Abstract
Erwinia asparaginase is an important component in the treatment of pediatric acute lymphoblastic leukemia. A large variability in serum concentrations has been observed after intravenous Erwinia asparaginase. Currently, Dutch Childhood Oncology Group protocols dose alterations are based on trough concentrations to ensure adequate asparaginase activity (≥100 IU/L). The aim of this study was to describe the population pharmacokinetics of intravenous Erwinia asparaginase to quantify and gather insight into inter-individual and inter-occasion variability. The starting dose was evaluated on the basis of the derived population pharmacokinetic parameters. In a multicenter prospective observational study, a total of 714 blood samples were collected from 51 children (age 1–17 years) with acute lymphoblastic leukemia. The starting dose was 20,000 IU/m2 three times a week and adjusted according to trough levels from week three onwards. A population pharmacokinetic model was developed using NONMEM®. A 2-compartment linear model with allometric scaling best described the data. Inter-individual and inter-occasion variability of clearance were 33% and 13%, respectively. Clearance in the first month of treatment was 14% higher (P<0.01). Monte Carlo simulations with our pharmacokinetic model demonstrated that patients with a low weight might require higher doses to achieve similar concentrations compared to patients with high weight. The current starting dose of 20,000 IU/m2 might result in inadequate concentrations, especially for smaller, lower weight patients, hence dose adjustments based on individual clearance are recommended. The protocols were approved by the institutional review boards. (Registered at NTR 3379 Dutch Trial Register; www.trialregister.nl).
Introduction
Asparaginase is an enzyme that catalyzes the hydrolysis of asparagine to aspartic acid and ammonia. Leukemic cells rely on exogenous supplies of asparagine for their protein synthesis. Hence the depletion of asparagine results in cell death.1 Asparaginase has become an important component in the treatment of pediatric acute lymphoblastic leukemia (ALL), and therefore every effort should be made to expose the patient to the protocol-prescribed cumulative dose.2–6 Erwinia asparaginase is derived from Erwinia chrysanthemi, whereas the other forms of asparaginase [native Escherichia coli (E. coli) and polyethylene glycol (PEG)-asparaginase] are derived from E. coli. Currently the E. coli derivatives are the first choice in treatment naïve patients, and the use of Erwinia asparaginase is indicated in patients who develop hypersensitivity to the E. coli-derived asparaginase or in the case of silent inactivation of E. coli asparaginase.4,7,8
Little is known about the pharmacokinetics (PK) of Erwinia asparaginase, especially after intravenous administration. Currently, in the Dutch Childhood Oncology Group (DCOG) ALL-11 protocol (and the preceding ALL-10), the interval and/or dose alterations of Erwinia asparaginase are based on therapeutic drug monitoring (TDM), with the aim of keeping the trough asparaginase activity above the 100 IU/L threshold, which leads to complete asparagine depletion.7,9–13 A concentration of 100 IU/L is considered safe concerning asparagine depletion; however, complete depletion has been observed at lower concentrations.14–16 Currently, the consensus for the target threshold is 100 IU/L.17 No evidence-based guidelines for increasing or decreasing the dose are available and dose-adaptations are based on empirical knowledge.
The PK data of different asparaginase forms are not transferrable. Intramuscular native E. coli asparaginase has an elimination half-life of 1.3 days,18 and intravenous recombinant and native E. coli asparaginase 17.3–19.0 hours (h),19,20 and follow linear elimination, whereas E. coli PEG-asparaginase has time-dependent pharmacokinetics and a half-life with an observed range of 2.4–11.8 days.9,18,21,22 Erwinia asparaginase has the shortest half-life with means of 12.6–22.1 h after intramuscular administration,7,9,23,24 and 6.4–7.6 h after intravenous administration.23,25
The aim of the present study was to describe the population PK of intravenously administered Erwinia asparaginase in pediatric ALL patients, to quantify the random parameters inter-individual (IIV), inter-occasion (IOV) and residual variability, and to determine the association between patients’ characteristics and the PK parameters. Quantification of random parameters is important for proper TDM, as IIV can be compensated by TDM, whereas IOV cannot. In addition, the current starting dose was evaluated considering a trough concentration above 100 IU/L.
Methods
Patients’ characteristics and treatment
The study was designed as a prospective multicenter study in 7 pediatric oncology centers in the Netherlands. Children aged 1–18 years with acute lymphoblastic leukemia (ALL) were eligible for the study when treated according to the DCOG ALL-10 (Nov 1, 2004–April 1, 2012) or ALL-11 (since April 1, 2012 and ongoing) protocols with Erwinia asparaginase (Erwinase®; EUSA Pharma, Europe) after the development of an allergy to E. coli-derived asparaginase or silent inactivation of E. coli-derived asparaginase. The starting dose of Erwinia asparaginase was 20,000 IU/m2 intravenously (i.v.) over 1 h thrice weekly (Mon/Wed/Fri). The dose was fixed for the first two weeks. Subsequently, if the 72 h concentration was more than 100 IU/L, the dose interval was adjusted to twice weekly. Additionally in ALL-11, the dose was increased based on clinical expertise if the 72 h concentration was less than 100 IU/L. When this was not sufficient, the dose interval was set to every other day. The TDM samples were collected between May 1, 2009 and February 5, 2015. These are trough samples prior to their next dose, predominantly 48 or 72 h after the last Erwinia asparaginase administration. For the purpose of this study, additional peak concentrations were collected between 1 and 4 h in a subset of patients. Erwinia asparaginase activity concentrations in serum were analyzed as previously described by us, with a lower limit of quantification (LLoQ) of less than 5 IU/L.26 The protocols were approved by a central Institutional Review Board.
