Abstract
First-line therapy for higher-risk myelodysplastic syndromes (MDS) includes decitabine (DAC) or azacitidine (AZA). Variables have not identified differential response rates between these. We assessed the influence of patient sex on outcomes including overall survival (OS) in 642 patients with higher-risk MDS treated with AZA or DAC. DAC-treated patients (35% of females, 31% of males) had marginally better OS than AZA-treated patients (p = .043), (median OS of 18.7 months versus 16.4 months), but the difference varied strongly by sex. Female patients treated with DAC had a longer median OS (21.1 months, 95% CI: 16.0–28.0) than female patients treated with AZA (13.2 months, 95% CI: 11.0–15.9; p = .0014), while for males there was no significant difference between HMAs (median OS 18.3 months with DAC versus 17.9 months for AZA, p = .59). The biological reason for this variability is unclear, but may be a consequence of differences in cytidine deaminase activity between men and women.
Keywords: Sex, hypomethylating agents, MDS, azacitidine, decitabine
Introduction
The myelodysplastic syndromes (MDS) are a heterogeneous collection of clonal hematopoietic malignancies that compromise a large subgroup of the myeloid neoplasms and collectively are the most common acquired adult bone marrow failure syndromes.[1] They are characterized by poor overall survival (OS) due to ineffective hematopoiesis, progressive cytopenias often requiring blood cell transfusions and putting patients at risk for complications such as infections, and variable risk of progression to acute myeloid leukemia (AML). The incidence rate of MDS is ~5 per 100,000, sufficient to rank MDS as the most commonly diagnosed myeloid neoplasm in USA and Europe.[2] Common risk factors for developing MDS include advanced age, male sex, and antecedent exposure to chemotherapy or radiation as treatment for other cancers.[3] Males, in addition to having a higher incidence of MDS, also have worse outcomes than females, for unclear reasons.[4] Analyzes looking at the differences between the sexes for treatment outcomes are useful to add clarity to this issue.
The most commonly used disease-modifying agents in higher-risk MDS are azacitidine (AZA) and decitabine (DAC). Each has been shown to decrease transfusion burden, improve quality of life, and delay progression to AML, and AZA has been additionally shown to extend survival.[5] AZA and DAC are 5-methylated cytidine analog drugs that exert their therapeutic effect though depletion of DNA methyltransferase-1 (DNMT1) after incorporation into DNA and subsequent alteration of methylation patterns across the genome, and are therefore commonly called “hypomethylating agents”.[6] Although similar in structure to DAC, AZA contains ribose (rather than deoxribose) and is incorporated primarily into RNA and to a much lesser extent into DNA. This difference may account for the ~10-fold higher potency of drug intensity of DAC compared with AZA.[7] It has been previously demonstrated in mice and in humans that increased levels of cytidine deaminase (CDA) rapidly inactivate these cytidine analog drugs, leading to decreased drug half-life, particularly. Increased CDA expression/activity in males contributes to decreased cytidine analog half-lives and may contribute to worse outcomes with AZA or DAC therapy in men compared to women.[8]
A risk-adapted treatment strategy is used in MDS to help determine the most appropriate therapies and treatment goals based on individual patient characteristics. Currently, the most commonly used MDS risk stratification tools include the International Prognostic Scoring System ± Revised (IPSS or the IPSS-R [9,10]) and the World Health Organization (WHO) 2008 disease classification,[1] which incorporate cytopenias, blast percentage, cytogenetic risk groups and age. While these systems are useful for guiding choice of therapy, they do not predict response to HMA therapy, and none incorporates sex as a prognostic variable. In this study, we looked at the impact of a patient’s sex on outcome after treatment with AZA or DAC in a cohort of patients evaluated at institutions of the MDS Clinical Research Consortium (CRC). The goal of this analysis was to ascertain if there is an outcome difference by HMA therapy between males and females.
