Abstract
Purpose
In the ‘placebo arm’ of a recent study we found that aerosol saline (sham treatment) produced substantial relief of laboratory-induced dyspnea (Breathing Discomfort – BD) in nearly half the subjects. The sham intervention included a physiological change, and instructions to subjects could have produced expectation of dyspnea relief. In the present study we attempted to discover whether the response to sham aerosol was driven by behavioral or physiological aspects of the intervention.
Methods
Dyspnea (air hunger) was evoked by constraining tidal volume during graded hypercapnia. We measured Petco2 vs BD relationship before and after aerosol saline. To minimize subjects’ expectations of dyspnea relief, participants in were clearly instructed that we would only deliver saline aerosol. In Protocol 1, we delivered aerosol saline with a ventilator (mimicking our prior study); in Protocol 2, we delivered aerosol without a ventilator.
Results
Administration of aerosol saline had little effect on BD in this group of subjects with one exception: one subject experienced appreciable reduction in BD in Protocol 1. This treatment effect was less in Protocol 2. The two most likely explanations are a) that procedures surrounding ventilator administration of aerosol produced a psychological placebo treatment effect even though the subject knew a drug was not given, b) there were behavioral changes in breathing undetected by our measurements of respiratory flow and volume that altered the subjects comfort.
Conclusion
When the expectation of treatment effect is minimized, a significant reduction in dyspnea in response to saline placebo is uncommon but not impossible.
Keywords: Dyspnea, placebo, symptom management, aerosol treatment
INTRODUCTION
Aerosolized furosemide been tested in clinical and laboratory settings as a palliative treatment for dyspnea [1–9]. Results remain highly variable - aerosolized furosemide has proven effective in reducing dyspnea in many individuals, but has been ineffective in many others. In the ‘placebo arm’ of our most recent study of aerosol furosemide, we found that aerosol saline produced substantial relief of laboratory-induced dyspnea in nearly half the subjects; the relief was comparable to that produced by furosemide, but usually not in the same subjects (unpublished data). Neither treatment produced worsening of dyspnea, thus results are unlikely to be due to random measurement noise. The frequent occurrence of a substantial positive treatment effect following saline puzzled us, as it had not been seen in prior studies of laboratory dyspnea in which the placebo arm was aerosol saline [2,3] or injected saline, [10]. Our recent study differed from earlier studies in that aerosol delivery was controlled by a mechanical ventilator, and in that subject expectations may have been heightened by our blinding procedures.
Because saline and drug were about equally effective, we hypothesized that saline administration via ventilator had either an unexpected physiological effect or a psychological ‘placebo’ effect, although a physiological effect of aerosol saline could not be entirely ruled out. Expectations play a large role in placebo effect [e.g., 11,12]. These expectations may have been accentuated in our earlier study by the subjects’ desire for the experiment to succeed in finding a treatment to relieve patient suffering. To balance expectations, subjects in that study were informed that we were investigating two drugs to palliate dyspnea –the subjects were informed that on each of the test days they would receive one of two drugs – furosemide or albuterol – in reality they received furosemide on one day and saline on the other. In our care to balance expectations between saline and drug, we may have biased the subjects to expect drug action on both days. In addition, the more elaborate aerosol administration procedures may have provided stronger cues for psychological placebo response.
The present study was an attempt to control for the effect of expectation, and to explore the effect of method of aerosol delivery, and to test the possibility of a physiological effect of saline. In one protocol, we delivered aerosol saline with a ventilator; in a second, we delivered the aerosol without a ventilator. Subjects in the present study clearly understood that they would only receive an inactive saline aerosol.
METHODS
Subjects and briefing
We studied 6 healthy subjects, 3 were women, and the average age was 31 years (one subject failed to follow instructions, and her data were excluded, thus 5 subjects were analyzed). Subjects were screened for chronic and acute medical problems and for the ability to consistently rate dyspnea. More detail about the screening criteria and subjects will be found in Online Resource Table S-1. Subjects were informed that the lab was preparing to do experiments on an aerosol drug, but needed to sort out the effect on perception of the method of administration before proceeding with the drug studies. Subjects were clearly informed during the consent process and in subsequent laboratory instructions that they would be getting only saline aerosol: “The main purpose of this study is to determine how different methods for delivering aerosol to the lung affect unpleasant breathing sensations (dyspnea or shortness of breath). Drugs are delivered using these methods, but in this study you will not inhale a drug. You will inhale an inactive saline mist.”
