Abstract
Plasma pharmacy is a subset of the broader field of plasma medicine. Although not strictly defined, the term aqueous plasma pharmacy (APP) is used to refer to the generation and distribution of reactive plasma-generated species in an aqueous solution followed by subsequent administration for therapeutic benefits. APP attempts to harness the therapeutic effects of plasma-generated oxidant species within aqueous solution in various applications, such as disinfectant solutions, cell proliferation related to wound healing, and cancer treatment. The subsequent use of plasma-generated solutions in the APP approach facilitates the delivery of reactive plasma species to internal locations within the body. Although significant efforts in the field of plasma medicine have concentrated on employing direct plasma plume exposure to cells or tissues, here we focus specifically on plasma discharge in aqueous solution to render the solution biologically active for subsequent application. Methods of plasma discharge in solution are reviewed, along with aqueous plasma chemistry and the applications for APP. The future of the field also is discussed regarding necessary research efforts that will enable commercialization for clinical deployment.
Keywords: plasma pharmacy, aqueous plasma pharmacy, plasma medicine, plasma-activated medium, plasma-stimulated medium, plasma-activated solution
I. Introduction
Aqueous plasma pharmacy (APP) falls within the field of plasma medicine, which has been growing rapidly over the past two decades; scientific advancements have revolved around the application of nonthermal plasma plume chemistries for a variety of clinical purposes. In this regard, APP differs from the topical use of plasma plumes onto the surface of body tissues, which has been more extensively studied and which generally includes the direct application of low-temperature (nonthermal or “cold”) atmospheric plasmas, such as jets, plumes, or dielectric barrier discharge systems. Ultimately fundamental plasma physics, life science research, and clinical medical applications all intersect in the APP field. Plasma is defined as an ionized, electronically excited gas containing ions, radicals, and electrons. Nonthermal plasma indicates that the kinetic energy of the gas atoms, molecules, and ions is lower than the electrons, which results in minimal temperature increase from room temperature.1 Plasma medicine approaches involve exposing cells or tissues to plasma species to induce destruction (in the case of bacteria and cancer cells) or to harness the therapeutic effects of oxidant plasma species (e.g., to promote angiogenesis and wound healing).2
Applications are generally categorized as follows: (1) dermatological applications. such as wound healing and treating scars, ulcers, acne, etc.; (2) selective cancer therapies wherein in vitro studies indicate promise toward selective treatment of cancer cells, but this approach is currently limited to easily accessible cancer cells such as melanoma; (3) disinfection and sterilization of planktonic and biofilm bacteria for a variety of surface and topical applications; (4) promotion of angiogenesis, or the growth of new blood vessels; (5) transdermal drug delivery methods, as the plasma plume induces poration and thus increases drug uptake; and (6) bleeding applications, which promotes coagulation during surgical procedures, or for cutting during electrosurgery.
Notably, many universities and institutes internationally are exploring the versatile application of plasma species for therapeutic action with emphasis on both fundamental plasma science and commercial clinical application. Many individual research groups and institutions have recognized the possibilities of plasma applications in health care. Major contributors to the plasma pharmacy field include laboratories at Drexel University, George Washington University, the Leibniz Institute, the Max Planck Institute, Nagoya University, Texas A&M University, and others. In addition to academic research, several companies specialize in plasma tools for use in electrosurgery, such as Bovie Medical Corporation, Olympus, Plasma Surgical, EP Technologies, and US Medical Innovations.
The AJ Drexel Nyheim Plasma Institute at Drexel University includes the Plasma Medicine Laboratory, led by a faculty team with expertise across the fields of biology and medicine as well as electrical, chemical, and mechanical engineering. The Plasma Medicine Laboratory has led the field with seminal publications on dielectric barrier discharge plasma plumes for sterilization, cancer treatment, bacterial inactivation, coagulation, angiogenesis, bone fusion, and a variety of dermal applications including wound, ulcer, and scar treatment.
Other translations work has been performed at George Washington University where Prof. Keidar's team in the Department of Mechanical and Aerospace Engineering has led versatile plasma research in the field of plasma medicine. More specifically much work has been published outlining the use of plasma plumes for wound healing; cancer treatment, and electrosurgery, as well as specialty plasma manufacturing applications.
Additionally, the Leibniz Institute for Plasma Science and Technology (INP Greifswald e.V.) has been working over the past decade to investigate the application of plasma-plume technology for disinfection, in vitro cancer treatment and cell modification, and a variety of topical dermal applications. Notably, the work at Leibniz has resulted in a spin-off company, Neoplas Tools, which specializes in the production and application of a proprietary hand-held plasma torch pen, the kINPen; they have three product offerings for dermal applications (e.g. disinfection, wound healing): (1) kINPen® MED for cold tissue-compatible plasma, (2) kINPen® DENT for dental medicine, and (3) kINPen® VET for veterinary applications.
Most of the work completed thus far in the field of plasma medicine falls within the following approaches (Fig. 1): (1) treatment of adhered cells in the absence of media; (2) treatment of cells in the presence of media where the cells are (a) adhered to a substrate or (b) suspended in the media; (3) treatment of media or saline, which is subsequently applied to cells; (4) direct treatment of xenografted tumors in animal models; and (5) direct dermal treatment of animal or human models. Notably, approaches 1–3 include in vitro studies, while 4 and 5 encompass in vivo work.
Fig. 1.
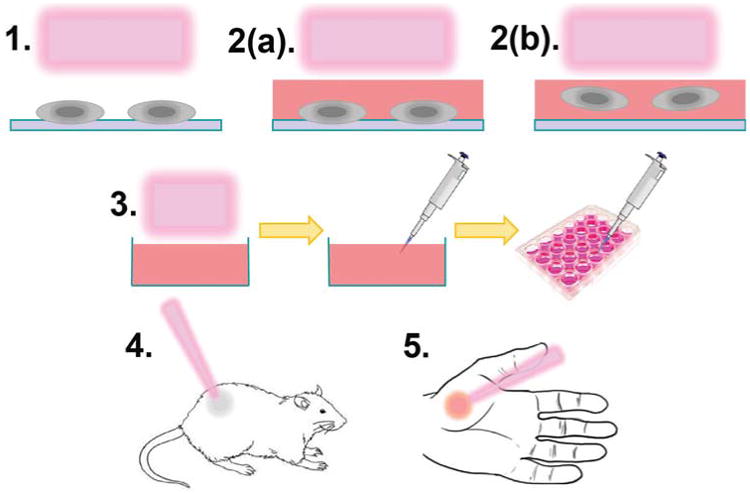
Approaches to plasma medicine include (1) direct plasma plume treatment of cells; (2) direct plasma plume treatment of cells in the presence of media where the cells are (a) adhered or (b) suspended in media; (3) plasma treatment of aqueous solution for secondary application to cells; (4) direct plasma treatment of xenografted tumors in animal models; and (5) direct dermal treatment of wounds and other topical ailments.
Many previous review articles have been published containing in-depth summaries of the plasma medicine field3–10; several reviews also specifically cover cancer treatment,11–19 dermatology,20,21 and wound healing.22 Thus, the field of plasma medicine related to plasma-plume applications directly to cells or tissues is becoming well established; however, applications of plasma-activated aqueous solutions have been explored less and represent a more recently emerging field. The focus of this review article is to highlight the recent advances in the plasma modification of aqueous solutions to result in biologically active liquids, which subsequently can be applied internally or externally for a variety of therapeutic applications, referred to as APP. The emerging field of APP is based upon the transfer of plasma species to aqueous solution, which can then be administered secondarily to yield therapeutic effects in cells and tissues.23
Herein, we present (a) methods for plasma discharge in aqueous solution, (b) the chemistry of plasma species in solution, (c) biomedical applications for plasma-pre-pared bioactive liquids, and (d) commercial applications and future directions for the field, all presented within the context of APP. A prior review on plasma pharmacy examined applications in the preparation of bioactive liquids, pharmaceutical preparations, drug transport, and biotechnological processes.23 However, since its publication in early 2013, much more has been learned about the preparation and application of bioactive liquids via plasma processes, as highlighted below. We also summarize the state of the field regarding methods for plasma discharge and subsequent solution chemistry, where most work has focused on applications in water treatment; however, these findings can be extended to apply to APP as well. Table 1 describes the major players in the field of plasma medicine and the multitude of applications for which plasma systems are being applied. Representative publications from each group are shown, and the major applications published by each group are highlighted.
Table 1.
A summary of the various laboratories and institutes involved in plasma medicine research, predominantly the application of plasma plume technologies for topical (e.g., dermal) applications, and/or to perform in vitro cell assay experiments
| University / Institute | Application | Ref. |
|---|---|---|
| Ajou University | Cancer (in vitro) | 24–30 |
| Changchun University of Science and Technology | Cancer (in vitro) | 31 |
| Clemson University | Melanoma (in vitro) | 32 |
| Conway Institute of Biomolecular and Biomedical Research | Cancer (in vitro) | 33 |
| CSIRO | Cancer (in vitro) | 34,35 |
| Drexel University | Dermal applications; disinfection; melanoma (in vivo); cancer (in vitro) | 36–48 |
| Dong-A University | Cancer (in vitro) | 49–52 |
| Eindhoven University of Technology | Disinfection, cancer (in vitro) | %53 |
| George Washington University | Fibroblast modification; wound healing; cancer (in vitro); electrosurgery | %54–%67 |
| Heinrich-Heine University | Skin infection, wound healing | %68 |
| Kanazawa University | Wound healing | %69 |
| Korea Advanced Institute of Science and Technology | Cancer (in vitro) | %70 |
| Kwangwoon University Plasma Bioscience Research Center | Cancer (in vitro) | %71–%73 |
| Leibniz Institute for Plasma Science and Technology | Dermal applications; cancer (in vitro) | %74–%88 |
| Max Planck Institute | Dermal applications; cancer (in vitro) | %89–%91 |
| McGill University | Cancer (in vitro) | %92,%93 |
| Nagoya University | Cancer (in vitro); anti-fungal; macular degeneration | %94–%104 |
| National Institute for Laser, Plasma, and Radiation Physics | Cancer (in vitro) | %105 |
| Old Dominion University | Fungal decontamination, cancer (in vitro) | %106,%107 |
| Plasma Engineering Research Lab, Texas A&M | Surface decontamination, antibiotic-resistant bacteria, wound treatment, cancer (in vitro) | %108–%117 |
| Pohang University | Melanoma | %118,%119 |
| Pusan National University | Melanoma | %120 |
| Queen's University Belfast Centre for Plasma Physics | Nosocomial infection prevention, biofilm eradication, cancer (in vitro) | %121–%128 |
| Shahid Beheshti University | Melanoma | %129 |
| Umversite' d'Orleans | Subcutaneous tumor treatement (murine model) | %130–%134 |
| University of Buffalo | Melanoma | %135,%136 |
| University of Campinas | Fibroblast modification | %137 |
| University of Iasi | Dermal applications | %138 |
| University of Notre Dame | Cancer (in vitro) | %139 |
| York Plasma Institute, University of York | Cancer (in vitro) | %140 |
II. Methods of Plasma Discharge in Solution
Several approaches to enabling plasma discharge near or directly submerged in an aqueous solution are presented in the literature. Much of this work stems from the application of plasmas for water treatment, where transfer of potent short- and long-lived oxidant species to solution can result in in situ treatment. Plasma systems represent an attractive synergistic approach to water treatment over conventional stand-alone advanced oxidation processes (i.e., ozone, UV, hydrogen peroxide) because multiple oxidation mechanisms are employed for in situ disinfection and organic compound oxidation.141-143 As such, a variety of plasma generation systems, including pulsed corona discharge, dielectric barrier discharge, and contact glow discharge electrolysis, have been investigated for water treatment applications.144 Additional applications for aqueous plasma include environmental, chemical, material functionalization, synthesis, and industrial clean-up applications.145,146 Comprehensive reviews are available in the literature that describe the fundamental physics of various systems;147 herein, we provide a condensed review of these methods within the context of APP.
There are many different methods of producing plasma discharge in solution, but they generally can be split into two main categories: (1) surface-liquid discharges, where plasma is discharged in a gas near the surface of the liquid, and (2) direct discharges. where plasma is discharged within the solution. Reviews specific to methods of plasma generation provide great detail regarding these methods.141,147 Some of the main methods for producing plasma in each of these categories are outlined below, although there are many possible variations and combinations of these systems. A summary of these methods is shown in Table 2. Any of the methods below are applicable to the production of plasma-activated solutions for use in APP.
Table 2. A summary of the main plasma discharge methods.
| Method | Characteristics | Advantages | Disadvantages |
|---|---|---|---|
| Gliding arc discharge | A gas/water mixture flows between two diverging electrodes | Improved sequestration of volatile reactants such as H2O2; continuous flow | Low liquid flow; more complex system; difficult to scale |
| Dielectric barrier discharge | Plasma discharges from an internal electrode through a porous dielectric barrier and into a gas/water mix | Exposes liquid to reactive species more effectively than point/plane-to-plane approaches; can be continuous flow; some scale-up potential | More complicated apparatus; higher power usage; lower liquid flow |
| Surface-water point-to-plane | Pointed electrode at high voltage above solution with a grounded plane electrode in solution | Simpler system that does not require as much power as direct discharges; easy to generate plasma; air as an electrode insulator | Plasma generated species only have contact with the surface of the solution; difficult to scale |
| Direct discharge without feed gas injection | Usually point-to-plane, with a vapor layer (formed at a certain voltage) coating electrode | Less complex system; feed gas or special electrodes not required; plasma-generated species have direct contact with solution | Joule heating required to produce vapor around electrodes for discharge facilitation; heavy electrode wear |
| Direct discharge with feed gas injection | Usually point-to-plane with special electrodes at high voltage that release gas to facilitate plasma | Bubbles enable better tuning of chemistry via feed gas and enhance diffusion of plasma into solution; Joule heating not required to induce vapor around electrode; can be modified into a continuous flow system | Extensive electrode wear; possible quenching of plasma from liquid |
A. Surface-Water Discharges
1. Gliding Arc Discharge
The gliding arc discharge method features the formation and movement of an arc discharge between two diverging electrodes in the presence of gas flow, illustrated in Fig. 2(a). The flow essentially drags the discharge upward, which causes the arc to extend laterally as it rises, eventually extinguishing as the gap between electrodes becomes too great. The discharge has arc and nonthermal glow properties due to its transient nature. Liquid can be introduced with the feed gas, in which case radicals and other oxidants are produced by the interaction of the arc, feed gas, and liquid.142 Additionally, it has been shown that H2O2, O3, HNO3, and other reactive species can be produced by a gliding arc discharge near the surface of an aqueous solution.148
Fig. 2.
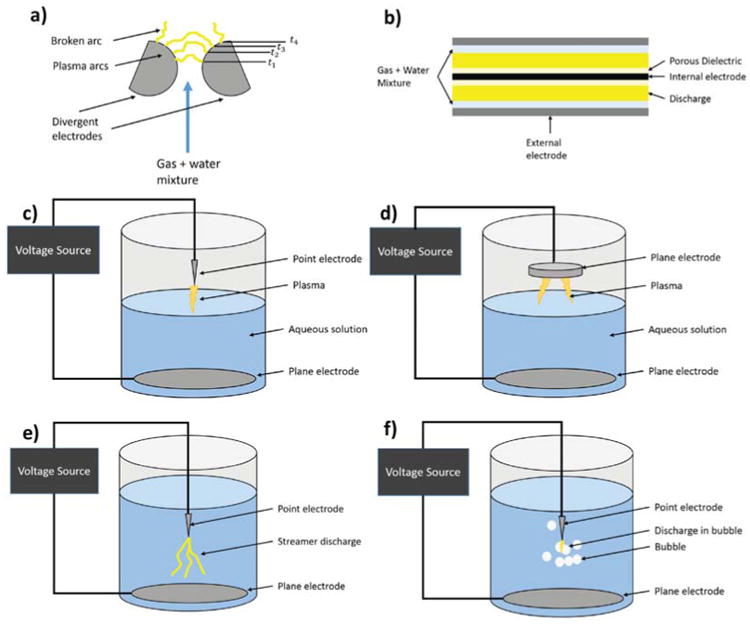
The following plasma discharges are pictorially represented: (a) gliding arc discharge, (b) dielectric barrier discharge, (c) surface-water point-to-plane glow discharge, (d) surface-water plane-to-plane glow discharge, (e) direct discharge without feed gas injection, and (f) direct discharge with feed gas injection.
This method has been shown to be effective when a spray of liquid is introduced with the feed gas, especially at generating H2O2 and other volatile species. A possible explanation is that the droplets are much more effective at sequestration of volatiles than a larger body of liquid due to the increased surface tension. The disadvantage of this type of system is that scaling to a larger volume of liquid treated is difficult due to the primarily gas phase nature of the system.