Pharmacokinetic analysis
A detailed description of the pharmacokinetic analysis methods is provided in the Online Supplementary Appendix. Time profiles of log transformed Erwinia asparaginase concentrations were analyzed using the non-linear effects modeling approach implemented in non-linear mixed effects modeling (NONMEM). The data were initially fitted to a 1-compartment linear model without an absorption compartment. Subsequently more complex models were evaluated; improvement of the fit was evaluated by the precision of the estimated PK parameters, change in the objective function values (OFV), goodness-of-fit plots (GOF), and visual predictive checks (VPC). A 3.84-point decrease in OFV for one degree of freedom was considered a significant improvement (P<0.05).
The data were obtained in a pediatric population, hence PK parameters were allometrically scaled to adequately describe the parameters across a wide range of body weights. For allometric scaling, standard fixed exponent values of 0.75 for the flow dependent physiological process parameters and 1 for volume-related parameters were used.27–29 Inter-patient and inter-occasion variability in clearances and volumes of distribution were characterized with exponential models. An additive error model was used to describe the residual error in plasma concentrations.
After the finalization of the structural model, covariate models were built by a stepwise forward inclusion procedure. The covariate with the greatest reduction in OFV was added to the base model. This was iterated over all the covariates until no statistically significant decrease in OFV occurred. For the internal validation of the model, non-parametric bootstrap procedures (n=1000) were performed and VPCs were obtained. The final model including covariates was used to perform Monte Carlo simulations (n=5000) for different doses and patient weight.
Results
Patients and samples
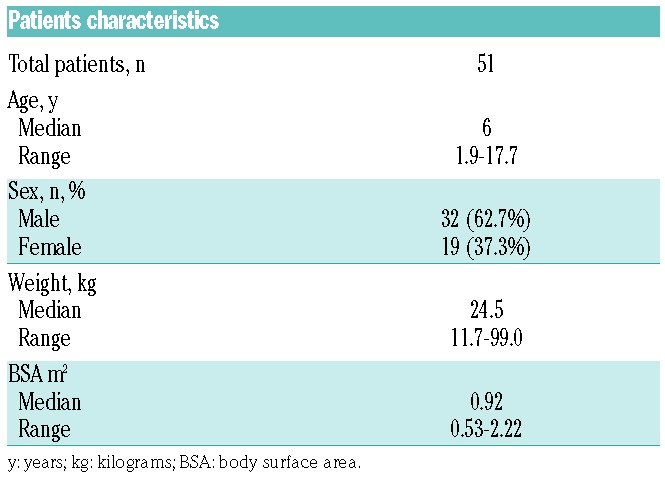
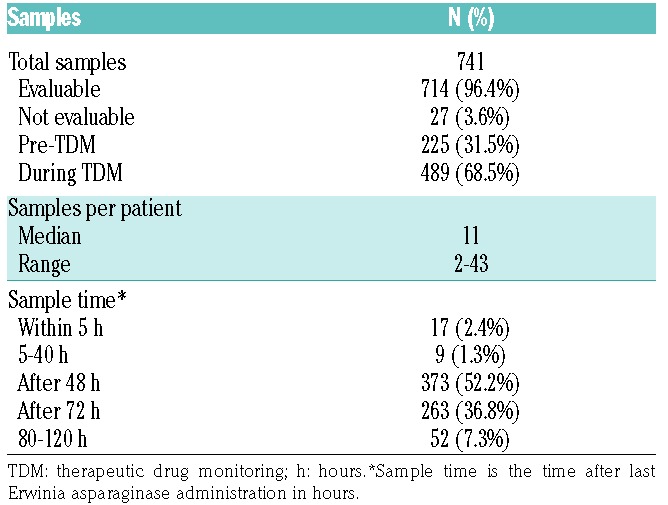
During the study period, 53 patients were switched from E. coli-derived asparaginase to Erwinia asparaginase due to allergic reactions or silent inactivation. Data from 51 of these 53 patients were included in the PK analysis. Two patients were excluded due to Erwinia asparaginase concentrations below the LLoQ. One of these patients had anti-Erwinia asparaginase antibodies neutralizing the drug and prohibiting sufficient exposure, for the other patient the reason for unmeasurable concentrations is not known. Both patients discontinued treatment with Erwinia asparaginase. A summary of patients’ characteristics is shown in Table 1. A total of 741 samples were available, with a median of 11 samples per patient (range 2–43 samples). Twenty-seven samples (3.6%) were excluded from the analysis due to: missing sampling data (n=20), no measurable asparaginase (n=4), or unrealistic high concentrations (n=3) due to sampling artefacts. The four unmeasurable trough concentrations were all from the same patient; however, this patient did have measurable Erwinia asparaginase concentrations within 24 h after administration.
Table 1.
Patients’ characteristics.
Samples were collected for two weeks up to 12 months after the start of Erwinia asparaginase treatment. Samples were predominantly trough concentrations taken around 48 h (52.2%) and 72 h (36.8%) after Erwinia asparaginase administration. Figure 1 shows the combined Erwinia asparaginase concentrations versus the time after dose for all patients throughout therapy, demonstrating a large variability. The concentrations can be stratified for the first two weeks of treatment (no dose adjustments), and after two weeks with potential adjustments (TDM) in Erwinia asparaginase dose frequency (both ALL-10 and ALL-11 protocol) and dose (ALL-11 protocol only). The median asparaginase trough concentrations two days after administration (range 42–50 h) for, respectively, the first two weeks and during TDM, were 166.2 IU/L [interquartile range (IQR) 103.4–270.1] and 191.0 IU/L (IQR 115.0–296.5); 75.4% and 82.6% of the patients had asparaginase trough concentrations of 100 IU/L or more; 90.16% and 91.30% had trough concentrations of 50 IU/L or more. Three days after administration (65–80 h), the median trough concentrations for, respectively, the first two weeks and during TDM, were 48.4 IU/L (IQR 29.9–104.7) and 83.7 IU/L (IQR 49.5–98.7); 26.4% and 39.5% of the patients had trough concentrations of 100 IU/L or more; 50% and 74.3% had trough concentrations of 50 IU/L or more.
Figure 1.