Materials and methods
Patient cohorts
All patients included in this series were diagnosed with MDS or CMML per the 2008 WHO criteria and were evaluated at MDS CRC participating institutions (Johns Hopkins, Cleveland Clinic, H. Lee Moffitt Cancer Center, MD Anderson Cancer Center, Dana-Farber Cancer Institute, and Weill-Cornell Medical College). Clinical data were collected from patients who had consented to medical record review under an Institutional Review Board (IRB) approved protocol at each institution in accordance with the Declaration of Helsinki. Patients were treated with AZA at 75 mg/m2 daily intravenously or subcutaneously for 5–7 days of a 28-day cycle, or with DAC at 20 mg/m2 intravenously for 5 days of a 28-day cycle. Patients were excluded if they received both drugs in succession. Blood product replacement and other supportive care measures were per individual institution practices. All patients had higher-risk MDS by IPSS (intermediate-2 or high risk) or IPSS-R (intermediate, high, or very high risk) calculated at the time of diagnosis and the majority (97.4%) were treated with AZA or DAC as first-line therapy.
Statistical analysis
Missing data were multiply imputed 100 times using the mice approach with random forest imputation per variable. Baseline characteristics and demographics are reported descriptively. Differences among variables were evaluated by the chi-square test and Mann–Whitney U-test for categorical and continuous variables, respectively, with continuous variables summarized by median and range. Responses to therapy were defined as per the International Working Group 2006 (IWG-2006) criteria.[11] According to the best achieved response, patients were categorized into responders (CR, partial response, hematologic improvement and marrow CR) and non-responders [stable disease and progressive disease (PD)]. Overall response rate (ORR) was defined as the sum of patients who were CR, partial response and hematologic improvement. OS was calculated from the time of start of HMA therapy to the time of death or last known follow-up. Propensity scores were calculated for DAC versus AZA using all variables available pretreatment. Propensity for being assigned to take AZA was estimated using a generalized boosting tree method as implemented in the R package twang. Twenty-seven pretreatment variables were considered in the propensity fitting. Propensity scores were estimated using the overall cohort and separately within males and females. All survival regression analyzes (Kaplan–Meier and Cox proportional hazards modeling) were stratified on clinical site and weighted using average treatment effect (ATE) weights from the respective propensity score models. Median OS estimates were also calculated using ATE propensity weights. Log-rank tests were used to compare survival curves and Wald tests were used assess significance of coefficients from Cox models. Survival time from start of HMA therapy was the primary outcome. All analyzes, including propensity estimation, were done within each of the 100 imputations, then combined using the standard multiple imputation combining rules to get final estimates. Fraction of missing information (FMI), which gives a measure of the amount of information for a parameter of interest lost due to the presence of missing data, was calculated for the beta coefficients in the Cox models and for median OS.
Results
Baseline characteristics
A total of 642 higher-risk MDS patients treated with AZA or DAC were included in the analysis. Baseline characteristics by gender and treatment group are shown in Table 1 with corresponding p values. Approximately one-third (33.7%) of the total cohort was female. Of female patients, 35% received DAC as front line HMA compared to 31% of male patients. The median age of female patients was 67.7 years (range 35–84) in the DAC-treated group and 68.9 years (36–91) in the AZA-treated group (p = .20). Cytopenias were comparable across both sexes and treatment cohorts. The groups were matched by IPSS and IPSS-R. By IPSS-R, 47.3% of the DAC-treated females and 43.1% of the AZA-treated females had very poor risk disease. In the female cohort, 46.5% of patients treated with DAC and 47.4% treated with AZA progressed to AML. In the male cohort, 44.8% of patients treated with DAC and 43.1% treated with AZA progressed to AML.
Table 1.
Patient characteristics by treatment with azacitdine (AZA) or DAC by sex at time of diagnosis.