2. Dielectric Barrier Discharge
In the dielectric barrier discharge (DBD) approach, the plasma is produced in a gas in close proximity to the surface of the liquid flowing through the apparatus (Fig. 2b). In its simplest configuration, DBD is the gas discharge between two electrodes, separated by one or more dielectric layers. The gap between electrodes is usually on the order of millimeters, with a broad range of voltages required for discharge depending on the configuration. The presence of the dielectric barrier inhibits the transition from glow to arc discharge, thus ensuring stable, nonthermal plasma.149 Reactive species produced in the gas interact with the liquid at the surface region.142 Researchers have found that these DBD plasmas can produce a variety of reactive species including, for example, hydrogen peroxide (H2O2), ozone (O3), and nitric acid (HNO3) in aqueous solutions, which have been shown to have efficacy for applications including wound treatment and sterilization.148
3. Point/Multi-Point/Plane-to-Plane Atmospheric Discharge
In the atmospheric discharge approach, sometimes called a glow discharge, a discharge is initiated between the surface of the liquid and an external electrode (Fig. 2c–d). If the solution contains electrolytes, the discharge current actually flows through the electrolytic solution. In other atmospheric discharge applications, the plasma is produced in general proximity to the surface of the liquid. In both cases, as current flows chemical reactions such as radical production can take place in both the liquid and the gas phase.141
The most common version of this plasma discharge method is the point-to-plane configuration. The advantages of this type of system include ease of electrode replacement, freedom to treat liquids independent of their composition, and ease of plasma generation. This method is effective because the air surrounding the point electrode acts as an insulator, which helps focus the current density to the electrode tip thereby generating plasma more readily. The disadvantage is that this approach creates essentially a surface treatment rather than a volumetric treatment, so to treat an entire liquid volume, the system must rely on diffusion or convection. This presents a problem for scale-up, although it has been proven to be effective at small-scale liquid treatment. More recently, it has been shown that placing the high-voltage electrode in the aqueous phase and the ground in the gas phase above produces larger amounts of hydrogen peroxide in the liquid phase and ozone in the gas phase,150 although this configuration still is not scalable for commercial use.
B. Direct Discharge Methods
1. Point-to-Plane Liquid Discharge
A method of achieving the direct interaction of plasma with liquid water is to produce the discharge within the liquid itself. This so-called direct injection method typically involves the application of a fast, high-voltage pulse between submerged electrodes.142 To produce a pulsed discharge in water, it is necessary to have a high-intensity electric field at the tip of the electrode. One of the major problems of the point-to-plane geometry used in these pulsed corona reactors is that the very large electric field at the high-voltage electrode (up to 109 V/m) necessary to produce the discharge causes extensive wear on the electrode. To help overcome this technical challenge, the most common method of direct discharge is the point-to-plane geometry, shown in Fig. 2(e-f). The concentrated electric field at the pointed electrode drives localized air bubble formation and subsequent electrical breakdown of the fluid between the electrodes, thereby inducing a plasma. This approach is also used with introduction of feed gas injection which facilitates the formation of gas bubbles in which plasma is discharged. The gas bubbles are distributed throughout aqueous solution, enabling higher surface area between the plasma/gas phase and the surrounding liquid, which facilitates transfer of the oxidant species to solution. Feed gas injection parameters include the choice of gas composition and gas flow rate, which subsequently impact the chemistry of the plasma produced, in addition to enhancing the diffusion of radicals into solution,141 which is addressed in the chemistry section of this review. Radicals are formed within the gas bubbles at the water–gas interface, and it has been demonstrated that radical formation increases greatly when gas was bubbled into the discharge region.151
A larger-scale solution to this was developed by Manolache et al. in the form of a dense-medium plasma reactor (DMPR), which is a direct discharge system with multiple electrodes in parallel.152–154 The voltage drop across the electrodes was equivalent, although the current density was significantly lower, which reduced wear on individual electrodes. Additionally, this approach provided a larger treatment and mass transfer area. Johnson et al. improved this with the tubular high-density plasma reactor (THDPR). which addressed the problems of scale-up and continuous flow for industrial operations, in addition to exposing reactive species created by plasma to a much larger volume of liquid.155 The THDPR has demonstrated excellent disinfection capabilities, with the ability to inactivate bacteria with contact times on the order of seconds for power plant cooling tower water applications on the order of 500 V or less.156 Another benefit to spreading the current density across multiple electrodes is the potential of reduced heat transfer, which is an important advantage for some possible plasma applications.
While surface-water systems have generally shown higher yields of reactive species produced by plasma on a g kW-1 hr-1 basis, the nature of their design makes scale-up to industrial levels difficult or costly. Direct discharge methods, particularly point-to-plane with the direct injection of gas and modifications such as multiple electrodes in a cylindrical system, have the greatest potential for scaling up to a more useful level and also overcome diffusion limitations associated with above-solution discharges. Additionally, more recent innovations such as electrode coatings or multiple parallel electrode configurations promise to help mitigate longevity problems such as electrode wear.142 Overall, many different electrical discharge methods are available for preparing plasma-activated solutions for subsequent therapeutic use. Such technologies enable plasma discharge above or directly in an aqueous solution, where oxidant plasma species can be transferred to the bulk solution. In subsequent sections, we describe in more detail which systems are being actively investigated; however, some of these systems have yet to be thoroughly investigated for APP applications.
III. Aqueous Plasma Chemistry
The complex chemistry that occurs in aqueous plasma systems is reactive in nature and is dependent on factors such as feed gas composition, aqueous solution composition, power input, and time parameters (e.g., plasma exposure time and lifetime of solution post-preparation). The complexity of plasma-generated solutions with regards to oxidant species formation, composition, reactivity, and stability not only presents an analytical challenge but also sets APP apart as a unique and synergistic approach to tailoring oxidant chemistries such that different therapeutic processes can be targeted. As such, the tunable combination of oxidant species in solution enables plasma-activated solutions to target multiple cellular pathways that would not otherwise be achieved by a single oxidant species alone or non–plasma-generated mixes of multiple oxidant species. Many fundamental studies have been performed to determine the physical properties of individual plasma systems; the goal of this review, therefore, is not to review each characterization study in depth but to generalize such findings to be relevant to the field of APP. Several reviews are available that describe the chemical characterization of species prepared via electrical discharges in solution;143,144,157,158 herein, we give a comprehensive overview of these findings.
In general, plasma discharges result in several different routes of action (Fig. 3), including (a) electromagnetic radiation (visible, IR, UV, EMF), (b) high-energy electrons, (c) intermediate chemical species (radicals, ions), and (d) stable chemical products (ions, low-molecular-weight products). The identity and quantity of these species is dependent upon variable system parameters, as discussed in more detail below. While all of these processes are important to determine which species form in solution during plasma treatment, the longer-lived oxidant species in solution will ultimately impact cellular function to impart therapeutic action. Thus, for APP applications, it is necessary to understand which plasma parameters will enhance the formation of species that are most therapeutically relevant.
Fig. 3.
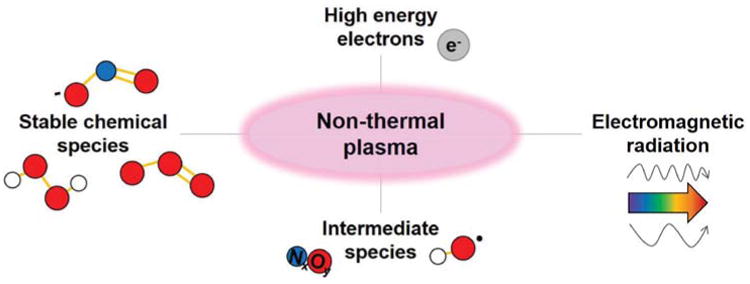
Plasma discharges initiate multiple mechanisms, including electromagnetic radiation, high-energy electrons, intermediate chemical species, and stable chemical species.
The multitude of reactions possible in plasma plumes and subsequently in aqueous solution are initiated by electron processes. The high-energy electrons produced experience inelastic collisions with ambient molecules, atoms, and ions to yield multiple reaction effects, including excitation (Eq. 1), dissociation (Eq. 2), electron capture (Eq. 3), and ionization (Eq. 4).
| (1) |
| (2) |
| (3) |
| (4) |
For example, energetic electrons result in water dissociation to form OH• and H•, among other physical processes.159 In nonthermal plasma, the plasma does not reach local thermal equilibrium, and high-energy electrons are present among non-excited atoms and molecules. The presence of energetic electrons and parent species (O2, N2, H2O) enables the formation of primary plasma species, such as radicals, that ultimately result in the formation of secondary species, namely reactive oxygen species (ROS) and reactive nitrogen species (RNS).
In general, species formed in the plasma include radicals (O•, OH•, N•, NO2•, NO•), excited state species (O3*, N2*, N*), cations (O2+, N2+, N+, O+, NO+), and anions (OH-, O2-, O-).144 Such species undergo interfacial transfer from the plasma phase to the liquid phase, where subsequent chemistries occur within solution to yield species in the aqueous solution (NO2-, NO3-). Additionally, high-energy electrons formed in the plasma phase can penetrate into the aqueous solution phase to form solvated electrons.160 The emission of UV light is due to the relaxation of excited state species, where the presence of UV light can result in subsequent reactions, such as the formation of H• and OH• from the photolysis of H2O. Predictive models demonstrate that hydrogen (H2) formation can occur via hydrogen radical recombination.161
For electrical discharges directly in aqueous solution, a variety of reactions occur between water molecules and electrons, including numerous rotational excitation, vibrational excitation, dissociation, ionization, and attachment processes.158 As such, direct aqueous discharges represent an attractive approach for the preparation of plasma-activated solutions. Electrical discharges produced directly in aerated water yield similar potent species (OH•, H•, O•) as well as stable oxidant products, such as ozone (O3) and hydrogen peroxide (H2O2). Combined, the oxidant species that form in plasma plumes and aqueous electrical discharges represent some of the most potent oxidant species due to their high oxidation potentials. Notably, the hydroxyl radical (OH•) is discussed widely in the literature as being a critical player for in situ reactivity as it has a high oxidation potential compared to other plasma species; although short-lived, OH• is a critical species formed in the plasma-gas phase, which can diffuse to the aqueous phase or form via subsequent aqueous reactions.162 Critical to solution reactivity is the presence of excess H2O, which results in a series of reactions that are described in Table 3 along with pertinent rate constants as reviewed by Malik et al.144 Overall, chemistries in plasma, gas, and aqueous phases, as generalized in Fig. 4, are versatile and dictated by interfacial diffusion processes. Considering the short lifetimes and high reactivities of the radicals and electrons formed, these processes occur on very short timescales.159
Table 3. Prevalent chemical reactions that occur during electrical discharge in aqueous solution and corresponding rate constants.
| Reaction | Rate constant (k) |
|---|---|
| H2O → OH• + H• | 9.25 × 10-10 M s-1 |
| 2H2O → H3O+ + eeq- + OH• | 2.35 × 10-9 M s-1 |
| H2O → ½H2O2 + ½H2 | 1.2 × 10-6 M s-1 |
| H• + O2 → HO2• | 1.0 × 1010 M-1 s-1 |
| H• + H2O2 → H2O + OH• | 1.0 × 1010 M-1 s-1 |
| H• + HO2• → H2O2 | 1.0 × 1010 M-1 s-1 |
| 2H• → H2 | 1.0 × 1010 M-1 s-1 |
| H• + OH• → H2O | 2.4 × 1010 M-1 s-1 |
| H• + eeq- + H2O → OH- + H2 | 2.5 × 1010 M-1 s-1 |
| OH• + H2O2 → H2O + HO2• | 5 × 107 M-1 s-1 |
| 2OH• → H2O2 | 4.0 × 109 M-1 s-1 |
| OH•+ HO2• → H2O + O2 | 1.0 × 1010 M-1 s-1 |
| OH• + eeq- → OH- | 3 × 1010 M-1 s-1 |
| 2HO2• → H2O2 + O2 | 2.0 × 106 M-1 s-1 |
| H2O2 + eeq- → OH- + OH• | 1.2 × 1010 M-1 s-1 |
| H3O+ + OH- → 2H2O | 3.0 × 1010 M-1 s-1 |
Fig. 4.
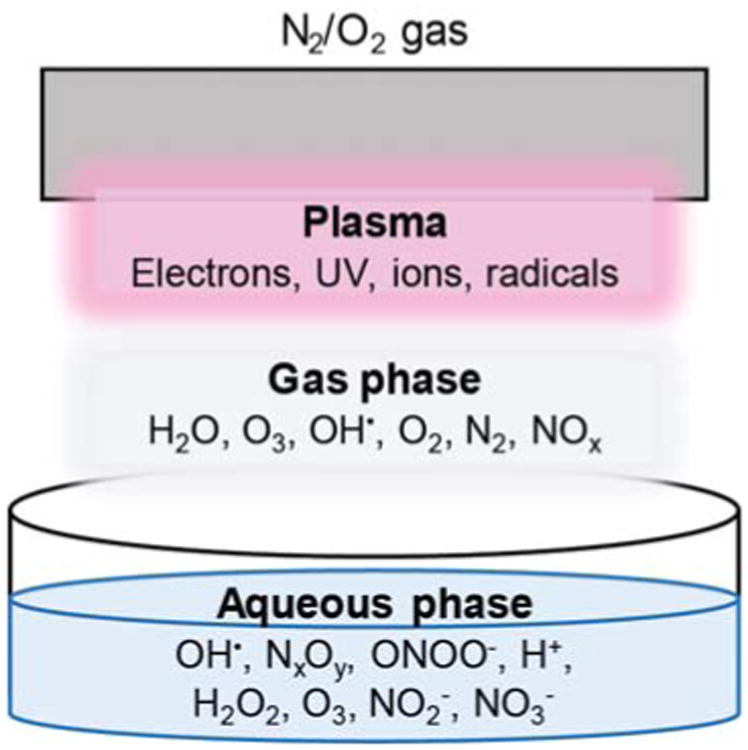
Variable species are formed in the plasma, gas, and aqueous phases to ultimately result in a variety of aqueous plasma species that can be applied for therapeutic action.
As a result of the complex chemistry ongoing in the aqueous phase during plasma discharge, many short-lived intermediates form, which ultimately result in the formation of stable end products, such as H2O2 and O3, which can go on to participate in oxidative effects for therapeutic or other applications. Ozone is formed predominantly due to the reaction of diatomic oxygen (O2) with oxygen radical (O•) in the presence of a third collision partner, M (such as O2 or N2), according to Eq. 5.163
| (5) |
Hydrogen peroxide formation in nonaerated, nonoxygenated systems typically proceeds via hydroxyl radical combination (Eq. 6); under oxygenated conditions, H2O2 formation is facilitated via hydroperoxyl radical recombination (Eq. 7).164 Once formed, O3 and H2O2 participate in a number of aqueous reactions that lead to additional species or can act directly on cells or biomolecules.
| (6) |
| (7) |
Additional reactions are possible due to the presence of N2 in systems that use air as the feed gas, ultimately resulting in production of NxOy species, both short-lived and stable. In plasma, nitrogen and oxygen gas combine to form nitric oxide (NO) according to Eq. 8. Further, NO reacts with O2 to form nitrogen dioxide (NO2) according to Eq. 9. In general, NOx species formed in the air discharge will transfer to the aqueous phase, where reaction with water results in NO2- and NO3- accompanied by H+ formation, which results in pH drop, as shown in Eq. 10 and Eq. 11.
| (8) |
| (9) |
| (10) |
| (11) |
Under these acidic conditions, HNO2 undergoes further reaction to result in the formation of nitric oxide (NO•), nitrogen dioxide (NO2•), nitrosonium ion (NO+), and other NxOy species (e.g., N2O, N2O3, and N2O4), including peroxynitrite (ONOO-) due to reaction of H2O2 and HNO2.165
In general, many factors impact the aqueous plasma species that form in solution. including (1) the choice of feed gas (if used), (2) choice of plasma system and the system power input, (3) the solution properties (pH, conductivity, and composition), and (4) time parameters, as discussed in detail below. Such parameters can be manipulated to tailor the resultant solution chemistries for subsequent therapeutic application.
A. Feed Gas
As described previously, OH•, H•, and O• production is enhanced for gas bubble configurations, where increased production correlated to increased rate of gas bubbling.144 Additionally, H• is favored with argon as the feed gas, but minimized when oxygen gas is used, where O• is instead the major radical product. As such, to enhance the formation of RNS, N2-containing feed gas sources can be used, such as pure N2 to maximize RNS formation, or house air, which contains both N2 and O2. To enhance chemistries involving O• for ROS species formation, oxygen feed gas is suggested, whereas inert argon gas favors H• chemistries. The use of aqueous bubble discharge systems has yielded a wide range of H2O2 generation rates, ranging from 0.002 to 2 g/h for Ar (pulsed corona and multi-electrode systems) to 0.01 g/h for air (DC discharge) and ∼2 g/h for O2 (pulsed systems).164 For saline solutions containing sodium chloride (NaCl), a DC microplasma jet discharge resulted in predominantly sodium hydroxide (NaOH) formation in solution with oxygen or argon gas, whereas nitric acid (HNO3) formation dominated for air or nitrogen gas.148 Thus, the use of O2 or Ar gas can lead to alkaline solutions, whereas air or N2 gas will yield acidic solutions Overall, the feed gas can be tuned to achieve different target therapeutic oxidant species that are favored for therapeutic action. For instance, to target a more highly oxidizing plasma-treated solution, oxygen or air feed gas can be used to favor ROS species, while nitrogen feed gas favors RNS.