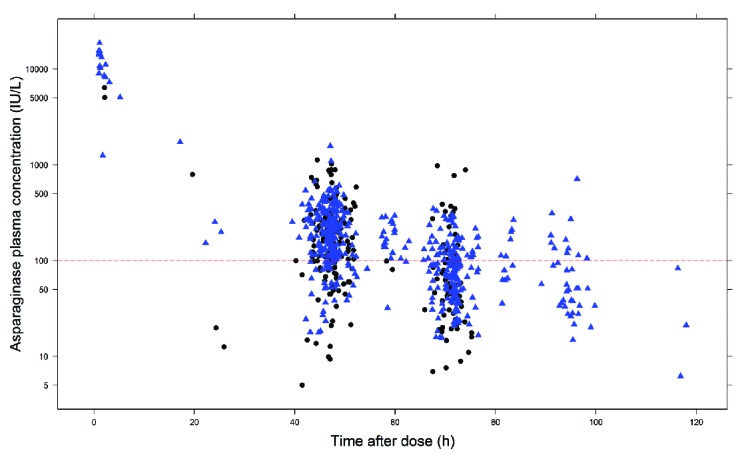
Asparaginase concentration (IU/L) versus time after dose in hours (h) for all patients and all occasions on a semi-log plot. This shows the large inter-patient variability in plasma concentrations as collected throughout treatment. Triangles (black) show concentrations prior to possible dose adjustments (first 2 weeks) and circles (blue) show observed concentrations with possible dose adjustments [therapeutic drug monitoring (TDM) after week two].
The summary of the number of samples and time points can be found in Table 2. A total of 311 samples (43.6%) were collected in the first month of Erwinia asparaginase treatment. The number of samples during the following months ranged from 86 (in month 2) to 1 (in month 12). Especially trough concentrations taken at 72 h frequently dropped below the desired therapeutic target threshold of 100 IU/L. Eleven patients (21.6%) were switched from thrice to twice weekly interval after asparaginase 72 h trough concentrations of more than 100 IU/L during their treatment. A total of 117 samples (15.5%) were drawn during a twice-weekly interval.
Table 2.
Samples for pharmokinetic analysis.
Pharmacokinetic model
Both 1- and 2-compartment models were evaluated. The estimated PK parameters were normalized to a weight of 70 kg using the ¾ allometric model. The 2-compartment model provided a better fit to the data than a 1-compartment model based on the OFV and the goodness-of-fit plots. The OFV significantly decreased 127.2 points from 366.5 to 239.3 (P<0.001). A slight decrease in additive error was observed from 0.70 IU/L (1-compartment model) to 0.64 IU/L (2-compartment model). Addition of a third compartment did not improve the model. A Michaelis-Menten elimination model did not improve the model either.
The estimated IIV on CL was 36%, whereas this parameter could not be estimated for distribution of the central compartment (Vc), intercompartmental clearance (Q) and distribution of the peripheral compartment (Vp). Complete removal of the a priori incorporated allometric scaling from the model, based on body weight (standardized for 70 kg) and fixed exponents, resulted in a worse model with a 8.3 points increase in OFV and an increase of the IIV for CL from 33% to 40%. Samples were collected throughout therapy on different occasions. Addition of IOV resulted in an improvement of the population model with an estimated value of 14%. By incorporation of the IOV, the OFV decreased with 44.9 points, additive error decreased from 0.64 to 0.57, and the IIV for CL decreased from 36% to 33%. The shrinkage was 6% for IIV on CL, 32% for IOV on CL and 9% for residual variability. This was considered the structural model and was used for the stepwise forward inclusion of covariates.
All covariates were tested one at a time for improvement of the structural model. The clearance in the first month was 14% higher in comparison to the subsequent months with a decrease of 17.0 points in OFV (P<0.001). Dose as a covariate on CL also improved the model (P<0.05). However, during TDM, the dose is adjusted according to the patients’ asparaginase concentrations and therefore indirectly for their clearance, which explains the correlation between dose and clearance. Similarly, an association between dose interval and clearance was detected. With dose interval as covariate, the OFV significantly decreased 6.11 points (P<0.05) and patients with twice weekly dosing (n=11) were associated with a 24% lower CL in comparison with patients on thrice weekly dosing (n=40). Due to TDM, dose and dose interval were not incorporated in the model. The other covariates [age, weight, height, BSA, sex, treatment protocol (ALL-10 or ALL-11) and treatment center] did not result in a significant improvement of the base model.
The final model was a 2-compartment model with fixed exponents allometric scaling, a correction factor for increased clearance in the first month, and inter-individual and inter-occasion variability on clearance. The parameter estimates of the final model were: CL 0.44 L/h/70kg, Vc (central compartment) 3.2 L/70kg, Q (intercompartmental clearance) 0.15 L/h/70kg and Vp (peripheral compartment) 2.9 L/70kg. The calculated half-lives (t1/2) for the 2-compartment model are T1/2,α 3.5 h and T1/2,β 19.6 h, which represent respectively the distribution phase and elimination phase. The inter-individual variability of clearance was 33%. There was an inter-occasion variability of 13% based on monthly intervals (Table 3). The basic goodness-of-fit plots (Figure 2) show good model performance. Individual predictions versus observation are well distributed around the unity line. The weighted residuals are within a good range (2 to −2) and evenly distributed. The population predictions show a deviation from the unity line for the lower concentrations where limited samples were available.
Table 3.
Population parameter estimates and non-parametric bootstrap.