| Parameters | Female (N = 216, 33.6%)
|
Male (N = 427, 66.3%)
|
||||
|---|---|---|---|---|---|---|
| DAC (N = 75, 35%) | AZA (N = 141, 65%) | p value | DAC (N = 133, 31%) | AZA (N = 294, 69%) | p value | |
| Age | 67.7 (35–84) | 68.9 (36–91) | .20 | 70 (38–89) | 70 (31–99) | .70 |
| Race | .34 | .56 | ||||
| Hispanic | 1, 1.3% | 2, 1.4% | 2, 1.5% | 5, 1.7% | ||
| Black | 6, 8% | 5, 3.6% | 8, 6.0% | 10, 3.4% | ||
| Other | 10, 13.3% | 11, 7.9% | 3, 2.3% | 12, 4.1% | ||
| White | 58, 77.3% | 122, 87.1% | 120, 90.2% | 267, 77.3% | ||
| ECOG PS | .34 | .09 | ||||
| 0 | 16, 21.6% | 22, 15.6% | 34, 25.6% | 61, 20.7% | ||
| 1 | 50, 67.6% | 101, 71.6% | 83, 62.4% | 212, 72.1% | ||
| 2 | 8, 6.1% | 17, 12.1% | 16, 12% | 18, 6.1% | ||
| 3 | 0, 0% | 1, 0.7% | 0, 0% | 2, 0.7% | ||
| WHO category | .89 | .05 | ||||
| RAEB-2 | 45, 59.2% | 75, 53.2% | 87, 65.4% | 143, 48.5% | ||
| RAEB-1 | 20, 26.3% | 38, 27% | 22,16.5% | 90, 30.5% | ||
| RCMD | 6, 7.9% | 16, 11.3% | 13, 9.8% | 42, 14.2% | ||
| RARS +del5q | 2, 2.6% | 4, 2.8% | 3, 2.3% | 5, 1.7% | ||
| Other | 3, 3.9% | 8, 5.7% | 8, 6% | 15, 5.1% | ||
| Cytogenetics | .29 | .06 | ||||
| Good | 19, 25.3% | 24, 17.1% | 40, 30.3% | 57, 19.4% | ||
| Intermediate | 16, 21.3% | 27,19.3% | 24,18.2% | 63, 21.4% | ||
| Poor | 40, 53.3% | 89, 63.1% | 68, 51.5% | 174, 59.2% | ||
| Transfusion dependent | 38, 50.7% | 111, 78.7% | <.001 | 73, 54.9% | 196, 66.7% | .028 |
| Hb | 9.47 (4.39–13.29) | 9.27 (4.83–13.58) | .51 | 9.40 (4.09–13.45) | 9.16 (3.00–14.96) | .16 |
| Plts | 58.13 (2.0–409.15) | 59.38 (4.93–652.2) | .97 | 56.50 (3.0–654.0) | 66.49 (2.49–460.69) | .23 |
| WBC | 2.85 (0.70–53.22) | 2.70 (0.72–69.13) | .60 | 2.61 (0.5–89.50) | 2.73 (0.50–60.27) | .69 |
| ANC | 1.09 (0.04–28.17) | 0.88 (0.09–36.95) | .30 | 1.04 (0.02–45.15) | 1.02 (0.01–45.19 | .76 |
| Marrow blasts | 12.3 (0.09–30.5) | 10 (0–84) | <.001 | 13 (0–28.97) | 9.78 (0–83) | <.001 |
| Peripheral blasts | 0.47 (0–22) | 0 (0–38) | .002 | 0 (0–19) | 0 (0–21.12) | <.001 |
| IPSS | .19 | .004 | ||||
| High | 28, 37.3% | 39, 27.9% | 55, 41.4% | 79, 26.9% | ||
| Intermediate-2 | 47, 62.7% | 101,72.1% | 78, 58.6% | 215, 73.1% | ||
| IPSS-R | .83 | .98 | ||||
| Very high | 39, 52% | 85, 60.3% | 76, 57.1% | 156, 53.1% | ||
| High | 26, 29.1% | 41, 29.1% | 42, 31.6% | 103, 35% | ||
| Intermediate | 10, 10.6% | 15, 10.6% | 15, 11.3% | 29, 9.9% | ||
| Treatment-related MDS | 25, 33.3% | 52, 37.1% | .55 | 38, 28.6% | 71, 24.1% | .31 |
| HMA as initial therapy | 73, 97.3% | 136, 96.5% | .79 | 104, 98.5% | 238, 97.3% | .49 |
RAEB: refractory anemia with excess blasts; RCMD: refractory cytopenias with multilineage dysplasis; RARS: refractory anemia with ring sinderoblasts; ECOG PS: Eastern Cooperative Oncology Group Performance status; HMA: hypomethylating agent; Hb: hemoglobin; Plts: platelets; WBC: white blood cells; ANC: absolute neutrophil count; IPSS: International Prognostic Scoring System; IPSS-R: Revised International Prognostic Scoring System. Categorical variables reported with ranges and continuous variables reported as percentages.