B. Plasma System and Power Input
In general, the concentration of primary reactive species formed in a plasma discharge, and subsequently the species formed in aqueous solution, is dependent upon the system energy input, but it must be balanced with the cost of power production and undesirable effects that may occur, such as thermal heating.143 As highlighted in a prior review focused on water treatment, chemically active species in solution were increased by the following discharge parameters: (a) increasing applied voltage, but optimized in the 15–25 kV range; (b) decreasing the radius of curvature of the needle electrode, if a needle electrode configuration is employed; and (c) using positive polarity rather than negative polarity for DC voltage.144 Ozone concentration increased with increasing applied voltage and gas residence time for a given N2/O2 feed gas system; additionally, for a dielectric barrier (DB) system, ozone generation was increased when using a single DB versus double DBs.166 Similar energy yields were required for H2O2 production across multiple plasma platforms, including RF, pulsed, AC, and DC, thus suggesting a radical quenching rate-limiting step at the plasma-liquid interface; the energy yield was improved by employing discharges in bubbles, presumably due to overcoming diffusion limitations by increasing the surface area of the plasma-liquid interface.164 Earlier studies employing a pulsed streamer corona discharge treatment of aqueous (DI water) solution indicated that the time-averaged power input greatly impacted the formation of plasma species; more specifically, OH• and e-(aq) concentrations increased in a nonlinear fashion with respect to average power input, while H2O2 formation increased linearly167 Other studies indicate a linear increase in O3 concentration when using a point-to-plane discharge in solution up to ∼20 kV, past which point a linear decrease in O3 concentration was observed.168 Notably, certain plasma setups, such as plasma discharge above solution, experience diffusion limitations that limit the concentration of plasma species formed in solution. However, certain setups that maximize the surface area between the plasma region and the aqueous phase result in more facile transfer of species to solution, such as systems that involve plasma discharge into gas bubbles.151 To maximize the oxidizing capacity of plasma-prepared solutions, the choice of plasma configuration and applied power is critical. For instance, O3 has been identified as a key species responsible for therapeutic activity, as described in later sections; thus, O3 levels can be increased by increasing applied voltage. As more is learned about the cellular mechanism of action and which species are most therapeutic, such parameters can be tuned accordingly.
C. Solution Composition
In general, the solution conductivity and the presence of any solutes will greatly dictate the resultant chemistry in solution. The conductivity of the aqueous solution has been shown to play an important role in the production of aqueous species.144 More specifically, weak radical (OH•) emission intensity was observed for low conductivity solutions (1 μS/cm), but the radical emission intensity and discharge properties strengthened and streamer channel length increased with an increase in the solution conductivity in the range 10–80 μS/cm, past which point the radical emission intensity weakened.151 Additionally, in the same study, the choice of KOH over KC1 as the solution ions resulted in stronger radical emission due to possible pH effects where OH• levels increase at neutral or alkaline pH. Other work has shown that, despite the use of different electrode geometries to facilitate corona discharge in solution, similar spectral features were generated for a given solution conductivity, thus implying that that solution chemistry may dictate aqueous plasma species more so than the choice of electrode configuration.169 A recent review outlined the variable levels of H2O2, NO2-, and NO3 formed in solution when exposed to a dielectric barrier discharge system at variable pH and salt content. H2O2 levels were ∼0.1 mM for deionized (DI) water, phosphate-buffered saline (PBS) solution, and saline; however, higher concentrations of NO2- and NO3- formed in PBS at pH 6.5 versus DI water at pH 2.7.165 Another review highlights several studies employing direct electrical discharges in solution, whereby the solution conductivity and pH along with power consumption led to variable levels of plasma species such as H2O2 and OH•.158 In the presence of saline solution containing NaCl, mechanisms have been deduced involving atomic oxygen reactions with chloride to yield •Cl2- or ClO- species, where the chlorine chemistries dominated H2O2-mediated reactions.170 In the presence of solutes in the aqueous phase, variable oxidation reactions occur mainly due to OH• reactions. In general, it is critical to recognize how the parameters of the initial aqueous solution, such as composition and pH, will impact which species form. For instance, as described below, much APP work related to cancer treatment has involved activation of cell media, where subsequent stability issues have been recognized. It will be critical to determine whether the plasma activation is most effective before or after the addition of reagents, and whether components, such as cell nutrients, are actually required.
D. Time
A recent review describes the time scales associated with variable plasma processes, such as diffusion, reaction, thermal conduction, speed of light, and radiation, all of which are critical to the time-dependent formation of variable primary and secondary plasma species.157 Aqueous concentrations of plasma-induced H2O2 and O3 increased with discharge time until eventually a saturation level was reached.169 For example, a study considering O3 production specifically indicated that O3 concentration in solution increased with increasing treatment time up to ∼20 min, past which point O3 generation was no longer favored and the O3 levels started to decrease due to purging of O3 into the gas phase via O2 bubbling. This observation was explained due to an increase in solution conductivity with increasing plasma treatment time due to dissolved ionic species that formed in solution; the increased conductivity of the solution thus reduced intensity of the spot discharge and increased streamer production, whereby O3 production was reduced.168 Other studies show a linear relationship between oxidant concentration (H2O2, NO3-, NO2-) and discharge treatment time for up to 30 min for an aqueous solution at pH 6.9 exposed to an air discharge plasma.165 Further, concentrations of H2O2, NO3-, NO2- were monitored up to 300 s post-discharge time where, at lower pH (3.3), H2O2 and NO2- levels dropped while NO3- levels increased; however, at higher solution pH, the oxidant levels remained constant post-plasma treatment. Other effects that are pronounced with increased plasma treatment include the acid effect, wherein a drop in solution pH occurs and can proceed on a rapid timescale (order of minutes).143 The plasma exposure time is critical to determine which species form and in what concentrations; additionally, the length of time between solution preparation and application to a cell or tissue will dictate which species remain in solution for subsequent therapeutic activity.
A multitude of complex chemical reactions occur in plasma plumes and subsequently the plasma-treated aqueous solution. Many factors impact the identity and concentration of species that form in solution, as well as their lifetime, including choice of plasma system, feed gas, power input, composition of the aqueous solution, and time parameters. The use of aqueous plasma systems should enable a specific set of reactions that can be harnessed for the most optimized and effective water treatment or use for subsequent therapeutic applications in the case of APP. However, because so many factors impact the subsequent solution chemistry, it is common to achieve competitive rather than specific reactions.143 Additionally, because the plasma chemistry is so diverse and complex, the ability to achieve specific chemical products can be difficult. However, using the aforementioned parameters as applied to water treatment, much can be learned about tuning solution chemistries for medical applications specifically. Notably, some of the plasma species discussed above have been characterized in different plasma plume and plasma-treated aqueous systems using various methods; however, much basic research is still needed to understand the complex reactivity and abundance of species present in activated solutions prepared by multiple different discharge methods.
IV. APP Applications
Along with the development of methods for facilitating plasma discharges into solutions and increased understanding of the relevant chemical reactions involved, fundamental advances also have been made in the field of APP in recent years. More specifically, work in the past 10 years has focused on the preparation of plasma-treated bioactive solutions for applications in disinfection/antiseptics, cell proliferation/growth inhibition relating to angiogenesis and wound healing, and selective cancer therapies. In this section, we concentrate on three main APP research areas: (1) disinfectant solutions, (2) cell proliferation related to wound healing, and (3) cancer treatment.
A. Disinfectant Solutions
In general, the use of plasma discharges in water for direct in situ disinfection operates on the multimechanistic approach in which active oxidant species, UV, ozone, and hydrogen peroxide all act in concert to inactivate microbes.146 In some studies, H2O2 has been identified as a critical oxidant species due to its ability to interact with UV radiation and shock waves to form OH• radicals in bulk solution,171 where OH• has been implicated as a major antimicrobial player.172 Other work has suggested O3 as the dominant species responsible for disinfection,173 while some studies suggest H2O2 and acidified nitrite (HNO2) as key players.165,174–176 One study found nitric oxides to be predominant long-lived species produced by plasmas for disinfection.114 However, work involving the application of plasma-treated water to wounds or surfaces for disinfection will likely harness the action of multiple stable oxidant species in solution, and will be dependent upon the types and concentrations of oxidants formed depending upon the initial solution and plasma parameters employed.
The use of “plasma activated water” (PAW) for secondary disinfection was investigated by Naitali et al. through a series of studies employing gliding electrical discharges, shown in Fig. 1(a). In 2007, they studied both direct (in situ treatment of contaminated aqueous solutions) and indirect (preparation of PAW for ex situ secondary aqueous disinfection) methods of bacterial inactivation and found that plasma treatment time was critical for both configurations.177 More specifically, for a given 20-min contact time for Hafnia alvei exposed to PAW, a longer plasma exposure period during initial PAW preparation yielded more effective secondary disinfection, such as a 2- or 10-min exposure time, resulting in 3.7- or 7-log disinfection, respectively. Follow-up work with H. alvei considered the impact of various exposure times using 5 min PAW as well as varying initial concentrations (CFU) of planktonic H. alvei.178 First-order inactivation kinetics were observed, most likely rate-limited by mass transfer of the active disinfection species into solution, where longer treatment times yielded a greater log-reduction in CFU; further, the starting concentration of H. alvei was critical, where a decrease in initial CFU concentration yielded faster inactivation kinetics. Further, bacteria adhered to substrates were less susceptible to treatment compared to planktonic bacteria in solution, which follows expectations because biofilm bacteria are usually more difficult to treat. Additional work considered the use of PAW to treat various bacteria strains, including S. epidermidis, L. mesenteroides, H. alvei, and a yeast model, S. cerevisiae.179 Again, treatment was generally more effective for the planktonic form of each strain compared to the adherent form; also, bacterial inactivation was more effective than yeast treatment. Another study implicated nitrite as a major species in PAW, while also considering a synergistic effect of nitrate and H2O2.180 In other discussions, Naitali has implicated ONOOH/ONOO- as a critical part of the disinfection mechanism.181
Plasma-activated saline solution has also been investigated for secondary disinfection applications. More specifically, work out of Leibniz Institute indicated that a surface dielectric barrier discharge treatment of a NaCl solution yielded at least 7-log disinfection of E. coli, which was similar to the disinfection kinetics observed for directly treated E. coli in saline solution.182 Further, a 30-min-old solution yielded less facile disinfection kinetics but still yielded >4-log reduction, thus indicating that the lifetime of the solution is an important parameter. This work further suggested NO2- and H2O2 as the major stable species, but other species such as O3, ONOO-, OH•, NO•, and NO2• were suggested. While the lifetime of radicals is very short, some of these species may be available for cellular action via decomposition of other oxidant species in solution.
The shelf life of the PAW solution is critical to understand the stability of plasma species in solution and subsequent antibacterial effects. Plasma-activated solutions have been demonstrated to maintain long-term antibacterial efficacy on the order of days.183 For a 3-h contact time, disinfection efficacy was maintained for 2-day-old PAW; however, efficacy dropped by ∼1 log by 4 days, and ∼2 log by 7 days. For a 15-min exposure time, however, efficacy dropped by ∼5 log for both 1- and 2-day-old solutions. Thus, disinfection efficacy can be maintained, but it is dependent upon contact time between PAW and E. coli. Over the course of the 7-day study, solution composition did vary in terms of NO2- and H2O2 levels dropping, which correlated to loss of disinfection efficacy; however, likely different species exist on different time scales that still enable disinfection for 2+ day-old PAW solutions. In other work, plasma-activated solutions of water and saline exhibited antimicrobial effects after 4 weeks of storage, despite significant loss of initial H2O2 and O3 by the end of the storage period.184 Additionally, preparing solutions of similar oxidant concentration did not yield the same disinfection effects as the plasma-activated solutions, indicating that the versatile chemistry induced by plasma is required for optimum effects. Other studies have indicated that the ratio of aqueous NO2- to H2O2 in addition to plasma discharge parameters are critical to establish long-term antibacterial effects of saline solutions.84 To further extend the shelf life of PAW solutions, it has been demonstrated that the addition of N-acetyl-cysteine (NAC) prior to plasma treatment resulted in solutions with a shelf life of 2 years; however, the mechanism is unclear.185 These results indicate the potential for these plasma-activated solutions to be prepared in advance for subsequent shipment, storage, and clinical use; however, if shelf-life limitations become apparent in future work, the commercial focus may shift to on-site generation for direct use upon preparation.
An additional benefit for using PAW is that these versatile oxidant-containing solutions are less prone to developing antibiotic resistance compared to traditional small-molecule antiseptics and antibiotics.84 This approach has been proposed in light of the complex plasma species involved that suggest multiple modes of disinfectant action, where plasma treatment in general has been implicated for broad spectrum bacterial kill.186 Antibacterial resistance is currently an overwhelming issue in the healthcare arena; thus, the development of plasma-activated aqueous solutions with the capability to address this problem and effectively reduce bacterial loads will be hugely beneficial.
Overall, the exact mechanism of PAW for disinfection is unknown. Variable plasma systems have been employed to prepare such solutions, and different species, such as H2O2, NO3-, and NO2- have been implicated, along with importance of the acid effect. Disinfection has been demonstrated to be more effective using PAW versus mock solutions prepared with individual or mixed species, thus demonstrating the versatile nature of the plasma activation that leads to disinfection. These solutions also have demonstated stability and bactericidal properties even after up to 4 weeks of storage, despite significant drops in measured oxidant concentrations. Accelerated aging studies further indicate that the presence of certain additives can extend the shelf life on the order of years. In general, much is still unknown about the exact mechanism(s) involved and more work is required to understand how systematically to prepare optimized PAW solutions for the best disenfection results.
B. Cell Proliferation Related to Wound Healing
The application of direct plasma plumes to dermal tissues has been of interest to promote wound healing. More specifically, plasma represents a synergistic approach to simultaneously reduce bacterial loads while also stimulating skin cells to initiate proliferation and angiogenesis to ultimately promote wound closure.88 In addition to the dermal application of plasma plumes, plasma-activated aqueous solutions also have indicated the ability to promote similar effects, as discussed below, where an aqueous medium contaming plasma species could be more effective at accessing internal sites within a wound to lead to more effective wound healing.
In 2010, work by Kalghatgi et al. described the effect of direct plasma treatment using a dielectric barrier discharge system on porcine aortic endothelial cells in the presence (50 μL) of medium to “prevent drying” of cells during treatment.40 Low doses of plasma (4 J cm-2 at up to 30 s treatment time) yielded cell proliferation after treatment, whereas higher plasma doses (8 J cm-2 at ≥60 s treatment time) yielded cell death. Furthermore, fibroblast growth factor-2 (FGF2) was enhanced after plasma treatment, likely due to ROS-mediated FGF2 release, thus yielding proliferative effects. This study demonstrates the direct correlation between treatment time and ROS concentration, thus yielding proliferative or inhibitive cellular effects related to angiogenesis applications.
Work by Hamaguchi used a low-frequency plasma jet with He feed gas, where the tip of the plasma jet was just in contact with the surface of 100 μL aliquots of Dulbecco's Modified Eagle Medium (DMEM) in a 96-well plate format.187 The effects of plasma-treated DMEM on human synoviocyte (HS) cells was explored in three different scenarios: (1) direct plasma exposure of DMEM containing cultured cells; (2) direct plasma exposure of DMEM containing adhered cells, whereby the media was immediately exchanged for fresh, non–plasma-treated DMEM; and (3) direct plasma treatment of DMEM only, followed by culture of cells in the plasma-treated medium. In each case, an optimized treatment time was found that would enhance cell proliferation (on the order of 60 s); however, plasma overexposure resulted in cell death. In general, a correlation between cell proliferation and solution oxidative stress was found where a subsequent simulation model indicated that an increase in ROS/RNS species (such as OH• and NO•) directly affected HS proliferation. As such, when considering the variety of solution chemistries that can be tuned by various plasma parameters (as discussed above), it is beneficial to target oxidant levels that will be beneficial to kill bacteria, while not harming healthy cells, similar to the below discussion regarding selective cancer cell treatment. Thus, for a given application, such as wound healing, target oxidant levels must be determined that will induce therapeutic effects while avoiding cell-damaging effects.