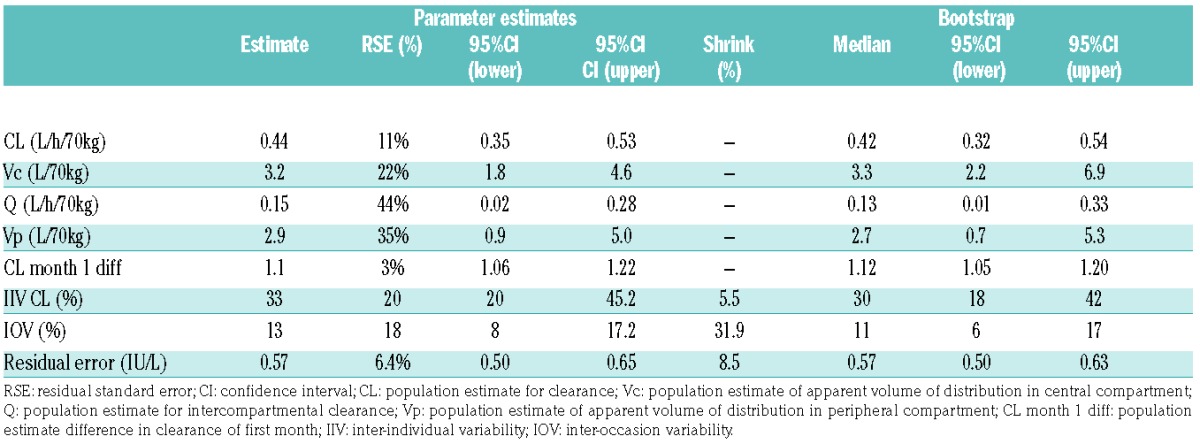
Figure 2.
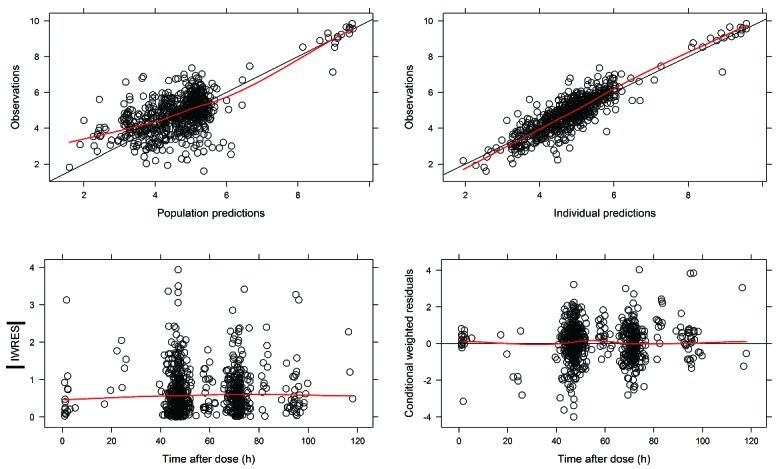
Goodness-of-fit plots final model. (Upper left) Predicted population concentrations versus observed concentrations of the final model. (Upper right) Predicted individual concentrations versus observed concentrations of the final model. (Lower left) Individual weighted residuals versus individual predictions, (lower right) conditional weighted residuals versus time after dose. h: time in hours; IWRES: individual weighted residual predictions.
Model validation
The non-parametric bootstrap procedure was performed to test the robustness of the model. A total 998 of the 1000 runs were successful. The results are shown in Table 3. The estimates of the final model are in accordance with the results from the 1000 bootstrap replicates. The plot of the prediction corrected visual predictive check (pcVPC) shows the median and 90% interval of the observed asparaginase concentrations (Figure 3). The model adequately predicts the time course of the asparaginase plasma concentration during the first three time frames (0–36 h, 36–60 h and 60–84 h). However, an under-prediction of median concentrations and 5th and 95th percentiles was observed in the 84–118 h timeframe.
Figure 3.
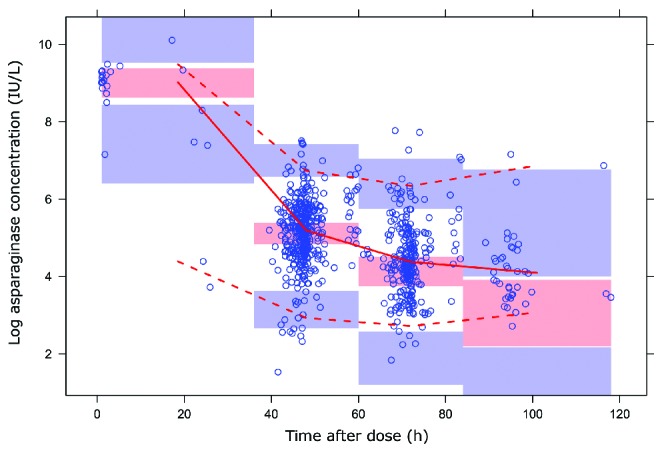
Visual predictive check. Prediction corrected (Pred Corr) visual prediction plot of observed log asparaginase concentrations versus time after last Erwinia asparaginase dose in hours (h) of the final model. Red solid line shows the median observed concentrations and the surrounding opaque red area the simulation based 95% interval for the median. Red dashed lines indicate the observed 5% and 95% percentiles; surrounding opaque blue areas show the simulated 95% confidence intervals for the corresponding predicted percentiles.