Basic Procedures
Dyspnea stimulus
We used the same laboratory model to evoke dyspnea as was used in our recent study of furosemide; this model has been used several times in prior studies [13,10,14]. In brief, we induced dyspnea in healthy subjects before and after drug administration by varying inspired Pco2 while restricting minute ventilation to 0.13 liters•min−1•kg−1 by limiting the flow into an anesthetic bag. Respiratory frequency (fR) was fixed by instructing the subject to breathe in time with a metronomic sound, thereby also fixing VT. Each new level of Petco2 was achieved as rapidly as possible (step change) by overshooting or undershooting the inspired level at the beginning of the step (See Fig S-1 in Online Resource). Subjects took 3 large breaths at step transitions to speed gas changes and to ‘reset’ sensation. Each level was approximately 3 min in duration, the shortest time to allow sensation to reach near steady state [15]. In contrast to our earlier study in which monotonic increase in stimulus was employed, we both raised and lowered the stimulus (Petco2) from one step to the next in order to reduce the subject’s expectation of stimulus order.
Dyspnea Measurement
Subjects rated “Breathing Discomfort” on a Visual Analog Scale (BDVAS; 0 was defined as no breathing discomfort, 100% of scale was defined as intolerable breathing discomfort). Subjects were asked to describe their breathing discomfort in the last 30 seconds of each run using the Multidimensional Dyspnea Profile [16].
Methods for aerosol administration
We utilized three ultrasonic screen nebulizers (Aeroneb Solo, Aerogen Ltd, Galway, Ireland) producing an aerosol of 3.4microns ±2.2GSD from 8 ml of saline. On separate days, we used two protocols for aerosol administration. Six subjects completed Protocol 1; all subjects except AF31 returned for Protocol 2.
Protocol 1 utilized the same aerosol administration method used in our recent study of furosemide. In brief, to optimize deposition we controlled inspiratory flow (300–500 ml/s) and tidal volume (15% predicted vital capacity) using a standard clinical ventilator (Siemens Servo 900c, Siemens Elema AB, Solna, Sweden). The nebulizers were attached to a manifold in series with the inspiratory line, which provided a collection space for aerosol accumulation during the expiratory phase thereby maximizing delivery of aerosol on the ensuing breath. Respiratory frequency was set at 13–15, yielding a mean minute ventilation of 10.5 L/min and mean Petco2 of 32.5 Torr at the end of administration.
Protocol 2, utilized the same nebulizers and manifold for aerosol administration, but in Protocol 2 subjects inspired the aerosol without using the ventilator. Flow was directed by one-way valves so that the aerosol accumulated during the expiratory phase was delivered with the following inspiration. Subjects were instructed to take slow breaths that were slightly bigger than normal; respiratory frequency ranged from 9–15, VT ranged from 0.63–1.0 L, yielding a mean minute ventilation of 10.2 L/min and mean Petco2 of 33.1 Torr at the end of administration.
Treatment Testing Procedure
As in prior studies, subjects were assessed for consistency of sensory ratings vs stimulus intensity, and were rehearsed on 1 or 2 ‘run-up’ days prior to treatment testing. Subjects who exhibited poor correlation (R<0.7) between stimulus (Petco2) and response (BDVAS) were excluded before treatment studies. (See the Online Resource section on Methods for account of excluded subjects.) Subjects participated in treatment test studies on 2 days and were informed that they would receive only saline on both days. The method of aerosol administration was the only difference between the 2 days. The protocol was double blinded in that the subject did not know what levels of Petco2 were being administered, and on treatment test days the operator administering the aerosolized saline did not know what responses (BDVAS ratings) the subject was giving following treatment.
Data quality control
The time traces of physiological variables and ratings were examined, and individual data points were excluded if any of the following a priori exceptions occurred during data point collection periods: 1) retention of air by the subject that raised end-expiratory volume by more than 50% of tidal volume, and consequently raised the subsequent end-inspiratory volumes (such volume increases reduce breathing discomfort [17]). 2) failure of the limiting bag to collapse on inspiration (at very low levels of stimulus drive), thus not fulfilling the criterion of limited ventilation. 3) failure of the subject to keep reasonable time with the metronomic sound – minor departures (e.g., to swallow) were accepted, but major departures (e.g., behavioral tachypnea) causing more than 20% change in fR (and consequent change in VT) were excluded. Subject AF 32 always exhibited behavioral tachypnea at higher stimuli, thus had to be excluded entirely.