Earlier work at the Leibniz Institute by Hoentsch et al. considered the effects of both direct and indirect argon plasma jet aqueous exposure of murine epithelial cells (mHepR1).188 For direct treatment, mHepR1 cells were suspended in cell culture medium (DMEM) for various plasma exposure times (30, 60, and 120 s), whereas the indirect treatment involved plasma treatment of DMEM alone (30, 60, and 120 s), followed by mHepR1 culture in the plasma-treated medium. The effects of plasma treatment times were assessed via cell viability and morphology. After only 30 s of mHepR1 direct plasma treatment in DMEM, significant morphological changes were observed. Whereby the cells were no longer adherent thus inhibiting monolayer formation; similar effects were found for indirect plasma-activated DMEM treatment alone for 30, 60, and 120 s before cell exposure. For both direct and indirect methods, an increase in plasma exposure time yielded lower cell viability, where effects were more pronounced for the direct method. In general, cellular impacts of plasma-treated media were established concerning impact on morphology, viability, and tight junction formation, but no analysis of the aqueous species was performed to inform which radicals or compounds were responsible for these effects. These results point to the need to better understand the relationship between aqueous plasma species in solution and cellular effects; if, for instance, direct plasma treatment of epithelial cells in media diminished cell viability, this level of plasma exposure could be detrimental to the epithelium and to wound healing suggesting that milder conditions would be more suitable. As a follow-up to this work, studies also were published considering the stability of plasma-treated DMEM for subsequent application to mHepR1 cells.81 Plasma-treated DMEM effects of changing cell morphology, viability, and tight junction formation were found for solutions even when stored for up to 7 days at 37°C prior to cell exposure in vitro. To inform the impact of medium composition on cells, pH, H2O2, and O2 were assessed immediately after plasma treatment and after 1 day of storage. Overall, the pH dropped slightly but remained rather consistent after storage, whereas H2O2 levels decreased dramatically (by ∼90%) after 24 h, and O2 levels in solution increased after storage. Based on these findings, it was assumed the RNS/ROS are critical to the bioactivity of the plasma-treated DMEM, but due to a sharp drop in H2O2 levels after storage but maintenance of the cellular effects, it was assumed the species other than H2O2 are critical to impact the cellular effects observed. Additionally, the observed effects on cellular morphology and viability are not desirable for wound healing applications. Thus, this suggests the need to further identify the exact composition of the solution and understand which configurations, such as <30 s of plasma treatment, will yield a solution that does not yield undesirable effects. However, this work was critical to establish the prolonged therapeutic effects that are possible for plasma-treated media even after storage for up to 1 week.
Further work by Winter et al. of the Leibniz Institute indicated that the concentration of H2O2 in the liquid phase after plasma treatment via an atmospheric pressure argon plasma jet correlated directly with the viability of adhered human skin cells.80 Complete cell-growth medium (RPMI) was plasma treated in a 60-mm-diameter Petri dish at variable treatment times ranging from 0 to 100 s, where the plasma jet was moved constantly across the medium surface at a nozzle-liquid distance of 9 mm. Additionally, the rate of H2O2 production in the gas phase matched that of net H2O2 production in the liquid phase, where the H2O2(g) solubility in the liquid phase was a key step. Other species studied by laser induced fluorescence (OF) and electron paramagnetic resonance (EPR) spectroscopies indicated that OH• and O2•- did not vary significantly with treatment, thus indicating H2O2 as a critical species in initiating proliferation of skin cells. Due to its longer half-life in solution compared to other shorter-lived species such as OH• or O2•-, H2O2 is able to remain in solution on the timescale required for direct cellular exposure. In this study, H2O2 concentrations in plasma-treated RPMI solution were achieved at 0.8, 2.9, and 9.0 mg/L due to variations in argon gas humidity and treatment time. H2O2 levels were maintained for up to 1.5 hours post-treatment regardless of initial H2O2 concentration; however, past 1.5 hours H2O2 concentration decreased based upon the starting concentration. The addition of H2O2 stabilizers significantly increased the stability of the H2O2 in solution. Despite experimental evidence indicating H2O2 as having a major role in initiating cell proliferation, additional species are also involved, but they were not quantified in this work. This research with human skin cells, wherein proliferative effects were observed, contrasts sharply with the Leibniz research in which plasma-treated media imparted undesirable morphological and viability effects, including loss of cell adhesion ability, for murine epithelial cells. Thus, these findings demonstrate the dependence of therapeutic effects on the cell line investigated, treatment time employed, and specific parameters of the system that will result in different plasma species in solution. Thus, a more thorough understanding of the aqueous plasma species and resultant solution chemistry is required to enable tailoring of the solution properties for a given application, as with traditional medicine approaches. While further exploratory work in the field is required, current findings also suggest a wide range of possibilities for future uses of plasma techniques for APP applications.
Reuter et al. further considered the impact of feed gas and ambient gas humidity on the aqueous plasma species formed and subsequent impact on human skin cells (HaCaT keratinocytes).189 In general, ozone levels decreased with increasing humidity, whereas H2O2 levels linearly increased with increasing humidity. The impact on HaCaT viability was unaffected based on varying humidity in the shielding gas, but a significant decrease in viability was found for increasing humidity in the feed gas. Specifically, for the feed gas humidity, the loss of HaCaT viability was attributed to HaCaT viability to decreased ozone and increased H2O2 production, thus implicating O3 and H2O2 as critical aqueous plasma species that impact skin cell viability. Earlier Leibniz work, also with HaCaT cells, indicated that direct cell treatment in the presence of medium to reduce cell viability was accompanied by a significant downregulation of E-cadherin and epidermal growth factor receptor (EGFR), with a lesser impact on α2- and β1-integrins, and no impact on intercellular adhesion molecule 1 (ICAM-1).190 Overall, this work informs the impact of plasma treatment on cell-surface adhesion molecules that are critical to wound healing.
In general, work on cell proliferation due to plasma-activated solutions has indicated that different routes of treatment can be employed (direct v. indirect), and that stable species, such as H2O2 and O3, are dominant in the resulting aqueous chemistry. Choice of feed gas, feed gas humidity levels, and treatment time are critical parameters to tune to achieve optimum proliferation related to wound healing and dermal applications. However, much remains unknown regarding the exact cellular mechanisms and all of the critical aqueous plasma species involved. Future systematic studies are required to determine how given plasma systems can be tuned to achieve chemistries that target specific desired cellular pathways.
C. Cancer Treatment
The clinical application of plasma species is of particular interest for selective cancer treatment. Despite significant advances in chemotherapeutic approaches over the past several decades with the advent of new drugs and advanced combinatorial therapies,191 cancer is still the second leading cause of death in the United States.192 A recent review highlighting emerging cancer therapies points out several limitations to current chemotherapeutic approaches in addition to limited efficacy, such as pathogenesis complications, drug resistance, cytotoxicity to normal healthy cells and tissues, side effects, inadequate delivery methods to the tumor site, and high recurrence rates of certain cancer types.193 As such, all of these combined factors imply the critical need for new multi-functional cancer treatments to overcome issues associated with traditional treatment options. The success of any new cancer treatment depends on its ability to selectively target cancer cells while minimizing cytotoxicity to normal cells; plasma-related treatment may be an effective and unique solution.
For plasma plume direct treatment of cancer and normal cells in vitro, many different cancer types have been considered, including cervical,24 colon,27,132 glioblastoma,132 head and neck,30 oral,28 liver,33 breast,42,49 bladder,50 pancreatic,75 prostate,127 and ovarian cancers,97 as well as melanoma32,34,129,135 and leukemia.111,116 General direct plasma plume work has indicated both cancer cell apoptosis and necrosis, where necrotic pathways were implicated at higher exposure times.111,116 However, most studies have indicated apoptotic mechanisms that involve oxidative stress, DNA damage,27,41,89,118,132 and mitochondrial dysfunction.24,30,118 These references, and others cited in Table 1, have generally indicated that the involvement of ROS and RNS result in the following effects on cancer cells: cell detachment, inhibition of cell growth, impact on cell migration and invasiveness, clonogenicity, dose-dependent induction of apoptosis and/or necrosis, cell cycle arrest, senescence, induction of intra- or extra-cellular ROS production, and down-regulation of integrins. In general, the extent of therapeutic anti-cancer effects has been directly correlated to the plasma treatment time as the most critical parameter, although the exact mechanism is unknown. It has been suggested that the plasma species, such as H2O2 and NO•,35,194 are able to interfere with the cancer cell-cycle regulation, as well as result in DNA damage, apoptosis, and cell membrane damage.18 Due to the variable ROS/RNS and subsequent routes of action, it has been suggested that the use of plasmas for cancer treatment is less likely to result in the development of drug resistance compared with other approaches.195
As discussed in a thorough review by Trachootham et al., the nature of oxidative stress on cancer cells, as induced by various ROS, is critical for the selective treatment of cancer cells.196 As shown in Fig. 5, normal cells maintain redox homeostasis through a balance of ROS generation via pro-oxidants and elimination via antioxidants to maintain basal ROS levels for proper physiological function. In general, moderate increases in ROS levels can promote helpful processes, such as cell proliferation and differentiation. However, too much ROS, as represented by the threshold value, will impart oxidative damage to lipids, proteins, and DNA, leading to toxic cellular effects. The quantitative values associated with the ROS levels that are damaging to cancer cells but innocuous to normal cells vary depending upon the specific cell types and the identity of the ROS involved. Thus, the y-axis in Fig. 5 conceptually depicts ROS levels from low to high, with a threshold above which anti-proliferative effects are observed for cancer cells but not for normal cells. Normal cells can be exposed to a given amount of exogenous ROS stress due to reserves of antioxidants to prevent cellular ROS levels from reaching the fatal threshold. Cancer cells already exist at an increased ROS generation due to metabolic abnormalities and oncogenic signaling which is believed to lead to many cancer characteristics, including cancer cell proliferation, disruption of cell death signaling, and chemoresistance. When both cancer and normal cells are exposed to exogenous ROS levels, normal cells are able to maintain homeostasis, whereas cancer cells are pushed past the ROS threshold. Overall, cancer cells are more vulnerable to external oxidative stress than normal cells, thus enabling selective cancer cell destruction in the presence of oxidative species, such as those provided by a plasma-treated aqueous solution. Key to the investigation of plasma-induced anti-cancer applications will be the identification of the threshold ROS values required to selectively target cancer cells while leaving normal cells unharmed, which will likely vary depending upon the specific type of cancer. Such ROS-mediated approaches to targeting cancer treatment may overcome issues with traditional chemotherapies, such as cytotoxicity and chemoresistance.
Fig. 5.
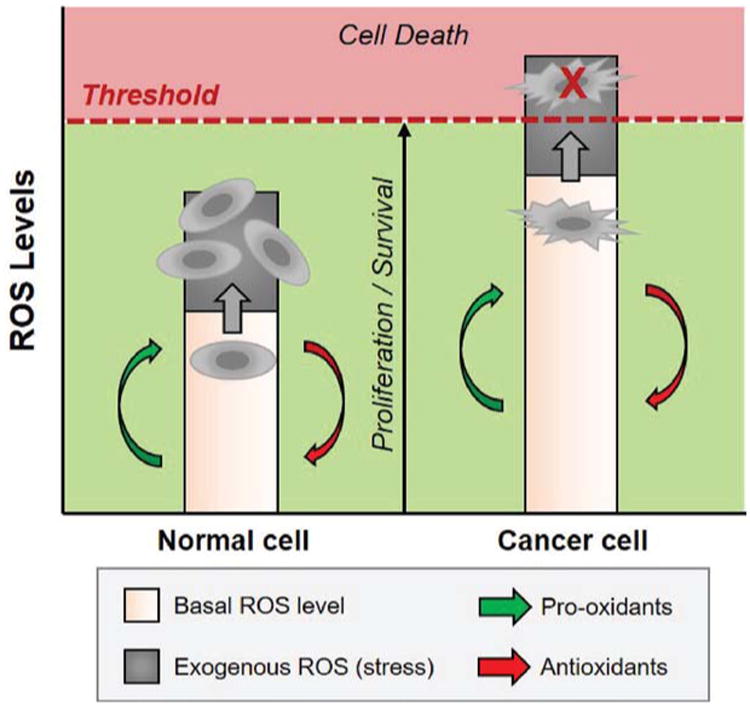
The basal ROS levels are higher for cancer cells compared to normal cells, thus application of exogenous ROS species in plasma-activated solutions will push cancer cells over the ROS threshold, while normal cells are able to maintain homeostasis via antioxidant pathways. The actual ROS threshold level will vary depending upon the cell type and specific ROS involved.
In general, direct plasma plume treatment has significant clinical limitations as its geometry makes it most suitable for treating surface sites, such as skin cancer. One review describing direct plasma applications for prostate cancer specifically proposes delivery of plasma plume species to a tumor site transperineally with an accompanying transrectal ultrasonography (TRUS) probe.19 However, this is a relatively complex mode of access to the tumor site, and one can imagine that general application of such a plasma plume approach to other types of internal cancer sites may be even more difficult compared to a prostate tumor. On the other hand, transfer of plasma species to the aqueous phase can create bioactive solutions that are more readily administered, even to difficult-to-reach places, such as internal tumors, or injected into the bloodstream for treating leukemia. Studies have indicated that plasma-activated aqueous solutions can perform just as effectively as direct application of plasma plume to cancer cells, and it has been further suggested that the transfer of plasma species to aqueous solution actually accesses certain chemistries and thus therapeutic effects that would not otherwise be possible with direct plasma plume treatment alone.23 Additionally, plasma-treated liquids may be safer because they avoid direct tissue exposure to potentially undesirable plasma mechanisms, such UV irradiation.
Initial APP research related to cancer treatment centered on exposing cell-containing solutions to plasma to understand the subsequent aqueous effects on cells. For example, a 2012 study by Graham et al. considered the impact of plasma-treating medium covering adhered breast cancer cells (Fig. 1, Scenario 2a), where DNA damage impacted cell viability in a dose-dependent manner.122 Other work led by Old Dominion University considered treating free-floating media suspensions of leukemia cells (Fig. 1, Scenario 2b) to induce cell morphology and viability results in a dose-dependent manner.107 However, as mentioned, the true goal of APP is to transfer plasma species to solution for subsequent application to cells or tissues. As such, below we present recent studies pertinent to the field for cancer treatment specifically. Table 4 highlights the major studies reviewed herein that focus on the plasma activation of cell media for secondary indirect application of plasma species to cancer cells, which have been investigated only within the past five years.
Table 4. Summary of the various studies considering plasma-activated media solutions for cancer treatment, both in vitro and in vivo.
| Institute | Year | Cancer Cell Line | Normal Cell Line | Result | Ref. |
|---|---|---|---|---|---|
| Nagoya University | 2011 | Glioblastoma, human (U251SP) | Normal brain astrocytes, human (ACBRI-371) | Selective apoptosis (caspase 3/7), AKT kinase down-regulation) | %95 |
| 2012 | Normal fibroblasts, human (WI-38) | Down-regulation of survival/proliferation signaling networks, selectivity | %96 | ||
| 2013 | Ovarian cancer (paclitaxel/cisplatin resistant) | N/A | Anti-tumor effects demonstrated, in vitro and in vivo | %98 | |
| 2014 | Ovarian clear cell carcinoma (TOV21G, ES-2, SKOV3) | Peritoneal mesothelial cells, human | CCC selective apoptosis | %99 | |
| 2015 | Lung (A549), liver (HepG2), and breast (MCF-7) cancer lines | N/A | Media composition effects; apoptosis (mitochondrial dysfunction) | %100 | |
| 2015 | Gastric cancer (NUGC4, CS-2-NU, MKN28, MKN45) | Normal fibroblasts, human (WI-38) | Selective apoptosis (caspase 3/7); cell line effects | %101 | |
| 2015 | Pancreatic cancer (PANC-1, Capan-2, BxPC-3, MIA PaCa-2) | Normal pancreatic duct epithelial cells, human (HPDE6/C7) | Selective apoptosis (caspase 3/7, cell morphology); in vitro and in vivo | %103 | |
| Leibniz Institute | 2015 | Melanoma (SK-Mel-147) | N/A | Cell migration and adhesion more affected than apoptosis | %83 |
| George Washington University | 2014 | Glioblastoma, human (U87) | N/A | FBS concentration and temperature storage effects | %61 |
| 2015 | Glioblastoma (U87) and breast cancer (MDA-MB-231, MCF-7) | N/A | Potency based on cell line, optimized plasma treatment parameters, cysteine involvement | %66 | |
| 2016 | Glioblastoma (U87), pancreatic, and (PA-TU-8988T) breast (MDA-MB-231) cancers | N/A | Amino acid scavenging of ROS and implications on potency and storage | %67 |
The term “plasma-activated medium” or “PAM” was coined by researchers at Nagoya University's Plasma Nanotechnology Research Center. Tanaka et al. have utilized a non-equilibrium atmospheric pressure plasma plume to expose cell media in a well plate format. In their seminal 2011 paper, PAM was demonstrated to selectively kill glioblastoma cells by inducing apoptosis via a caspase 3/7 pathway95 Additionally, the application of PAM to the cancer cells resulted in the downregulation of AKT kinase, thus impacting the survival signal transduction pathway. Cell viability also correlated with plasma treatment time, where lesser survival was found for a 5-min treatment time versus 1 min. No impact was observed on the normal brain astrocyte control line employed, suggesting selective cancer treatment. In terms of shelf life, the PAM lost its anti-tumor effects after 18 h, where the growth effects of the medium were restored, suggesting that it had no major impact on the composition of the growth medium. The limited 18-h window, past which point the solution lost its potency, suggests that on-site production of the PAM may be necessary for commercialization and clinical use. A follow-up publication in 2012 explored the mechanism of action against glioblastoma cells in more detail; major findings indicated complete downregulation of survival and proliferation signaling networks (e.g., AKT, ERK, and mTOR survival/proliferation pathways; CD44 membrane-bound receptor), while the control fibroblasts were comparably unaffected.96 Other work has indicated similar findings for paclitaxel/cisplatin-resistant ovarian cancer cells, where the addition of N-acetyl-cysteine inhibited antitumor effects, presumably due to ROS scavenging, thus implicating ROS as the main players.98 This study explored both in vitro effects as well as the treatment of xenografted tumors in an in vivo nude mouse model, where significant reduction in tumor growth was exhibited. Further work indicated the selective PAM treatment of ovarian clear cell carcinoma, which is particularly unaffected by conventional chemotherapies.99
Recently, three additional studies were published by the Nagoya team. In one study, the effects of PAM on lung, liver, and breast cancer lines were investigated based upon variations in the medium composition.100 Pyruvate presence in the media decreased ROS availability, leading to less effective antitumor effects, where H2O2 was identified as a critical player in apoptosis. In contrast to the 2011 glioblastoma work, this study indicated a caspase-independent apoptotic pathway, where mitochondrial stress and membrane effects were instead indicated due to H2O2 and other reactive species. Furthermore, PAM maintained its potent therapeutic effects after 1 week of storage at -80 °C, thus indicating the possibility of storing bioactive solutions for later administration. Because H2O2 was identified in this study as a critical oxidant species, storage of the solution at extremely low temperature (-80°C) in the dark would facilitate stabilization. Another 2015 study considered the impact of PAM on the cell morphology and proliferation rate of gastric cancer cells.101 The impact on cancer cells was dependent upon plasma exposure time during PAM preparation, the cell line, and the initial cell concentration. Some cell lines were more resistant to PAM treatment, but this could be overcome by increasing the plasma treatment time during preparation. In this work, the caspase 3/7 pathway was activated by PAM, leading to selective apoptosis; glutathione synthesis was also implicated. Caspases represent a family of protease enzymes that are essential to programmed cell death, including either initiator or executioner roles in apoptosis. The most recent Nagoya study investigated four different pancreatic cancer cell lines in vitro, where apoptosis was implicated via morphological changes and caspase 3/7 activation.103 Additional in vivo studies using a xenograft mouse model demonstrated significant tumor reduction (similar to their 2013 study) upon subcutaneous injection of PAM at the tumor site. The Nagoya team has indicated that further research is required to understand the exact mechanism of action by which PAM is effective against cancer cells.104
Other work has investigated the plasma treatment of cell media to prepare bioactive solutions for administration to cancer cells. For example, Schmidt et al. recently reported that, more so than metabolic, apoptotic, and cell-cycle effects, plasma-treated medium impacted tumor cell motility and colony formation.83 Gene analysis implied disorganization of the actin cytoskeleton via multiple signaling pathways, where interestingly genes related to cell adhesion, structure, and cell junction activity were more highly affected than those related to apoptotic signaling.
