Abstract
Hematopoietic stem cells can be mobilized from healthy donors using single-agent plerixafor without granulocyte colony-stimulating factor and, following allogeneic transplantation, can result in sustained donor-derived hematopoiesis. However, when a single dose of plerixafor is administered at a conventional 240 μg/kg dose, approximately one-third of donors will fail to mobilize the minimally acceptable dose of CD34+ cells needed for allogeneic transplantation. We conducted an open-label, randomized trial to assess the safety and activity of high-dose (480 μg/kg) plerixafor in CD34+ cell mobilization in healthy donors. Subjects were randomly assigned to receive either a high dose or a conventional dose (240 μg/kg) of plerixafor, given as a single subcutaneous injection, in a two-sequence, two-period, crossover design. Each treatment period was separated by a 2-week minimum washout period. The primary endpoint was the peak CD34+ count in the blood, with secondary endpoints of CD34+ cell area under the curve (AUC), CD34+ count at 24 hours, and time to peak CD34+ following the administration of plerixafor. We randomized 23 subjects to the two treatment sequences and 20 subjects received both doses of plerixafor. Peak CD34+ count in the blood was significantly increased (mean 32.2 versus 27.8 cells/μL, P=0.0009) and CD34+ cell AUC over 24 hours was significantly increased (mean 553 versus 446 h cells/μL, P<0.0001) following the administration of the 480 μg/kg dose of plerixafor compared with the 240 μg/kg dose. Remarkably, of seven subjects who mobilized poorly (peak CD34+ ≤20 cells/μL) after the 240 μg/kg dose of plerixafor, six achieved higher peak CD34+ cell numbers and all achieved higher CD34+ AUC over 24 hours after the 480 μg/kg dose. No grade 3 or worse drug-related adverse events were observed. This study establishes that high-dose plerixafor can be safely administered in healthy donors and mobilizes greater numbers of CD34+ cells than conventional-dose plerixafor, which may improve CD34+ graft yields and reduce the number of apheresis procedures needed to collect sufficient stem cells for allogeneic transplantation. (ClinicalTrials.gov, identifier: NCT00322127)
Introduction
Granulocyte colony-stimulating factor (G-CSF) is the most commonly used cytokine to mobilize and collect peripheral blood progenitor cells from healthy donors for allogeneic transplantation. Although hematopoietic progenitors in G-CSF-mobilized grafts differ from bone-marrow harvested grafts in several respects, the long-term engraftment potential of G-CSF-mobilized peripheral blood progenitor cell grafts is well documented.1 Reductions in bone marrow stromal cell derived factor-1 and up-regulation in CXCR4 constitute one of the multiple mechanisms through which G-CSF mobilizes CD34+ cells into the circulation.2 Although G-CSF successfully mobilizes peripheral blood progenitor cells in most healthy donors,3 this agent often leads to inadequate mobilization of autologous peripheral blood progenitor cells in patients with hematologic malignancies who have received prior chemotherapy.4–7
Plerixafor is a bicyclam compound that inhibits the binding of stromal cell derived factor-1 to its cognate receptor CXCR4. Following administration of plerixafor, CD34+ cells are rapidly released into the circulation and can be collected by apheresis.8–11 The Food and Drug Administration has approved plerixafor at the 240 μg/kg dose for use in conjunction with G-CSF to mobilize autografts for transplantation in patients with non-Hodgkin lymphoma and multiple myeloma.12 Investigators have recently shown that allogeneic grafts can also be mobilized from healthy donors and non-human primates using single-agent plerixafor without G-CSF and, following transplantation, can result in sustained donor-derived hematopoiesis.10,13–15 However, when a single dose of plerixafor is administered at a conventional 240 μg/kg dose, approximately one-third of donors will fail to mobilize the minimally acceptable dose of CD34+ cells needed for allogeneic transplantation.14,16
Healthy volunteers tolerate plerixafor without experiencing many of the adverse events associated with G-CSF administration.10,16,17 Following a 240 μg/kg subcutaneous (sc) plerixafor injection, white blood cell counts peak at 6–10 h and return to baseline levels by approximately 24 h.10,16 Additionally, plerixafor has a short half-life10,18 and following administration for 3 consecutive days, a consistent and reversible increase in peripheral blood CD34+ cells can be achieved,19 with a rapid pharmacodynamic washout effect.
