Abstract
Objectives. To evaluate the effect of levothyroxine (LT4) replacement therapy on nonalcoholic fatty liver disease (NAFLD) in subclinical hypothyroidism (SCH) patients. Methods. This study was a post hoc analysis of a randomized controlled trial and involved 33 significant and 330 mild SCH patients. All of the significant SCH patients received LT4 supplement. The mild SCH patients were grouped as LT4 treated or not. After 15 months of follow-up, prevalence of NAFLD in each group was reevaluated. Subgroup analysis was conducted in mild SCH patients with dyslipidemia. Results. After treatment with LT4, the prevalence of NAFLD in significant SCH patients reduced from 48.5% to 24.2% (p = 0.041). In mild SCH patients, prevalence of NAFLD and serum alanine aminotransferase (ALT) was not significantly affected by LT4 supplementation. Nonetheless, mild SCH patients with dyslipidemia who received LT4 treatment experienced decreases in the prevalence of NAFLD and serum ALT levels (p < 0.05 for both). In contrast, these parameters remained comparably stable in patients who were not treated. Conclusion. LT4 supplementation has benefits on NAFLD in significant SCH patients or mild SCH patients with dyslipidemia. For NAFLD patients with SCH, appropriate supplementation of LT4 may be an effective means of controlling NAFLD. The original trial was registered with ClinicalTrials.gov (NCT01848171).
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is defined as extrahepatic accumulation of fat in the absence of excess alcohol consumption. This condition is the most common cause of asymptomatic abnormal liver function tests [1], with its prevalence worldwide estimated to be 20% to 30% [2, 3], and the burden of this disease is translating to an increasing demand for liver transplantation [4]. Moreover, NAFLD is closely associated with components of metabolic syndrome, including visceral obesity, dyslipidemia, and insulin resistance and is indicative of an increased risk of cardiovascular disease and type 2 diabetes [5, 6]. Despite gratifying progress in the etiology of NAFLD in recent years, the mainstay of therapy remains weight loss through modification of diet and lifestyle [7]. However, even with an intensified lifestyle modification program, not all patients could achieve remission of NAFLD [8], underscoring the need for more specific targeted therapies.
Subclinical hypothyroidism (SCH) is an early manifestation of thyroid underactivity, which is characterized by elevated serum thyroid-stimulating hormone (TSH) levels, whereas serum-free thyroxine (FT4) levels are within the normal range [9]. It is a common medical condition with a prevalence ranging from 4% to 20% of adults, and the prevalence is progressively increasing [10, 11]. As stated by Lancet, “The world faces a burden of thyroid disease that has reached epidemic proportions” [12]. Remarkably, a growing body of evidence has led to the hypothesis that SCH is an independent risk factor of NAFLD [13–16]. A cross-sectional study reported that the prevalence of NAFLD in SCH patients was significantly increased compared with that of subjects with euthyroidism (29.9% versus 19.5%, p < 0.001) [13]. Moreover, population-based studies have demonstrated that increased TSH levels indicate an elevated incidence of NAFLD [14]. In addition, our group observed that TSH could directly regulate hepatic cholesterol and triglyceride metabolism and subsequently exacerbate the accumulation of fat in the liver [17–19]. Levothyroxine (LT4) is the standard medication for treatment of hypothyroidism and is associated with a significant decrease in body weight and serum lipids [20, 21]. Therefore, does the treatment of SCH patients to normalize elevated serum TSH levels via appropriate LT4 supplementation yield any benefits on NAFLD? No interventional study is currently focusing on this issue.
We performed this post hoc analysis of a randomized controlled trial to evaluate the effect of SCH treatment on NAFLD, aiming to provide a new choice for the treatment of NAFLD. In addition, SCH is closely associated with disturbances in lipid metabolism [22, 23], and dyslipidemia is a risk factor of NAFLD [24–26]. We further conducted subgroup analysis to assess the effect of LT4 supplementation on NAFLD in mild SCH patients with dyslipidemia.
2. Materials and Methods
2.1. Study Design and Patients
The design of the original study has been described elsewhere [21]. Briefly, the original study was an open-label, randomized, controlled trial designed to assess the effects of LT4 replacement therapy on lipid profiles in SCH patients. Subjects were recruited from Ningyang County, Shandong Province, China. All registered Chinese civilians aged 40 years or older who had lived in Ningyang County for at least five years were invited by telephone or door-to-door visits to undergo the screening program for SCH, which began in July 2013. Subjects diagnosed with SCH (TSH ≥ 4.2 mIU/L with normal serum FT4 confirmed on the basis of at least two hormonal assays with a three-month interval [27]) and in the absence of previous or ongoing treatment for hypothyroidism were included in the trial. Exclusion criteria were as follows: (1) pregnancy or breast-feeding, (2) complications or conditions that affect thyroid status or lipid metabolism, (3) taking any medicine that affects the thyroid or lipid metabolism in the previous three months, and (4) obviously poor compliance. Finally, 415 SCH patients, including 37 significant SCH (TSH ≥ 10 mIU/L) and 378 mild SCH (TSH of 4.2–10 mIU/L), were enrolled in the trial. All of the participants completed abdominal ultrasonography at enrollment.
All patients provided informed written consent. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, was approved by the Ethics Committee of the Provincial Hospital affiliated to Shandong University, and was registered with ClinicalTrials.gov (NCT01848171).
2.2. Randomization
According to the guideline for hypothyroidism cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association [27], significant SCH patients were all treated with LT4 (Euthyrox, 50 μg per tablet, Merck Serono, Darmstadt, Germany). Mild SCH patients were randomized in a 1.5 : 1 fashion to LT4 replacement therapy or no treatment. Randomization was performed through the use of computer-generated random numbers. Neither patients nor investigators involved in the study were masked to treatment allocation.
