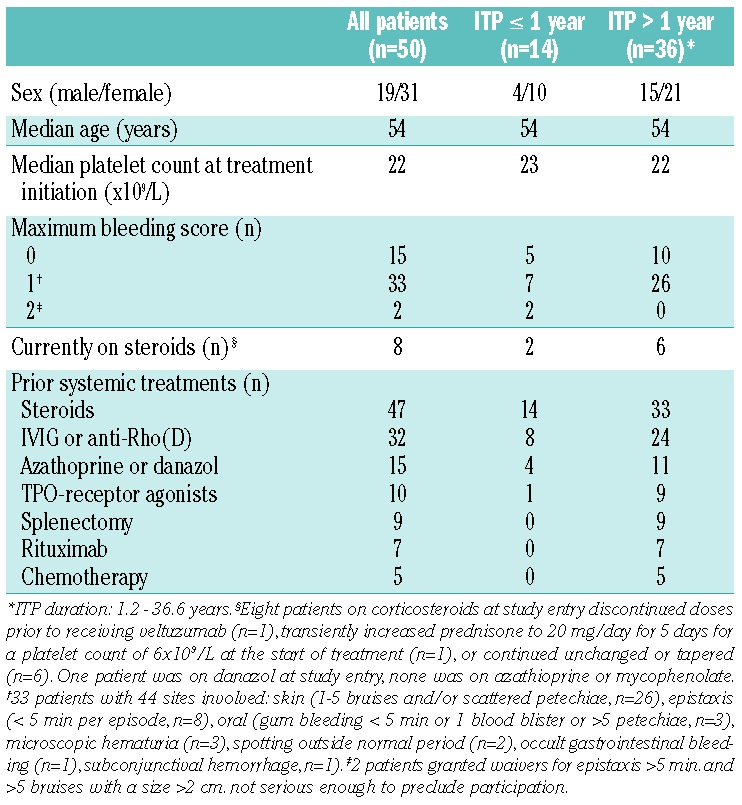
Fifty patients received veltuzumab administered by SC injection. Only two patients had newly-diagnosed ITP (<3 months), while 12 had persistent ITP (3–12 months). Since these 14 patients had previously been treated with only steroids and/or immunoglobulins, they were combined into one group (ITP ≤1 year) for the purposes of comparison with the remaining 36 patients who had chronic disease (ITP >1 year) and had received additional therapies. The demographics and baseline characteristics of the patients are summarized in Table 1. All 50 patients completed their scheduled treatment, receiving veltuzumab twice, 2 weeks apart, at a dose of 80 (n=9), 160 (n=10), or 320 mg (n=15), or once-weekly 320 mg doses for 4 consecutive weeks (n=16). At the investigators’ discretion, 44% of patients (22/50) received acetaminophen alone or with diphenhydramine prior to at least one injection, and two patients also received benzodiazepines for anxiety, but no steroids or other premedication was given.
Table 1.
Demographics and baseline characteristics of the patients.
Platelet response
Three patients, while receiving SC injections, initiated high-dose prednisone, cyclosporine, or romiplostim because of their extremely low platelet counts which continued to decrease at the start of treatment. This early use of rescue medications resulted in prolonged platelet improvements but precluded unequivocal assessment of the contribution of veltuzumab to the response. The other 47 patients could be evaluated for platelet response to veltuzumab. These included 23 responders who did not receive any rescue medications or other treatment interventions until relapse (n=18) or who received limited immunoglobulins (n=2), a brief course of steroids (n=2), or platelet transfusions (n=1) during treatment for low platelet levels with improvement continuing substantially beyond any expected transient response to these agents and without further interventions. The remaining 24 patients either had no platelet response after completing the 12-week, post-treatment evaluation period (n=9) or initiated other treatments after having no improvement in platelet counts 1 – 8 weeks following veltuzumab treatment (n=15).