Initial phase I studies suggested optimal CD34+ cell mobilization was achieved following the sc administration of plerixafor 240 μg/kg. We performed a subsequent dose escalation study and observed that a single dose of plerixafor up to a dose of 480 μg/kg sc was safe in humans, and had a higher plerixafor Cmax and CD34+ cell area under the curve (AUC). However, the small number of subjects in that trial precluded determination of whether the efficacy of CD34+ cell mobilization was improved with the higher 480 μg/kg dose of plerixafor.16 To test whether high-dose plerixafor is safe and more efficient at mobilizing stem cells for allogeneic transplantation compared to conventional-dose plerixafor, we conducted a randomized, crossover trial comparing CD34+ mobilization in healthy subjects mobilized with a single dose of sc plerixafor given at either a high dose (480 μg/kg) or a conventional dose (240 μg/kg).
Methods
Study design and participants
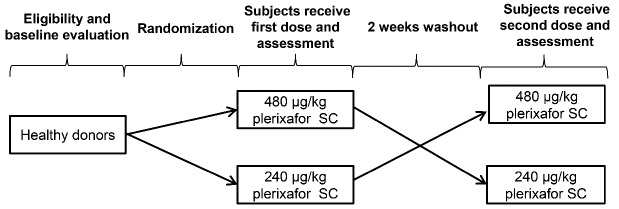
This was a phase II, open-label, single-center, investigator-initiated, randomized, crossover trial, conducted between October 12, 2010, and June 8, 2012, in healthy stem cell donors at the National Institutes of Health Clinical Center in Bethesda (MD, USA). Study subjects were randomly assigned in a 1:1 ratio to receive an initial dose of either 240 μg/kg plerixafor (followed by crossover to a 480 μg/kg dose of plerixafor) or an initial dose of 480 μg/kg plerixafor (followed by crossover to 240 μg/kg plerixafor) as shown in Figure 1. Each treatment period was separated by a minimum washout period of 2 weeks. Eligibility criteria were age between 18 and 50 years and normal renal, hepatic, and hematologic function. Exclusion criteria were cerebrovascular disease, cardiac disease, a Framingham 10-year coronary risk of >10%, positive human immunodeficiency virus status, a history of hepatitis B or C infection, a history of cancer in the preceding 5 years, a history of autoimmune disease, pregnant and lactating women, or a history of stroke or transient ischemic attack.
Figure 1.
Study design.
We selected a crossover trial design to evaluate the difference in activity endpoints between high-dose and conventional-dose plerixafor based on within-subject comparisons because CD34+ cell mobilization varies considerably between stem cell donors.16 The short half-life and rapid washout of plerixafor enable the use of this randomized crossover design with a minimum of a 2-week washout period to avoid a carryover effect and allow for an estimate of the direct treatment effect of high-dose plerixafor on CD34+ cell mobilization.
This trial conformed with the principles of the Declaration of Helsinki and was approved by the National Heart, Lung, and Blood Institute Institutional Review Board. All participants provided written informed consent.
Procedures
Healthy stem cell donors were screened and assessed for eligibility. Eligible study participants were admitted to the National Institutes of Health Clinical Center for dosing and phlebotomy and underwent continuous cardiac telemetry monitoring for 24 h after each injection of plerixafor. Complete blood counts and serum chemistry panels were assessed in all subjects within 24 h prior to each dose of plerixafor and 1 week after each administration. Electrocardiography was done prior to dosing and before discharge. Complete blood counts and CD34+ cell counts were enumerated immediately before (time 0) and 2, 4, 6, 8, 10, 12, 14, 18 and 24 h after each sc injection of plerixafor. Blood samples were evaluated for CD34+ cell content using the International Society of Hematotherapy and Graft Engineering guidelines.20 The absolute number of circulating CD34+ cells/μL was determined by standard flow cytometry techniques. Fluorochromes used for cell surface staining were CD45 APC-Cy7 and CD34 APC (IgG1 class antibody, BD Biosciences, San Jose, CA, USA) and cells were identified using the BD FACSCanto instrument (BD Biosciences). FACSDiva software (BD Biosciences) was used for the analysis. To assess colony-forming units (CFU), peripheral blood samples obtained immediately before and 6 h after dosing with plerixafor were compared, as described previously.16
Outcomes
The primary endpoint was the peak CD34+ cell count in the blood within 24 h following plerixafor injection. Secondary endpoints were CD34+ cell AUC over 24 h, CD34+ count at 24 h, time to peak CD34+ cell count, and CFU quantified from peripheral blood samples. CD34+ cell AUC was calculated using the trapezoidal rule. The safety endpoint was the tolerability and toxicity, including incidence and severity of adverse events, evaluated after each injection of plerixafor up until 1 week following its administration. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3. Treatment-related serious adverse events were reviewed by an independent medical monitor.