Focusing on differences in variables between sexes for each treatment, bone marrow blast percentage at diagnosis was higher in the female DAC-treated group 12.3% (0.09–30.5%) compared to 10% (0–84%) in the AZA group (p ≤ .001), while there were more patients with RAEB-2 in the male DAC group compared to the AZA group (65.4 versus 48.55%, p = .05).
Responses to AZA and DAC: patient outcomes
Clinical site and transfusion dependency at start of HMA therapy remained imbalanced between the AZA and DAC groups both overall and within sex subsets after propensity weighting and were included in the Cox regression modeling. All other pretreatment variables showed good balance between the AZA and DAC groups after propensity weighting. DAC-treated females were on treatment longer than AZA-treated females. The time on therapy for females was 6 months (0.13–30.1) for DAC treatment and 4.2 months (range 0.14–41.3) for AZA treatment. In the male cohort, it was 4.7 (0.10–38.8) months on DAC and 5.4 months (range 0.03–55) on AZA.
The ORR for the entire cohort to any hypomethylating therapy when best objective response was evaluated was 42.1% (CR, 21.0%; partial response, 9.8%; hematologic improvement, 11.3%), whereas in 57.9% no objective response was achieved (stable disease, 38.4%; PD, 19.5%). The ORR for AZA-treated females was 40% compared to 53.3% for DAC-treated females (p <.001) and the ORR for AZA-treated males was 38.4% compared to 47.4% for DAC-treated males (p <.001).
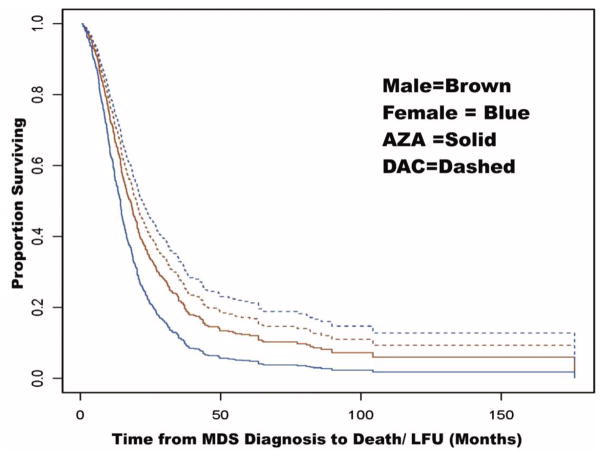
The median OS from the start of HMA therapy for the cohort was 15.1 months (95% CI: 13.8–16.4, FMI = 0.06); 15.9 months for males (95% CI: 14.5–17.4, FMI = 0.03) and 13.0 months for females (95% CI: 10.9–15.5, FMI = 0.05). DAC-treated patients had marginally better OS than AZA patients (p = .140), with a median OS of 16.8 months (95% CI: 13.9–20.3, FMI= 0.03) compared to 14.4 months (95% CI: 13.2–15.6, FMI = 0.11), respectively. Female patients treated with DAC had significantly better OS than female patients treated with AZA (p = .0031), with a median OS of 18.9 months (95% CI: 13.8–26.1, FMI = 0.08) versus 11.4 months (95% CI: 9.6–13.7, FMI = 0.05), respectively. Males patients treated with AZA and DAC had similar OS (p = .78), with a median OS of 16.3 months (95% CI: 12.7–20.9, FMI = .01) compared to 15.6 months (95% CI: 14.0–17.3, FMI = .04), respectively (Figure 1). Table 2 shows the hazard ratio (HR) estimates of DAC versus AZA from Cox proportional hazard models, which were propensity-weighted and stratified by site. Females treated with DAC continued to have improved OS compared to females treated with AZA in a Cox proportional hazard analysis (Table 2, HR = 0.57, p = .051) after adjusting for transfusion dependence at the start of HMA therapy, which along with site were the only pretreatment covariates that showed significant imbalance across treatment groups after propensity correction.
Figure 1.
OS by treatment with AZA or DAC by sex from time of disease diagnosis.
Table 2.