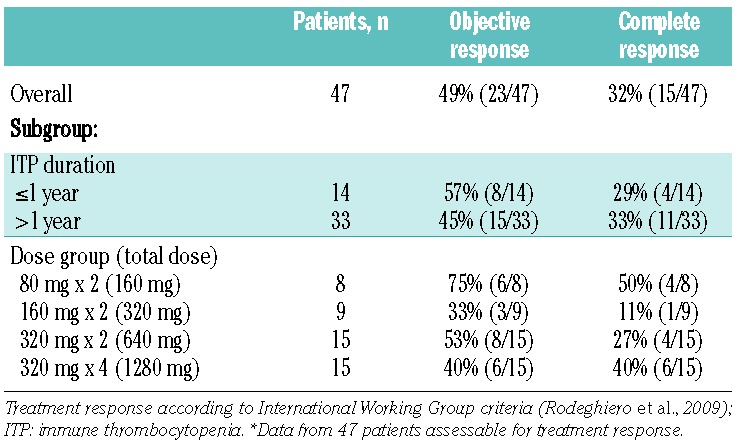
Among the 47 patients whose response to veltuzumab could be assessed, there were 49% (23/47) objective responses, including 32% (15/47) complete responses. Response rates (including complete responses) were generally comparable regardless of whether patients had ITP for ≤1 year or longer, and responses were variable across the four dose groups as expected with small numbers of patients with no clear evidence of a dose-response relationship (Table 2). The median time from first veltuzumab dose to response onset was 26 days (range, 7 – 264), with a median time of 38 days (7 – 189) to first occurrence of platelet counts ≥ 100×109/L for patients with complete responses. There was no clear evidence that response onset varied with dose group (data not shown).
Table 2.
Response rates.*
Of the 23 responders, one patient currently remains in long-term follow-up with a response still ongoing 2.6 years after treatment initiation, 17 have relapsed, and the other five are off study with continuing responses as of last evaluation (lost to follow up after 0.8, 2.7, and 3.2 years; withdrawn after 1.6 years for scheduled surgery; concluded the 5-year study follow-up period). The median time from treatment initiation to relapse for all 23 responders was 1.3 years. As shown in Figure 1, the duration of a response increased with higher veltuzumab doses, with the median time from treatment initiation to relapse reaching 2.7 years in the patients treated with four weekly 320-mg doses. Responders who achieved complete responses or had ITP ≤1 year also showed trends towards having more durable responses (Online Supplementary Figure S1).
Figure 1.
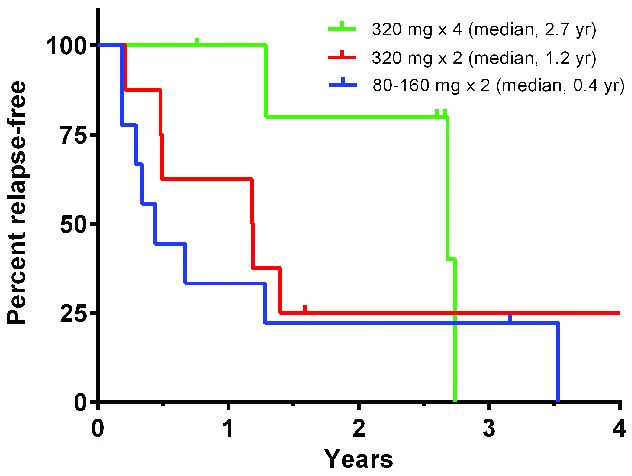
Kaplan-Meier estimates of increasing durability of responses with higher veltzumab dose levels. Time to relapse in each responder was measured from the initial treatment dose to first occurrence of a platelet count <30 × 109/L, but was censored at the time of last evaluation (ticks) if discontinued from the study prior to relapse. Results show percentages of responders continuing relapse-free after receiving either 80 (n=6) or 160 (n=3) mg doses twice 2 weeks apart [pooled for clarity], 320 mg (n=8) doses twice weekly 2 weeks apart, or 320 mg doses (n=6) once-weekly for 4 consecutive weeks (pairwise log-rank tests: 320 mg × 4 vs. 320 mg × 2, P= 0.22; 320 mg × 4 vs. 80–160 mg, P=0.17; both 320 mg doses vs. 80–160 mg, P=0.16).