Statistical analysis
This randomized crossover study was designed to determine whether the 480 μg/kg dose of plerixafor would mobilize higher peak CD34+ cell numbers in the blood compared to the conventional 240 μg/kg dose. A sample size of 20 subjects was determined to have 96% power to detect a difference of 10 cells/μL (SD of the difference 12) in peak CD34+ counts using a two-sided paired t-test at the 0.05 significance level. Efficacy was analyzed on an intention-to-treat basis and all randomized participants were included. The analysis of activity endpoints based on paired differences was also assessed in all participants who received both doses of study drug. All study subjects were included in the safety analysis.
Repeated measures analysis of variance was used for the primary efficacy analysis to compare circulating CD34+ cell numbers and AUC between the two plerixafor dose levels. The secondary analysis of paired data used paired t-tests or Wilcoxon signed rank tests for non-normally distributed data. The period effect and carryover effect were tested using unpaired t-tests or Mann-Whitney U tests to examine whether the order of administration of the two plerixafor doses affected the overall activities and differences in CD34+ cell mobilization, respectively. The incidences of adverse events between the two dose groups were compared by the Fisher exact test. R statistical software (version 3.2.2) was used for all statistical analyses.
Results
Subjects’ characteristics
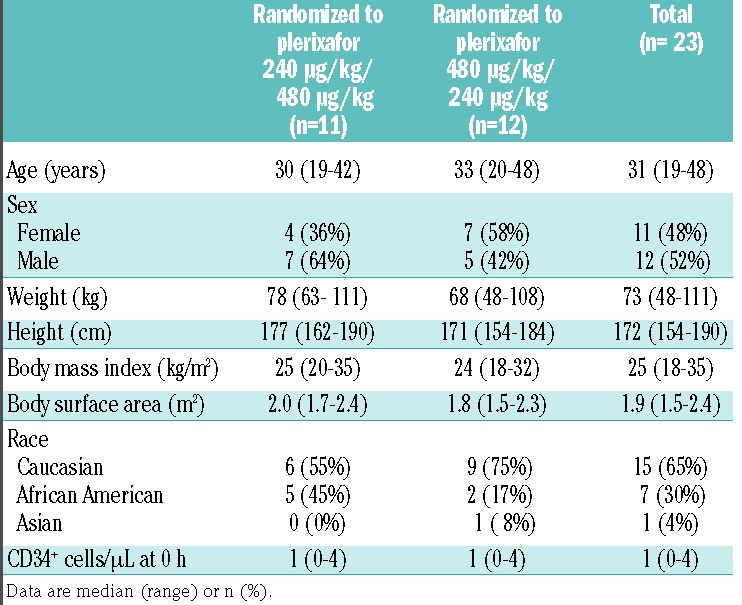
We screened 28 healthy stem cell donors and enrolled 23 into the trial, comprising 11 women and 12 men, with a median age of 31 years (range, 19–48) and a median weight of 73 kg (range, 48–111). Table 1 summarizes the subjects’ baseline characteristics which were comparable between the two randomization groups. Three subjects discontinued the study after receiving the initial dose of plerixafor. One subject failed to return after receiving the 240 μg/kg dose, and two subjects were withdrawn from the study after receiving the 480 μg/kg dose; neither withdrawal was attributed to plerixafor administration. Therefore, 20 subjects received both doses of plerixafor and completed the study.
Table 1.
Baseline characteristics.