HR estimates of DAC versus AZA.
| Subset | Model | Predictor | HR | 95% CI for HR | p value | FMI |
|---|---|---|---|---|---|---|
| Overall | TD + gender + HMA | Transfusion dependent at start | 1.78 | (1.41, 2.25) | <.001 | 0.15 |
| Male versus female | 0.95 | (0.76, 1.19) | .68 | 0.15 | ||
| DAC versus AZA | 0.87 | (0.67, 1.15) | .33 | 0.14 | ||
| Females | HMA only | DAC versus AZA | 0.57 | (0.38, 0.86) | .0075 | 0.11 |
| TD + HMA | Transfusion dependent at start | 1.96 | (1.32, 2.92) | <.001 | 0.14 | |
| DAC versus AZA | 0.67 | (0.45, 1.00) | .051 | 0.14 | ||
| Males | HMA only | DAC versus AZA | 0.98 | (0.69, 1.38) | .89 | 0.10 |
| TD + HMA | Transfusion dependent at start | 1.60 | (1.23, 2.09) | <.001 | 0.11 | |
| DAC versus AZA | 0.96 | (0.68, 1.35) | .80 | 0.12 |
TD: transfusion dependence; HMA: hypomethylating agent.
HR estimates from propensity-weighted stratified (by site) cox proportional hazards models of survival time from start of HMA therapy. Propensity weights re-estimated within each subset.
Discussion
Current therapies for MDS are inadequate and research into new treatments is needed. Knowledge about the molecular biology of MDS has far outstripped the availability of novel therapies directed at identified candidate clonal driver mutations. For example, while it has been determined that patients with TET2 mutations may be more responsive to HMAs than patients whose marrow cells are TET2 wild-type, ultimately almost all patients with higher-risk disease will be treated with these drugs, since HMAs have been shown to delay disease progression and improve survival and there are few effective alternatives.[12–14]
DAC and AZA have never been compared head-to-head in a published prospective trial in higher-risk MDS, and as both drugs are at the end of their patent life, it seems unlikely that such a trial will ever be conducted. Clinicians currently choose between AZA and DAC based on comparison of data across studies with varying design, or based on factors unrelated to effectiveness such as treatment scheduling or economic considerations. Identifying a reliable variable that can be used at MDS diagnosis to choose between the two available HMAs could be clinically useful.
We used a large dataset of patients assembled from six centers to determine whether differences existed in response and outcome, between sexes, treated with AZA or DAC. No differences were observed in median OS across sex, and DAC-treated patients had marginally better OS than AZA patients. Female DAC-treated patients had much better OS than female AZA-treated patients, but for males there was no difference in outcome based on HMA choice. The improvement in survival of female DAC-treated patients remained significant in a Cox PH analysis after adjusting for other variables including cytogenetic category and bone marrow blast percentage at diagnosis. It should be noted, however, that AZA-treated female patient cohort had a significant percentage of patients with poor cytogenetics, transfusion dependence, and very high risk IPSS-R scores; these negative prognostic markers were more prevalent than in the DAC-treated female cohort. When transfusion dependence was included in the multivariate analysis, the negative effect of AZA in female patients was not found. Additionally the females treated with DAC were on therapy longer than those on AZA. It is unclear in this restrospective analysis if this is the cause or consequence of the outcome difference for HMA therapy in females as all patients’ outcomes were not examined at the same time from start of therapy.
These sex differences in survival have a potential biologic rationale. Higher CDA expression within malignant myeloid cells from males has been hypothesized to contribute to worse outcomes, as upregulation of CDA expression in malignant cells can lower intracellular cytidine analog levels and has been implicated as a mechanism of resistance to HMAs [8,15–17]. It is possible that CDA inactivates AZA more rapidly than DAC in females and thus shows a more pronounced effect in women compared to men. It is theoretically possible that adjusting drug doses based on sex could overcome these differences, though it is unknown whether this would be clinically feasible with the expected increase in attendant side effects with higher drug doses. This would likely be only one facet of personalizing therapy though, given that DAC is more dose-intense. Other possible mechanisms for a sex difference in HMA effectiveness include altered drug metabolism or dosage of genes encoded on the region of the pseudo-autosomal region of the X chromosome, some of which contribute to DNA methylation or chromatin conformation.