Nine responders were retreated at the investigators’ discretion after relapsing. Five patients did not respond when retreated again twice, 2 weeks apart, with the same or higher dose. One patient retreated with four weekly 320-mg doses required rescue medications during retreatment which precluded response assessment. The three other patients responded to retreatment, as follows. One patient who had an initial partial response lasting 3 months responded with a similar duration when retreated with two 320-mg doses 2 weeks apart. Another patient who had an initial complete response lasting 6 months was retreated three times with two 320-mg doses 2 weeks apart and a fourth time with four weekly 320-mg doses, each time responding with a complete response of similar duration. The remaining patient who had an initial complete response lasting 2.7 years was retreated with four weekly 320-mg doses and achieved a response which is currently ongoing 10 months later.
Bleeding reduction
At treatment initiation, 68% (34/50) of all patients had one or more sites of bleeding; this percentage progressively decreased to 29% (12/42) of patients assessed at the end of the 12-week, post-treatment evaluation period. Bleeding primarily involved the skin, oral cavity, and epistaxis, with few occurrences at other anatomic sites and no cases of intracranial, intraocular, or pulmonary bleeding. Most bleeding was minor (IBLS grade 1) with marked bleeding (IBLS grade 2) limited to ~10% of patients or less at any evaluation. Bleeding reduction following treatment occurred in all dose groups without evidence of a dose-response relationship and was primarily limited to patients achieving objective responses (Figure 2).
Figure 2.
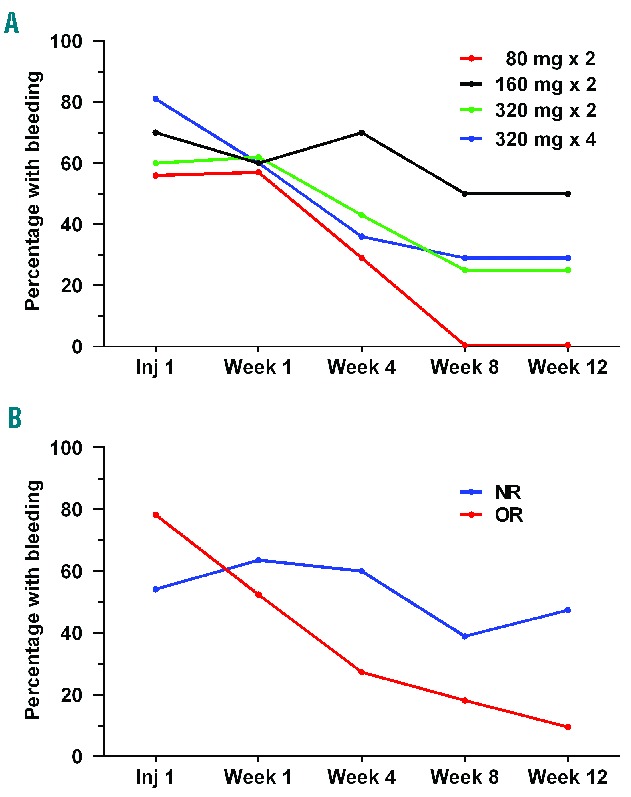
Percentage of patients with any bleeding at first injection and then at 1, 4, 8 and 12 weeks following treatment with SC veltuzumab. The ITP Bleeding Scale was used to evaluate any bleeding at any site. (A) Results by dose group for patients receiving 80 mg (n=9), 160 mg (n=10), or 320 mg (n=15) doses twice 2 weeks apart or 320 mg doses (n=16) once-weekly for 4 consecutive weeks. (B) Results by platelet response for patients who achieved an objective response (OR, n=23) or were non-responders (NR, n=24). In patients with platelet responses continuing beyond 12 weeks, 84% (16/19) were free from bleeding at 24 weeks and 100% (16/16) at 48 weeks.
Immunological changes
The first dose of SC veltuzumab effectively depleted peripheral blood B cells in most patients, with median B-cell levels decreasing from 284 cells/μL before treatment to 4 cells/μL by the second dose. Only four patients (all treated twice, 2 weeks apart) did not achieve B-cell levels ≤20 cells/μL by 4 weeks after treatment; however, their B-cell counts decreased 74–94% from baseline and two patients achieved objective responses (both complete responses). B-cell depletion appeared comparable across the four dose groups but recovery towards baseline levels appeared slower among patients treated with the higher doses (Online Supplementary Figure S2). T cells and serum immunoglobulin levels evaluated 4 weeks after treatment showed no consistent pattern of change from baseline, either for patients treated with two doses, 2 weeks apart, or those given four doses of 320 mg at weekly intervals (data not shown).