Analysis
To obtain a single metric for statistical testing of response to treatment, we used the same analysis approach established a priori for our earlier studies of IV morphine and aerosol furosemide [10]. In brief, using linear regression we found the Petco2 at which BDVAS=60%Full Scale (%FS) prior to treatment (excluding points at Petco2 exceeding 1.5 torr below resting). We required a minimum of 4 data points for each regression, and required good correlation between stimulus and response (R2 ≥.49) prior to treatment. After treatment, we determined BDVAS at the same Petco2, again using linear regression. The post-pre treatment difference in BDVAS at this Petco2 was defined as “treatment effect” – a negative number indicating that the treatment reduced breathing discomfort. In addition, we examined the time traces of physiological records and the full data plots before and after treatment. In examining these plots and the associated time traces, we noticed that the BDVAS response to Petco2 downsteps was often slower than the response to upsteps; therefore BDVAS in these steps did not approach steady state. As a result the correlation between stimulus and response was often unacceptable (R≪0.49). Consequently, the main analysis presented here excludes the down steps, but the results including downsteps are shown in the Online Resource.
RESULTS
Protocol 1
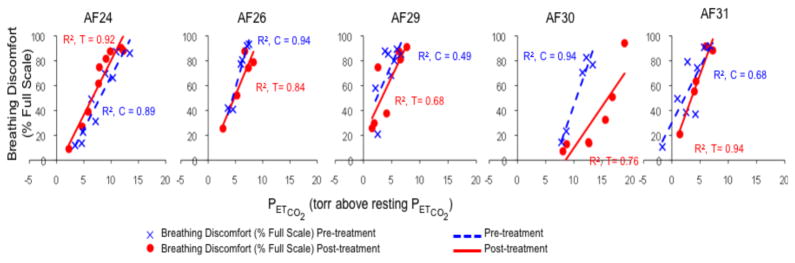
With a single exception, administration of aerosol saline by ventilator had little effect on breathing discomfort in this group of subjects. Examination of plots (See Fig 1) showed substantial overlap between pre- and post-treatment data points. Subject AF30 showed a substantial treatment effect with ventilator delivered aerosol, and examination of the plot reveals very good rating consistency and good separation between pre- and post-treatment data points.
Fig. 1.
Individual results for Protocol 1. Plots depict all up-step data points, regression lines, and coefficients of determination (r2). Blue indicates pre-treatment values, red indicates post-treatment values. In Protocol 1, saline aerosol was delivered with a ventilator to control inspiratory flow and volume.
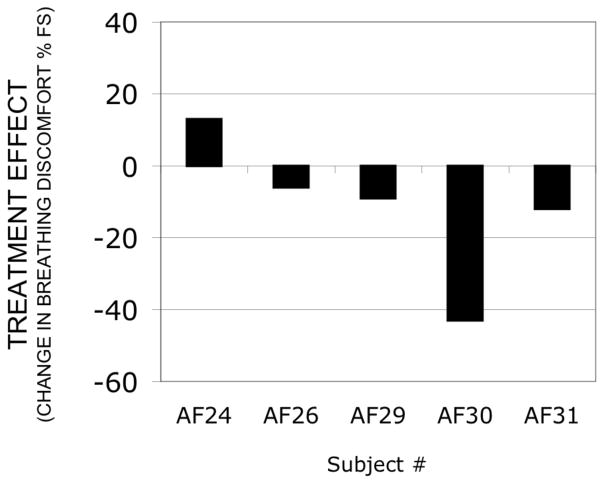
Treatment effect calculation allows us to provide a single number to compare subjects responses. As shown in Fig 2 treatment effect was small in 4 of 5 of the subjects (12%FS or less).
Fig. 2.
Treatment effect of aerosol saline delivered by ventilator to 5 subjects (Protocol 1). Negative values represent relief of dyspnea by treatment. Maximum possible treatment effects range from +40 to −60. Subject AF 32 was excluded because of tachypnea during stimulus presentation.
Removal of downsteps, while considerably improving stimulus-response correlations, had no effect on conclusions – see Online Resource Fig S-3. As previously described, one subject (AF32) was excluded because elimination of data points due to pronounced tachypnea left insufficient data to analyze.