Evaluation of the starting dose
To investigate the appropriateness of the starting dose, Monte Carlo simulations with varying weights and doses were performed utilizing the developed population PK model. Based on the simulations, patients with a lower body weight appeared to require higher weight-normalized starting doses to achieve sufficient Erwinia asparaginase concentrations after 48 h. Patients weighing 100 kg require 500 IU/kg compared to doses exceeding 1000 IU/kg for patients with a body weight below 20 kg in order to have Erwinia asparaginase concentrations of 100 IU/L or more in 75% of the patients at 48 h after administration (Figures 4 and 5). With the current starting dose of 20,000 IU/m2, approximately 75% of the patients with a body weight of more than 50 kg would have concentrations of 100 IU/L or more after 48 h. For patients with a body weight between 30–50 kg, the suggested starting dose would be 25,000 IU/m2, and for patients with a body weight of 10–30 kg doses of 25,000–37,000 IU/m2 to achieve Erwinia asparaginase concentrations of 100 IU/L or more after 48 h in at least 75% of the patients after the first dose. To achieve this in 90% of the patients, starting dose for all patients would have to be above 33,000 IU/m2. The dose in IU/kg was converted to IU/m2 utilizing corresponding BSA to weight for pediatric oncology patients.30 Figures 5 and 6 show required starting dose (in IU/kg and IU/m2) versus weight to achieve 48 h-trough concentration of 100 IU/L or more (Figure 5) and of 50 IU/L or more (Figure 6) in a percentage of patients ranging from 10% to 90%. The simulations with the final PK model are in accordance with the observations made in the patient data. Starting doses of 36 patients were evaluable (Erwinia asparaginase dose of 20,000 IU/m2 and an available 48 h sample after administration of the first dose). With respect to weight, 12 of 31 patients (39%) weighing less than 50 kg and and 1 of 5 patients (20%) weighing 50 kg or more had 48 h trough concentrations below 100 IU/L. Although these numbers are small, it shows the same trend in weight-concentration relationship as the Monte Carlo simulations.
Figure 4.
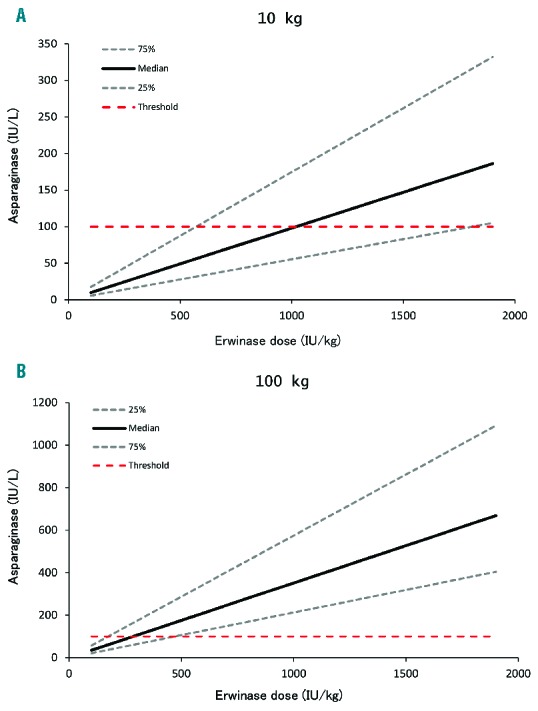
Simulated Erwinia asparaginase concentrations for a 10 kg and a 100 kg patient. Median and the 25% and 75% percentiles of the patients who achieve asparaginase concentrations (y-axis) after 48 hours for different Erwinia asparaginase doses (x-axis). (A) Concentrations for a 10 kg patient and (B) a 100 kg patient. Red dashed line is the target trough concentration of 100 IU/L.
Figure 5.
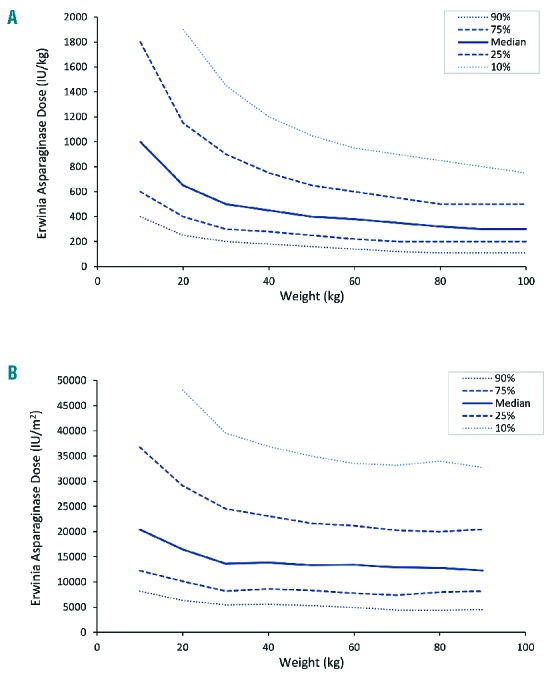
Erwinia asparaginase starting dose versus patient weight to achieve 100 IU/L or more after 48 hours (h). (A) Required starting dose in IU/kg and (B) IU/m2 versus weight of patients in kilograms (kg) to achieve 100 IU/L or more after 48 h. Median (solid line), 25% and 75% percentiles (dashed line) and 10% and 90% percentiles (dotted line) of the patients with asparaginase concentrations of 100 IU/L or more with different weight (x-axis) and different starting doses (y-axis).
Figure 6.
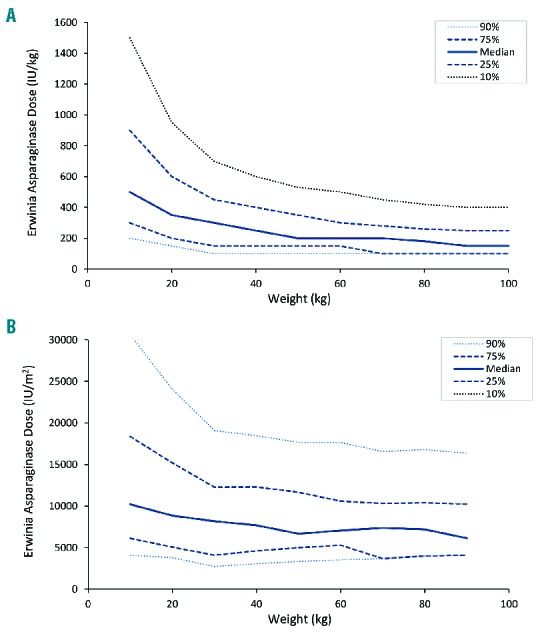
Erwinia asparaginase starting dose versus patient body surface area to achieve 50 IU/L or more after 48 hours (h). (A) Required starting dose in IU/kg and (B) IU/m2 versus weight of patients in kilograms (kg) to achieve 50 IU/L or more after 48 h. The median (solid line), 25% and 75% percentiles (dashed line) and 10% and 90% percentiles (dotted line) of the patients with asparaginase concentrations of 50 IU/L or more with different weight (x-axis) and different starting doses (y-axis).