2.3. Intervention
For patients treated with LT4, the initial dosage was 25 μg/day. Treatments were administered with water between 30 and 60 minutes prior to eating breakfast [27]. Thyroid function was re-evaluated six weeks later based on a test of serum TSH and FT4 levels, and the dosage of LT4 was adjusted accordingly. The adjustment continued until euthyroidism (TSH of 0.27–4.2 mIU/L with a normal serum FT4 level) was reached, and the dosage that achieved euthyroidism was subsequently maintained. Finally, the median LT4 dosage was 75 μg daily for significant SCH patients and 50 μg daily for mild SCH patients who received LT4 replacement therapy. All recruited subjects were interviewed every three months. Updates on patient status were recorded at each visit, including any diseases, drugs, operations, and obvious changes in lifestyle (e.g., vegetarian diet or intense exercise). In addition, patients who received LT4 replacement therapy were advised to return any unused tablets at each visit to allow monitoring of the compliance with the allocated medication. All of the participants received follow-up for 15 months.
2.4. Study Endpoints
The primary endpoint of the original trial was serum total cholesterol (TC) concentration change from baseline to end-of-study. The secondary endpoints were the changes in other serum lipid parameters during the study course, including low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG). In the present post hoc analysis, the primary endpoint was the effect of LT4 replacement therapy on the prevalence of NAFLD. Secondary endpoints were changes in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels.
2.5. Follow-Up Assessments
All subjects completed a self-report questionnaire, including information on demographic characteristics, medical history, and lifestyle, by trained interviewers at recruitment. Clinical assessment was performed at baseline and end-of-study and included measurements of weight, height, waist circumference (WC), blood pressure, and liver ultrasound. Weight (kilograms) and standing height (meters) were measured between 8:00 a.m. and 10:00 a.m. after a minimum 10-hour fasting in light clothing and without shoes. Body mass index (BMI) was calculated by dividing weight by the square of the height. The methods for determining WC and blood pressure were similar to those used in the national survey of the prevalence of diabetes in 2010 [28].
Liver ultrasound was performed using a 3.75 MHz curvilinear probe (model CC-15M71-MA; Toshiba, Tokyo, Japan) by the same experienced radiologist throughout the study. The radiologist was blind to the study design and laboratory values of the participants. The diagnostic criteria for fatty liver by ultrasonography included the following items: (1) diffuse enhancement of near field echogenicity in the hepatic region (compared with that in the kidney and spleen region) and gradual attenuation of the far field echogenicity, (2) unclear display of intrahepatic lacuna structure, (3) mild to moderate hepatomegaly with a round and blunt border, (4) color doppler ultrasonography shows a reduction of the blood flow signal in the liver or it is even hard to display, but the distribution of blood flow is normal, and (5) blurred or nonintact display of the envelope of the right liver lobe and diaphragm. Fatty liver was diagnosed if item 1 and any one or more of items 2–4 are matched [29, 30]. NAFLD was diagnosed based on the criteria suggested by the Chinese Society of Hepatology, Chinese Medical Association [30], as fatty liver confirmed by ultrasonic imaging and in the absence of the following: (1) excess alcohol consumption (>70 g per week for women and >140 g per week for men), (2) diagnosis of viral hepatitis, (3) usage of hepatotoxic drugs, and (4) other diseases that might cause fatty liver [31].
Venous blood samples were drawn between 8:00 a.m. and 10:00 a.m. after a minimum 10-hour fasting. Serum-free triiodothyronine (FT3), FT4, and TSH levels were measured by chemiluminescence methods (Cobas E601; Roche, Basel, Switzerland). The serum lipid profiles, fasting plasma glucose (FPG), liver enzymes, and renal functions were quantified using a Beckman Chemistry Analyzer AU5800 System (Beckman Coulter, Tokyo, Japan). Non-HDL cholesterol (non-HDL-C) was calculated by subtracting HDL-C from TC. The examinations of these variables were all completed at the clinical laboratory of the Shandong Provincial Hospital. The laboratory reference ranges were 3.1–6.8 pmol/L (2.01–4.42 pg/mL) for FT3, 12–22 pmol/L (0.94–1.72 ng/dL) for FT4, 0.27–4.2 mIU/L for TSH, 9–50 IU/L for ALT, and 15–40 IU/L for AST. Diabetes was diagnosed based on the World Health Organization (WHO) 1999 criteria, as FPG concentration ≥ 7.0 mmol/L and/or self-reported history of type 2 diabetes [32].
2.6. Statistical Analysis
Quantitative data are expressed as the mean ± standard deviation or median (interquartile range) according to their distributions, and categorical data are presented as a number (percentage). Comparisons of variables among the groups were performed using one-way ANOVA, independent Student's t-test, or the Mann-Whitney U test. A paired-samples t-test or Wilcoxon paired rank test was used for within-group comparisons (baseline and end-of-study variables in each group). Differences in categorical data were evaluated by chi-squared test. A two-tailed value of p < 0.05 was regarded as significant. All statistical analyses were performed using SPSS version 22.0 for Windows (Chicago, IL, USA). All authors had access to the study data and reviewed and approved the final manuscript.
Given the high prevalence of dyslipidemia in SCH patients and the role of dyslipidemia as an important risk factor of NAFLD, we performed subgroup analysis to evaluate the effect of LT4 replacement therapy on NAFLD in mild SCH patients with dyslipidemia at baseline. Dyslipidemia was defined as abnormalities in the serum levels of lipids, including TG ≥ 1.70 mmol/L or TC ≥ 6.22 mmol/L or LDL-C ≥ 4.14 mmol/L or HDL-C < 1.04 mmol/L for men and <1.30 mmol/L for women [33].
