Abstract
Improvement of graft-versus-host disease prophylaxis remains an important goal in allogeneic hematopoietic stem cell transplantation. Based on reports of possibly preferential properties of sirolimus, we compared the standard regimen of cyclosporine and methotrexate (n=106) with a combination of tacrolimus and sirolimus (n=103) as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation in a prospective, open, randomized trial. The hypothesis was that the tacrolimus/sirolimus regimen would lead to less acute graft-versus-host disease and reduced transplant-related mortality. There was no significant difference in the cumulative incidence of acute graft-versus-host disease of grades II–IV (41% vs. 51%; P=0.19) or grades III–IV (13% vs. 7%; P=0.09) between the groups. Time to neutrophil engraftment (18 days vs. 17 days; P=0.24) was similar, but time to platelet engraftment was longer in cyclosporine/methotrexate patients (14 vs. 12 days; P<0.01). No significant differences in incidence of oropharyngeal mucositis, time to full donor chimerism, or number of cytomegalovirus infections were seen between the two treatment arms, and transplant-related toxicities were equally distributed. Triglyceride (P=0.005) and cholesterol (P=0.009) levels were higher in tacrolimus/sirolimus patients. Transplant-related mortality (18% vs. 12%; P=0.40) and 5-year overall survival (72% vs. 71%; P=0.71) were similar. Five-year relapse-free survival in patients with malignant diagnoses was 65% in the cyclosporine/methotrexate group and 63% in the tacrolimus/sirolimus group (P=0.73). We conclude that tacrolimus/sirolimus remains a valid and safe alternative to cyclosporine/methotrexate as graft-versus-host disease prophylaxis after allogeneic hematopoietic stem cell transplantation, with comparable transplant-related outcomes. The trial was registered at clinicaltrials.gov identifier: 00993343.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an established treatment for a series of otherwise lethal hematopoietic disorders.1–3 Continuous refinements of transplant procedures, e.g. in the areas of human leukocyte antigen (HLA)-typing, expanded donor registries, conditioning regimens and supportive care, have steadily improved patient outcome after HSCT.4,5 Despite these advances, graft-versus-host disease (GvHD) remains a frequent and serious complication, affecting 30%–50% of matched sibling transplantations and 40%–70% of matched unrelated HSCT recipients. Both acute and chronic GvHD contribute significantly to morbidity, and are associated with high mortality.6,7 Ambitious efforts to address this issue have included substitution of immunosuppressive pharmaceuticals, graft engineering, and cellular therapies.8–10 In parallel, new immunosuppressive strategies have been evaluated in solid organ transplantation, making way for their introduction in HSCT.11,12 A regimen that has shown promising results in prevention of GvHD is the combination of sirolimus and tacrolimus.13,14 This regimen differs in action from the most commonly used GvHD prophylaxis in HSCT today, cyclosporine in combination with methotrexate.15,16 Sirolimus has been of interest to the HSCT field due to its promising mechanisms, which in theory offer potential advantages over the immunosuppressive agents currently in use. Its actions include immunosuppression through inhibition of T-cell and dendritic cell activity, while promoting regulatory T cells.17,18 Furthermore, sirolimus has antifibrotic, antineoplastic, antiviral, and antifungal activities, as well as synergistic action when combined with tacrolimus, but has limited toxicity in relation to calcineurin inhibitors.19–22 With this knowledge, we performed a prospective randomized trial to determine whether an immunosuppressive regimen of tacrolimus/sirolimus (Tac/Sir) is better than the established prophylaxis with cyclosporine/methotrexate (CsA/Mtx) in HSCT, hypothesizing that the combination of Tac/Sir would lead to less acute GvHD and reduced transplant-related mortality (TRM). To the best of our knowledge, the regimens of CsA/Mtx and Tac/Sir have not been compared head-to-head in any previous prospective randomized trial of GvHD prophylaxis.6,23
Methods
Study design
The study was designed to compare two GvHD prophylaxis regimens: CsA/Mtx versus Tac/Sir. It was preceded by a safety pilot study using Tac/Sir in 24 HSCT patients,24 and was performed as a prospective, randomized, open-label, phase III, multicenter trial. Study end points are presented in Figure 1. The aim was to include 200 patients, 100 in each arm; this would have sufficient power to detect a difference in incidence of acute GvHD of 24 percentage points between the treatment groups with a probability of P<0.05. The level of effect was set since the incidence of grade II–IV GvHD in our patient material at the time was 34% using CsA/Mtx prophylaxis, and the corresponding incidence in the published pilot study of Tac/Sir by Cutler et al. was 10%.13
Figure 1.
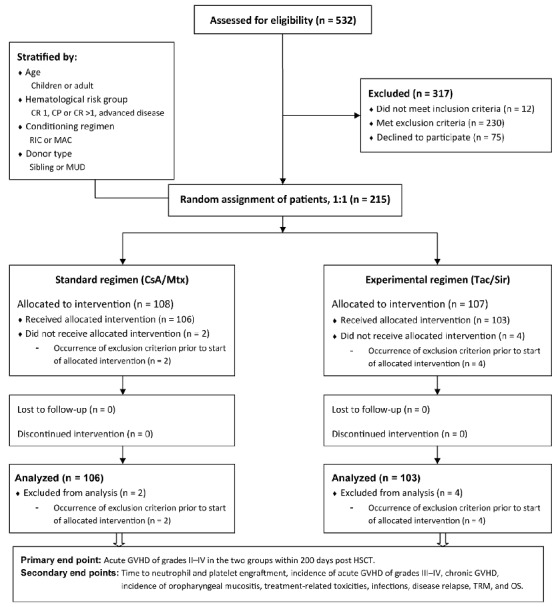
CONSORT diagram and end points. Flow of patients enrolled in the trial. CR: complete remission; CP: chronic phase; RIC: reduced intensity conditioning; MAC: myeloablative conditioning; MUD: matched unrelated donor; CsA: cyclosporine; Mtx: methotrexate; Tac: tacrolimus; Sir: sirolimus; GvHD: graft-versus-host disease; HSCT: allogeneic hematopoietic stem cell transplantation; TRM: transplant-related mortality; OS: overall survival.