Work from the Keidar group at The George Washington University has examined the use of cold atmospheric plasma (CAP) to treat media, referred to as “plasma-stimulated medium” (PSM). They describe the PSM as a cocktail of various reactive species, which selectively targets cancer cells. One study indicated effects on cancer cell treatment due to the levels of fetal bovine serum (FBS) in the media.61 In general, increased levels of FBS in solution increased the cell viability when exposed to PSM, while, for a given FBS concentration, increased plasma treatment time decreased cell viability. It was therefore concluded that the presence of FBS plays a protective role for the cancer cells. Considering the known effect of plasma species on amino acids (e.g., preferential reactions that decrease sulfur-containing and aromatic amino acids),197 it is possible that the plasma treatment of media will result in ROS scavenging during treatment due to higher FBS levels, which will result in lower levels of ROS ultimately available to cells during exposure. Additionally, plasma impacts on the proteins and additives in solution could subsequently impact availability of cell nutrients.
Other work by Yan et al. demonstrated that PSM targeted both glioblastoma and breast cancer cells, where glioblastoma cells were more resistant to treatment due to faster consumption of effective reactive species.66 It was suggested that H2O2 is a critical ROS in PSM responsible for anti-cancer effects. Key to this work was a systematic review of several operating parameters during PSM preparation that optimized the potency of the resultant solution. More specifically, increasing the volume of media during-treatment yielded lower ROS/RNS levels, whereas using a larger well for a given medium volume enhanced solution potency, presumably due to an increase in the interfacial surface area between the plasma plume and medium volume during treatment which enhanced plasma-to-liquid transfer of species. Similarly, holding the plasma plume closer to the medium during treatment enhanced PSM potency, again due to interfacial transfer effects. Another key feature of this work considered which ammo acid residues would consume relevant plasma species required for subsequent therapeutic action of PSM. Of the 20 amino acids studied, cysteine and tryptophan exhibited the strongest reactivity towards PSM plasma species, and cysteine prevented anti-cancer effects the best. Follow-up work identified methionine in addition to cysteine as medium-containing amino acids that scavenged plasma species most effectively.67
Additional effects of temperature during storage preadministration to glioblastoma cells have been considered.61 PSM was held at room temperature (RT) or frozen (-20°C) for 1 and 2 days. For PSM supplemented with FBS prior to plasma treatment, no significant difference in cell viability between RT and -20°C stored PSM was found, but 1 day stored had more impact on cancer cells than 2 days. For media not supplemented with FBS and stored for 2 days, RT PSM yielded better anti-cancer effects than -20°C stored PSM. This is in contrast to the earlier described Nagoya work where freezing (-80°C) the PAM solution significantly increased the shelf life. However, it must be considered that the composition of media varies widely depending upon the cells that are being cultared; thus, it is expected that aqueous chemistries, and thus stabilities, will vary among media types and additives. More recently, methods for stabilizing PSM were considered in relation to amino acid scavenging effects.67 The use of phosphate-buffered saline (PBS) or cysteine/methionine-free DMEM enabled anti-cancer effects after 3 days of storage at 8 and -25°C, whereas the addition of a tyrosine derivative to the unmodified DMEM enabled stable storage for 3 days at 8°C. These experiments have widespread applications to the general shelf life and storage considerations of plasma-activated solutions. Overall, the storage considerations for plasma-activated solutions are complex and application specific, and they depend upon solution generation parameters. Some points are indicated by prior studies, such as the elimination of cysteine and tryptophan from PAM formulations to minimize ROS reactivity; however, additional studies are required to understand how stabilization of these solutions can be achieved to enable shelf lives that are commercially relevant.
Overall, the use of plasma-activated solutions for subsequent application to cancer cells (in vivo or in vitro) has been indicated to yield selective antitumor effects across a range of cancer types and has been attributed to ROS effects. In general, the potency of the bioactive solution is related to plasma exposure time, but the effects also vary by cell line. Preliminary knowledge has been gathered about the mechanism of action due to apoptosis; however, much work is still required to identify the exact mechamsm(s) involved based upon differences in the plasma treatment, aqueous solution composition, and cancer cell line of interest. Additionally, stability and subsequent anti-cancer effects are dependent upon the amino acid compositions of the media, and temperature also has an impact on the stability of the solution during storage.
V. Future Directions in APP
Together, much of the aforementioned research has highlighted the importance of multiple aqueous plasma species that can result in subsequent therapeutic action. The combination of plasma species appears to be more potent than any individual chemical component or mixtures of certain compounds when added versus being plasma generated, which high-lights the importance of such a synergistic, multifaceted therapeutic approach. Because much is still unknown about the exact mechanism of action for applications in disinfection, cell modification, and cancer treatment, it is critical to identify these species and subsequently understand which are most critical to the end application such that plasma chemistry can be tuned to favor the species and solution conditions that are most effective.
A recent book chapter by von Woedtke discussed one of the main hurdles in the field of plasma pharmacy, the ability to measure the aqueous plasma species with accuracy.174 For plasma-prepared bioactive solutions to succeed in commercial applications, it will be critical to identify exactly the composition of these solutions for their subsequent therapeutic use. This research will require advanced analytical methods that are selective and sensitive for a given species of interest, thus enabling a detailed understanding of the plasma-solution mechanisms that give rise to therapeutic effects. The quantification of an oxidant species, such as H2O2, in the presence of other oxidant species, such as O3 is compounded in difficulty for this application due to the multiple transient species, including radicals and short-lived intermediates, which may be present in the plasma-activated solution as a function of solution lifetime. Thus, the analytical methods of choice must be performed to capture an understanding of these system complexities while being selective and sensitive. A thorough understanding of solution composition, mechanism of action, and therapeutic indications will be critical to pass regulatory review, such as via the U.S. Food & Drug Administration (FDA). The aqueous chemistry will be heavily dependent upon the initial solution composition. Most of the work to date has been performed with saline solutions or well-established cell media formulations; it will be of great interest to explore additional aqueous formulations, such as plasma treatment of water alone where cell nutrient additives and additional reagents are added post-treatment. The identity of solution additives and whether they are added pre- or post-plasma treatment will greatly impact solution chemistry.
Part of understanding the exact chemistries and mechanisms of action will be a more thorough evaluation of different aqueous plasma discharge methods, as discussed in section II of this review. More specifically, most of the PAM/PSM studies related to cancer have been performed using liquid surface plasma plume exposure. Instead, however, it may lead to more promising chemistries and more effective therapeutics to use methods that directly discharge plasma into solution, with or without bubbles. Exploring various plasma methods and devices also will require thorough plasma diagnostics to understand the electrical, optical, thermal, and chemical properties of each system and subsequent impact on aqueous chemistry and the interfacial transfer kinetics that impact the concentration of plasma species that make it to the aqueous phase. The development of various commercial devices for generating plasma-activated solutions also will require attention to regulatory aspects.
An additional hurdle for the field will be to develop commercialization strategies that enable aqueous plasma systems and/or solutions to be available for clinical use. Such strategies can include off-site generation of activated solutions, which can be shipped and stored prior to use. For this approach to work, much more work is needed to understand the stability of aqueous plasma species and the time effects on the solution composition and subsequent therapeutic efficacy for a number of applications. This includes a better understanding of preparation and storage conditions that will enable longer shelf life of the aqueous solutions such that they can be stored and shipped to clinicians. Another obstacle to commercialization is the small scale of current plasma-activated solution production methods. The creation of systems that can treat higher volumes of solution will be critical to producing plasma-activated species on a useful scale, which requires a better understanding of the plasma physics.
In theory, the use of plasma-activated solutions represents more versatile routes of administration compared to direct plasma plumes with the ability to access internal sites through the bloodstream. Plasma plumes have mostly been suggested for surface disinfection and dermal applications, such as wounds and skin cancer. It has been demonstrated that nonthermal plasma plumes can be operated without dermal safety concerns,47,198,199 but they are only sufficient to penetrate and thus treat the top 3–5 cellular layers.75 Direct application of plasma plume species to a prostate tumor site has been suggested via transperineal administration;19 however, many internal tumors are not so easily accessible as the prostate is rectally. Thus, if stability and composition hurdles can be overcome, it would be more convenient and cost-effective to sell bags of activated solution for administration. Administration of aqueous solutions includes intra-artenal and intraperitoneal routes, among others, where the solution can be delivered at the site of interest, such as a tumor. It is also possible to sell small plasma devices for on-site generation of plasma-prepared bioactive solutions in a systematic and reproducible fashion, where the bioactive solution can be prepared on-site immediately prior to patient administration.
Looking ahead, it is anticipated that the field of APP will extend beyond the applications described above. Plasma-treated solutions have been implicated in electrosurgery applications,159,200 as well as to enhance the germination, growth rates, and overall nutritional quality of various plants.201 Many other disease states are known to be related to oxidative stress, such as neurological, cardiovascular, and gastrointestinal disorders in addition to inflammatory and degenerative diseases.202 Thus, APP may eventually be applied to a variety of indications, such as cardiovascular disease, diabetes, asthma, stroke, arthritis, and renal failure. As an example, a variety of NOx species are involved in NO pathways that are relevant to a variety of vascular applications, such as anti-platelet, antibacterial, vasodilatory, angiogenic, neurotransmitive, and wound-healing mechanisms.203
Other potential opportunities include combining plasma-activated solutions with already established small-molecule drugs. For example, the direct plasma treatment of pancreatic cancer cells improved cellular association with subsequent gemcitabine chemotherapeutic treatment;133 other studies have further indicated combinatorial approaches for direct plasma plumes as anti-cancer therapies.86 Gold nanoparticles have been separately investigated for cancer treatment; Cheng et al. demonstrated the effective plasma plume treatment of gold nanoparticles to maximize anticancer efficiency.204 Other work by Daeschlein et al. indicated that cold plasma therapy can be combined with electrochemotherapy for enhanced therapy for melanoma treatment.78 Beyond implying combinatorial cancer treatments, work has also suggested that the plasma plume treatment of chemoresistant cancer cells will restore cell responsiveness, thus overcoming chemoresistance issues.90 These combined and enhanced chemotherapy applications can certainly be applied to plasma-activated solutions.
In conclusion, the field of APP is still in its early stages, but plasma-activated solutions have shown promise for a variety of therapeutic applications, including potential to combat chemoresistant cancers and antibiotic-resistant bacteria. The complex aqueous chemistry yields a multitude of plasma species that perform more effectively than any single oxidant species alone. Furthermore, the transfer of plasma species to the aqueous phase represents a more effective approach than plasma plume exposure alone, and also represents better commercialization potential with the capability to reach internal sites that plasma plumes would not be able to access. More work is required to thoroughly understand the plasma science, chemistry, composition, and mechanism of cellular action for a variety of therapeutic applications (Fig. 6). The work presented to date indicates that this is a very promising field with the ability to address significant widespread unmet clinical needs, such as antibiotic resistance and more effective, selective chemotherapies.
Fig. 6.
Future work in the field of APP requires more research regarding plasma treatment, aqueous chemistry, and bioapplications.
Acknowledgments
The authors gratefully acknowledge support from the National Science Foundation (NSF grant no. 1256582), the American Society for Engineering Education/NSF Small Business Postdoctoral Research Diversity Fellowship, and the National Institutes of Health National Cancer Institute (NIH grant no. R43CA203273) and National Institute of General Medical Sciences (NIH grant no. R43GM121092).