Pharmacokinetics
Veltuzumab serum levels were measured on treatment days and then 1, 2, 3, 4, 8, and 12 weeks after the last dose, with values generally increasing by dose group and reaching a maximum value 1 week after the last treatment dose before subsequently slowly declining over this period (Online Supplementary Figure S3). Evaluation of post-treatment pharmacokinetic parameters showed that the peak value (Cmax) and area-under-the-curve (AUC) increased with dose group, as expected, while the terminal half-life and clearance from the blood differed little across the dose groups (Online Supplementary Table S1).
Safety
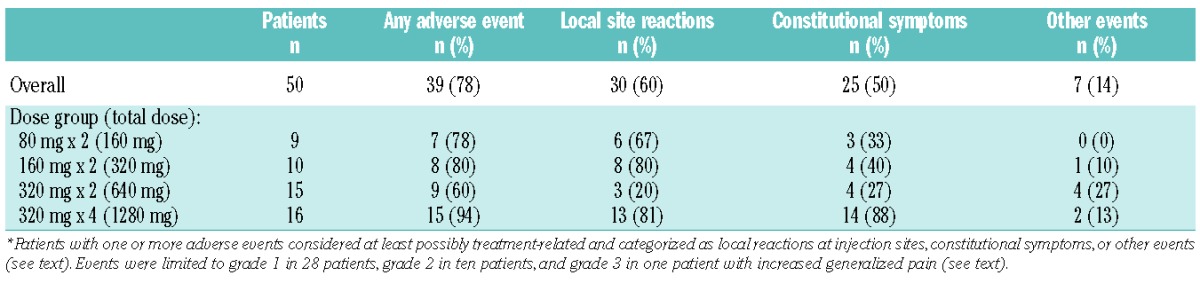
There was only one serious adverse event, grade 3 viral gastroenteritis, which occurred 6 months after treatment and was considered unrelated to the treatment. One patient had increased generalized pain 10 days after the first veltuzumab injection which was considered grade 3 and possibly treatment-related, although the patient had a history of chronic pain with herniated discs and multiple prior surgeries for pain relief. Otherwise, all treatment-related adverse events were grade 1–2 (mild-moderate) and occurred in 39 patients (28 with only grade 1 events), including 30 patients with local injection site reactions (pain, burning or soreness, bruising, rash, erythema), 26 patients with constitutional symptoms (body aches or generalized pain, chills, low-grade fever, headache, fatigue, nausea, rash), and seven patients with other events considered at least possibly related [sore throat, increased thirst and urination, fatigue, headache, edema, abdominal bloating, knee pain, and electrocardiogram abnormalities in one patient (see below)]. Most reactions resolved spontaneously or with acetaminophen on the day of injection, and no patient required steroids. Treatment-related adverse events did not appear to be dose-dependent for patients treated twice, 2 weeks apart, and increases in patients treated with four weekly doses were not unexpected since these patients received double the number of injections (Table 3). Other adverse events included minor infections (predominantly of the upper respiratory tract or sinusitis) in 14 patients, ≥1 minor bleeding events (conjunctival × 3; gingival × 2; mucosal purpura, hemoptysis, hematemesis, epistaxis) in seven patients, palpitations at second injection in two patients with a history of hypertension which resolved spontaneously, and brief episodes of tachycardia several weeks after treatment in two patients which were considered unrelated to veltuzumab. Electrocardiography was required in all patients at the time of the last injection; the only finding of clinical significance was asymptomatic atrial fibrillation of uncertain etiology which occurred in one patient with a cardiac history and resolved spontaneously without treatment or recurrence on subsequent examination. Other than platelet counts, routine hematology and serum chemistry tests showed no consistent pattern of change from baseline (data not shown), and there were no cases of serum sickness, hypogammaglobulinemia, delayed neutropenia, or other side effects that had been previously reported with rituximab.
Table 3.