Patients were enrolled at two participating centers (Stockholm and Turku) between September 2007 and January 2014. Randomization occurred 4–7 days before HSCT graft infusion. It was performed at a ratio of 1:1 with the use of random block sizes, stratified by age (children or adult), hematologic risk group (CR 1, CP or >CR 1, advanced disease; see below for explanation of abbreviations), conditioning regimen [reduced-intensity conditioning (RIC) or myeloablative conditioning (MAC)], and donor type [sibling or matched unrelated donor (MUD)]. Patients with non-malignant disease were included in the low hematologic risk group. No blinding was attempted after randomization.
The study protocol was approved by the Ethical Review Boards in Stockholm (DNR 2006/1430-31/3) and Helsinki (#541/2007, DNR 360/E5/07), and the Swedish and Finnish Medical Products Agencies (DNR 151:2007/38987 and KLNR 57/2008, respectively). The study was registered at clinicaltrials.gov identifer: 00993343 and the European Clinical Trials Database n. 2006-006577-25, and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient, or from parents/guardians of patients who were under 18 years of age, before the start of HSCT conditioning treatment. All the authors vouch for the accuracy of the data reported, and for adherence to the study protocol.
Patient eligibility and random assignment
Patients eligible for inclusion were 0.5–75 years of age and subject to treatment with HSCT. Inclusion and exclusion criteria are described in Table 1. Due to the risk of toxicity, the intention to use a MAC regimen of busulphan and cyclophosphamide (BuCy) was added as an exclusion criterion during the trial (see below).
Table 1.
Study criteria, patient eligibility.

After assessment for eligibility, 215 patients were randomized in the trial. The flow of enrolled patients is shown in Figure 1. Two patients with symptoms of uncontrolled infection before HSCT, 2 patients with delayed notification of previous inclusion in a different trial with another investigational drug, and 2 patients scheduled for BuCy conditioning (at the time point of introduction of the additional exclusion criterion; see below) were excluded from the trial after randomization but before administration of their assigned GvHD prophylaxis. Inclusion of these 6 patients was considered as protocol violations, and they were excluded from further analysis.
Transplant procedures
All patients and donors were typed using molecular high-resolution typing (PCR-SSP) for both HLA class I and II antigens.25 Pre-transplantation conditioning regimens depended on disease, age, and standard criteria at the participating centers.
Myeloablative conditioning consisted of cyclophosphamide (Cy) 50 mg/kg/d for 4 days, or Cy 60 mg/kg/d for 2 days in combination with fractionated total body irradiation (TBI) with 12 Gy given in 3 fractions over 4 days26,27 (Online Supplementary Table S1). Initially, a MAC regimen of Cy, 60 mg/kg/d for 2 days, in combination with busulphan (Bu), 4 mg/kg/d for 4 days (BuCy) was permitted in the trial. Of the first 6 patients who received this regimen in combination with Tac/Sir prophylaxis, 2 developed early signs of excess toxicity and veno-occlusive disease (VOD)28 of the liver, and 2 developed signs of thrombotic microangiopathy (TMA),29 reported as severe adverse events. These findings coincided with the publication of similar discoveries in a comparable trial conducted by Cutler et al.30,31 Following a review of our data at the time, it was decided by the sponsor and the principal investigator to stop further recruitment of patients receiving BuCy conditioning to the trial.
Reduced-intensity conditioning consisted of fludarabine, 30 mg/m2/d for 3–6 days, in combination with either Cy 60 mg/kg/d for 2 days, 2×3 Gy TBI and Cy 60 mg/kg/d for 2 days, 2 Gy TBI, treosulphan 14 g/m2 for 3 days, or Bu 4 mg/kg/d for 2 days32–34 (Online Supplementary Table S1).
Antithymocyte globulin (ATG) was given as part of the conditioning regimen to patients receiving grafts from an unrelated donor, and to patients with non-malignant disorders. A total dose of 4–8 mg/kg was given; 4 mg/kg to malignant diseases receiving RIC, 6 mg/kg to malignant diseases receiving MAC, and 8 mg/kg to non-malignant diseases or HLA-mismatched grafts.33,35 The dose was administered in consecutive doses of 2 mg/kg/day, with the last dose given the day prior to graft infusion. Supportive care followed previously described institutional standards.36 The source of stem cells was peripheral blood progenitor cells (PBSCs) or bone marrow.
Graft-versus-host disease prophylaxis consisted of CsA/Mtx or Tac/Sir. Patients in the standard arm started CsA on day −1 (the day before graft infusion) and Mtx 15 mg/m2 was given on day +1, with consecutive doses of 10 mg/m2 given on days +3, +6, and +11 for all diagnoses. CsA was given twice a day (mainly orally). During the first two months, monitored plasma concentration levels were kept between 80–100 ng/mL in patients who received grafts from HLA-identical siblings, and between 150–250 ng/mL in MUD transplants. CsA was discontinued after tapering 3–4 months after HSCT in recipients of HLA-identical sibling grafts, and after six months in recipients of MUD transplants, in the absence of GvHD. Patients in the experimental arm started Tac/Sir in combination on day −3 before graft infusion. Sirolimus was given orally once daily, starting with a bolus dose of 6 mg in adults and 0.1 mg/kg in children, followed by continuous individual adjustment with monitored plasma target levels of 3–12 ng/mL. Tacrolimus was given orally twice a day, starting at 0.15 mg/kg/day, with a target plasma concentration of 5–15 ng/mL. Sirolimus was discontinued after tapering during the third month after HSCT in all patients in the absence of GvHD. In recipients of HLA-identical sibling grafts, tacrolimus was tapered in month 3 and discontinued in month 4 in the absence of GvHD. For MUD transplants, tacrolimus was discontinued after tapering six months after HSCT in the absence of GvHD. Dose modification guidelines for both immunosuppressive regimens included adjustments for toxicity, GvHD, and relapse.
Definitions and outcome assessment
Acute GvHD was diagnosed clinically and graded by the attending physicians from 0 to IV according to previously published criteria.37 Chronic GvHD was graded using the National Institutes of Health consensus criteria for clinical trials.38 Neutrophil engraftment was defined as the first of three consecutive daily measurements with a neutrophil count of more than 0.5×109/L, and platelet engraftment as the first of three consecutive daily measurements with a platelet count of more than 20×109/L without transfusions.