References
- 1.Tendero C, Tixier C, Tnstant P, Desmaison J, Leprince P. Atmospheric pressure plasmas: a review. Spectrochim Acta B. 2006;61:2–30. [Google Scholar]
- 2.Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Applied plasma medicine. Plasma Process Polym. 2008;5:503–33. [Google Scholar]
- 3.Kalghatgi S, Dobrynin D, Fridman G, Cooper M, Nagaraj G, Peddinghaus L, Balasubramanian M, Barbee K, Brooks A, Vasilets A, Gutsol A, Fridman A, Friedman G. Plasma Assisted Decontamination of Biological and Chemical Agents. New York: Springer; 2008. Applications of non thermal atmospheric pressure plasma in medicine; pp. 173–81. [Google Scholar]
- 4.Kong MG, Kroesen G, Morfill G, Nosenko T, Shimizu T, van Dijk J, Zimmermann XL. Plasma medicine: an introductory review. New J Phys. 2009;11:115012. [Google Scholar]
- 5.Weltmann K, Kindel E, von Woedtke T, Hahnel M, Stieber M, Brandenburg R. Atmospheric-pressure plasma sources: prospective tools for plasma medicine. Pure Appl Chem. 2010;82:1223–37. [Google Scholar]
- 6.Park GY, Park SJ, Choi MY, Koo IG, Byun JH, Hong JW, Sim JY, Collins GJ, Lee JK. Atmospheric-pressure plasma sources for biomedical applications. Plasma Sources Sci Technol. 2012;21:043001. [Google Scholar]
- 7.Isbary G, Zimmermann JL, Shimizu T, Li Y, Morfill GE, Thomas HM, Steffes B, Heinlin J, Karrer S, Stolz W. Non-thermal plasma—more than five years of clinical experience. Clin Plasma Med. 2013;1:19–23. [Google Scholar]
- 8.von Woedtke T, Reuter S, Masur K, Weltmann K. Plasmas for medicine. Phys Rep. 2013;530:291–320. [Google Scholar]
- 9.von Woedtke T, Metelmann H, Weltmann K. Clinical plasma medicine: state and perspectives of in vivo application of cold atmospheric plasma. Contrib Plasma Phys. 2014;54:104–17. [Google Scholar]
- 10.Keidar M, Robert E. Preface to special topic: plasmas for medical applications. Phys Plasmas. 2015;22:121901. [Google Scholar]
- 11.Schlegel J, Koritzer J, Boxhammer V. Plasma in cancer treatment. Clin Plasma Med. 2013;1:2–7. [Google Scholar]
- 12.Keidar M, Shashunn A, Volotskova O, Stepp M, Srinivasan P. Cold atmospheric plasma in cancer therapy. Phys Plasmas. 2013;20:057101. [Google Scholar]
- 13.Kajiyama H, Nakamura K, Utsumi F, Tanaka H, Hon M, Kikkawa F. Perspective of strategic plasma therapy in patients with epithelial ovarian cancer: a short review of plasma in cancer treatment. Japan J Appl Phys. 2014;53:05FA. [Google Scholar]
- 14.Song K, Li G, Ma Y. A review on the selective apoptotic effect of nonthermal atmospheric-pressure plasma on cancer cells. Plasma Med. 2014;4:193–209. [Google Scholar]
- 15.Ratovitski EA, Cheng X, Yan D, Sherman JH, Canady J, Trink B, Keidar M. Anti-cancer therapies of 21st century: novel approach to treat human cancers using cold atmospheric plasma. Plasma Process Polym. 2014;11:1128–37. [Google Scholar]
- 16.Babington P, Rajjoub K, Canady J, Siu A, Keidar M, Sherman JH. Use of cold atmospheric plasma in the treatment of cancer. Biointerphases. 2015;10:029403. doi: 10.1116/1.4915264. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka H, Mizuno M, Toyokuni S, Maruyama S, Kodera Y, Terasaki H, Adachi T, Kato M, Kikkawa F, Hori M. Cancer therapy using non-thermal atmospheric pressure plasma with ultra-high electron density. Phys Plasmas. 2015;22:122004. [Google Scholar]
- 18.Keidar M. Plasma for cancer treatment. Plasma Sources Sci Technol. 2015;24:033001. [Google Scholar]
- 19.Hirst AM, Frame FM, Maitland NJ, O'Connell D. Low temperature plasma: a novel focal therapy for localized prostate cancer? Biomed Res Int. 2014;2014:878319. doi: 10.1155/2014/878319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emmert S, Brehmer F, Hanble H, Helmke A, Mertens N, Ahmed R, Simon D, Wandke D, Maus-Friedrichs W, Daschlein G, Schon MP, Viol W. Atmospheric pressure plasma in dermatology: ulcus treatment and much more. Clin Plasma Med. 2013;1:24–9. [Google Scholar]
- 21.Tiede R, Hirschberg J, Daeschlein G, von Woedtke T, Vioel W, Emmert S. Plasma applications: a dermatological view. Contrib Plasma Phys. 2014;54:118–30. [Google Scholar]
- 22.Haertel B, von Woedtke T, Weltmann K, Lindequist U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol Ther. 2014;22:477–90. doi: 10.4062/biomolther.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Woedtke T, Haertel B, Weltmann K, Lindequist U. Plasma pharmacy—physical plasma in pharmaceutical applications. Pharmazie. 2013;68:492–8. [PubMed] [Google Scholar]
- 24.Ahn HJ, Kim KI, Kim G, Moon E, Yang SS, Lee JS. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE. 2011;6:e28154. doi: 10.1371/journal.pone.0028154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim CH, Kwon S, Bahn JH, Lee K, Jun SI, Rack PD, Baek SJ. Effects of atmospheric nonthermal plasma on invasion of colorectal cancer cells. Appl Phys Lett. 2010;96:243701. doi: 10.1063/1.3449575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim CH, Bahn JH, Lee SH, Kim GY, Jun SI, Lee K, Baek SJ. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J Biotechnol. 2010;150:530. doi: 10.1016/j.jbiotec.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Kim K, Choi JD, Hong YC, Kim G, Noh EJ, Lee JS, Yang SS. Atmospheric-pressure plasma-jet from micronozzle array and its biological effects on living cells for cancer therapy. Appl Phys Lett. 2011;98:073701. [Google Scholar]
- 28.Chang JW, Kang SU, Shin YS, Kim KI, Seo SJ, Yang SS, Lee JS, Moon E, Baek SJ, Lee K, Kim CH. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: involvement of DNA-damage-triggering sub-G1 arrest via the ATM/p53 pathway. Arch Biochem Biophys. 2014;545:133. doi: 10.1016/j.abb.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Kim K, Ahn HJ, Lee JH, Kim JH, Yang SS, Lee JS. Cellular membrane collapse by atmospheric-pressure plasma jet. Appl Phys Lett. 2014;104:013701. [Google Scholar]
- 30.Kang SU, Cho JH, Chang JW, Shin YS, Kim KI, Park JK, Yang SS, Lee JS, Moon E, Lee K, Kim CH. Nonthermal plasma induces head and neck cancer cell death: the potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014;5:e1056. doi: 10.1038/cddis.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Li M, Zhou R, Feng K, Yang S. Ablation of liver cancer cells in vitro by a plasma needle. Appl Phys Lett. 2008;93:021502. [Google Scholar]
- 32.Kim JY, Wei Y, Li J, Kim S. 15-um-sized single-cellular-level and cell-manipulatable microplasma jet in cancer therapies. Biosens Bioelectron. 2010;26:555. doi: 10.1016/j.bios.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 33.Naciri M, Dowling D, Al-Rubeai M. Differential sensitivity of mammalian cell lines to non-thermal atmospheric plasma. Plasma Processes Polym. 2014;11:391–400. [Google Scholar]
- 34.Ishaq M, Kumar S, Varinli H, Han ZJ, Rider AE, Evans MDM, Murphy AB, Ostrikov K. Atmospheric gas plasma-induced ROS production activates TNF-ASK1 pathway for the induction of melanoma cancer cell apoptosis. Mol Biol Cell. 2014;25:1523. doi: 10.1091/mbc.E13-10-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan X, Xiong Z, Zou F, Zhao S, Lu X, Yang G, He G, Ostrikov K. Plasma-induced death of HepG2 cancer cells: Intracellular effects of reactive species. Plasms Process Polym. 2012;9:59. [Google Scholar]
- 36.Fridman G, Peddinghaus M, Ayan H, Fridman A, Balasubramanian M, Gutsol A, Brooks A. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem Plasma Process. 2006;26:425–42. [Google Scholar]
- 37.Fridman G, Shereshevsky A, Jost MM, Brooks AD, Fridman A, Gutsol A, Vasilets V, Friedman G. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem Plasma Process. 2007;27:163–76. [Google Scholar]
- 38.Kalghatgi SU, Fridman G, Cooper M, Nagaraj G, Peddinghaus M, Balasubramanian M, Vasilets VN, Gutsol AF, Fridman A, Friedman G. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans Plasma Sci. 2007;35:1559–66. [Google Scholar]
- 39.Joshi SG, Paff M, Friedman G, Fridman G, Fridman A, Brooks AD. Control of methicillin-resistant S. aureus in planktonic form and biofilms: a biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am J Infect Control. 2010;38:293–301. doi: 10.1016/j.ajic.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Kalghatgi S, Friedman G, Fridman A, Clyne AM. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann Biomed Eng. 2010;38:748–57. doi: 10.1007/s10439-009-9868-x. [DOI] [PubMed] [Google Scholar]
- 41.Sensenig R, Kalghatgi S, Cerchar E, Fridman G, Shereshevsky A, Torabi B, Arjunan KP, Podolsky E, Fridman A, Friedman G, Azizkhan-Clifford J, Brooks AD. Non-thermal plasma induces apoptosis in melanoma cells via production of intracellular reactive oxygen species. Ann Biomed Eng. 2011;39(2):674–87. doi: 10.1007/s10439-010-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Kalghatgi S, Kelly CM, Cerchar E, Torabi B, Alekseev O, Fridman A, Friedman G, Azizkhan-Clifford J. Effects of non-thermal plasma on mammalian cells. PLoS One. 2011;6(1):e16270. doi: 10.1371/journal.pone.0016270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalghatgi S, Kelly C, Cerchar E, Azizkhan-Clifford J. Selectivity of non-thermal atmospheric-pressure microsecond-pulsed dielectric barrier discharge plasma induced apoptosis in tumor cells over healthy cells. Plasma Med. 2011;1:249–63. [Google Scholar]
- 44.Dobrynin D, Wasko K, Friedman G, Fridman A, Fndman G. Cold plasma sterilization of open wounds: Live rat model. Plasma Med. 2011;1:109–14. [Google Scholar]
- 45.Chakravarthy K, Dobrynin D, Fridman G, Friedman G, Murthy S, Fridman A. Cold spark discharge plasma treatment of inflammatory bowel disease in an animal model of ulcerative colitis. Plasma Med. 2011;1:3–19. [Google Scholar]
- 46.Arjunan KP, Friedman G, Fridman A, Clyne AM. Non-thermal dielectric barrier discharge plasma induced angiogenesis through reactive oxygen species. J R Soc Interface. 2012;9:147–57. doi: 10.1098/rsif.2011.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu AS, Kalghatgi S, Dobrynin D, Sensenig R, Cerchar E, Podolsky E, Dulaimi E, Paff M, Wasko K, Arjunan KP, Garcia K, Fridman G, Balasubramanian M, Ownbey R, Barbee KA, Fridman A, Friedman G, Joshi SG, Brooks AD. Porcine intact and wounded skin responses to atmospheric nonthermal plasma. J Surg Res. 2013;179:E1–E12. doi: 10.1016/j.jss.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 48.Lin A, Truong B, Pappas A, Kirifides L, Oubarri A, Chen S, Lin S, Dobrynin D, Fridman G, Fridman A, Sang N, Miller V. Uniform nanosecond pulsed dielectric barrier discharge plasma enhances anti-tumor effects by induction of immunogenic cell death in tumors and stimulation of macrophages. Plasma Process Polym. 2015;12:1392–9. [Google Scholar]
- 49.Kim S, Chung T, Bae S, Leem S. Induction of apoptosis in human breast cancer cells by a pulsed atmospheric pressure plasma jet. Appl Phys Lett. 2010;97:023702. [Google Scholar]
- 50.Joh H, Kim S, Chung T, Leem S. Reactive oxygen species-related plasma effects on the apoptosis of human bladder cancer cells in atmospheric pressure pulsed plasma jets. Appl Phys Lett. 2012;101:053703. [Google Scholar]
- 51.Kim S, Joh H, Chung TH. Production of intracellular reactive oxygen species and change of cell viability induced by atmospheric pressure plasma in normal and cancer cells. Appl Phys Lett. 2013;103:153705. [Google Scholar]
- 52.Joh H, Kim S, Chung TH, Leem SH. Comparison of the characteristics of atmospheric pressure plasma jets using different working gases and applications to plasma-cancer cell interactions. AIP Adv. 2013;3:092128. [Google Scholar]
- 53.Stoffels E, Kieft I, Sladek R, van den Bedem L, van der Laan E, Steinbuch M. Plasma needle for in vivo medical treatment: Recent developments and perspectives. Plasma Sources Sci Technol. 2006;15:S169. [Google Scholar]
- 54.Shashurin A, Stepp M, Hawley TS, Pal-Ghosh S, Brieda L, Bronnikov S, Jurjus RA, Keidar M. Influence of cold plasma atmospheric jet on surface integrin expression of living cells. Plasma Process Polym. 2010;7:294–300. [Google Scholar]
- 55.Keidar M, Walk R, Shashurin A, Srinivasan P, Sandler A, Dasgupta A, Ravi R, Guerrero-Preston R, Trink B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Brit J Cancer. 2011;105:1295–301. doi: 10.1038/bjc.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Volotskova O, Hawley TS, Stepp MA, Keidar M. Targeting the cancer cell cycle by cold atmospheric plasma. Sci Rep. 2012;2:636. doi: 10.1038/srep00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keidar M, Shashurin A, Volotskova O, Stepp M, Srinivasan P, Sandler A, Trink B. Cold atmospheric plasma in cancer therapy. Phys Plasmas. 2013;20:057101. [Google Scholar]
- 58.Alhabshan R, Belyea D, Stepp M, Barratt J, Grewal S, Shashurin A, Keidar M. Effects of in vivo application of cold atmospheric plasma on corneal wound healing in New Zealand white rabbits. Int J Ophthalmic Pathol. 2013;2:1000118. [Google Scholar]
- 59.Walk RM, Snyder JA, Srinivasan P, Kirsch J, Diaz SO, Blanco FC, Shashurin A, Keidar M, Sandler AD. Cold atmospheric plasma for the ablative treatment of neuroblastoma. J Pediatr Surg. 2013;48:67–73. doi: 10.1016/j.jpedsurg.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 60.Wang M, Holmes B, Cheng X, Zhu W, Keidar M, Zhang L. Cold atmospheric plasma from selectively ablating metastatic breast cancer cells. PLoS ONE. 2013;8:e73741. doi: 10.1371/journal.pone.0073741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yan D, Sherman JH, Cheng X, Ratovitski E, Canady J, Keidar M. Controlling plasma stimulated media in cancer treatment application. Appl Phys Lett. 2014;105:244101. [Google Scholar]
- 62.Cheng X, Sherman J, Murphy W, Ratovitski E, Canady J, Keidar M. The effect of turning cold plasma composition on glioblastoma cell viability. PLoS ONE. 2014;9:e98652. doi: 10.1371/journal.pone.0098652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guerrero-Preston R, Ogawa T, Uemura M, Shumulinsky G, Valle BL, Pirini F, Ravi R, Sidransky D, Keidar M, Trink B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int J Molec Med. 2014;34:941–6. doi: 10.3892/ijmm.2014.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shashurin A, Scott D, Zhuang T, Canady J, Beilis II, Keidar M. Electric discharge during electrosurgery. Sci Rep. 2015;4:9946. doi: 10.1038/srep09946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siu A, Volotskova O, Cheng X, Khalsa SS, Bian K, Murad F, Keidar M, Sherman JH. Differential effects of cold atmospheric plasma in the treatment of malignant glioma. PLoS One. 2015;10:0126313. doi: 10.1371/journal.pone.0126313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan D, Talbot A, Nourmohammadi N, Cheng X, Canady J, Sherman J, Keidar M. Principles of using cold atmospheric plasma stimulated media for cancer treatment. Sci Rep. 2015;5:18339. doi: 10.1038/srep18339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan D, Nourmohammadi N, Bian K, Murad F, Sherman JH, Keidar M. Stabilizing the cold plasma-stimulated medium by regulating medium's composition. Sci Rep. 2016;6:26016. doi: 10.1038/srep26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heuer K, Hoffmanns MA, Demir E, Baldus S, Volkmar CM, Rohle M, Fuchs PC, Awakowicz P, Suschek CV, Oplander C. The topical use of non-thermal dielectric batter discharge (DBD): Nitric oxide related effects on human skin. Nitric Oxide. 2015;44:52. doi: 10.1016/j.niox.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 69.Nasruddin, Nakajima Y, Mukai K, Rahayu H, Nur M, Ishijima T, Enomoto H, Uesugi Y, Sugama J, Nakatam T. Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clin Plasma Med. 2014;2:28. [Google Scholar]
- 70.Kim D, Gweon B, Kim DB, Choe W, Shin JH. A feasibility study for the cancer therapy using cold plasma. ICBME Proc. 2009;23:355. [Google Scholar]