3. Results
3.1. Baseline Characteristics
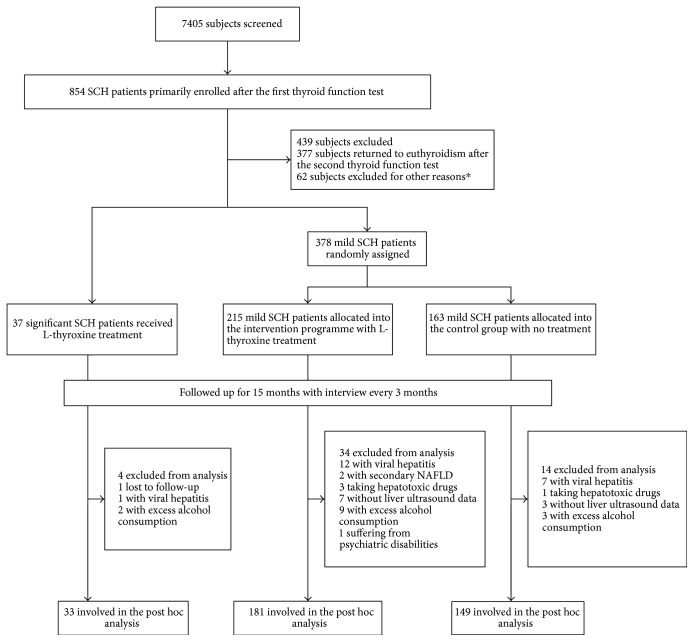
Based on the self-reported medical history, subjects with viral hepatitis, secondary causes of NAFLD, and use of hepatotoxic drugs, as well as with excess alcohol consumption or incomplete abdominal ultrasound data, were further excluded from the analysis (n = 52). Finally, this post hoc analysis involved 363 participants, including 33 significant SCH patients and 330 mild SCH patients (Figure 1). Among the mild SCH patients, 181 were treated with LT4 (mild SCH-LT4 group) and 149 were not treated (mild SCH-control group). Table 1 presents the baseline characteristics of the participants in three groups. The cohort was middle aged with a predominance of females, and the majority of the participants were overweight or obese (BMI ≥ 24 kg/m2), [34]. Notably, approximately two-thirds of the subjects also had dyslipidemia. Nine (27.3%) out of the 33 significant SCH patients suffered from diabetes at enrollment. The prevalence of diabetes in the mild SCH-LT4 group and the mild SCH-control group were 13.8% and 16.8% (p = 0.455), respectively. The prevalence of NAFLD in the significant SCH patients was 48.5%. In mild SCH patients, the prevalence of NAFLD in the LT4 group and the control group was 44.2% and 39.6%, respectively. The prevalence of NAFLD in the studied SCH patients was considerably increased compared with that reported in general population which was estimated at 20% to 30% [2, 3]. The baseline demographic, anthropometric, biochemical, and clinical variables were evenly matched in the two arms of mild SCH patients.
Figure 1.
Flow diagram. ∗Reasons for exclusion are given in Materials and Methods. SCH: subclinical hypothyroidism; NAFLD: nonalcoholic fatty liver disease.
Table 1.
Baseline characteristics of participants.
| Variables | Significant SCH-LT4 (n = 33) | Mild SCH patients | ||
|---|---|---|---|---|
| LT4 group (n = 181) | Control group (n = 149) | p value∗ | ||
| Age (year) | 57.79 ± 8.51 | 55.04 ± 7.85 | 56.59 ± 8.31 | 0.074 |
| Female, n (%) | 27 (81.8) | 135 (74.6) | 115 (77.2) | 0.584 |
| BMI (kg/m2) | 25.88 ± 3.15 | 26.12 ± 3.11 | 25.72 ± 3.58 | 0.372 |
| Weight (kg) | 66.13 ± 10.26 | 66.46 ± 10.40 | 65.17 ± 11.24 | 0.308 |
| WC (cm) | 91.53 ± 9.17 | 90.81 ± 9.44 | 91.19 ± 10.52 | 0.675 |
| ALT (IU/L) | 18.61 ± 7.67 | 19.09 ± 9.00 | 17.20 ± 8.35 | 0.050 |
| AST (IU/L) | 26.12 ± 5.83 | 26.08 ± 6.80 | 24.65 ± 5.77 | 0.057 |
| TC (mmol/L) | 5.68 ± 0.93 | 5.91 ± 1.13 | 5.70 ± 1.05 | 0.086 |
| HDL-C (mmol/L) | 1.35 ± 0.27 | 1.41 ± 0.33 | 1.41 ± 0.32 | 0.923 |
| LDL-C (mmol/L) | 3.37 ± 0.91 | 3.40 ± 0.82 | 3.29 ± 0.80 | 0.222 |
| Non-HDL-C (mmol/L) | 4.33 ± 0.92 | 4.50 ± 1.09 | 4.29 ± 1.02 | 0.071 |
| TG (mmol/L) | 1.35 (0.78) | 1.37 (1.00) | 1.38 (1.02) | 0.712 |
| SBP (mmHg) | 149.13 ± 19.61 | 149.92 ± 19.81 | 151.75 ± 23.80 | 0.435 |
| DBP (mmHg) | 82.16 ± 10.34 | 85.21 ± 11.66 | 86.01 ± 11.77 | 0.519 |
| FPG (mmol/L) | 6.98 ± 2.93 | 6.28 ± 1.68 | 6.34 ± 1.88 | 0.799 |
| Obesity, n (%) | 0.440 | |||
| Normal (BMI< 24) | 10 (30.3) | 46 (25.4) | 46 (30.9) | |
| Overweight (24 ≤ BMI< 28) | 14 (42.4) | 92 (50.8) | 66 (44.3) | |
| Obese (BMI ≥ 28) | 9 (27.3) | 43 (23.8) | 37 (24.8) | |
| Dyslipidemia, n (%) | 22 (66.7) | 116 (64.1) | 91 (61.1) | 0.573 |
| NAFLD, n (%) | 16 (48.5) | 80 (44.2) | 59 (39.6) | 0.400 |
| Diabetes, n (%) | 9 (27.3) | 25 (13.8) | 25 (16.8) | 0.455 |
Values for quantitative data are expressed as mean ± standard deviation or median (interquartile range); values for categorical variables are expressed as number (percentage).
∗ p value for comparing variables between mild SCH-LT4 group and mild SCH-control group.
SCH: subclinical hypothyroidism; LT4: levothyroxine; BMI: body mass index; WC: waist circumference; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; non-HDL-C: nonhigh-density lipoprotein cholesterol; TG: triglyceride; SBP: systolic blood pressure; DBP: diastolic blood pressure; FPG: fasting plasma glucose; NAFLD: nonalcoholic fatty liver disease.
3.2. Efficacy of LT4 on Thyroid Function
Thyroid functions throughout the study were shown in Supplementary Table 1 available online at https://doi.org/10.1155/2017/5753039. At end-of-study, all of the SCH patients that received LT4 treatment, including the significant SCH patients and the mild SCH patients in the mild SCH-LT4 group, achieved normalization of thyroid function, with a significant decrease in TSH level and a significant increase in FT4 level compared with that at baseline (p < 0.05 for all). In the mild SCH-control group, serum FT4 level remained stable throughout the course of the study. Although the serum TSH level in the mild SCH-control group exhibited a naturally slight decrease at end-of-study, it was still higher than the normal range (0.27–4.2 mIU/L). Serum FT3 was not significantly altered throughout the study in each group.