Discussion
The PK of Erwinia asparaginase was best described with a 2-compartment model with linear elimination and, therefore, more similar to native asparaginase than PEGylated asparaginase which has time-dependent elimination, probably due to the polyethylene glycol.21 There appears to be a negative correlation between weight and weight-normalized clearance, where the patients with a lower weight require higher weight-normalized doses based on Monte Carlo simulations with the final PK model. The same trend was observed in the actual patient data; however, only a small number of patients had a body weight of more than 50 kg. In addition, clearance in the first month was significantly higher.
Asparaginase is an important component in the treatment of pediatric ALL, where it contributes 10%–20% to the total treatment outcome.6,11,12,19,31,32 However, treatment with asparaginase at suboptimal dose schedules leads to an inferior outcome.11–13,32 Asparaginase is available in different molecular forms (eg. PEGylated or native). The PK properties of these different forms are, however, not similar, and therefore can not be used interchangeably.9,18,33 Erwinia asparaginase has a shorter half-life in comparison with the other asparaginase forms; this results in lower concentrations and exposure when administered at equal dose schedules and, consequently, worse outcome.11–13
In North-America, intramuscular injection of Erwinia asparaginase was the only Food and Drug Administration (FDA)-approved method of administration, but this has recently been extended with i.v. administration.34 In Europe, the predominant method of Erwinia asparaginase administration is i.v. administration. Several PK studies of intramuscular administration have been published.8,9,23,24 This is important in studying the PK because, in addition to the asparaginase molecule or pharmaceutical form, the route of administration may also influence the PK. The PK of intramuscular-administered Erwinia asparaginase differs from i.v. administration due to the presence of a rate-limiting step in the absorption phase.23,24,34 Bypassing the absorption from the muscle will result in faster elimination and probably more predictable concentrations, as variability in absorption rate is eliminated. The calculated terminal half-life was 19.6 h, which is similar to the terminal half-life of the i.v.-administered native E. coli asparaginase of 19.0 h.22 Previously published studies with i.v. Erwinia asparaginase showed a half-life of 6.4 h and 7.5 h.23,25 However, our study uses a 2-compartment model which has a fast elimination half-life of 3.5 h during the distribution phase and a slower elimination half-life of 19.6 h for the terminal elimination phase. This presents itself in a concentration time curve with a steep decline in the first phase followed by a slower decline in the second; this can also be observed in Figures 1 and 3.
During TDM, a large variability in Erwinia asparaginase concentrations was also observed after i.v.-administered Erwinia asparaginase (Figure 1). Hence the population PK of Erwinia asparaginase was studied to evaluate the elimination of Erwinia asparaginase from the body in a quantitative manner, and to explain and quantify the variability in order to improve individual dosing to achieve sufficient asparaginase concentrations prior to their next dose. PK-based TDM dosing can compensate for IIV, but not for IOV. In this study, the IIV was 33% and the IOV was 13%, which is favorable for PK-based dosing.
The development of the PK model was successful despite the limited number of peak concentrations. The parameters were estimated with good precision; the shrinkage of IIV and the residual error was small. The bootstrap estimates were also in accordance with the model estimates. The VPC showed the model predictions to be in line with the observations, except for the last time frame (84–118 h), which showed a slight under-prediction (Figure 3). However, samples in this timeframe were patients who were switched to twice-weekly Erwinia asparaginase administration due to high asparaginase con centrations, hence presumably characterized by a lower clearance. Calculations based on the total population would, therefore, over-predict the clearance in this group, resulting in the under-prediction of the Erwinia asparaginase concentrations. When the predictions are corrected for the dose interval, the under-prediction disappears and is in accordance with the observations (Online Supplementary Figure S1).
Monte Carlo simulations of patients with different body weights and starting doses of Erwinia asparaginase were performed aiming at trough concentrations of 100 IU/L or more. Based on the simulations, the current starting dose of 20,000 IU/m2 seems rather low, especially for children with a body weight less than 50 kg. If this starting dose is used, close monitoring of the patient is required to ensure sufficient Erwinia asparaginase concentrations. The PK model uses the standard allometric scaling based on weight, which is the gold standard for allometric scaling in population PK. Unlike weight-based scaling, it is unclear how scaling based on BSA should be implemented. The implementation of BSA in the PK model might depend on the chosen method of BSA calculation (e.g. Mosteller, Dubois & Dubois, Haycock), as these methods use different internal (exponential) correction factors.35–37 However, to our knowledge, this has still not been studied. Therefore, weight-based allometric scaling was used for the development of the PK model. Monte Carlo simulations were also expressed on a per weight basis. Additional simulations were performed with scaling based on BSA resulting in similar results. For clinical convenience, the dose was converted to IU/m2 using the corresponding BSA to weight in pediatric oncology patients for the representation of Figures 5B and 6B.30 With the registered dose of 25,000 IU/m2, approximately 75% of the patients have Erwinia asparaginase concentrations above 100 IU/L 48 h after the first dose and 90%–100% of the patients above 50 IU/L.
When increasing the dose to achieve sufficient trough concentrations, one has to keep in mind that the peak concentrations (Cmax) and exposure (AUC) increase as well. This might lead to side effects which include hypersensitivity or infusion reactions, pancreatitis, liver abnormalities, central neurotoxicities, glucose intolerance or coagulation abnormalities,24,38,39 although we showed no significant correlation between asparaginase activity concentrations and pancreatitis, thrombosis or central neurotoxicities.39 High concentrations of asparaginase were associated with high triglyceride and high cholesterol concentrations, and were more pronounced in children aged ten years or older. This might be explained by the lower weight normalized clearance in older children.39 Information concerning specific toxic concentrations was not available therefore maximum concentrations (Cmax) and exposure (area under the curve) were not evaluated. In addition, due to the increased clearance in the first month, the plasma concentrations will be lower compared to the following months. Hence increasing the dose might not be necessary.