- 71.Panngom K, Baik KY, Nam MK, Han JH, Rhim H, Choi EH. Preferential killing of human lung cancer cell lines with mitochondrial dysfunction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 2013;4:e642. doi: 10.1038/cddis.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaushik NK, Attri P, Kaushik N, Choi E. A preliminary study of the effect of DBD plasma and osmolytes on T98G brain cancer and HEK non-malignant cells. Molecules. 2013;18:4917. doi: 10.3390/molecules18054917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaushik N, Uhm H, Choi E. Micronucleus formation induced by dielectric barrier discharge plasma exposure in brain cancer cells. Appl Phys Lett. 2012;100:084102. [Google Scholar]
- 74.Oehmigen K, Hoder T, Wilke C, Brandenburg R, Hahnel M, Weltmann K, von Woedtke T. Volume effects of atmospheric-pressure plasma in liquids. IEEE Trans Plasma Sci. 2011;39:2646–7. [Google Scholar]
- 75.Partecke L, Evert K, Haugk J, Doering F, Normann L, Diedrich S, Weiss FU, Evert M, Huebner NO, Guenther C, Heidecke CD, Kramer A, Bussiahn R, Weltmann KD, Pati O, Bender C, von Bernstorff W. Tissue tolerable plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer. 2012;12:473. doi: 10.1186/1471-2407-12-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Metelmann H, Vu T, Do H, Le T, Hoang T, Phi T, Luong T, Doan V, Nguyen T, Nguyen T, Nguyen T, Le D, Le T, von Woedtke T, Bussiahn R, Weltmann K, Khalili R, Podmelle F. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: a clinical long term observation. Clin Plasma Med. 2013;1:30–5. [Google Scholar]
- 77.Schmidt A, Wende K, Bekeschus S, Bundscherer L, Barton A, Ottmuller K, Weltmann KD, Masur K. Non-thermal plasma treatment is associated with change in transcriptome of human epithelial skin cells. Free Radical Res. 2013;47:577–92. doi: 10.3109/10715762.2013.804623. [DOI] [PubMed] [Google Scholar]
- 78.Daeschlem G, Scholz S, Lutze S, Arnold A, von Podewrls S, Kiefer T, Tueting T, Hardt O, Haase H, Grisk O, Langner S, Ritter C, von Woedtke T, Jünger M. Comparison between cold plasma, electrochemotherapy and combined therapy in a melanoma mouse model. Exp Dermatol. 2013;22:582–6. doi: 10.1111/exd.12201. [DOI] [PubMed] [Google Scholar]
- 79.Tresp H, Hammer MU, Weltmann K, Reuter S. Effects of atmospheric composition and liquid type on plasma-generated reactive species in biologically relevant solutions. Plasma Med. 2013;3:45–55. [Google Scholar]
- 80.Winter J, Tresp H, Hammer MU, Insei S, Kupsch S, Schmidt-Bleker S, Wende K, Dunnbier M, Masur K, Weltmann K, Reuter S. Tracking plasma generated H2O2 from gas into liquid phase and revealing its dominant impact on human skin cells. J Phys D: Appl Phys. 2014;47:285401. [Google Scholar]
- 81.Hoentsch M, Bussiahn R, Rebl H, Bergemann C, Eggert M, Frank M, von Woedtke T, Nebe B. Persistent effectivity of gas plasma-treated, long time-stored liquid on epithelial cell adhesion capacity and membrane morphology. PLoS ONE. 2014;9:e104559. doi: 10.1371/journal.pone.0104559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wende K, Williams P, Dalluge J, Van Gaens W, Aboubakr H, Bischof J, von Woedtke T, Goyal SM, Weltmann KD, Bogaerts A, Masur K, Bruggeman PJ. Identification of the biologically active liquid chemistry induced by a nonthermal atmospheric pressure plasma jet. Biointerphases. 2015;10:029518. doi: 10.1116/1.4919710. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt A, Bekeschus S, won Woedtke T, Hasse S. Cell migration and adhesion of a human melanoma cell line is decrease by cold plasma treatment. Clin Plasma Med. 2015;3:24–31. [Google Scholar]
- 84.Hansch M, Mann M, Weltmann K, von Woedtke T. Analysis of antibacterial efficacy of plasma-treated sodium chloride solutions. J Phys Chem D: Appl Phys. 2015:48. [Google Scholar]
- 85.Matthes R, Jablonowski L, Koban I, Quade A, Hubner N, Schlueter R, Weltmann KD, von Woedtke T, Kramer A, Kocher T. In vitro treatment of Candida albicans biofilms on denture base material with volume dielectric barrier discharge plasma (VDBD) compared with common chemical antiseptics. Clin Oral Invest. 2015;19:2319–26. doi: 10.1007/s00784-015-1463-y. [DOI] [PubMed] [Google Scholar]
- 86.Masur K, von Behr M, Bekeschus S, Weltmann K, Hackbarth C, Heidecke C, von Bernstorff W, von Woedtke T, Partecke LI. Synergistic inhibition of tumor cell proliferation by cold plasma and gemcitabine. Plasma Process Polym. 2015;12:1377–82. [Google Scholar]
- 87.Bekeschus S, Schmidt A, Bethge L, Masur K, von Woedtke T, Hasse S, Wende K. Redox stimulation of human THP-1 monocytes in response to cold physical plasma. Oxid Med Cell Longevity. 2016;2016:5910695. doi: 10.1155/2016/5910695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hasse S, Tran T, Hahn O, Kindler S, Metelmann H, von Woedtke T, Masur K. Induction of proliferation of basal epidermal keratinocytes by cold atmospheric-pressure plasma. Clin Exp Dermatol. 2016;41:202–9. doi: 10.1111/ced.12735. [DOI] [PubMed] [Google Scholar]
- 89.Arndt S, Wacker E, Li Y, Shimizu T, Thomas HM, Morfill GE, Karrer S, Zimmermann JL, Bosserhoff AK. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp Dermatol. 2013;22:284. doi: 10.1111/exd.12127. [DOI] [PubMed] [Google Scholar]
- 90.Kontzer J, Boxhammer V, Schafer A, Shimizu T, Klampfl TG, Li YF, Welz C, Schwenk-Zieger S, Morfill GE, Zimmermann JL, Schlegel J. Restoration of sensitivity in chemo-resistant glioma cells by cold atmospheric plasma. PLoS ONE. 2013;8:e64498. doi: 10.1371/journal.pone.0064498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Isbary G, Koritzer J, Mitra A, Li Y, Shimizu T, Schroeder J, Schlegel J, Morfill GE, Stolz W, Zimmermann JL. Ex vivo human skin experiments for the evaluation of safety of new cold atmospheric plasma devices. Clin Plasma Med. 2013;1:36. [Google Scholar]
- 92.Yonson S, Coulombe S, Leveille V, Leask RL. Cell treatment and surface functionalization using a miniature atmospheric pressure glow discharge plasma torch. J Phys D Appl Phys. 2006;39:3508. [Google Scholar]
- 93.Leduc M, Guay D, Leask RL, Coulombe S. Cell permeabilization using a non-thermal plasma. New J Phys. 2009;11:115021. [Google Scholar]
- 94.Iseki S, Ohta T, Aomatsu A, Ito M, Kano H, Higashijima Y, Hori M. Rapid inactivation of Penicillium digitatum spores using high-density nonequilibrium atmospheric pressure plasma. Appl Phys Lett. 2010;96:153704. doi: 10.1063/1.3399265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tanaka H, Mizuno M, Ishikawa K, Nakamura K, Kajiyama H, Kano H, Kikkawa F, Hon M. Plasma-activated medium selectively kills glioblastoma brain tumor cells by down-regulating a survival signaling molecule, AKT kinase. Plasma Med. 2011;1:265–77. [Google Scholar]
- 96.Tanaka H, Mizuno M, Ishikawa K, Nakamura K, Utsumi F, Kajiyama H, Kano H, Maruyama S, Kikkawa F, Hon M. Cell survival and proliferation signaling pathways are downregulated by plasma-activated medium in glioblastoma brain tumor cells. Plasma Med. 2012;2:207–20. [Google Scholar]
- 97.Iseki S, Nakamura K, Hayashi M, Tanaka H, Kondo H, Kajiyama H, Kano H, Kikkawa F, Hori M. Selective killing of ovarian cancer cells through induction of apoptosis by nonequilibrium atmospheric pressure plasma. Appl Phys Lett. 2012;100:113702. [Google Scholar]
- 98.Utsumi F, Kajiyama H, Nakamura K, Tanaka H, Mizuno M, Ishikawa K, Kondo H, Kano H, Hon M, Kikkawa F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS One. 2013;8:e81576. doi: 10.1371/journal.pone.0081576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Utsumi F, Kajiyama H, Nakamura K, Tanaka H, Hori M, Kikkawa F. Selective cytotoxicity of indirect nonequilibrium atmospheric pressure plasma against ovarian clear-cell carcinoma. SpingerPlus. 2014;3:398. doi: 10.1186/2193-1801-3-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Adachi T, Tanaka H, Nonomura S, Hara H, Kondo S, Hori M. Plasma-activated medium induces A549 cell injury via a spiral apoptotic cascade involving the mitochondrial-nuclear network. Free Radical Biol Med. 2015;79:28–44. doi: 10.1016/j.freeradbiomed.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 101.Torii K, Yamada S, Nakamura K, Tanaka H, Kajiyama H, Tanahashi K, Iwata N, Kanda M, Kobayashi D, Tanaka C, Fujii T, Nakayama G, Koike M, Sugimoto H, Nomoto S, Natsume A, Fujiwara M, Mizuno M, Hon M, Saya H, Kodera Y. Effectiveness of plasma treatment on gastric cancer cells. Gastric Cancer. 2015;18:635–43. doi: 10.1007/s10120-014-0395-6. [DOI] [PubMed] [Google Scholar]
- 102.Ye F, Kaneko H, Nagasaka Y, Ijima R, Nakamura K, Nagaya M, Takayama K, Kajiyama H, Senga T, Tanaka H, Mizuno M, Kikkawa F, Hori M, Terasaki H. Plasma-activated medium suppresses choroidal neovascularization in mice: A new therapeutic concept for age-related macular degeneration. Sci Rep. 2015;5:7705. doi: 10.1038/srep07705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hattori N, Yamada Y, Torii K, Takeda S, Nakamura K, Tanaka H, Kajiyama H, Kanda M, Fujii T, Nakayama G, Sugimoto H, Koike M, Nomoto S, Fujiwara M, Mizuno M, Hori M, Kodera Y. Effectiveness of plasma treatment on pancreatic cancer cells. Int J Oncol. 2015;47:1655–62. doi: 10.3892/ijo.2015.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanaka H, Mizuno M, Ishikawa K, Takeda K, Nakamura K, Utsumi F, Kajiyama H, Kano H, Okazaki Y, Toyokum S, Maruyama S, Kikkawa F, Hori M. Plasma medical science for cancer therapy: Toward cancer therapy using nonthermal atmospheric pressure plasma. IEEE Trans Plasma Sci. 2014;42:3760–4. [Google Scholar]
- 105.Georgescu N, Lupu AR. Tumoral and normal cells treatment with high-voltage pulsed cold atmospheric plasma jets. IEEE Trans Plasma Sci. 2010;38:1949. [Google Scholar]
- 106.Kolb JF, Mohamed A, Price RO, Swanson RJ, Bowman A, Chiavarini RL, Stacey M, Schoenbach KH. Cold atmospheric pressure air plasma jet for medical applications. Appl Phys Lett. 2008;92:241501. [Google Scholar]
- 107.Barekzi N, Laroussi M. Dose-dependent killing of leukemia cells by low-temperature plasma. J Phys D Appl Phys. 2012;45:422002. [Google Scholar]
- 108.Thiyagarajan M, Alexeff I, Parameswaran S, Beebe S. Atmospheric pressure resistive barrier cold plasma for biological decontamination. IEEE Trans Plasma Sci. 2005;33:322–3. [Google Scholar]
- 109.Thiyagarajan M, Sarani A, Gonzales X. Characterization of portable resistive barrier plasma jet and its direct and indirect treatment for antibiotic resistant bacteria and THP-1 leukemia cancer cells. IEEE Trans Plasma Sci. 2012;40:3533–45. [Google Scholar]
- 110.Thiyagarajan M, Waldbeser L. Portable plasma medical device for infection treatment. Stud Health Technol Inform. 2012;173:518–20. [PubMed] [Google Scholar]
- 111.Thiyagarajan M, Waldbeser L, Whitmill A. THP-1 leukemia cancer treatment using a portable plasma device. Stud Health Technol Inform. 2012;173:515. [PubMed] [Google Scholar]
- 112.Thiyagarajan M, Gonzales XF, Anderson H. Regulated cellular exposure to non-thermal plasma allows preferentially directed apoptosis in acute monocytic leukemia cells. Stud Health Technol Inform. 2013;184:436–42. [PubMed] [Google Scholar]
- 113.Thiyagarajan M, Sarani A, Gonzales XF. Characterization of an atmospheric pressure plasma jet and its applications for disinfection and cancer treatment. Stud Health Technol Inform. 2013;184:443–9. [PubMed] [Google Scholar]
- 114.Thiyagarajan M, Sarani A, Gonzales X. Atmospheric pressure resistive barrier air plasma jet induced bacterial inactivation in aqueous environment. J Appl Phys. 2013;113:093302. [Google Scholar]
- 115.Thiyagarajan M. A portable atmospheric air plasma device for biomedical treatment applications. J Med Dev Trans ASME. 2013;7:011007. [Google Scholar]
- 116.Thiyagarajan M, Anderson H, Gonzales X. Induction of apoptosis in human myeloid leukemia cells by remote exposure of resistive barrier cold plasma. Biotech Bioeng. 2014;111:535. doi: 10.1002/bit.25114. [DOI] [PubMed] [Google Scholar]
- 117.Sarani A, Nicula C, Gonzales XF, Thiyagarajan M. Characterization of kilohertz-ignited nonthermal He and He/O2 plasma pencil for biomedical applications. IEEE Trans Plasma Sci. 2014;42:3148–60. [Google Scholar]
- 118.Kim GJ, Kim W, Kim KT, Lee JK. DNA damage and mitochondria dysfunction in cell apoptosis induced by nonthermal air plasma. Appl Phys Lett. 2010;96:021502. [Google Scholar]
- 119.Kim GJ, Park SR, Kim GC, Lee JK. Targeted cancer treatment using anti-EGFR and -TFR antibody-conjugated gold nanoparticles stimulated by nonthermal air plasma. Plasma Med. 2011;1:45. [Google Scholar]
- 120.Lee HJ, Shon CH, Kim YS, Kim S, Kim GC, Kong MG. Degradation of adhesion molecules of G361 melanoma cells by a non-thermal atmospheric pressure microplasma. New J Phys. 2009;11:115026. [Google Scholar]
- 121.Alkawareek MY, Algwari QT, Gorman SP, Graham WG, O'Connell D, Gilmore BF. Application of atmospheric pressure nonthermal plasma for the in vitro eradication of bacterial biofilms. FEMS Immunol Med Microbiol. 2012;65:381–4. doi: 10.1111/j.1574-695X.2012.00942.x. [DOI] [PubMed] [Google Scholar]
- 122.Graham WG, Schaper L, Muir M, Currell FJ. The effect of electrical discharges in the cell media on their viability and DNA damage and comparison with the effect of X-rays. Plasma Med. 2012;2:169–78. [Google Scholar]
- 123.Alkawareek MY, Algwari QT, Laverty G, Gorman SP, Graham WG, O'Connell D, Gilmore BF. Eradication of Pseudomonas aeruginosa biofilms by atmospheric pressure non-thermal plasma. PLoS One. 2012;7:e44289. doi: 10.1371/journal.pone.0044289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alshraiedeh NH, Alkawareek MY, Gorman SP, Graham WG, Gilmore BF. Atmospheric pressure, nonthermal plasma inactivation of MS2 bacteriophage: effect of oxygen concentration on virucidal activity. J Appl Microbiol. 2013;115:1420–6. doi: 10.1111/jam.12331. [DOI] [PubMed] [Google Scholar]
- 125.Alkawareek MY, Gorman SP, Graham WG, Gilmore BF. Potential cellular targets and antibacterial efficacy of atmospheric pressure non-thermal plasma. Int J Antimicrob Agents. 2014;43:154–60. doi: 10.1016/j.ijantimicag.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 126.Alkawareek MY, Alshraiedeh NH, Higginbotham S, Flynn PB, Algwan QT, Gorman SP, Graham WG, Gilmore BF. Plasmid DNA damage following exposure to atmospheric pressure nonthermal plasma: kinetics and influence of oxygen admixture. Plasma Med. 2014;4:211–9. [Google Scholar]
- 127.Gibson AR, McCarthy HO, Ali AA, O'Connell D, Graham WG. Interactions of a non-thermal atmospheric pressure plasma effluent with PC-3 prostate cancer cells. Plasma Process Polym. 2014;11:1142. [Google Scholar]
- 128.Flynn PB, Higginbotham S, Alshraiedeh NH, Gorman SP, Graham WG, Gilmore BF. Bactericidal efficacy of atmospheric pressure non-thermal plasma (APNTP) against the ESKAPE pathogens. Int J Antimicrob Agents. 2015;46:101–7. doi: 10.1016/j.ijantimicag.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 129.Mashayekh S, Rajaee H, Akhlaghi M, Shokn B, Hassan Z. Atmospheric-pressure plasma jet characterization and applications on melanoma cancer treatment (B/16-F10) Phys Plasmas. 2015;22:093508. [Google Scholar]
- 130.Vandamme M, Robert E, Pesnel S, Barbosa E, Dozias S, Sobilo J, Lerondel S, Le Pape A, Pouvesle JM. Antitumor effect of plasma treatment on U87 glioma xenografts: preliminary results. Plasma Process Polym. 2010;7:264. [Google Scholar]
- 131.Vandamme M, Robert E, Dozias S, Sobilo J, Lerondel S, Le Pape A, Pouvesle JM. Response of human glioma U87 xenografted on mice to non thermal plasma treatment. Plasma Med. 2011;1:27. [Google Scholar]
- 132.Vandamme M, Robert E, Lerondel S, Sarron V, Ries D, Dozias S, Sobilo J, Gosset D, Kieda C, Legrain B, Pouvesle JM, Pape AL. ROS implication in a new antitumor strategy based on non-thermal plasma. Int J Cancer. 2012;130:2185. doi: 10.1002/ijc.26252. [DOI] [PubMed] [Google Scholar]