Protocol 2
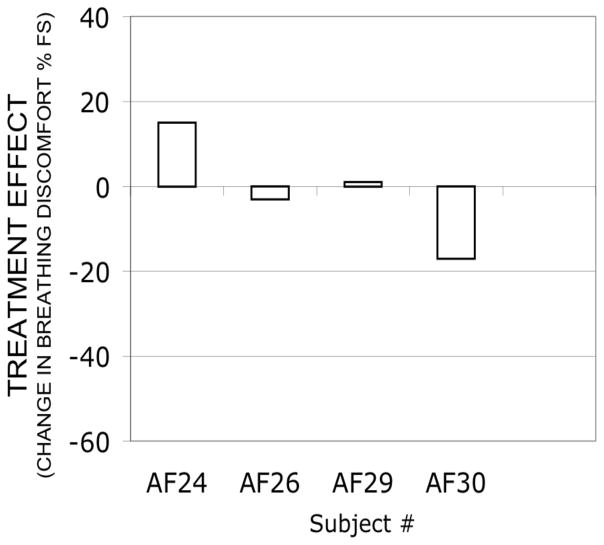
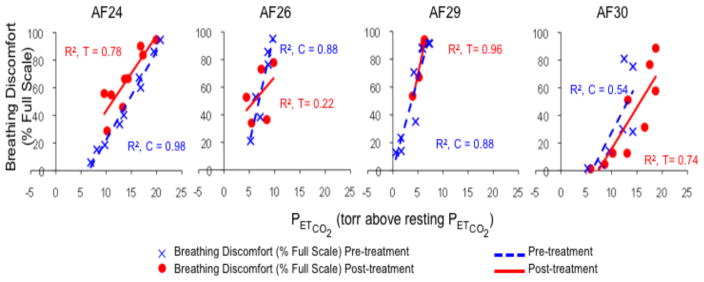
Administration of aerosol saline by spontaneous inhalation had little effect on breathing discomfort in this group of subjects. Treatment effect was small (Fig 3) and examination of plots (Fig 4) showed substantial overlap between pre- and post-treatment data points in all subjects, although subject AF24 exhibited a slight ‘wrong way’ effect, and subject AF30 exhibited a slight treatment effect.
Fig. 3.
Treatment effect of aerosol saline inhaled by 4 subjects (Protocol 2). Negative values represent relief of dyspnea by treatment. Maximum possible treatment effects range from +40 to −60. Subject AF 32 was excluded because of tachypnea; Subject AF31 dropped out before this protocol.
Fig. 4.
Individual results for Protocol 2. Plots depict all up-step data points, regression lines, and coefficients of determination (r2). Blue indicates pre-treatment values, red indicates post-treatment values. In Protocol 2, subjects inspired the saline aerosol having been instructed to take slow, deep breaths.
Multidimensional description subjects’ experience
Results of the MDP from practice or pre-treatment trials are shown in Online Resource Figure S-2 to better describe the stimulus. The ‘Air hunger’ descriptor cluster (I am not getting enough air or I am smothering or I feel hunger for air) was chosen as the most apt descriptor of discomfort 25 times in 31 opportunities when overall discomfort was ≥5. ‘My breathing requires mental effort or concentration’ was chosen as the most apt descriptor of discomfort 5/9 opportunities when overall discomfort was <5. The description did not change with aerosol saline treatment – in 14/17 opportunities subject chose air hunger when overall discomfort was ≥5.
DISCUSSION
With a single exception, the administration of saline aerosol had no appreciable effect on breathing discomfort in these subjects who were instructed that the purpose of the study was to test aerosol delivery methods using a neutral isotonic saline solution. Although the small number of subjects does not allow us to estimate the frequency of placebo responders in the population, our finding of one in five agrees with estimates from the pain literature suggesting that about 30% of patients respond to placebo with a measurable effect [18,19].
In the single subject (AF30) there was an appreciable treatment effect when aerosol was administered via ventilator. This treatment effect was less when the subject inhaled saline aerosol without the ventilator. Subject AF30 provided similar MDP description of the sensory quality experience as the other subjects (mainly air hunger), but he had a tendency to rate breathing-related anxiety greater than average at a given overall breathing discomfort (A1); this was true both before and after treatment; however, he was not the subject who rated the highest breathing-related anxiety.
There are several possible explanations for this single substantial treatment effect in our study:
Mechanical ventilation had some effect on breathing discomfort in this subject alone that lasted from 10 to 50 min after ventilation was discontinued;
This was a random variation in subject report; i.e., ‘noise’.