Sex differences in OS have been observed in other MDS patient cohorts: In 856 mostly untreated patients with MDS (50% did not receive any therapy, 17% received therapy such as HMAs), significantly worse OS was observed in males, even those treated with HMAs.[18] In another cohort of 897 untreated patients with MDS, significantly poorer OS was again observed in males.[19] Within HMA-treated cohorts, there has also been a noted difference in median survival times between the sexes with shorter median OS in males compared to females. In 99 DAC-treated patients with MDS, the median OS in males was 399 days compared with 529 days in females (no p value reported).[20] In another study of 177 DAC-treated patients with MDS, the OS in males compared with females was 14 versus 17 months, though this difference was not statistically significant (p = .41).[21] Our study differs from these previously done in that it was multicenter and looked specifically in the multivariable analysis to determine sex differences between the two available HMAs. The sex differences in OS documented in untreated patients and in MDS incidence also suggest that there are likely additional unknown factors beyond higher CDA expression that contribute to poorer OS in males. Observing differential survival of men compared to women who receive the investigational HMA SGI-110 (guadecitabine) in ongoing trials in MDS and AML may give further insight into the likelihood of this mechanism, as SGI-110 is designed to neutralize CDA.
This observed difference in outcome by sex could be explored prospectively, for example by using bio-markers of the intended pharmacodynamic effect (e.g. DNA methylation or DNMT1 levels), to guide adjustments to therapy. Pharmacodynamic biomarkers could simultaneously account for the effects of other pharmacogenetic factors, such as the CDA single nucleotide polymorphism A79C, which influences CDA activity,[8] especially in females. Measuring CDA enzyme activity has also been proposed as a guide to dose modification with other chemotherapy.[22] A complementary approach would be to dampen the influence of CDA altogether, by combination therapy with a CDA inhibitor such as CDAi tetrahydrouridine.[23,24] There is an active clinical trial investigation of a newer CDA inhibitor being administered concomitantly with DAC to enhance its bioavailability, whose preliminary results appear promising.[25]
In conclusion, we observed that men treated with DAC or AZA may have inferior outcomes to women treated similarly and that women treated with DAC had better outcomes than women treated with AZA. Importantly, one of the potential mechanisms for worse outcomes should be amenable to rational modifications to treatment dose, schedule, or formulation in order to ultimately improve patient outcomes in the MDS community.
Supplementary Material
Acknowledgments
We would like to thank the patients who contributed to this large database and their families, as well the research coordinators in the six sites who worked to construct the database. The authors would also like to thank the Edward P. Evans Foundation and the Aplastic Anemia and Myelodysplastic Syndromes Foundation for supporting the research activities of the MDS Clinical Research Consortium. This research was presented in part at the American Society of Hematology 57th Annual meeting in Orlando, FL, USA in December 2015.
Footnotes
Preliminary results were presented in abstract form at the 2015 American Society of Hematology (ASH) Annual Meeting in Orlando, Florida.
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at http://dx.doi.org/10.1080/10428194.2016.1246726.
Supplemental data for this article can be accessed here.
References
- 1.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 2.Cogle CR, Iannacone MR, Yu D, et al. High rate of uncaptured myelodysplastic syndrome cases and an improved method of case ascertainment. Leuk Res. 2014;38:71–75. doi: 10.1016/j.leukres.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 3.Sekeres MA. Epidemiology, natural history, and practice patterns of patients with myelodysplastic syndromes in 2010. JNCCN. 2011;9:57–63. doi: 10.6004/jnccn.2011.0006. [DOI] [PubMed] [Google Scholar]
- 4.Sekeres MA. The myelodysplastic syndromes. Exp Opin Biol Ther. 2007;7:369–377. doi: 10.1517/14712598.7.3.369. [DOI] [PubMed] [Google Scholar]