- 133.Brulle L, Vandamme M, Ries D, Martel E, Robert E, Lerondel S, Trichet V, Richard S, Pouvesle JM. Le Pape A. Effects of non thermal plasma treatment alone or in combination with gemcitabine in a MIA PaCa2-Iuc orthotopic pancreatic carcinoma model. PLoS One. 2012;7:e52653. doi: 10.1371/journal.pone.0052653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Robert E, Vandamme M, Brulle L, Lerondel S, Le Pape A, Sarron V, Ries D, Darny T, Dozias S, Collet G, Kieda C, Pouvesle JM. Perspectives of endoscopic plasma applications. Clin Plasma Med. 2013;1:8. [Google Scholar]
- 135.Zucker SN, Zirnheld J, Bagati A, DiSanto TM, Des Soye B, Wawrzymak JA, Etemadi K, Nikiforov M, Berezney R. Preferential induction of apoptotic cell death m melanoma cells as compared with normal keratinocytes using a non-thermal plasma torch. Cancer Biol Ther. 2012;13:1299. doi: 10.4161/cbt.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zirnheld JL, Zucker SN, DiSanto TM, Berezney R, Etemadi K. Nonthermal plasma needle: development and targeting of melanoma cells. IEEE Trans Plasma Sci. 2010;38:948. [Google Scholar]
- 137.Lopes B, Kraft M, Rehder J, Batista F, Puzzi M. The interactions between non-thermal atmospheric pressure plasma and ex-vivo dermal fibroblasts. Procedia Eng. 2013;59:92. [Google Scholar]
- 138.Nastuta AV, Pohoata V, Topala I. Atmospheric pressure plasma jet—living tissue interaction: electrical, optical, and spectral characterization. J Appl Phys. 2013;113:183302. [Google Scholar]
- 139.Han X, Klas M, Liu Y, Stack MS, Ptasinska S. DNA damage in oral cancer cells induced by nitrogen atmospheric pressure plasma jets. Appl Phys Lett. 2013;102:233703. [Google Scholar]
- 140.Hirst AM, Frame FM, Maitland NJ, O'Connell D. Low temperature plasma causes double-strand break DNA damage in primary epithelial cells cultured from a human prostate tumor. IEEE Trans Plasma Sci. 2014;42:2740–1. doi: 10.1109/TPS.2014.2351453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Foster J, Sommers BS, Gucker SN, Blankson IM, Adamovsky G. Perspectives on the interaction of plasmas with liquid water for water purification. IEEE Trans Plasma Sci. 2012;40:1311–23. [Google Scholar]
- 142.Locke BR, Sato M, Sunka P, Hoffmann MR, Chang JS. Electrohydraulic discharge and nonthermal plasma for water treatment. Ind Eng Chem Res. 2006;45:882–905. [Google Scholar]
- 143.Brisset J, Moussa D, Doubla A, Hnatiuc E, Hnatiuc B, Youbi GK, Herry JM, Naitali M, Bellon-Fontaine MN. Chemical reactivity of discharges and temporal post-discharges in plasma treatment of aqueous media: examples of gliding discharge treated solutions. Ind Eng Chem Res. 2008;47:5761–81. [Google Scholar]
- 144.Malik MA, Ghaffar A, Malik SA. Water purification by electrical discharges. Plasma Sources Sci Technol. 2001;10:82–91. [Google Scholar]
- 145.Bruggeman PJ, Locke BR. Assessment of potential applications of plasma with liquid water. In: Chu PK, Lu X, editors. Low Temperature Plasma Technology: Methods and Applications. New York: Taylor and Francis; 2013. pp. 367–99. [Google Scholar]
- 146.Yang Y, Fridman A, Cho YI. Advances in Heat Transfer. Vol. 42. New York: Elsevier; 2010. Plasma discharge in water; pp. 179–292. [Google Scholar]
- 147.Joshi RP, Thagard SM. Streamer-like electrical discharges in water: Part I. Fundamental mechanisms. Plasma Chem Plasma Process. 2013;33:1–15. [Google Scholar]
- 148.Rumbach P, Witzke M, Sankaran RM, Go DB. Plasma-liquid interactions: separating electrolytic reactions from plasma/gas phase reactions. Proc ESA Annual Meeting on Electrostatics. 2013:1–8. [Google Scholar]
- 149.Mastanaiah N, Johnson JA, Roy S. Effect of dielectric and liquid on plasma sterilization using dielectric barrier discharge plasma. PLoS ONE. 2013;8:e70840. doi: 10.1371/journal.pone.0070840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Locke BR, Sato M, Sunka P, Hoffmann MR, Change J. Electrohydraulic discharge and nonthermal plasma for water treatment. Ind Eng Chem Res. 2006;45:882–905. [Google Scholar]
- 151.Sun B, Sato M, Clements JS. Optical study of active species produced by a pulsed streamer corona discharge in water. J Electros! 1997;39:189–202. [Google Scholar]
- 152.Manolache S, Somers EB, Wong ACL, Shamamian V, Denes F. Dense medium plasma environments: a new approach for the disinfection of water. Environ Sci Technol. 2001;35(18):3780–5. doi: 10.1021/es010704o. [DOI] [PubMed] [Google Scholar]
- 153.Johnson DC, Shamamian VA, Callahan JH, Denes FS, Manolache SO, Dandy DS. Treatment of methyl tert-butyl ether contaminated water using a dense medium plasma reactor: a mechanistic and kinetic investigation. Environ Sci Technol. 2003;37:4804–10. doi: 10.1021/es0263487. [DOI] [PubMed] [Google Scholar]
- 154.Johnson DC, Dandy DS. A computational fluid dynamics investigation of fluid flow and prediction of reaction kinetics in a dense medium plasma reactor. J Phys D: Appl Phys. 2007;40:488–500. [Google Scholar]
- 155.Johnson DC, Dandy DS, Shamamian VA. Development of a tubular high-density plasma reactor for water treatment. Water Res. 2006;40:311–22. doi: 10.1016/j.watres.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 156.Johnson DC, Bzdek JP, Fahrenbruck CR, Chandler JC, Bisha B, Goodridge LD, Hybertson BM. An innovative non-thermal plasma reactor to eliminate microorganisms in water. Desalin Water Treat. 2015:1–12. [Google Scholar]
- 157.Locke BR, Thagard SM. Analysis and review of chemical reactions and transport processes in pulsed electrical discharge plasma formed directly in liquid water. Plasma Chem Plasma Process. 2012;32:875–917. [Google Scholar]
- 158.Joshi RP, Thagard SM. Streamer-like electrical discharges in water: Part II. Environmental applications. Plasma Chem Plasma Process. 2013;33:17–49. [Google Scholar]
- 159.Graham WG, Stalder KR. Plasma in saline solution. J Phys: Conf Ser. 2007:012013. [Google Scholar]
- 160.Rumbach P, Bartels DM, Sankaran RM, Go DB. The solvation of electrons by an atmospheric-pressure plasma. Nat Commun. 2014;6:7248. doi: 10.1038/ncomms8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mededovic S, Locke BR. Primary chemical reactions in pulsed electrical discharge channels in water. J Phys D: Appl Phys. 2007;40:7734–46. [Google Scholar]
- 162.Kanazawa S, Kawano H, Watanabe S, Furuki T, Akamine S, Ichiki R, Ohkubo T, Kocik M, Mzeraczyk J. Observation of OH radicals produced by pulsed discharges on the surface of a liquid. Plasma Sources Sci Technol. 2011;20:034010. [Google Scholar]
- 163.Eliasson B, Hirth M, Kogelschatz U. Ozone synthesis from oxygen in dielectric barrier discharges. J Phys D: Appl Phys. 1987;20:1421–37. [Google Scholar]
- 164.Locke BR, Shih K. Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma Sources Sci Technol. 2011;20:034006. [Google Scholar]
- 165.Lukes P, Dolezalova E, Sisrova I, Clupek M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxymtrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci Technol. 2014;23:015019. [Google Scholar]
- 166.Chang MB, Wu S. Experimental study of ozone synthesis via dielectric barrier discharges. Ozone: Sci Eng. 1997;19:241–54. [Google Scholar]
- 167.Joshi AA, Locke BR, Arce P, Finney WC. Formation of hydroxyl radicals, hydrogen peroxide and aqueous electrons by pulsed streamer corona discharge in aqueous solution. J Hazard Mater. 1995;41:3–30. [Google Scholar]
- 168.Clements JS, Sato M, Davis RH. Preliminary investigation of prebreakdown phenomena and chemical reactions using a pulsed high-voltage discharge in water. IEEE Trans Ind Appl. 1987;IA-23:224–35. [Google Scholar]
- 169.Sunka P, Babicky V, Clupek M, Lukes P, Simek M, Schmidt J, Cernak M. Generation of chemically active species by electrical discharges in water. Plasma Sources Sci Technol. 1999;8:258–65. [Google Scholar]
- 170.Wende K, Williams P, Lukes P, Van Gaens W, Aboubakr H, von Woedtke T, Bogaerts A, Masur K, Bruggeman PJ. Atomic oxygen-a potent precursor in RF plasma induced liquid chemistry. 22nd International Symposium on Plasma Chemistry; Antwerp, Belgium. 2015. [Google Scholar]
- 171.Gupta SB, Bluhm H. The potential of pulsed underwater streamer discharges as a disinfection technique. IEEE Trans Plasma Sci. 2008;36:1621–32. [Google Scholar]
- 172.Hahnel M, von Woedtke T, Weltmann K. Influence of the air humidity on the reduction of Bacillus spores in a defined environment at atmospheric pressure using a dielectric barrier surface discharge. Plasma Process Polym. 2010;7:244–9. [Google Scholar]
- 173.Pavlovich MJ, Chang H, Sakiyama Y, Clark DS, Graves DB. Ozone correlates with antibacterial effects from indirect air dielectric barrier discharge treatment of water. J Phys D: Appl Phys. 2013;46:145202. [Google Scholar]
- 174.von Woedtke T, Oehmigen K, Brandenburg R, Hoder T, Wilke C, Hahnel M, Weltmann KD. Plasma-liquid interactions: chemistry and antimicrobial effects. In: Machala Z, Hensel K, Akishev Y, editors. Plasma for Bio-Decontamination, Medicine and Food Security. Dordrecht, The Netherlands: Springer; 2012. pp. 67–78. [Google Scholar]
- 175.Oehmigen K, Hahnel M, Brandenburg R, Wilke C, Weltmann K, von Woedtke T. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Process Polym. 2010;7:250–7. [Google Scholar]
- 176.van Gils CAJ, Hofmann S, Boekema B, Brandenburg R, Bruggeman PJ. Mechanisms of bacterial inactivation in the liquid phase induced by a remote RF cold atmospheric pressure plasma jet. J Phys D: Appl Phys. 2013;46:175203. [Google Scholar]
- 177.Kamgang-Youbi G, Herry J, Bellon-Fontaine M, Brisset J, Doubla A, Naitali M. Evidence of temporal postdischarge decontamination of bacteria by gliding electric discharges: Application to Hafnia alvei. Appl Environ Microbiol. 2007;73:4791–6. doi: 10.1128/AEM.00120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kamgang-Youbi G, Herry J, Brisset J, Bellon-Fontaine M, Doubla A, Naitali M. Impact on disinfection efficiency of cell load and of planktonic/adherent/detached state: case of Hafnia alvei inactivation by plasma activated water. Appl Microbiol Biotechnol. 2008;81:449–57. doi: 10.1007/s00253-008-1641-9. [DOI] [PubMed] [Google Scholar]
- 179.Kamgang-Youbi G, Herry J, Meylheuc T, Brisset J, Bellon-Fontaine M, Doubla A, Naitali M. Microbial inactivation using plasma-activated water obtained by gliding electric discharges. Lett Appl Mcrobiol. 2009;48:13–8. doi: 10.1111/j.1472-765X.2008.02476.x. [DOI] [PubMed] [Google Scholar]
- 180.Naitali M, Kamgang-Youbi G, Herry JM, Bellon-Fontaine MN, Brisset JL. Combined effects of long-living chemical species dunng microbial inactivation using atmospheric plasma-treated water. Appl Environ Mcrobiol. 2010;76:7662–4. doi: 10.1128/AEM.01615-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Naitali M, Herry J, Hnatiuc E, Kamgang G, Bnsset J. Kinetics and bacterial inactivation induced by peroxynitrite in electric discharges in air. Plasma Chem Plasma Process. 2012;32:675–92. [Google Scholar]
- 182.Oehmigen K, Winter J, Hahnel M, Wilke C, Brandenburg R, Weltmann K, von Woedtke T. Estimation of possible mechanisms of Escherichia coli inactivation by plasma treated sodium chloride solution. Plasma Process Polym. 2011;8:904–13. [Google Scholar]
- 183.Traylor MJ, Pavlovich MJ, Karim S, Halt P, Sakiyama Y, Clark DS, Graves DB. Long-term antibacterial efficacy of air plasma-activated water. J Phys D: Appl Phys. 2011;44:472001. [Google Scholar]
- 184.Julak J, Scholtz V, Kotucova S, Janouskova O. The persistent microbicidal effect in water exposed to the corona discharge. Physica Medica. 2012;28:230–9. doi: 10.1016/j.ejmp.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 185.Ercan UK, Wang H, Ji HF, Fridman G, Brooks AD, Joshi SG. Nonequilibrium plasma-activated anti-microbial solutions are broad-spectrum and retain their efficacies for extended period of time. Plasma Processes Polym. 2013;10:544–55. [Google Scholar]
- 186.Daeschlem G, Scholz S, Arnold A, von Podewils S, Haase H, Emmert S, von Woedtke T, Weltmann KD, Junger M. In vitro susceptibility of important skin and wound pathogens against low temperature atmosphere pressure plasma jet (APPJ) and dielectric barrier discharge plasma (DBD) Plasma Process Polym. 2012;9:380–9. [Google Scholar]
- 187.Hamaguchi S. Chemically reactive species in liquids generated by atmospheric-pressure plasmas and their role in plasma medicine. AIP Conf Proc. 2013;1545:214–22. [Google Scholar]
- 188.Hoentsch M, von Woedtke T, Weltmann K, Nebe JB. Time dependent effects of low-temperature atmospheric-pressure argon plasma on epithelial cell attachment, viability and tight junction formation in vitro. J Phys D: Appl Phys. 2012;45:025206. [Google Scholar]
- 189.Reuter S, Winter J, Iseni S, Schmidt-Bleker A, Dunnbier M, Masur K, Wende K, Weltmann KD. The influence of feed gas humidity versus ambient humidity on atmospheric pressure plasma jet-effluent chemistry and skin cell viability. IEEE Trans Plasma Sci. 2015;43:3185–92. [Google Scholar]
- 190.Haertel B, Hahnel M, Blackert S, Wende K, von Woedtke T, Lindequist U. Surface molecules on HaCaT keratinocytes after interaction with non-thermal atmospheric pressure plasma. Cell Biol Int. 2012;36:1217–22. doi: 10.1042/CBI20120139. [DOI] [PubMed] [Google Scholar]
- 191.DeSantis CE, Lin CC, Mariotto AB, Sigel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 192.Centers for Disease Control and Prevention. Centers for Disease Control and Prevention; 2014. Leading Causes of Death. http://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm: Available from: http://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. [Google Scholar]
- 193.Ediriwickrema A, Saltzman WM. Nanotherapy for cancer: targeting and multifunctionality in the future of cancer therapies. ACS Biomater Sci Eng. 2015;1:64–78. doi: 10.1021/ab500084g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bekeschus S, Kolata J, Winterbourn C, Kramer A, Turner R, Weltmann K, Broker B, Masur K. Hydrogen peroxide: a central player in physical plasma-induced oxidative stress in human blood cells. Free Rad Res. 2014;48:542–9. doi: 10.3109/10715762.2014.892937. [DOI] [PubMed] [Google Scholar]
- 195.Graves DP. Reactive species from cold atmospheric plasma: implications for cancer therapy. Plasma Process Polym. 2014;11:1120–7. [Google Scholar]
- 196.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Drug Discovery. 2009;8:579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 197.Takai E, Kitamura T, Kuwabara J, Ikawa S, Yoshizawa S, Shiraki K, Kawasaki H, Arakawa R, Kitano K. Chemical modification of amino acids by atmospheric-pressure cold plasma in aqueous solution. J Phys D: Appl Phys. 2014;47:285403. [Google Scholar]
- 198.Dobrynin D, Wu A, Kalghatgi S, Park S, Shainsky N, Wasko K, Dumani E, Ownbey R, Joshi S, Sensenig R, Brooks AD. Live pig skin tissue and wound toxicity of cold plasma treatment. Plasma Med. 2011;1:93–108. [Google Scholar]
- 199.Wende K, Bekeschus S, Schmidt A, Jatsch L, Hasse S, Weltmann K, Masur K, von Woedtke T. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutat Res. 2016;798–799:48–54. doi: 10.1016/j.mrgentox.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 200.Stalder KR, Woloszko J, Brown IG, Smith CD. Repetitive plasma discharges in saline solutions. Appl Phys Lett. 2001;79:4503–5. [Google Scholar]
- 201.Park DP, Davis K, Gilani S, Alonzo C, Dobrynin D, Friedman G, Fridman A, Rabinovich A, Fridman G. Reactive nitrogen species produced in water by non-equilibrium plasma increase plants growth rate and nutritional yield. Curr Appl Phys. 2013;13:S19–S29. [Google Scholar]
- 202.Pincemail J. Free radicals and antioxidants in human diseases. In: Favier AE, Cadet J, Kalyanaraman B, Fontecave M, Pierre JL, editors. Analysis of Free Radicals in Biological Systems. Basel, Switzerland: Birkhauser Verlag; 1995. pp. 83–98. [Google Scholar]
- 203.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 204.Cheng X, Rajjoub K, Sherman J, Canady J, Recek N, Yan D, Bian K, Murad F, Keidar M. Cold plasma accelerates the uptake of gold nanoparticles into glioblastoma cells. Plasma Process Polym. 2015;12:1364–9. [Google Scholar]