The saline itself had a physiological effect reducing breathing discomfort;
There were behavioral changes in breathing undetected by our measurements that altered the subjects comfort. For example, retention of volume would cause greater lung stretch receptor activity, which is known to reduce air hunger. An increase in end-expiratory volume has been shown to relieve dyspnea in a similar model [17].
The procedures and instructions surrounding ventilator administration of aerosol produced a psychological placebo treatment effect even though the subject knew a drug was not given.
We think that #1 is very unlikely – although the aerosol delivery ventilation settings produce mild hypocapnia, 10 minutes elapsed between administration and the first post-test data point, Petco2 was controlled by the stimulus system during data collection, subjects breathed a hyperoxic gas mixture throughout testing, and time was allowed for the perceptual response to reach steady state at each up step [15]. All subjects underwent the same procedure. The relief of dyspnea due to mechanoreceptor activation persists for only 30 to 60 seconds [20]. We think that #2 is plausible but unlikely – the high correlation of ratings to stimulus both before and after aerosol argues against random noise. This subject had a high correlation between stimulus and response during baseline conditions on the run-up day (r=0.93), on the Protocol 1 day (r=0.97) and on the Protocol 2 day (r=.82); correlation remained high after both treatments (r=.87 and .86). We think also that #3 is plausible but unlikely – although reduction in dyspnea following saline (but not sham aerosol) has been reported in COPD patients [21,22], it has been attributed to clearance of secretions, not an issue in this healthy subject. Experiments in dogs show no reflex or sensory nerve effect of isotonic saline [23]. Regarding #4, our pneumotach/integrator system had insufficient stability to detect changes in volume with a time course of a minute or more, so a sufficiently slow change could have been undetected – this would be difficult but not impossible for the subject to perform. Possibilities #4 and #5 remain.
Overall the results show that when the subjects are reliable raters of discomfort and expectation of treatment effect is minimized, a significant reduction in dyspnea in response to saline placebo is infrequent; but the finding of a single result in a subject who was a consistent rater argues that such events will occur. Our small sample allows us to estimate with 90% confidence that the proportion of responders in the population is below 50%.
Supplementary Material
Acknowledgments
The authors wish to thank Heather Bernstein, Dora Huang, Victoria Molina, and Andrew Sheridan for superb technical support.
This study was funded by grant R01-NR12009 from the National Institutes of Health, National Institute for Nursing Research
Footnotes
Ethical approval
All procedures performed in these studies were in accordance with the ethical standards of the BIDMC Committee on Clinical Investigations (institutional review board), which approved the research, and in accordance with the 1964 Helsinki declaration and its later amendments.”
Conflict of Interest: None
References
- 1.Stone P, Kurowska A, Tookman A. Nebulized frusemide for dyspnoea. Palliat Med. 1994;8(3):258. doi: 10.1177/026921639400800315. [DOI] [PubMed] [Google Scholar]
- 2.Moosavi SH, Binks AP, Lansing RW, et al. Effect of inhaled furosemide on air hunger induced in healthy humans. Respir Physiol Neurobiol. 2007;156(1):1–8. doi: 10.1016/j.resp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Nishino T, Ide T, Sudo T, et al. Inhaled furosemide greatly alleviates the sensation of experimentally induced dyspnea. Am J Respir Crit Care Med. 2000;161(6):1963–1967. doi: 10.1164/ajrccm.161.6.9910009. [DOI] [PubMed] [Google Scholar]
- 4.Shimoyama N, Shimoyama M. Nebulized furosemide as a novel treatment for dyspnea in terminal cancer patients. J Pain Symptom Manage. 2002;23(1):73–76. doi: 10.1016/s0885-3924(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 5.Kohara H, Ueoka H, Aoe K, et al. Effect of nebulized furosemide in terminally ill cancer patients with dyspnea. J Pain Symptom Manage. 2003;26(4):962–967. doi: 10.1016/s0885-3924(03)00322-1. [DOI] [PubMed] [Google Scholar]