3.3. Primary Endpoint: Prevalence of NAFLD
Within the significant SCH group, the prevalence of NAFLD was reduced from 48.5% to 24.2% (p = 0.041) during the course of the study (Table 2). The prevalence of NAFLD in both arms of mild SCH patient showed moderate but not statistically significant reduction. In the mild SCH-LT4 group, the prevalence of NAFLD decreased from 44.2% to 35.9% (p = 0.108). The prevalence of NAFLD in the mild SCH-control group also reduced (39.6% to 34.9%, p = 0.402). As can be seen, the reduction in the prevalence of NAFLD tended to be smaller in the mild SCH-control group compared with that in the mild SCH-LT4 group (4.7% versus 8.3%), though the difference did not reach the statistically significant level (p = 0.432) (Table 2). These results indicated that clinicians should be alert to the possibility of SCH in NAFLD patients. Regarding NAFLD patients with significant SCH, LT4 supplementation may be an effective means for controlling NAFLD.
Table 2.
Prevalence of NAFLD and serum liver enzymes throughout the study in SCH patients.
| Variables | Significant SCH-LT4 (n = 33) |
Mild SCH patients | ||
|---|---|---|---|---|
| LT4 group (n = 181) |
Control group (n = 149) |
p value∗ | ||
| Prevalence of NAFLD, n (%) | ||||
| Baseline | 16 (48.5) | 80 (44.2) | 59 (39.6) | 0.400 |
| End-of-study | 8 (24.2) | 65 (35.9) | 52 (34.9) | 0.848 |
| p value# | 0.041 | 0.108 | 0.402 | — |
| ALT (IU/L) | ||||
| Baseline | 18.61 ± 7.67 | 19.09 ± 9.00 | 17.20 ± 8.35 | 0.050 |
| End-of-study | 17.15 ± 7.34 | 17.95 ± 7.71 | 17.29 ± 7.68 | 0.410 |
| p value# | 0.383 | 0.087 | 0.906 | — |
| Change in ALT | −1.46 ± 9.44 | −1.14 ± 8.77 | 0.09 ± 9.01 | 0.217 |
| AST (IU/L) | ||||
| Baseline | 26.12 ± 5.83 | 26.08 ± 6.80 | 24.65 ± 5.77 | 0.057 |
| End-of-study | 20.52 ± 6.69 | 21.22 ± 6.88 | 21.36 ± 6.48 | 0.854 |
| p value# | <0.001 | <0.001 | <0.001 | — |
| Change in AST | −5.61 ± 1.27 | −4.86 ± 7.34 | −3.29 ± 6.65 | 0.046 |
Values for quantitative data are expressed as mean ± standard deviation; values for categorical variables are expressed as number (percentage).
∗ p value for comparing variables between mild SCH-LT4 group and mild SCH-control group.
# p value for comparing variables between baseline and end-of-study within each group.
NAFLD: nonalcoholic fatty liver disease; SCH: subclinical hypothyroidism; LT4: levothyroxine; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
3.4. Secondary Endpoint: Serum Liver Enzymes
Baseline and end-of-study data for liver function in each group are shown in Table 2. After LT4 treatment, significant SCH patients experienced a decrease of 5.61 IU/L in serum AST (p < 0.001) and a moderate but not statistically significant decrease in serum ALT.
Patients in the mild SCH-LT4 group showed a marginally significant reduction in serum ALT from 19.09 IU/L to 17.95 IU/L (p = 0.087), whereas serum ALT in the mild SCH-control group was quite stable in the study. Additionally, although there were reductions in serum AST within the two groups of mild SCH patients, the mild SCH-LT4 group reported a remarkably greater reduction than the mild SCH-control group (−4.86 versus −3.29 IU/L, p = 0.046).
3.5. Changes in Metabolic Characteristics in the Cohort
Details are presented in the Supplementary Material (Supplementary results, Supplementary Table 2, and Supplementary Table 3).
3.6. Subgroup Analysis: Mild SCH Patients Combined with Dyslipidemia
The prevalence of dyslipidemia in the studied mild SCH patients was as high as 62.7% (207 of 330). In addition, dyslipidemia is a risk factor of NAFLD, so we performed subgroup analysis to evaluate the effect of LT4 supplementation on NAFLD in mild SCH patients with dyslipidemia. The 207 mild patients with dyslipidemia at baseline were involved in this subgroup analysis and were grouped as the sub-LT4 group and subcontrol group according to whether they received LT4 replacement therapy. Baseline characteristics for these patients are displayed in Supplementary Table 4. The demographic, anthropometric, biochemical, and clinical characteristics were statistically matched between the sub-LT4 group and subcontrol group at baseline.