CD34+ cell mobilization
Table 2 shows the results of primary and secondary outcomes in all 23 randomized subjects. Compared to the 240 μg/kg dose, the 480 μg/kg dose of plerixafor resulted in: higher peak circulating CD34+ cell numbers [mean 32.2 versus 27.8 cells/μL; mean difference 4.6 cells/μL (95% CI: 2.3−6.9), P=0.0009], higher circulating CD34+ cell numbers at 24 h [mean 17.8 versus 10.7 cells/μL; mean difference 7.3 cells/μL (95% CI: 4.7−9.9), P<0.0001], and a greater circulating CD34+ AUC over 24 h [mean 553 versus 446 h cells/μL; mean difference 113 h cells/μL (95% CI: 79−148), P<0.0001]. In addition, the time to achieve this higher peak in the peripheral blood CD34+ count with the 480 μg/kg dose was longer by an average of 2.1 h; CD34+ cells peaked at a mean of 10.5 h (range 6–18, median 10) after the 480 μg/kg dose compared with a mean of 8.4 h (range 6–12, median 8) after the 240 μg/kg dose (P=0.012). Figure 2 and Online Supplementary Tables S1 and S2 show the analysis of paired data from 20 individual subjects who received both doses of plerixafor, with each line connecting the same subject at the two dose levels. In most subjects, all of these measures were greater following the administration of the 480 μg/kg dose compared to the 240 μg/kg dose. The peak circulating CD34+ counts were higher in 16 (same in one and lower in three) out of 20 subjects following the administration of plerixafor at the 480 μg/kg dose compared to the 240 μg/kg dose. Other exceptions included one subject who had a higher CD34+ AUC, two subjects who had a higher CD34+ cell number at 24 h, and three subjects who had a longer time to peak in circulating CD34+ cell numbers with the 240 μg/kg of plerixafor. Of note, no evidence of a period effect (P=0.76) or a carryover effect (P=0.59) was detected on the primary endpoint of peak CD34+ count. These tests were not statistically significant (all P>0.1) for other secondary endpoints. Thus the observed differences in CD34+ cell mobilization represented a direct treatment effect and were not related to the order of administration of the two plerixafor doses.
Table 2.
Treatment effects on primary and secondary outcomes.
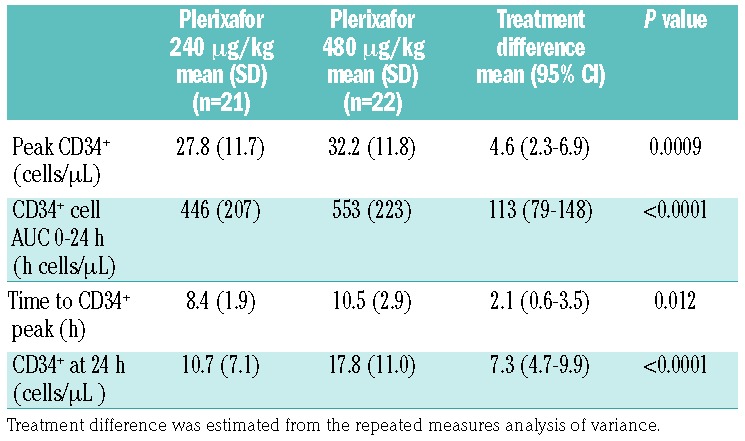
Figure 2.
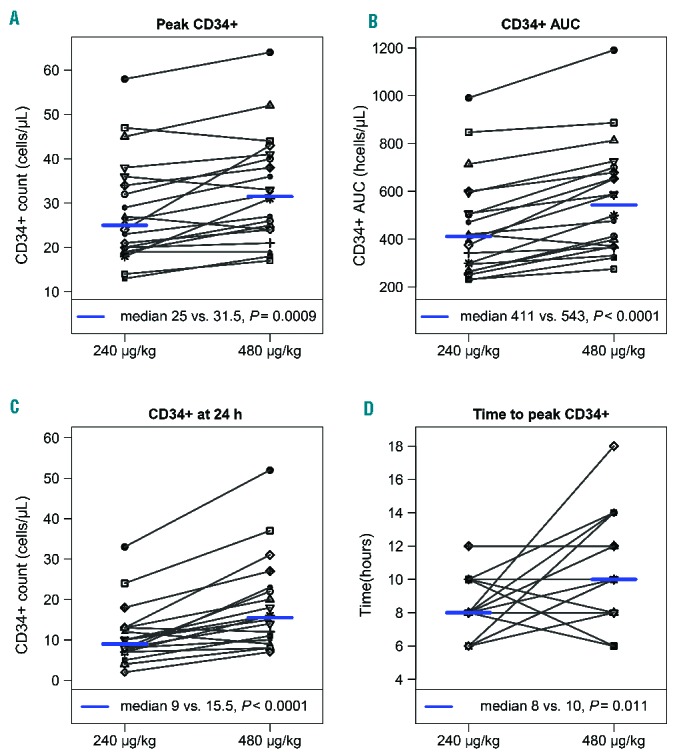
CD34+ cells in the peripheral blood in 20 subjects who received both doses of plerixafor. Individual subject plots with each line connecting the same subject at both dose levels representing the peak CD34+ peripheral blood count, CD34+ AUC over 24 h, CD34+ count 24 h after plerixafor administration, and time to peak CD34+ count.