A potential limitation of this study was that samples were collected during the TDM procedure and predominantly withdrawn at 48 and 72 h after administration. Additional peak samples were collected during the first hours after administration. Patients were at home in the period between the peak (first hours after dose) and trough concentrations (prior to the next dose), therefore Erwinia asparaginase concentrations within this timeframe were not available. However, with Erwinia asparaginase dosing, the aim is to achieve sufficient trough concentrations to assure complete asparagine depletion prior to the next dose (which is after 48 or 72 h), and the dose will be adjusted according to those time points. Two patients were excluded from the analysis due to non-measurable asparaginase levels. One patient had antibodies, which could explain the lack of asparaginase; the other patient might have had a very fast asparaginase clearance. Excluding these patients could result in a lower variability and estimated clearance.
With the registered Erwinia asparaginase dose of 25,000 IU/m2, approximately 25% of the patients will not have 48 h trough concentrations above 100 IU/L after their first dose. This will be more pronounced in those patients with a low body weight. However, the PK analysis showed an increased clearance in the first month, therefore Erwinia asparaginase concentrations will increase after the first month. Monitoring the plasma concentrations and adjusting the dose for the individual patients presented with concentrations below target threshold is recommended. Asparagine is completely depleted with Erwinia asparaginase concentrations of 100 IU/L or more, although some studies show complete depletion at lower concentrations.7,9,13,15,16,40 Increasing the dose for the group as a whole might lead to unnecessarily high concentrations in the majority of the patients with concentrations already over 100 IU/L, hence resulting in possible (long-term) side effects and unnecessary costs. Therefore, individual dose adjustments are recommended.
The optimal treatment would be dose adjustments based on patients’ individual PK parameters. With the PK model developed in this study, the individualized dose requirements can be calculated via post-hoc Bayesian analysis. This might reduce possible under-exposure that could potentially result in relapse, as well as reduce high concentrations. A prospective randomized controlled trial could compare conventional dosing versus individualized PK-based Erwinia asparaginase dosing to evaluate whether individual Erwinia asparaginase concentrations would improve and whether this affects treatment outcome. However, due to the limited number of Erwinia asparaginase-treated patients in the Netherlands, this is not possible and should be performed on an international level. A rational approach should be adopted for dose management to ensure adequate trough concentrations.
Supplementary Material
Acknowledgments
We thank the patients and their parents, participating centers and (research) nurses for their help. We thank the pediatric oncology/ hematology laboratory of the Erasmus MC – Sophia Children’s Hospital in Rotterdam, the Netherlands, for the analysis of the samples.
This study was funded by the Kinderen Kankervrij (KiKa) Foundation and the Go4Children Foundation.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/3/552
References
- 1.Dubbers A, Wurthwein G, Muller HJ, et al. Asparagine synthetase activity in paediatric acute leukaemias: AML-M5 subtype shows lowest activity. Br J Haematol. 2000; 109(2):427–429. [DOI] [PubMed] [Google Scholar]
- 2.Capizzi RL, Bertino JR, Skeel RT, et al. Clinical, Biochemical, Pharmacological, and Immunological Studies. Ann Intern Med. 1971;74(6):893–901. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe N, Traggis D, Das L, et al. L-asparaginase in the treatment of neoplastic diseases in children. Cancer Res. 1971;31(7):942–949. [PubMed] [Google Scholar]
- 4.Pieters R, Hunger SP, Boos J, et al. L-asparaginase treatment in acute lymphoblastic leukemia. Cancer. 2011;117: 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pession A. Long-Term Results of a Randomized Trial on Extended Use of High Dose L-Asparaginase for Standard Risk Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2005;23(28):7161–7167. [DOI] [PubMed] [Google Scholar]
- 6.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91–01. Blood. 2001;97(5):1211–1218. [DOI] [PubMed] [Google Scholar]
- 7.Salzer WL, Asselin B, Supko JG, et al. Erwinia asparaginase achieves therapeutic activity after pegaspargase allergy: A report from the Children’s Oncology Group. Blood. 2013;122(4):507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salzer W, Seibel N, Smith M. Erwinia asparaginase in pediatric acute lymphoblastic leukemia. Expert Opin Biol Ther. 2012; 12(10):1407–1414. [DOI] [PubMed] [Google Scholar]
- 9.Avramis VI, Spence SA. Clinical pharmacology of asparaginases in the United States: asparaginase population pharmacokinetic and pharmacodynamic (PK-PD) models (NONMEM) in adult and pediatric ALL patients. J Pediatr Hematol Oncol. 2007; 29(4):239–247. [DOI] [PubMed] [Google Scholar]
- 10.Tong WH, Pieters R, Kaspers GJL, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014;123(13):2026–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duval M, Suciu S, Ferster A, et al. Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children’s Leukemia Group phase 3 trial. Blood. 2002;99(8):2734–2739. [DOI] [PubMed] [Google Scholar]
- 12.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109(3):896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwok CS, Kham SK, Ariffin H, Lin HP, Quah TC, Yeoh AE. Minimal residual disease (MRD) measurement as a tool to compare the efficacy of chemotherapeutic drug regimens using Escherichia Coli-asparaginase or Erwinia-asparaginase in childhood acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer. 2006;47(3):299–304. [DOI] [PubMed] [Google Scholar]