Treatment-related adverse events.*
Immunogenicity
Eleven patients had elevated HAHA titers, which subsequently resolved or decreased in patients with available follow-up, without apparent clinical sequelae. Two patients previously treated with rituximab were already HAHA-positive at baseline (titers, 52 and 1006 ng/mL). Both achieved complete responses following two 80-mg doses of SC veltuzumab 2 weeks apart, but one patient who was retreated after relapse did not respond to a second course of treatment. Nine other patients were treated either twice 2 weeks apart with 80 (n=2), 160 (n=3), or 320 mg (n=1) doses or with four weekly 320-mg doses (n=3) and developed elevated titers after receiving veltuzumab (HAHA, 18%). These included eight patients with HAHA detectable after initial treatment (peak titers, 61 – 2190 ng/mL) and one patient who only had HAHA after being retreated (peak titer, 152 ng/mL). Of the nine patients, six achieved objective responses following initial treatment, including five with complete responses, but none of three patients retreated after relapse responded to a second course of treatment.
Discussion
Since the B-cell antigen burden in patients with ITP is widely believed to be less than that seen in patients with B-cell malignancies, we hypothesized that low doses of veltuzumab would likely be effective in this disease, thus allowing for anti-CD20 therapy by more convenient subcutaneous injections. Following a dosing schedule used with rituximab in other autoimmune diseases such as rheumatoid arthritis, we previously reported that in ITP, two doses of low-dose veltuzumab given either by intravenous infusion or subcutaneous injection 2 weeks apart had activity.7 With this dosing schedule, there was activity at the lowest dose level of 80 mg veltuzumab × 2. Furthermore there was no clear dose dependence or evidence of greater response at the highest level explored with 320-mg doses. We subsequently treated an additional 16 patients with 320-mg doses of SC veltuzumab given once-weekly for 4 consecutive weeks to determine whether the increased dosing frequency, and especially greater cumulative dose delivered with this dosing schedule, which is often used in oncology, would lead to increased activity or any safety issues when used with SC veltuzumab in ITP. With long-term responses and other study data now mature, this report provides the final results from all SC dose groups.
Compared to two doses of 80–320 mg SC veltuzumab given 2 weeks apart (total dose, 160–640 mg), there were no increased safety concerns with four weekly 320-mg SC doses (total dose, 1280 mg). The only treatment-related adverse events with SC veltuzumab administered in ITP with either dosing regimen were limited to mild-moderate (predominately grade 1) transient injection reactions, either local reactions at the injection site or constitutional symptoms, most of which resolved spontaneously or with acetaminophen, but did not require steroids. Routine laboratory tests remained unremarkable and no other safety concerns have emerged with SC veltuzumab.
Eleven patients had elevated HAHA titers which subsequently resolved or decreased without apparent clinical sequelae. These patients including two who had been previously treated with rituximab and had measurable titers at study entry. Both patients previously treated with rituximab who were already HAHA-positive at study entry achieved complete responses when treated with SC veltuzumab, consistent with case reports of veltuzumab being effective in patients who had become resistant to rituximab.5,6 The other nine patients developed elevated titers after receiving veltuzumab (8 after initial treatment, 1 after retreatment) without any obvious pattern regarding dose group or dosing regimen (HAHA, 18%). While immunogenicity rates with rituximab are not available in ITP, this frequency is comparable to rates reported with rituximab in rheumatoid arthritis (11%)11 and systemic lupus erythematosus (26%).12 Importantly, six of the nine patients achieved objective responses to veltuzumab with initial treatment, including five who had complete responses, consistent with other studies in cancer patients correlating immunogenicity with anti-B-cell antibodies to enhanced outcomes.13,14 However, of four patients with HAHA who received a second course of treatment with veltuzumab, none responded to retreatment, which would lower expectations for continued treatment in HAHA-positive patients.
Among evaluable patients, the objective response rate to initial treatment with SC veltuzumab was 49% (complete response rate, 32%) with a median time to relapse of 1.3 years from the first dose. Of nine responders retreated at relapse, three treated at higher levels again responded, including one patient who was retreated four times. This overall result is generally similar to what we reported earlier with intravenous or SC veltuzumab given twice 2 weeks apart.7 Although limited by small numbers, it still appears that complete responses are more durable than partial responses, in agreement with findings reported with rituximab,15 and that responses in adults with long-standing ITP are likely less durable as has also been reported for rituximab in at least one study.16 It is difficult to compare the overall response results with veltuzumab with those reported for rituximab, given the wide range of the latter, which may be due to heterogeneity of the patients studied, ITP severity and duration, and treatment patterns, as well as publication bias, and differing response criteria,17 including recent rituximab studies using treatment failure endpoints other than objective response rates alone.18,19 We evaluated bleeding as an endpoint in this study, finding that treatment with SC veltuzumab was often effective in reducing the mostly minor cases of bleeding that occurred in this population, particularly in patients achieving objective responses and therefore most likely an expected consequence of improved platelet counts.