Oropharyngeal mucositis was assessed three times a week, and graded according to the International Classification of Diseases from the WHO39 until day +24 or until hospital discharge.
Cytomegalovirus (CMV) infection was defined as detection of CMV DNA in whole blood by real-time PCR. A PCR result of more than 1000 CMV DNA copies/mL (changed to more than 2000 CMV DNA copies/mL in 2009) was considered to be a clinically relevant CMV-viremia, and initiation of pre-emptive CMV therapy was used as a basis for analysis between the groups. Patients were monitored with CMV-PCR once a week for three months after HSCT. Subsequent monitoring was individually prescribed for each patient at the discretion of the treating physician, according to standard operating protocols.
Chimerism analyses of peripheral blood and/or bone marrow were performed on all patients at regular intervals after HSCT, according to standard operating protocols, or at the discretion of the treating physician. The cell lineages analyzed were T lymphocytes (CD3), B lymphocytes (CD19), myeloid cells (CD33), and hematopoietic precursors (CD34; applicable to bone marrow samples). Analyses were performed with real-time PCR based on single nucleotide polymorphisms.40 Full donor chimerism was defined as more than 95% donor-derived cells in all lineages, and mixed chimerism was assumed when more than 5% but less than 95% donor-derived cells were present in the cell lineages analyzed.
Transplant-related mortality was defined as death from any cause without relapse. Relapse was defined as recurrent disease after complete remission, or disease progression after partial remission or stable disease. Relapse-free survival (RFS) was defined as survival without any sign of hematologic relapse.
Statistical analysis
Data that were current in February 2015 (7.5 and 1.1 years after the first and last patient enrollment, respectively) were used in the intention-to-treat analysis. Categorical variables were compared using the χ2 method and continuous variables were compared using the Mann-Whitney U test. Overall survival and RFS were calculated using the Kaplan-Meier method and compared using the log rank test. Survival time was calculated from the day of transplantation until death or last follow up. The incidences of GvHD, TRM, and relapse were obtained using an estimator of cumulative incidence curves. Patients were censored at time of death or last follow up. Multivariate analyses for OS and RFS were performed with the Cox proportional hazards model. Subgroup analysis was performed for patients with malignant diagnoses. End point analyses were statistically corrected for patient group characteristics, presented as additional hazard ratios (HR).
All analyses were performed using the cmprsk package (Gray, 2001), Splus 6.2 software (Insightful, Seattle, WA, USA) and Statistica software (Statsoft, Tulsa, OK, USA).
Results
Patients’ characteristics
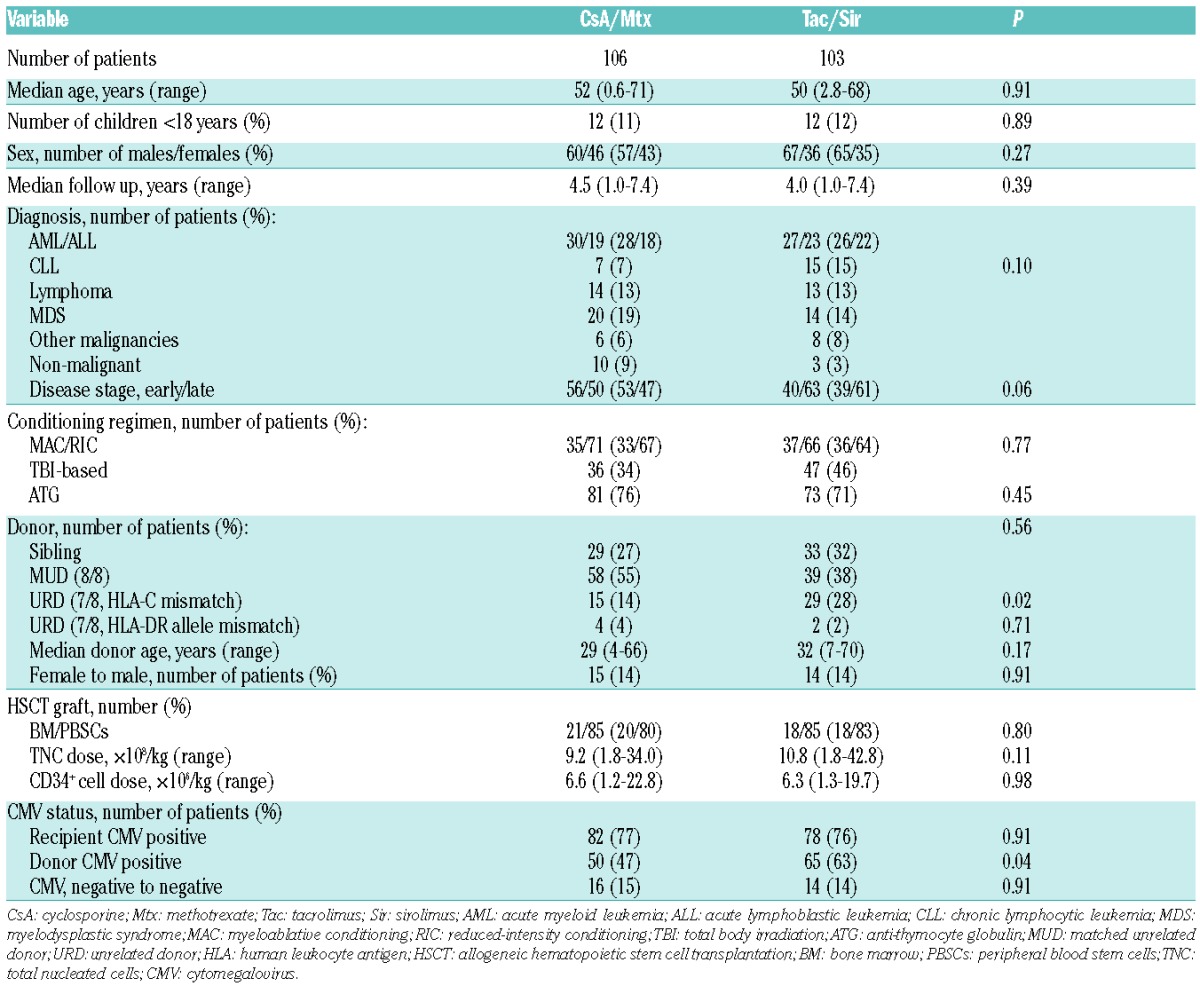
Patients’ and transplant characteristics are given in Table 2; 106 patients were analyzed in the standard arm (CsA/Mtx) and 103 in the experimental arm (Tac/Sir). Median follow up of the cohorts was 4.0 and 4.5 years, respectively, with a minimum follow up of 1.0 year in both groups at the time of data assessment. No statistically significant differences were seen between the groups for patients’ characteristics regarding age, diagnosis, or conditioning regimen, with the exception of an excess of HLA-C mismatched grafts (P=0.02) and CMV-seropositive donors in the CsA/Mtx arm (P=0.04).