3.6.1. Morbidity of NAFLD
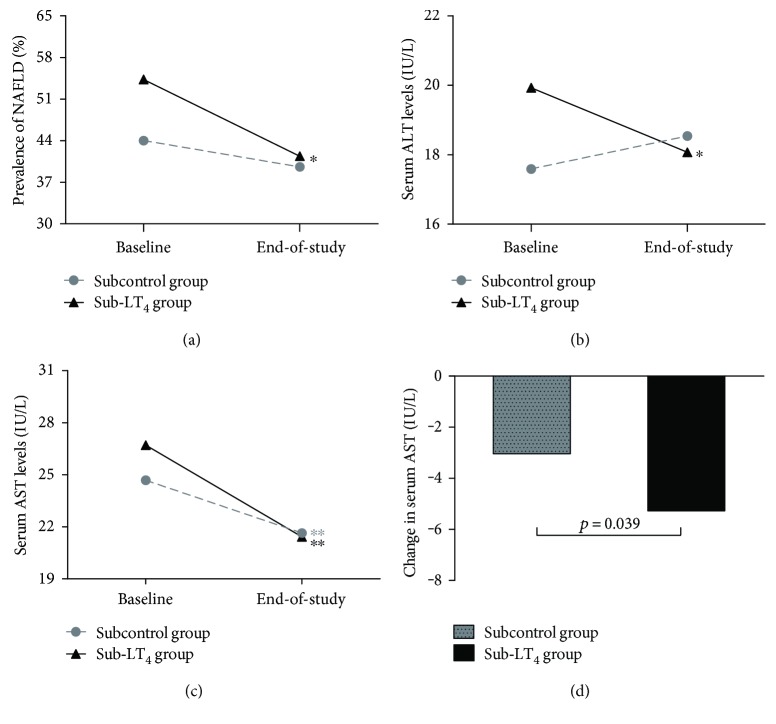
The prevalence of NAFLD was statistically comparable between the sub-LT4 group and subcontrol group at baseline (p = 0.139). Within the sub-LT4 group, the prevalence of NAFLD decreased from baseline to end-of-study (54.3% to 40.5%, p = 0.035) (Figure 2(a)). Although the prevalence of NAFLD also decreased in the subcontrol group during the study, the reduction did not reach significance (44.0% to 39.6%, p = 0.548) (Figure 2(a)). In addition, we further analyzed the remission rate of NAFLD among patients suffering from NAFLD at baseline and the incidence rate of NAFLD among patients without NAFLD at baseline. In the sub-LT4 group, 26 (41.3%) of 63 patients with NAFLD at baseline reverted to the normal liver at end-of-study, and the level tended to be higher compared with that of the subcontrol group (35.0%, p = 0.525). Among patients without NAFLD at baseline, the incidence of NAFLD at end-of-study in the sub-LT4 group was 18.9%, which is slightly lower compared with that in the subcontrol group (19.6%, p = 0.924) (Supplementary Table 5).
Figure 2.
Effects of LT4 replacement therapy on prevalence of NAFLD and serum liver enzymes in mild SCH patients with dyslipidemia. (a) Prevalence of NAFLD for each group at baseline and end-of-study. (b–d) Changes in serum liver enzymes for each group. Data are presented as means. (b) Serum ALT at baseline and end-of-study. (c) Serum AST at baseline and end-of-study. (d) Reductions in AST during the course of study for each group. ∗p < 0.05 and ∗∗p < 0.01 for comparison between baseline and end-of-study, respectively. SCH: subclinical hypothyroidism; NAFLD: nonalcoholic fatty liver disease; LT4: levothyroxine; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
3.6.2. Serum Liver Enzymes
Regarding liver enzymes, there was no difference in serum ALT or AST levels between the sub-LT4 group and the subcontrol group at baseline (p > 0.05 for all). Within-group comparisons revealed that serum ALT decreased from 19.93 IU/L to 18.07 IU/L (p = 0.043) in the sub-LT4 group but increased marginally in the subcontrol group (17.59 IU/L to 18.54 IU/L, p = 0.282) (Figure 2(b)). Moreover, serum AST declined in both groups during the study, but the reduction in the sub-LT4 group was significantly greater than that in the subcontrol group (−5.28 IU/L versus −3.04 IU/L, p = 0.039) (Figures 2(c) and 2(d)).
3.6.3. Metabolic Response
Statistically significant reductions (p < 0.05) in mean body weight and BMI were noted in patients treated with LT4, whereas patients who were not treated exhibited comparatively stable body weight and BMI (Table 3). Moreover, there was a trend toward a greater decrease in body weight in patients who experienced remission of NAFLD (n = 40) compared with those without remission of NAFLD (n = 63) (−1.65 versus −0.60 kg, p = 0.212), and the same trend of decrease was reported for BMI (−0.36 versus −0.17 kg/m2, p = 0.586). These results indicated that improvement of NAFLD in patients treated with LT4 might be associated with weight loss. Regarding serum lipids, patients treated with LT4 experienced a more profound reduction in serum TC compared with patients who were not treated. A similar decreasing trend was observed for serum LDL-C and non-HDL-C, whereas serum TG and HDL-C were not significantly affected by LT4 (Table 3). Glucose metabolism was not altered by LT4 supplementation given that FPG was the same at baseline and end-of-study within both groups.
Table 3.
Change from baseline in metabolic variables in mild SCH patients combined with dyslipidemia.
| Variables | Sub-LT4 group (n = 116) | Subcontrol group (n = 91) | p value∗ | ||||
|---|---|---|---|---|---|---|---|
| Baseline | End-of-study | p value# | Baseline | End-of-study | p value# | ||
| BMI (kg/m2) | 26.48 ± 3.13 | 26.00 ± 3.27 | 0.001 | 26.17 ± 3.68 | 26.20 ± 3.88 | 0.865 | — |
| Weight (kg) | 66.92 ± 10.27 | 65.44 ± 9.97 | <0.001 | 66.12 ± 11.57 | 66.12 ± 12.03 | 0.993 | — |
| TC (mmol/L) | 6.27 ± 1.21 | 5.69 ± 1.15 | <0.001 | 5.99 ± 1.16 | 5.74 ± 1.14 | 0.026 | — |
| HDL-C (mmol/L) | 1.33 ± 0.36 | 1.31 ± 0.31 | 0.301 | 1.31 ± 0.34 | 1.42 ± 0.37 | <0.001 | — |
| LDL-C (mmol/L) | 3.67 ± 0.83 | 3.43 ± 0.91 | <0.001 | 3.55 ± 0.85 | 3.35 ± 0.94 | 0.015 | — |
| Non-HDL-C (mmol/L) | 4.93 ± 1.08 | 4.37 ± 1.03 | <0.001 | 4.68 ± 1.04 | 4.32 ± 1.02 | 0.001 | — |
| TG (mmol/L) | 1.82 (1.27) | 1.49 (1.04) | <0.001 | 1.84 (1.29) | 1.46 (0.90) | <0.001 | — |
| FPG (mmol/L) | 6.43 ± 1.54 | 6.35 ± 1.83 | 0.329 | 6.65 ± 2.04 | 6.50 ± 2.07 | 0.229 | — |
| Change in TC | −0.58 ± 0.92 | −0.25 ± 1.06 | 0.018 | ||||
| Change in LDL | −0.25 ± 0.63 | −0.20 ± 0.77 | 0.595 | ||||
| Change in Non-HDL-C | −0.56 ± 0.84 | −0.36 ± 0.95 | 0.102 | ||||
| Change in TG | −0.32 (0.88) | −0.34 (0.85) | 0.392 | ||||
Values are expressed as mean ± standard deviation or median (interquartile range).