SC veltuzumab was pharmacodynamically active with peripheral blood B cells depleted rapidly in most patients even after one dose of 80 mg veltuzumab, and meaningful platelet responses (including complete responses) were achieved already at the lowest dose group of two 80 mg SC doses given 2 weeks apart. Veltuzumab serum levels increased with each dose group according to the total dose administered, but the terminal half-life and clearance of veltuzumab from the blood after treatment appeared generally comparable across all dose groups. While there was no clear evidence of a platelet response relationship among the four dose groups, the duration of the platelet responses progressively increased with greater total dose, achieving a median duration of 2.7 years in responders following 4 weekly 320-mg doses, including one patient with response still ongoing at last evaluation at 2.6 years. Furthermore, the platelet responses to retreatment occurred in patients treated both initially and at relapse with higher (320 mg) doses.
The small numbers of patients make it difficult to draw firm conclusions regarding the most appropriate SC veltuzumab dosing regimen for further consideration despite the possible longer duration of responses at the highest doses. Two other studies with SC veltuzumab also administered 80, 160 or 320 mg SC doses, again finding activity in all dose groups with increased serum levels and no obvious exposure-response relationship, but these were studies in B-cell malignancies and with too few patients to evaluate whether response duration improved with higher doses.3,4 One tentative explanation for the apparent lack of dose-dependence of responses might be that even 80-mg doses of SC veltuzumab result in B-cell depletion in peripheral blood irrespective of response. In this model, response would depend on whether B-cell clearance leads to platelet response. Further, even 80-mg doses of veltuzumab may already effectively saturate the mechanisms of action usually considered for immunotherapy (complement dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity, apoptosis) with little room for improvement at higher doses. It is also possible (at least in chronic lymphocytic leukemia) that increasing doses might be counterproductive by resulting in removal of CD20 from B-cell surfaces (trogocytosis)20 or by exhausting complement and cell-mediated effector systems needed for effective cytotoxicity.21
Even with rituximab, years after the drug’s initial approval, there is still no clear explanation for why some patients respond and others do not, or why increased doses do not improve response rates. In ITP, 100-mg doses of rituximab may be active,22 but other low doses have not been evaluated, and while different rituximab dosing regimens have been compared,23 the 375 or 750 mg/m2 doses are higher than the 80 – 320 mg doses of veltuzumab in this study. Furthermore for rituximab, the consensus of the limited studies in ITP is that the response rate at the lower doses (100 mg × 4) is the same as that at higher doses, but the duration of the responses is not as long, which is quite similar to the veltuzumab data reported here.
In summary, SC veltuzumab was convenient, well-tolerated and did not cause significant safety concerns, with platelet responses and bleeding reduction occurring in all dose groups. Based on a possible longer duration of responses with higher doses, higher SC doses may be warranted and a more concentrated formulation of veltuzumab has recently been developed for this purpose.