Table 2.
Patients’, donor, and transplant characteristics, according to treatment arm.
Graft-versus-host disease
The cumulative incidence of acute GvHD of grades II–IV in the CsA/Mtx arm was 41% [(95% confidence interval (CI): 32%–50%)] as compared to 51% (95%CI: 41%–61%) in the Tac/Sir arm (P=0.19) (Figure 2A). Corrected (for patients’ characteristics) effect of CsA/Mtx versus Tac/Sir showed HR 0.85 (95%CI: 0.55–1.31; P=0.46). There was no difference in the cumulative incidence of acute GvHD of grades III–IV in the CsA/Mtx group and the Tac/Sir group [13% (7%–19%), and 7% (2%–12%), respectively; P=0.09] (Figure 2B). Corrected effect of CsA/Mtx showed HR 2.31 (95%CI: 0.88–6.10; P=0.09). Median time to development of acute GvHD was 26 days in CsA/Mtx patients and 31 days in Tac/Sir patients (P=0.22). In patients with malignant diagnoses (n=96 in the CsA/Mtx arm and n=100 in the Tac/Sir arm), 14% (7%–21%) in the CsA/Mtx arm and 7% (2%–12%) in the Tac/Sir arm developed acute GvHD of grades III–IV (P=0.06). Rates of acute GvHD did not differ significantly by donor type (siblings 48%, MUDs 46%), stem cell source (PBSC 47%, bone marrow 44%), HLA-match (7/8 37%, 8/8 50%) or conditioning intensity (RIC 47%, MAC 45%). Multivariate analysis for acute GvHD grades II–IV showed higher risk for patients with malignant diagnoses [(risk ratio (RR) 9.39, 95%CI: 1.30–67.9; P=0.03)] and female donor to male recipient transplants [(RR 2.44), 95%CI: 1.17–5.05; P=0.02)].
Figure 2.
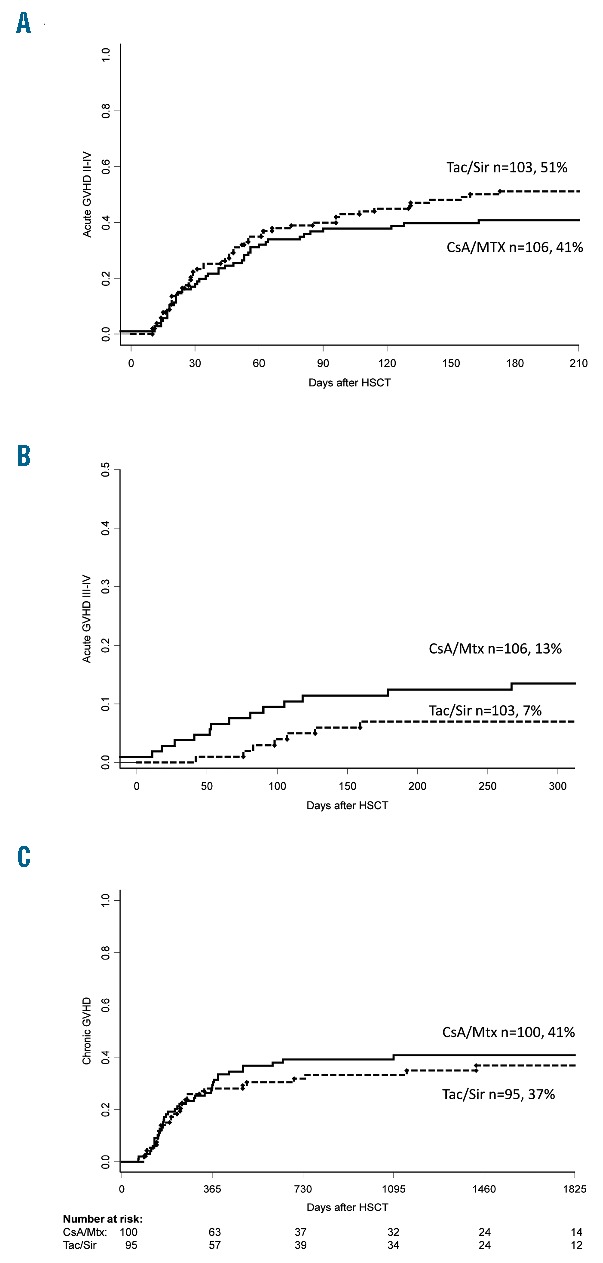
Graft-versus-host disease (GvHD) outcomes. (A) Cumulative incidence of acute GvHD of grades II–IV. (B) Cumulative incidence of acute GvHD of grades III–IV. (C) Cumulative incidence of chronic GvHD. CsA: cyclosporine; Mtx: methotrexate; Tac: tacrolimus; Sir: sirolimus; GvHD: graft-versus-host disease; HSCT: allogeneic hematopoietic stem cell transplantation.
In the patients who developed acute GvHD, there were no significant differences in the incidence of skin, gut, and liver manifestations between the two groups (Online Supplementary Figure S1).