∗ p value for comparing variables between sub-LT4 group and subcontrol group.
# p value for comparing variables between baseline and end-of-study within each group.
SCH: subclinical hypothyroidism; LT4: levothyroxine; BMI: body mass index; FPG: fasting plasma glucose; TC: total cholesterol; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; non-HDL-C: nonhigh-density lipoprotein cholesterol; TG: triglyceride.
4. Discussion
To our knowledge, this study is the first to evaluate the efficacy of LT4 replacement on NAFLD in SCH patients. We reported here that LT4 replacement treatment resulted in a reduction in the prevalence of NAFLD in significant SCH patients. Although we were not able to prove remarkable benefits of LT4 treatment on the prevalence of NAFLD and serum liver enzymes in mild SCH patients, subgroup analysis revealed that mild SCH patients with dyslipidemia could benefit from LT4 supplementation with profound reductions in the prevalence of NAFLD and serum liver enzymes. We also reported in the subgroup analysis that patients who received LT4 treatment exhibited trends of higher NAFLD remission rates and lower NAFLD incidence rates compared with patients who were not treated, albeit not statistically significant.
A number of studies have provided evidence supporting the role of SCH as an independent risk factor of NAFLD [13–16]. The mechanism underlying the association of SCH and NAFLD may be mediated by the direct regulation of TSH on liver lipid metabolism. Our group first proved that hepatocytes had functional TSH receptor (TSHR) expression, and the expression was not a case of illegitimate transcription [17]. Subsequently, we further demonstrated that TSH, by binding to TSHR on hepatocytes, upregulated the expression of hepatic 3-hydroxy-3-methyl-glutaryl coenzyme A reductase (HMGCR), which is the rate-limiting enzyme in cholesterol synthesis, and subsequently increased the cholesterol content in the liver [19]. In addition to the regulation of TSH on liver cholesterol metabolism, we also discovered a role of TSH in regulating triglyceride metabolism. We observed that TSH could aggravate the triglyceride accumulation in hepatocytes induced by a high-fat diet in mice through the activation of sterol regulatory element binding protein 1c (SREBP-1c), and TSHR knockout mice (Tshr−/−) exhibited a relatively lower degree of liver steatosis [18]. The present study was based on SCH patients and demonstrated that normalizing the elevated level of TSH in significant SCH patients or mild SCH patients with dyslipidemia via LT4 replacement therapy could reduce the prevalence of NAFLD. The results of this study were consistent with previous studies that revealed the independent role of elevated TSH in the course of NAFLD. This finding suggests that, when engaged with NAFLD patients, clinicians should be alert to the possibility of combination of SCH. For NAFLD patients suspected of secondary to SCH, normalizing thyroid function by appropriate supplementation with LT4 may be an effective means for controlling NAFLD.
Several lines of evidence have indicated that SCH is associated with dyslipidemia. The prevalence of dyslipidemia is higher in SCH patients compared with euthyroid subjects [35, 36]. Moreover, serum TSH levels are positively associated with serum lipid profiles independent of thyroid hormones [22]. In this study, the prevalence of dyslipidemia was as high as 62.7% in mild SCH patients, which is considerably increased compared with that reported in general Chinese population (estimated as 30% to 50%) [37, 38]. On the other hand, dyslipidemia is a risk factor associated with NAFLD [24–26]. A cross-sectional, community-based study conducted in Taiwan reported that elevated serum TG levels greater than 150 mg/dL were associated with an increased risk for NAFLD (odds ratio, 2.48; 95% confidence interval, 1.42–4.32; p = 0.001) among nonobese subjects [25]. In this study, we performed subgroup analysis in mild SCH patients with dyslipidemia and reported a significant decline in prevalence of NAFLD in subjects who received LT4 treatment compared with the control group. The benefits of LT4 supplementation on NAFLD found in the subgroup analysis suggests that LT4 replacement therapy should be suggested for mild SCH patients with dyslipidemia.
Previously, abnormalities in serum TG were more closely associated with NAFLD than serum cholesterol [39]. In recent years, evidence has suggested that atherogenic cholesterols were related to NAFLD either. Chan et al. reported that a diet enriched with cholesterol was involved in and essential for the development of liver steatosis in animal models [40]. Additionally, disturbances in liver cholesterol metabolism were common among NAFLD patients, suggesting a crucial role of cholesterol metabolism in the progression of NAFLD [41]. Furthermore, a prospective cohort study has indicated that non-HDL-C independently predicted new onset of NAFLD after adjusting for other confounders and was a stronger predictor for NAFLD than TG and LDL-C [42]. In this study, the subgroup analysis revealed that patients treated with LT4 experienced a significantly more profound reduction in serum TC compared with patients who were not treated, whereas serum TG levels were not significantly affected by LT4. Overall, we extrapolated that the decreased atherogenic cholesterol levels might contribute to the improvement of NAFLD.
Another interesting result of the subgroup analysis was that subjects who received LT4 exhibited a significant decrease in weight and BMI. Furthermore, patients who experienced remission of NAFLD exhibited greater decreases in body weight and BMI compared to those without remission of NAFLD. It has been well established that obesity has an independent effect on liver fat [43], and trial data have demonstrated that reduction in weight was associated with a significant improvement in the histological severity of NAFLD [8, 44]. It is plausible that LT4 supplementation could facilitate weight control by increasing the basal metabolic rate and subsequently ameliorating NAFLD. Further studies are needed to illuminate the precise mechanism under the benefits of LT4 supplementation on NAFLD in SCH patients.
There are some limitations of this study. First, this study was a retrospective post hoc analysis of the original randomized controlled trial; thus, the LT4 replacement therapy was not randomized in analyzed subjects. However, the subjects involved in this analysis were well balanced for baseline characteristics among treatment arms. Second, the diagnosis of NAFLD in this study was based on liver ultrasonographic examination. The optimal threshold for detecting steatosis on ultrasonographic imaging is about 30% in fat content [45, 46], which means that hepatic ultrasonography is not sufficiently sensitive to detect mild change in fatty infiltration. Also, ultrasonography has intrapractitioner variability in making a diagnosis. Since the trial was conducted in the rural area, we chose ultrasonography as the preferred method for diagnosis of the fatty liver given its advantage of being widely available and cost effective. Another limitation was that most of the patients in this study exhibited normal serum liver enzymes at enrollment. As a consequence, although we observed statistically significant reductions in serum liver enzymes in LT4-treated SCH patients, the reductions were relatively minor. Well-conducted trials with histological endpoints can provide more profound evidence of the benefits of LT4 on NAFLD.