Supplementary Material
Acknowledgments
The authors thank Lucy Lee, PharmD, Kiril Gordeyev, BS, and Robert M. Sharkey, PhD, for their contributions to the pharmacokinetic and immunoassay analyses.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/11/1327
References
- 1.Goldenberg DM, Morschhauser F, Wegener WA. Veltuzumab (humanized anti-CD20 monoclonal antibody): characterization, current clinical results, and future prospects. Leuk Lymphoma. 2010;51(5):747–755. [DOI] [PubMed] [Google Scholar]
- 2.Morschhauser F, Leonard JP, Fayad L, et al. Humanized anti-CD20 antibody, veltuzumab, in refractory/recurrent non-Hodgkin’s lymphoma: phase I/II results. J Clin Oncol. 2009;27(20):3346–3353. [DOI] [PubMed] [Google Scholar]
- 3.Negrea GO, Elstrom R, Allen SL, et al. Subcutaneous injections of low-dose veltuzumab (humanized anti-CD20 antibody) are safe and active in patients with indolent non-Hodgkin’s lymphoma. Haematologica. 2011;96(4):567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalaycio ME, Negrea OG, Allen SL, et al. Subcutaneous injections of low doses of humanized anti-CD20 veltuzumab: a phase I study in chronic lymphocytic leukemia. Leuk Lymphoma. 2016;57(4):803–811. [DOI] [PubMed] [Google Scholar]
- 5.Tahir H, Bhatia A, Wegener WA, Isenberg DA. Humanised anti-CD20 monoclonal antibody in the treatment of severe resistant systemic lupus erythematosus in a patient with antibody against rituximab. Rheumatology. 2005;44(4):561–562. [DOI] [PubMed] [Google Scholar]
- 6.Ellebrecht CT, Choi EJ, Allman DM, et al. Subcutaneous veltuzumab, a humanized anti-CD20 antibody, in the treatment of refractory pemphigus vulgaris. JAMA Dermatol. 2014;150(12):1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liebman HA, Saleh MN, Bussel JB, et al. Low-dose anti-CD20 veltuzumab given intravenously or subcutaneously is active in relapsed immune thrombocytopenia: a phase I study. Br J Haematol. 2013;162(5):693–701. [DOI] [PubMed] [Google Scholar]
- 8.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. [DOI] [PubMed] [Google Scholar]
- 9.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions, and outcome in idiopathic thrombocytopenic purpura (ITP) of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. [DOI] [PubMed] [Google Scholar]
- 10.Page L, Psaila B, Provan D, et al. The immune thrombocytopenic purpura (ITP) bleeding score: assessment of bleeding in patients with ITP. Br J Haematol. 2007;138(2):245–248. [DOI] [PubMed] [Google Scholar]
- 11.van Vollenhoven RF, Emery P, Bingham CO, 3rd, et al. Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J Rheumatol. 2010;37(3):558–567. [DOI] [PubMed] [Google Scholar]
- 12.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62(1):222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miotti S, Negri DR, Valota O, et al. Level of anti-mouse-antibody response induced by bi-specific monoclonal antibody OC/TR in ovarian-carcinoma patients is associated with longer survival. Int J Cancer. 1999;84(1):62–68. [DOI] [PubMed] [Google Scholar]
- 14.Azinovic I, DeNardo GL, Lamborn KR, et al. Survival benefit associated with human anti-mouse antibody (HAMA) in patients with B-cell malignancies. Cancer Immunol Immunother. 2006;55(12):1451–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel VL, Mahévas M, Lee SY, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012; 119(25):5989–5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper N, Stasi R, Cunningham-Rundles S, et al. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;125(2):232–239. [DOI] [PubMed] [Google Scholar]
- 17.Arnold DM, Dentali F, Crowther MA, et al. Systematic review: efficacy and safety of rituximab for adults with idiopathic thrombocytopenic purpura. Ann Intern Med. 2007;46(1):25–33. [DOI] [PubMed] [Google Scholar]
- 18.Arnold DM, Heddle NM, Carruthers J, et al. A pilot randomized trial of adjuvant rituximab or placebo for nonsplenectomized patients with immune thrombocytopenia. Blood. 2012;119(6):1356–1362. [DOI] [PubMed] [Google Scholar]
- 19.Ghanima W, Khelif A, Waage A, et al. Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9978):1653–1661. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy AD, Beum PV, Solga MD, et al. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol. 2004;172(5):3280–3288. [DOI] [PubMed] [Google Scholar]
- 21.Beurskens FJ, Lindorfer MA, Farooqui M, et al. Exhaustion of cytotoxic effector systems may limit monoclonal antibody-based immunotherapy in cancer patients. J Immunol. 2012;188(7):3532–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaja F, Vianelli N, Volpetti S, et al. Low-dose rituximab in adult patients with primary immune thrombocytopenia. Eur J Haematol. 2010;85(4):329–334. [DOI] [PubMed] [Google Scholar]
- 23.Zwaginga JJ, van der Holt B, Te Boekhorst PA, et al. Multi-center randomized open label phase II trial on three rituximab dosing schemes in immune thrombocytopenia patients. Haematologica. 2015;100(3):e90–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