- 6.Wilcock A, Walton A, Manderson C, et al. Randomised, placebo controlled trial of nebulised furosemide for breathlessness in patients with cancer. Thorax. 2008;63(10):872–875. doi: 10.1136/thx.2007.091538. [DOI] [PubMed] [Google Scholar]
- 7.Ong KC, Kor AC, Chong WF, et al. Effects of inhaled furosemide on exertional dyspnea in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169(9):1028–1033. doi: 10.1164/rccm.200308-1171OC. [DOI] [PubMed] [Google Scholar]
- 8.Jensen D, Amjadi K, Harris-McAllister V, et al. Mechanisms of dyspnoea relief and improved exercise endurance after furosemide inhalation in COPD. Thorax. 2008;63(7):606–613. doi: 10.1136/thx.2007.085993. [DOI] [PubMed] [Google Scholar]
- 9.Panahi Y, Motiei-Langroudi R, Alaeddini F, et al. Furosemide inhalation in dyspnea of mustard gas-exposed patients: a triple-blind randomized study. Inhal Toxicol. 2008;20(9):873–877. doi: 10.1080/08958370701861520. [DOI] [PubMed] [Google Scholar]
- 10.Banzett RB, Adams L, O’Donnell CR, et al. Using laboratory models to test treatment: morphine reduces dyspnea and hypercapnic ventilatory response. Am J Respir Crit Care Med. 2011;184(8):920–927. doi: 10.1164/rccm.201101-0005OC. 201101-0005OC [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wall PD. Pain and the placebo response. Ciba Found Symp. 1993;174:187–211. discussion 212–186. [PubMed] [Google Scholar]
- 12.Reicherts P, Gerdes AB, Pauli P, et al. Psychological Placebo and Nocebo Effects on Pain Rely on Expectation and Previous Experience. J Pain. 2016;17(2):203–214. doi: 10.1016/j.jpain.2015.10.010. S1526-5900(15)00925-6 [pii] [DOI] [PubMed] [Google Scholar]
- 13.O’Donnell CR, Schwartzstein RM, Lansing RW, et al. Dyspnea affective response: comparing COPD patients with healthy volunteers and laboratory model with activities of daily living. BMC Pulm Med. 2013;13:27. doi: 10.1186/1471-2466-13-27. 1471-2466-13-27 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moosavi SH, Golestanian E, Binks AP, et al. Hypoxic and hypercapnic drives to breathe generate equivalent levels of air hunger in humans. J Appl Physiol. 2003;94(1):141–154. doi: 10.1152/japplphysiol.00594.2002. [DOI] [PubMed] [Google Scholar]
- 15.Banzett RB. Dynamic response characteristics of CO2-induced air hunger. Respir Physiol. 1996;105(1–2):47–55. doi: 10.1016/0034-5687(96)00042-4. [DOI] [PubMed] [Google Scholar]
- 16.Banzett RB, O’Donnell CR, Guilfoyle TE, et al. Multidimensional Dyspnea Profile: an instrument for clinical and laboratory research. Eur Respir J. 2015;45(6):1681–1691. doi: 10.1183/09031936.00038914. 09031936.00038914 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vovk A, Binks AP. Raising end-expiratory volume relieves air hunger in mechanically ventilated healthy adults. J Appl Physiol. 2007;103(3):779–786. doi: 10.1152/japplphysiol.01185.2006. 01185.2006 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Holmes RD, Tiwari AK, Kennedy JL. Mechanisms of the placebo effect in pain and psychiatric disorders. Pharmacogenomics J. 2016 doi: 10.1038/tpj.2016.15. tpj201615 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 20.Evans KC, Banzett RB, Adams L, et al. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol. 2002;88(3):1500–1511. doi: 10.1152/jn.2002.88.3.1500. [DOI] [PubMed] [Google Scholar]
- 21.Poole PJ, Brodie SM, Stewart JM, et al. The effects of nebulised isotonic saline and terbutaline on breathlessness in severe chronic obstructive pulmonary disease (COPD) Aust N Z J Med. 1998;28(3):322–326. doi: 10.1111/j.1445-5994.1998.tb01956.x. [DOI] [PubMed] [Google Scholar]
- 22.Khan SY, O’Driscoll BR. Is nebulized saline a placebo in COPD? BMC Pulm Med. 2004;4:9. doi: 10.1186/1471-2466-4-9. 1471-2466-4-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pisarri TE, Jonzon A, Coleridge HM, et al. Vagal afferent and reflex responses to changes in surface osmolarity in lower airways of dogs. J Appl Physiol (1985) 1992;73(6):2305–2313. doi: 10.1152/jappl.1992.73.6.2305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.