The 5-year cumulative incidence of chronic GvHD in the CsA/Mtx arm was 41% (95%CI: 31%–51%), compared to 37% (95%CI: 26%–48%) in the Tac/Sir arm (P=0.51) (Figure 2C). Corrected effect of CsA/Mtx showed HR 1.52 (95%CI: 0.94–2.58; P=0.10). Median time to development of chronic GvHD was 214 days in CsA/Mtx patients and 204 days in Tac/Sir patients (P=0.72). Subgroup analysis of patients with malignant diagnoses showed no significant difference in moderate-to-severe chronic GvHD [18% (95%CI: 10–26%) in CsA/Mtx patients and 9% (95%CI: 3–15%) in Tac/Sir patients; P=0.09].
Tapering of GvHD prophylaxis occurred according to protocol, with a median treatment period of 191 days for cyclosporine, 150 days for tacrolimus and 68 days for sirolimus. At the time of last follow up, 61 CsA/Mtx patients and 63 Tac/Sir patients were alive and off their assigned immunosuppression.
Engraftment
There was no difference in the time to neutrophil engraftment [18 (10–305) days with CsA/Mtx as opposed to 17 (11–32) days with Tac/Sir; P=0.24] (Figure 3A). The median time to platelet engraftment was longer in CsA/Mtx patients [14 (0–190) days compared to 12 (0–68) days in CsA/Mtx patients; P=0.008] (Figure 3B).
Figure 3.
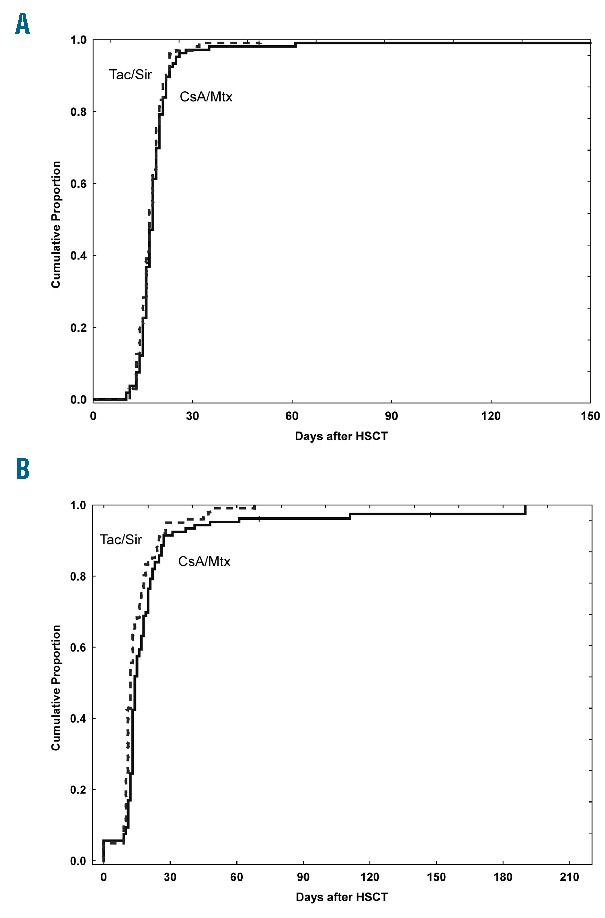
Engraftment outcomes. (A) Cumulative incidence of neutrophil engraftment. (B) Cumulative incidence of platelet engraftment. CsA: cyclosporine; Mtx: methotrexate; Tac: tacrolimus; Sir: sirolimus; HSCT: allogeneic hematopoietic stem cell transplantation.
Transplant-related outcomes
After exclusion of the BuCy regimen from the study, no additional patients fulfilled strict VOD criteria. In the CsA/Mtx group, no patient developed TMA during the first three months after HSCT, as compared to 2 in the Tac/Sir group. Thrombotic thrombocytopenic purpura (TTP, defined as TMA with severe acquired ADAMTS13 deficiency) was diagnosed in one CsA/Mtx patient and 2 Tac/Sir patients. Minor liver and renal toxicities were equally distributed.
In the Tac/Sir group, 7% of the patients developed suspected sirolimus-induced lesions in mouth, which healed after discontinuation of sirolimus administration. There was no significant difference in incidence or severity of oropharyngeal mucositis between the two groups.
The percentage of patients with elevated serum lipids was higher in the Tac/Sir arm during the first three months after HSCT. For triglycerides, the percentage of patients with any blood sample reaching a level of twice the upper limit of normal (ULN) was 17% in the CsA/Mtx group and 34% in the Tac/Sir group (P=0.005). For cholesterol, 9% and 22% of patients, respectively, had levels above the ULN (P=0.009). There was no significant difference in the number of days on total parenteral nutrition: median for CsA/Mtx patients was 3 (0–66) days as compared to 1.5 (0–194) for Tac/Sir patients (P=0.51). There was no significant difference in mean time to discharge between the two groups: 21 (1–45) days and 20 (3–59) days, respectively (P=0.15).
There were no significant differences in the number of patients who received pre-emptive therapy for CMV infection after HSCT in the two treatment arms. The incidence of post-transplant lymphoproliferative disorder,41,42 invasive fungal infections, and bloodstream infections were equal (Online Supplementary Table S2).
Chimerism analysis showed no significant differences between the groups regarding the number of patients classified as full donor or mixed chimerism at standard time points after HSCT (Online Supplementary Figure S2).
Subgroup analysis comparing MAC and RIC patients in the study groups did not show any significant differences in any of the analyzed end points.
Transplant-related mortality, survival, and relapse
The transplant-related mortality three years after HSCT was similar: 18% (95%CI: 11%–25%) in CsA/Mtx patients and 12% (95%CI: 6%–18%) in Tac/Sir patients (P=0.40), and corrected effect of CsA/Mtx showed HR 0.63 (95%CI: 0.30–1.31; P=0.70). The overall survival at five years after transplantation was 72% (95%CI: 63%–81%) in the CsA/Mtx group and 71% (95%CI: 62%–80%) in the Tac/Sir group (P=0.71) (Figure 4A). Corrected effect of CsA/Mtx showed HR 0.89 (95%CI: 0.49–1.59; P=0.67). Multivariate analysis identified allele-mismatched grafts (RR 8.40, 95%CI: 2.95–23.91; P<0.01) and patient age at the time of HSCT (RR 1.49, 95%CI: 1.22–1.91; P<0.01) as significant risk factors for mortality. Reduced-intensity conditioning was identified as a significant protective factor for mortality (RR 0.41, 95%CI: 0.20–0.81; P=0.01). Relapse of malignant disease was the most frequent cause of death in both groups (39% of deaths in CsA/Mtx patients and 50% of deaths in Tac/Sir patients).