- 14.Rizzari C, Citterio M, Zucchetti M, et al. A pharmacological study on pegylated asparaginase used in front-line treatment of children with acute lymphoblastic leukemia. Haematologica. 2006;91(1):24–31. [PubMed] [Google Scholar]
- 15.Ahlke E, Nowak-Göttl U, Schulze-Westhoff P, et al. Dose reduction of asparaginase under pharmacokinetic and pharmacodynamic control during induction therapy in children with acute lymphoblastic leukaemia. Br J Haematol. 1997; 96(4):675–681. [DOI] [PubMed] [Google Scholar]
- 16.Rizzari C, Zucchetti M, Conter V, et al. L-asparagine depletion and L-asparaginase activity in children with acute lymphoblastic leukemia receiving i.m. or i.v. Erwinia C. or E. coli L-asparaginase as first exposure. Ann Oncol. 2000;11(2):189–193. [DOI] [PubMed] [Google Scholar]
- 17.van der Sluis IM, Vrooman LM, Pieters R, et al. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica. 2016;101(3): 279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ. Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol. 1993;11(9): 1780–1786. [DOI] [PubMed] [Google Scholar]
- 19.Pieters R, Appel I, Kuehnel H-J, et al. Pharmacokinetics, pharmacodynamics, efficacy, and safety of a new recombinant asparaginase preparation in children with previously untreated acute lymphoblastic leukemia: a randomized phase 2 clinical trial. Blood. 2008;112(13):4832–4838. [DOI] [PubMed] [Google Scholar]
- 20.Borghorst S, Pieters R, Kuehnel H-J, Boos J, Hempel G. Population pharmacokinetics of native Escherichia coli asparaginase. Pediatr Hematol Oncol. Taylor & Francis; 2012; 29(2):154–165. [DOI] [PubMed] [Google Scholar]
- 21.Hempel G, Müller HJ, Lanvers-Kaminsky C, Würthwein G, Hoppe A, Boos J. A population pharmacokinetic model for pegylated-asparaginase in children. Br J Haematol. 2010;148(1):119–125. [DOI] [PubMed] [Google Scholar]
- 22.Borghorst S, Pieters R, Kuehnel H-J, Boos J, Hempel G. Population pharmacokinetics of native Escherichia coli asparaginase. Pediatr Hematol Oncol. Taylor & Francis; 2012; 29(2):154–165. [DOI] [PubMed] [Google Scholar]
- 23.Albertsen BK, Jakobsen P, Schrøder H, Schmiegelow K, Carlsen NT. Pharmacokinetics of Erwinia asparaginase after intravenous and intramuscular administration. Cancer Chemother Pharmacol. 2001;48(1):77–82. [DOI] [PubMed] [Google Scholar]
- 24.Albertsen BK, Schrøder H, Ingerslev J, et al. Comparison of intramuscular therapy with Erwinia asparaginase and asparaginase Medac: Pharmacokinetics, pharmacodynamics, formation of antibodies and influence on the coagulation system. Br J Haematol. 2001;115(4):983–990. [DOI] [PubMed] [Google Scholar]
- 25.Vrooman LM, Kirov II, Dreyer ZE, et al. Activity and Toxicity of Intravenous Erwinia Asparaginase Following Allergy to E. coli-Derived Asparaginase in Children and Adolescents With Acute Lymphoblastic Leukemia. Pediatr Blood Cancer. 2016;63(2):228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong WH, Pieters R, Hop WCJ, Lanvers-Kaminsky C, Boos J, van der Sluis IM. No evidence of increased asparagine levels in the bone marrow of patients with acute lymphoblastic leukemia during asparaginase therapy. Pediatr Blood Cancer. 2013; 60(2):258–261. [DOI] [PubMed] [Google Scholar]
- 27.Anderson BJ, Holford NHG. Tips and traps analyzing pediatric PK data. Paediatr Anaesth. 2011;21(3):222–237. [DOI] [PubMed] [Google Scholar]
- 28.Holford NH. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996;30(5): 329–332. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Peeters MYM, Allegaert K, et al. A bodyweight-dependent allometric exponent for scaling clearance across the human life-span. Pharm Res. 2012;29(6):1570–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharkey I, Boddy AV, Wallace H, et al. Body surface area estimation in children using weight alone: application in paediatric oncology. Br J Cancer. 2001;85(1):23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abshire TC, Pollock BH, Billett AL, Bradley P, Buchanan GR. Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: a Pediatric Oncology Group Study. Blood. 2000;96(5):1709–1715. [PubMed] [Google Scholar]
- 32.Paolucci G, Vecchi V, Favre C, et al. Treatment of childhood acute lymphoblastic leukemia. Long-term results of the AIEOP-ALL 87 study. Haematologica. 2001;86(5):478–484. [PubMed] [Google Scholar]
- 33.Boos J, Werber G, Ahlke E, et al. Monitoring of asparaginase activity and asparagine levels in children on different asparaginase preparations. Eur J Cancer. 1996;32A(9):1544–1550. [DOI] [PubMed] [Google Scholar]
- 34.Vrooman LM, Kirov II, Dreyer ZE, et al. Activity and toxicity of intravenous Erwinia asparaginase following allergy to E. coli-derived asparaginase in children and adolescents with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2016; 63(2):228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987; 317(17):1098. [DOI] [PubMed] [Google Scholar]
- 36.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93(1):62–66. [DOI] [PubMed] [Google Scholar]
- 37.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–311. [PubMed] [Google Scholar]
- 38.Erwinaze® (asparaginase Erwinia Chrysanthemi). [Package insert] Palo Alto, CA: Jazz Pharmaceuticals, Inc; [Internet]. 2014. [cited 29 June 2016]. Available from: http://www.accessdata.fda.gov/drugsatf-da_docs/label/2014/125359s085lbl.pdf Last accessed: 29 June 2016. [Google Scholar]
- 39.Tong WH, Pieters R, de Groot-Kruseman HA, et al. The toxicity of very prolonged courses of PEGasparaginase or Erwinia asparaginase in relation to asparaginase activity, with a special focus on dyslipidemia. Haematologica. 2014;99(11):1716–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rizzari C, Citterio M, Zucchetti M, et al. A pharmacological study on pegylated asparaginase used in front-line treatment of children with acute lymphoblastic leukemia. Haematologica. 2006;91(1):24–31. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