- 5.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomized, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santi DV, Garrett CE, Barr PJ. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell. 1983;33:9–10. doi: 10.1016/0092-8674(83)90327-6. [DOI] [PubMed] [Google Scholar]
- 7.Creusot F, Acs G, Christman JK. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2′-deoxycytidine. J Biol Chem. 1982;257:2041–2048. [PubMed] [Google Scholar]
- 8.Mahfouz RZ, Jankowska A, Ebrahem Q, et al. Increased CDA expression/activity in males contributes to decreased cytidine analog half-life and likely contributes to worse outcomes with 5-azacytidine or decitabine therapy. Clin Cancer Res. 2013;19:938–948. doi: 10.1158/1078-0432.CCR-12-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 10.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 12.Itzykson R, Thepot S, Berthon C, et al. Azacitidine for the treatment of relapsed and refractory AML in older patients. Leuk Res. 2015;39:124–130. doi: 10.1016/j.leukres.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Bejar R. Myelodysplastic syndromes diagnosis: what is the role of molecular testing? Curr Hematol Malign Rep. 2015;10:282–291. doi: 10.1007/s11899-015-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bejar R. Clinical and genetic predictors of prognosis in myelodysplastic syndromes. Haematologica. 2014;99:956–964. doi: 10.3324/haematol.2013.085217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steuart CD, Burke PJ. Cytidine deaminase and the development of resistance to arabinosyl cytosine. Nat New Biol. 1971;233:109–110. doi: 10.1038/newbio233109a0. [DOI] [PubMed] [Google Scholar]
- 16.Braess J, Pfortner J, Kern W, et al. Cytidine deaminase - the methodological relevance of AraC deamination for ex vivo experiments using cultured cell lines, fresh leukemic blasts, and normal bone marrow cells. Ann Hematol. 1999;78:514–520. doi: 10.1007/s002770050548. [DOI] [PubMed] [Google Scholar]
- 17.Ohta T, Hori H, Ogawa M, et al. Impact of cytidine deaminase activity on intrinsic resistance to cytarabine in carcinoma cells. Oncol Rep. 2004;12:1115–1120. [PubMed] [Google Scholar]
- 18.Garcia-Manero G, Shan J, Faderl S, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22:538–543. doi: 10.1038/sj.leu.2405070. [DOI] [PubMed] [Google Scholar]
- 19.Nosslinger T, Tuchler H, Germing U, et al. Prognostic impact of age and gender in 897 untreated patients with primary myelodysplastic syndromes. Ann Oncol. 2010;21:120–125. doi: 10.1093/annonc/mdp264. [DOI] [PubMed] [Google Scholar]
- 20.Pitako JA, Haas PS, Van den Bosch J, et al. Quantification of outpatient management and hospitalization of patients with high-risk myelodysplastic syndrome treated with low-dose decitabine. Ann Hematol. 2005;84(Suppl 1):25–31. doi: 10.1007/s00277-005-0007-y. [DOI] [PubMed] [Google Scholar]
- 21.Wijermans PW, Lubbert M, Verhoef G, et al. An epigenetic approach to the treatment of advanced MDS; the experience with the DNA demethylating agent 5-aza-2′-deoxycytidine (decitabine) in 177 patients. Ann Hematol. 2005;84(Suppl 1):9–17. doi: 10.1007/s00277-005-0012-1. [DOI] [PubMed] [Google Scholar]
- 22.Ciccolini J, Dahan L, Andre N, et al. Cytidine deaminase residual activity in serum is a predictive marker of early severe toxicities in adults after gemcitabine-based chemotherapies. J Clin Oncol. 2010;28:160–165. doi: 10.1200/JCO.2009.24.4491. [DOI] [PubMed] [Google Scholar]
- 23.Beumer JH, Eiseman JL, Parise RA, et al. Modulation of gemcitabine (2′,2′-difluoro-2′-deoxycytidine) pharmacokinetics, metabolism, and bioavailability in mice by 3,4,5,6-tetrahydrouridine. Clin Cancer Res. 2008;14:3529–3535. doi: 10.1158/1078-0432.CCR-07-4885. [DOI] [PubMed] [Google Scholar]
- 24.Beumer JH, Eiseman JL, Gilbert JA, et al. Plasma pharmacokinetics and oral bioavailability of the 3,4,5,6-tetrahydrouridine (THU) prodrug, triacetyl-THU (taTHU), in mice. Cancer Chemother Pharmacol. 2011;67:421–430. doi: 10.1007/s00280-010-1337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savona MR, Odenike O, Amrein PC, et al. Results of first in human (FIH) phase 1 pharmacokinetic (PK) guided dose-escalation study of ASTX727, a combination of the oral cytidine deaminase inhibitor (CDAi) E7727 with oral decitabine in subjects with myelodysplastic syndromes (MDS) Blood. 2015;126 Abstract 1683. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.