Figure 4.
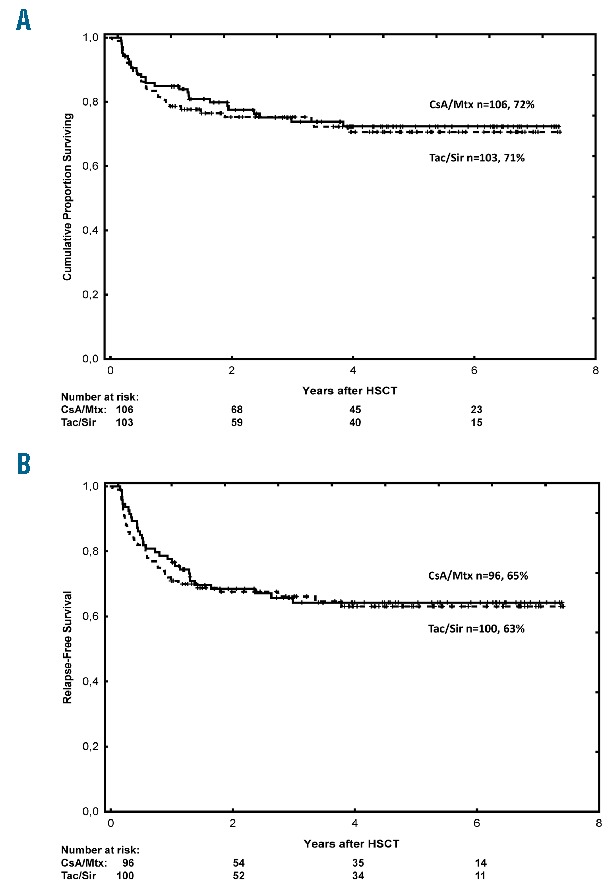
Survival outcomes. (A) Overall survival (all patients). (B) Relapse-free survival (malignant diagnoses). CsA: cyclosporine; Mtx: methotrexate; Tac: tacrolimus; Sir: sirolimus; HSCT: allogeneic hematopoietic stem cell transplantation.
Subgroup analysis of patients with malignant diagnoses showed no significant differences in relapse or RFS after HSCT (Figure 4B and Online Supplementary Figure S3). Five-year RFS in this cohort was 65% (95%CI: 55%–75%) in CsA/Mtx patients and 63% (95%CI: 53%–73%) in Tac/Sir patients (P=0.73). Corrected effect of CsA/Mtx showed HR 0.91 (95%CI: 0.54–1.54; P=0.72). Multivariate analysis showed that allele-mismatched grafts (RR 4.16, 95%CI: 1.48–11.69; P<0.01) and patient age at the time of HSCT (RR 1.16, 95%CI: 0.99–1.35; P=0.05) were the strongest predictors of lower RFS.
Subgroup analysis of patients receiving ATG as part of their conditioning regimen showed no significant differences in HSCT outcomes between the treatment groups, with the exception of incidence in acute GvHD grades III–IV (13% of ATG treated CsA/Mtx patients, and 4% of ATG treated Tac/Sir patients; P=0.04). No significant differences in outcome were seen in subgroup analysis of graft source.
Discussion
In this prospective, randomized, clinical trial we compared CsA/Mtx with Tac/Sir as GvHD prophylaxis after HSCT. We did not find any statistical difference in the cumulative incidence of acute GvHD of grades II–IV in the two groups. Similar results were seen in an equivalent randomized study published in 2014 by Cutler et al., which compared Tac/Sir with Tac/Mtx after matched, related HSCT.31 They found no significant increase in grades II–IV acute GvHD-free survival after HSCT between the two groups, but less oropharyngeal mucositis in the Tac/Sir group. Thus, despite our inclusion of MUD recipients and patients receiving allele-mismatched grafts, we did not see any benefit of Tac/Sir regarding the incidence of acute GvHD. This finding is somewhat contrary to another comparable study in children who received unrelated donor grafts, in which addition of sirolimus was associated with a lower incidence of GvHD of grades II–IV.43 One explanation of the discrepancy may have been our relatively heterogeneous patient population, possibly limiting the detection of a beneficial effect for particular diagnoses. Most of our patients also received ATG as part of their conditioning regimen, which reduces acute GvHD and TRM, and hence may have evened out differences in GvHD incidence.44,45 Despite the use of ATG, we observed a higher rate of acute GvHD in this trial when compared to the trial by Cutler et al. One reason might be that in the clinical setting for our patients, the objective was to achieve a grade I (-II) acute GvHD in patients with malignant diagnoses to reduce the risk of relapse. This was primarily achieved by a lower concentration of immunosuppression and ATG in both groups.5 For example, in the study plan, there was a wide concentration range for tacrolimus (5–15 ng/mL), but in the clinical situation, the aim was rather a concentration less than 10 ng/mL (no patient exceeded this level). Hence, the focus was rather to reduce the incidence of grades III–IV acute GvHD, which is reflected by a low incidence of this complication in our trial.
In a recent publication by Pidala, prolonged sirolimus administration (≥ 1 year) after HSCT was associated with a reduced risk of moderate-to-severe chronic GvHD in Tac/Sir patients compared to Tac/Mtx patients (34% vs. 65%; P<0.01).46 Prolonged treatment with cyclosporine also reduces chronic GvHD.47 In our study, none of the participants remained on sirolimus for more than six months (median 68 days).