5. Conclusions
In conclusion, this study demonstrated a beneficial effect of LT4 replacement therapy on NAFLD in patients with SCH, especially in significant SCH patients or mild SCH patients with dyslipidemia, with a decrease in the prevalence of NAFLD and serum liver enzymes. Our findings support the independent role of SCH for NAFLD and suggest the importance of early detection of thyroid function in patients with NAFLD. Moreover, based on our results, treatment of SCH with appropriate LT4 supplementation may be an effective means for controlling NAFLD. Double-blind, placebo-controlled trials with histological endpoints are needed to further validate the clinical benefits of LT4 supplementation on NAFLD.
Supplementary Material
The information of supplementary materials are as follows: S1 file. Sup. Table 1 Efficacy of LT4 on Thyroid Function; S2 file. Changes in Metabolic Characteristics in the Cohort; S3 file. Sup. Table 4 Baseline characteristics for subgroup analysis; S4 file. Sup. Table 5 Number of NAFLD in mild SCH patients with dyslipidemia.
Acknowledgments
The authors acknowledge the financial support from the National Basic Research Program (2012CB524900); the National Natural Science Foundation of China (30901461, 81230018, 81670721, 81370891, and 81270869); the Science and Technology Enabling Program of Shandong Province (2015GGH318016); and the Scientific & Technologic Development Program of Shandong Province (2012GSF11824); the Clinical Research Fund of Chinese Medical Association (15010010589); and the Special Scientific Research Fund of Public Welfare Profession of China (201402005).
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Alimentary Pharmacology and Therapeutics. 2007;25(8):883–889. doi: 10.1111/j.1365-2036.2007.03246.x. [DOI] [PubMed] [Google Scholar]
- 2.Frith J., Day C. P., Henderson E., Burt A. D., Newton J. L. Non-alcoholic fatty liver disease in older people. Gerontology. 2009;55(6):607–613. doi: 10.1159/000235677. [DOI] [PubMed] [Google Scholar]
- 3.Zelber-Sagi S., Nitzan-Kaluski D., Halpern Z., Oren R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver International. 2006;26(7):856–863. doi: 10.1111/j.1478-3231.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 4.Charlton M. R., Burns J. M., Pedersen R. A., Watt K. D., Heimbach J. K., Dierkhising R. A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 5.Liu H., Lu H. Y. Nonalcoholic fatty liver disease and cardiovascular disease. World Journal of Gastroenterology. 2014;20(26):8407–8415. doi: 10.3748/wjg.v20.i26.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung K. C., Kim S. H. Interrelationship between fatty liver and insulin resistance in the development of type 2 diabetes. Journal of Clinical Endocrinology and Metabolism. 2011;96(4):1093–1097. doi: 10.1210/jc.2010-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy T., Anstee Q. M., Day C. P. Nonalcoholic fatty liver disease: new treatments. Current Opinion in Gastroenterology. 2015;31(3):175–183. doi: 10.1097/MOG.0000000000000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong V. W., Chan R. S., Wong G. L., et al. Community-based lifestyle modification programme for non-alcoholic fatty liver disease: a randomized controlled trial. Journal of Hepatology. 2013;59(3):536–542. doi: 10.1016/j.jhep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Weetman A. P. Hypothyroidism: screening and subclinical disease. BMJ. 1997;314(7088):1175–1178. doi: 10.1136/bmj.314.7088.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tunbridge W. M., Evered D. C., Hall R., et al. The spectrum of thyroid disease in a community: the Whickham survey. Clinical Endocrinology. 1977;7(6):481–493. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 11.Marwaha R. K., Tandon N., Ganie M. A., et al. Status of thyroid function in Indian adults: two decades after universal salt iodization. Journal of the Association of Physicians of India. 2012;60:32–36. doi: 10.1002/ece3.2740. [DOI] [PubMed] [Google Scholar]
- 12.Thyroid disease—more research needed. Lancet. 2012;379(9821):p. 1076. doi: 10.1016/S0140-6736(12)60445-0. [DOI] [PubMed] [Google Scholar]
- 13.Chung G. E., Kim D., Kim W., et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. Journal of Hepatology. 2012;57(1):150–156. doi: 10.1016/j.jhep.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Xu L., Ma H., Miao M., Li Y. Impact of subclinical hypothyroidism on the development of non-alcoholic fatty liver disease: a prospective case-control study. Journal of Hepatology. 2012;57(5):1153–1154. doi: 10.1016/j.jhep.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Xu C., Xu L., Yu C., Miao M., Li Y. Association between thyroid function and nonalcoholic fatty liver disease in euthyroid elderly Chinese. Clinical Endocrinology. 2011;75(2):240–246. doi: 10.1111/j.1365-2265.2011.04016.x. [DOI] [PubMed] [Google Scholar]
- 16.Ding W. J., Wang M. M., Wang G. S., Shen F., Qin J. J., Fan J. G. Thyroid function is associated with non-alcoholic fatty liver disease in chronic hepatitis B-infected subjects. Journal of Gastroenterology and Hepatology. 2015;30(12):1753–1758. doi: 10.1111/jgh.12998. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W., Tian L. M., Han Y., et al. Presence of thyrotropin receptor in hepatocytes: not a case of illegitimate transcription. Journal of Cellular and Molecular Medicine. 2009;13(11-12):4636–4642. doi: 10.1111/j.1582-4934.2008.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan F., Wang Q., Lu M., et al. Thyrotropin increases hepatic triglyceride content through upregulation of SREBP-1c activity. Journal of Hepatology. 2014;61(6):1358–1364. doi: 10.1016/j.jhep.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 19.Tian L., Song Y., Xing M., et al. A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology. 2010;52(4):1401–1409. doi: 10.1002/hep.23800. [DOI] [PubMed] [Google Scholar]
- 20.Karmisholt J., Andersen S., Laurberg P. Weight loss after therapy of hypothyroidism is mainly caused by excretion of excess body water associated with myxoedema. Journal of Clinical Endocrinology and Metabolism. 2011;96(1):E99–103. doi: 10.1210/jc.2010-1521. [DOI] [PubMed] [Google Scholar]