A suspected increase in VOD and TMA in patients in the Tac/Sir arm was noted prior to exclusion of patients receiving the MAC regimen of BuCy. Similar findings were published by Cutler et al. in 2008.30 When used with Bu-based conditioning, sirolimus was associated with a higher rate of VOD (OR 8.8; P<0.01). In a prospective randomized study, it was found that patients treated with Bu had a cumulative incidence of VOD of 12%, as compared to 1% in patients who were treated with total body irradiation (P=0.009).26 The use of calcineurin inhibitors and/or sirolimus has also been suggested to be a potential risk factor for TMA, but the effect of the Tac/Sir combination is not well defined. Labrador et al. retrospectively analyzed the incidence of TMA in 102 HSCT recipients receiving Tac/Sir (n=68) or Tac/Mtx ±ATG (n=34) as GvHD prophylaxis, and no significant difference in the incidence of TMA was seen between the groups (7.4% vs. 8.8%; P=0.8).48 Accordingly, TMA in this setting might primarily be an effect of high-dose Bu-treatment, especially in combination with tacrolimus-based GvHD prophylaxis. Monitoring and adjustment of Bu concentrations in vivo, and modulation of the glutathione cellular content in the liver during conditioning, might reduce the risk of these side-effects.49
The number of post-transplant infections was similar in both groups. This is of interest, as development of certain infections after HSCT may be considered to be a surrogate indicator of immune competence. We did not detect any discrepancy in the numbers of clinically relevant CMV infections between the Tac/Sir and CsA/Mtx groups. A previous publication showed that sirolimus administration had little effect on induction of immediate-early gene expression in experimentally latent dendritic cells or cells from naturally latent individuals.50 This suggests that favorable CMV outcomes associated with sirolimus may be attributable to more indirect effects that influence CMV reactivation rather than a direct mechanistic action against CMV itself.
We acknowledge that our study has limitations, since it was performed in a relatively small patient cohort with notable patient- and transplant-related variations. Furthermore, no stratification of the patient material was carried out during randomization for graft source or level of HLA-match, factors recognized as risk factors for GvHD. This could have impaired observations of differences in incidence between grades III–IV GvHD between the study groups. At the same time, GvHD remains a major risk for HSCT patients regardless of diagnosis and/or transplant setting, and the inclusion of MUD recipients in the study make the results more valid, not least since there have been relatively few prospective randomized trials in this research area.
In summary, this study did not show any significant advantages of Tac/Sir over CsA/Mtx as GvHD prophylaxis after HSCT. However, our results confirm that Tac/Sir is a valid and safe GvHD prophylaxis option, with transplant-related outcomes comparable to those with CsA/Mtx. Hence, this study expands the randomized trial data in the realm of unrelated donor HSCT, but further studies in more homogenous patient settings, including similar or novel GvHD prophylaxis regimens, are required to investigate if specific patient groups benefit from a certain type of GvHD-prophylaxis, as the further refinement of HSCT procedures to improve outcome continues.
Supplementary Material
Acknowledgments
The authors would like to thank Björn Skölving, formerly working at Wyeth AB, for excellent collaboration when initiating the study, and we thank the medical, nursing, and laboratory staff of the Center for Allogeneic Stem Cell Transplantation (CAST), the Division of Hematology, and the Division of Pediatrics, at the Karolinska University Hospital, and the staff of the Department of Hematology at Turku University Hospital for their invaluable contributions to the study through compassionate care of the patients. We also thank the research nurses at CAST for excellent assistance with study records, and especially Eva Martell for first-rate help in retrieval of patient data.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/11/1417
Funding
JM was supported by grants from the Swedish Cancer Society (CF 2014–2016), the Swedish Children’s Cancer Foundation (PR2013-0022 and KF2013-0011), the Marianne and Marcus Wallenberg Foundation (2013.0117), and by grants provided by the Stockholm County Council (ALF project 20140451).
This study was supported in part by research funding from Astellas Pharma A/S (SE-09-RG-50) and Wyeth AB/Pfizer AB (#0468x1-3329) to OR and JM.
References
- 1.Negrin RS. Introduction to the review series on “Advances in hematopoietic cell transplantation”. Blood. 2014;124(3):307. [DOI] [PubMed] [Google Scholar]
- 2.Goldstone AH, Rowe JM. Transplantation in adult ALL. Hematology Am Soc Hematol Educ Program. 2009:593–601. [DOI] [PubMed] [Google Scholar]
- 3.Sureda A, Bader P, Cesaro S, et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant. 2015;50(8):1037–1056. [DOI] [PubMed] [Google Scholar]
- 4.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remberger M, Ackefors M, Berglund S, et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant. 2011; 17(11):1688–1697. [DOI] [PubMed] [Google Scholar]
- 6.Ziakas PD, Zervou FN, Zacharioudakis IM, Mylonakis E. Graft-versus-host disease prophylaxis after transplantation: a network meta-analysis. PLoS ONE. 2014; 9(12):e114735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109(10):4119–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perkins J, Field T, Kim J, et al. A randomized phase II trial comparing tacrolimus and mycophenolate mofetil to tacrolimus and methotrexate for acute graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2010;16(7):937–947. [DOI] [PubMed] [Google Scholar]
- 9.Reshef R. Prevention of graft-versus-host disease. Clin Adv Hematol Oncol. 2012;10(10):663–665. [PubMed] [Google Scholar]
- 10.Ringden O, Erkers T, Nava S, et al. Fetal membrane cells for treatment of steroid-refractory acute graft-versus-host disease. Stem Cells. 2013;31(3):592–601. [DOI] [PubMed] [Google Scholar]
- 11.Macdonald AS. Use of mTOR inhibitors in human organ transplantation. Expert Rev Clin Immunol. 2007;3(3):423–436. [DOI] [PubMed] [Google Scholar]
- 12.Knoll GA, Kokolo MB, Mallick R, et al. Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ. 2014;349:g6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutler C, Kim HT, Hochberg E, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):328–336. [DOI] [PubMed] [Google Scholar]
- 14.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storb R, Deeg HJ, Fisher L, et al. Cyclosporine v methotrexate for graft-v-host disease prevention in patients given marrow grafts for leukemia: long-term follow-up of three controlled trials. Blood. 1988;71(2):293–298. [PubMed] [Google Scholar]
- 16.Ringden O, Horowitz MM, Sondel P, et al. Methotrexate, cyclosporine, or both to prevent graft-versus-host disease after HLA-identical sibling bone marrow transplants for early leukemia¿ Blood. 1993;81(4):1094–1101. [PubMed] [Google Scholar]
- 17.Koenen HJ, Michielsen EC, Verstappen J, Fasse E, Joosten I. Superior T-cell suppression by rapamycin and FK506 over rapamycin and cyclosporine A because of abrogated cytotoxic T-lymphocyte induction, impaired memory responses, and persistent apoptosis. Transplantation. 2003;75(9):1581–1590. [DOI] [PubMed] [Google Scholar]
- 18.Hackstein H, Taner T, Zahorchak AF, et al. Rapamycin inhibits IL-4–induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101(11):4457–4463. [DOI] [PubMed] [Google Scholar]
- 19.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35(3 Suppl):7S–14S. [DOI] [PubMed] [Google Scholar]
- 20.Zaytseva YY, Valentino JD, Gulhati P, Evers BM. mTOR inhibitors in cancer therapy. Cancer Lett. 2012;319(1):1–7. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab. 2014;19(3):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng L, Jun S, Jianfeng L, Lianghui G. The effect of sirolimus-based immunosuppression vs. conventional prophylaxis therapy on cytomegalovirus infection after liver transplantation. Clin Transplant. 2015;29(6):555–559. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Gu Z, Zhai R, et al. The efficacy and safety of sirolimus-based graft-versus-host disease prophylaxis in patients undergoing allogeneic hematopoietic stem cell transplantation: a meta-analysis of randomized controlled trials. Transfusion. 2015;55(9):2134–2141. [DOI] [PubMed] [Google Scholar]
- 24.Ringden O, Remberger M, Dahllof G, et al. Sirolimus and tacrolimus as immune prophylaxis compared to cyclosporine with or without methotrexate in patients undergoing allogeneic haematopoietic stem cell transplantation for non-malignant disorders. Eur J Haematol. 2011;87(6):503–509. [DOI] [PubMed] [Google Scholar]
- 25.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. 1992;39(5):225–235. [DOI] [PubMed] [Google Scholar]
- 26.Ringden O, Ruutu T, Remberger M, et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood. 1994;83(9):2723–2730. [PubMed] [Google Scholar]
- 27.Champlin RE, Perez WS, Passweg JR, et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109(10):4582–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carreras E. Veno-occlusive disease of the liver after hemopoietic cell transplantation. Eur J Haematol. 2000;64(5):281–291. [DOI] [PubMed] [Google Scholar]
- 29.Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571–575. [DOI] [PubMed] [Google Scholar]
- 30.Cutler C, Stevenson K, Kim HT, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112(12):4425–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs. tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niederwieser D, Maris M, Shizuru JA, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and post-grafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101(4):1620–1629. [DOI] [PubMed] [Google Scholar]
- 33.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91(3):756–763. [PubMed] [Google Scholar]
- 34.Uzunel M, Remberger M, Sairafi D, et al. Unrelated versus related allogeneic stem cell transplantation after reduced intensity conditioning. Transplantation. 2006; 82(7):913–919. [DOI] [PubMed] [Google Scholar]
- 35.Remberger M, Svahn BM, Hentschke P, Lofgren C, Ringden O. Effect on cytokine release and graft-versus-host disease of different anti-T cell antibodies during conditioning for unrelated haematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;24(8):823–830. [DOI] [PubMed] [Google Scholar]
- 36.Forslow U, Mattsson J, Ringden O, Klominek J, Remberger M. Decreasing mortality rate in early pneumonia following hematopoietic stem cell transplantation. Scand J Infect Dis. 2006;38(11–12):970–976. [DOI] [PubMed] [Google Scholar]
- 37.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 38.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. WHO handbook for reporting results of cancer treatment. Geneva Albany, N.Y.: World Health Organization; sold by WHO Publications Centre USA, 1979. [Google Scholar]
- 40.Alizadeh M, Bernard M, Danic B, et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood. 2002;99(12):4618–4625. [DOI] [PubMed] [Google Scholar]
- 41.Loren AW, Porter DL, Stadtmauer EA, Tsai DE. Post-transplant lymphoproliferative disorder: a review. Bone Marrow Transplant. 2003;31(3):145–155. [DOI] [PubMed] [Google Scholar]
- 42.Sundin M, Le Blanc K, Ringden O, et al. The role of HLA mismatch, splenectomy and recipient Epstein-Barr virus seronegativity as risk factors in post-transplant lymphoproliferative disorder following allogeneic hematopoietic stem cell transplantation. Haematologica. 2006;91(8):1059–1067. [PubMed] [Google Scholar]
- 43.Pulsipher MA, Langholz B, Wall DA, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children’s Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood. 2014;123(13):2017–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Remberger M, Svahn BM, Mattsson J, Ringden O. Dose study of thymoglobulin during conditioning for unrelated donor allogeneic stem-cell transplantation. Transplantation. 2004;78(1):122–127. [PubMed] [Google Scholar]
- 45.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10(9):855–864. [DOI] [PubMed] [Google Scholar]
- 46.Pidala J, Kim J, Alsina M, et al. Prolonged sirolimus administration after allogeneic hematopoietic cell transplantation is associated with decreased risk for moderate-severe chronic graft-versus-host disease. Haematologica. 2015;100(7):970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lonnqvist B, Aschan J, Ljungman P, Ringden O. Long-term cyclosporin therapy may decrease the risk of chronic graft-versus-host disease. Br J Haematol. 1990;74(4):547–548. [DOI] [PubMed] [Google Scholar]
- 48.Labrador J, Lopez-Corral L, Lopez-Godino O, et al. Risk factors for thrombotic microangiopathy in allogeneic hematopoietic stem cell recipients receiving GVHD prophylaxis with tacrolimus plus MTX or sirolimus. Bone Marrow Transplant. 2014;49(5):684–690. [DOI] [PubMed] [Google Scholar]
- 49.Hassan Z, Hellstrom-Lindberg E, Alsadi S, Edgren M, Hagglund H, Hassan M. The effect of modulation of glutathione cellular content on busulphan-induced cytotoxicity on hematopoietic cells in vitro and in vivo. Bone Marrow Transplant. 2002;30(3):141–147. [DOI] [PubMed] [Google Scholar]
- 50.Glover TE, Kew VG, Reeves MB. Rapamycin does not inhibit human cytomegalovirus reactivation from dendritic cells in vitro. J Gen Virol. 2014;95(Pt 10):2260–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