- 21.Zhao M., Liu L., Wang F., et al. A worthy finding: decrease in total cholesterol and low-density lipoprotein cholesterol in treated mild subclinical hypothyroidism. Thyroid. 2016;26(8):1019–1029. doi: 10.1016/j.wneu.2017.02.106. [DOI] [PubMed] [Google Scholar]
- 22.Wang F., Tan Y., Wang C., et al. Thyroid-stimulating hormone levels within the reference range are associated with serum lipid profiles independent of thyroid hormones. Journal of Clinical Endocrinology and Metabolism. 2012;97(8):2724–2731. doi: 10.1210/jc.2012-1133. [DOI] [PubMed] [Google Scholar]
- 23.Xu C., Yang X., Liu W., et al. Thyroid stimulating hormone, independent of thyroid hormone, can elevate the serum total cholesterol level in patients with coronary heart disease: a cross-sectional design. Nutrition & Metabolism (London) 2012;9(1):p. 44. doi: 10.1371/journal.ppat.1006266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assy N., Kaita K., Mymin D., Levy C., Rosser B., Minuk G. Fatty infiltration of liver in hyperlipidemic patients. Digestive Diseases and Sciences. 2000;45(10):1929–1934. doi: 10.1023/A:1005661516165. [DOI] [PubMed] [Google Scholar]
- 25.Chen C. H., Huang M. H., Yang J. C., et al. Prevalence and risk factors of nonalcoholic fatty liver disease in an adult population of Taiwan: metabolic significance of nonalcoholic fatty liver disease in nonobese adults. Journal of Clinical Gastroenterology. 2006;40(8):745–752. doi: 10.1097/00004836-200609000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Hamaguchi M., Kojima T., Takeda N., et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Annals of Internal Medicine. 2005;143(10):722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 27.Garber J. R., Cobin R. H., Gharib H., et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Practice. 2012;18(6):988–1028. doi: 10.4158/EP12280.GL. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y., Wang L., He J., et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 29.Hamer O. W., Aguirre D. A., Casola G., Lavine J. E., Woenckhaus M., Sirlin C. B. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26(6):1637–1653. doi: 10.1148/rg.266065004. [DOI] [PubMed] [Google Scholar]
- 30.Zeng M. D., Fan J. G., Lu L. G., et al. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. Journal of Digestive Diseases. 2008;9(2):108–112. doi: 10.1111/j.1751-2980.2008.00331.x. [DOI] [PubMed] [Google Scholar]
- 31.Joy D., Thava V. R., Scott B. B. Diagnosis of fatty liver disease: is biopsy necessary? European Journal of Gastroenterology and Hepatology. 2003;15(5):539–543. doi: 10.1097/01.meg.0000059112.41030.2e. [DOI] [PubMed] [Google Scholar]
- 32.Alberti K. G., Zimmet P. Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 33.E. National Cholesterol Education Program Expert Panel on Detection, A. Treatment of High Blood Cholesterol. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 34.Hsueh K. L., Chen C. M. Guideline for Prevention and Control of Overweight and Obesity in Adult. Beijing: People's Medical Publishing House; 2006. [Google Scholar]
- 35.Velkoska Nakova V., Krstevska B., Bosevski M., Dimitrovski C., Serafimoski V. Dyslipidaemia and hypertension in patients with subclinical hypothyroidism. Prilozi. Contributions. 2009;30(2):93–102. [PubMed] [Google Scholar]
- 36.Bauer D. C., Ettinger B., Browner W. S. Thyroid functions and serum lipids in older women: a population-based study. American Journal of Medicine. 1998;104(6):546–551. doi: 10.1016/S0002-9343(98)00116-8. [DOI] [PubMed] [Google Scholar]
- 37.Wu J. Y., Duan X. Y., Li L., et al. Dyslipidemia in shanghai, China. Preventive Medicine. 2010;51(5):412–415. doi: 10.1016/j.ypmed.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Fu Y. Y., Yu J. M., Wang J. H., et al. Prevalence and awareness of dyslipidemia among overweight and obese population in Beijing community. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38(6):562–566. [PubMed] [Google Scholar]
- 39.Speliotes E. K., Massaro J. M., Hoffmann U., et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51(6):1979–1987. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan J., Sharkey F. E., Kushwaha R. S., Vande Berg J. F., Vande Berg J. L. Steatohepatitis in laboratory opossums exhibiting a high lipemic response to dietary cholesterol and fat. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2012;303(1):G12–G19. doi: 10.1152/ajpgi.00415.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simonen P., Kotronen A., Hallikainen M., et al. Cholesterol synthesis is increased and absorption decreased in non-alcoholic fatty liver disease independent of obesity. Journal of Hepatology. 2011;54(1):153–159. doi: 10.1016/j.jhep.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 42.Zelber-Sagi S., Salomone F., Yeshua H., et al. Non-high-density lipoprotein cholesterol independently predicts new onset of non-alcoholic fatty liver disease. Liver International. 2014;34(6):e128–e135. doi: 10.1111/liv.12318. [DOI] [PubMed] [Google Scholar]
- 43.Milic S., Lulic D., Stimac D. Non-alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World Journal of Gastroenterology. 2014;20(28):9330–9337. doi: 10.3748/wjg.v20.i28.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Promrat K., Kleiner D. E., Niemeier H. M., et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saadeh S., Younossi Z. M., Remer E. M., et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 46.Lee J. Y., Kim K. M., Lee S. G., et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. Journal of Hepatology. 2007;47(2):239–244. doi: 10.1016/j.jhep.2007.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The information of supplementary materials are as follows: S1 file. Sup. Table 1 Efficacy of LT4 on Thyroid Function; S2 file. Changes in Metabolic Characteristics in the Cohort; S3 file. Sup. Table 4 Baseline characteristics for subgroup analysis; S4 file. Sup. Table 5 Number of NAFLD in mild SCH patients with dyslipidemia.