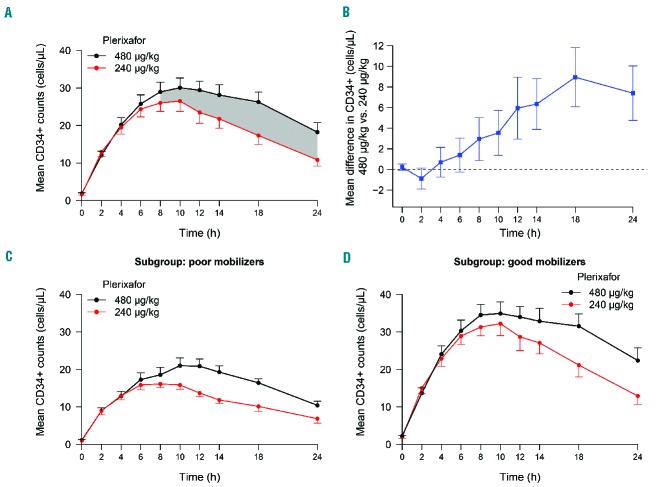
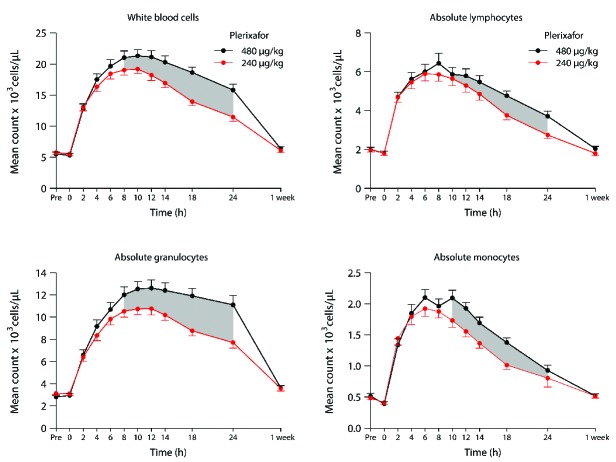
An analysis of the mean number of CD34+ cells mobilized into the circulation over time (24 h) for both dose cohorts demonstrated the CD34+ cell AUC was greater with the 480 μg/kg plerixafor dose (Figure 3). This, combined with the observation that CD34+ cells peaked 2 h later with the higher plerixafor dose, would affect the timing and duration of apheresis procedures to optimize the CD34+ cell yield collected after administering the 480 μg/kg plerixafor dose. As shown in Figure 3A where higher sustained CD34+ circulating numbers were observed following high-dose plerixafor, a large-volume apheresis spanning ≥4 h initiated 8 h after administration of plerixafor 480 μg/kg would be predicted to provide a greater collection yield of CD34+ cells compared to a volume-matched apheresis collected over the same amount of time, starting at any time point, following the 240 μg/kg dose of plerixafor. When we examined paired differences between peripheral blood CD34+ counts following administration of both plerixafor doses over time in individual subjects (Figure 3B), we observed that after 2 h there was a steady increase in the difference between CD34+ counts in subjects who received both plerixafor doses at each sampled time-point, with these differences being statistically significant over the 8 to 24 h time-frame (shaded regions between curves in Figure 3A). Seven (35%) of the 20 subjects had peak CD34+ counts ≤20 cells/μL after the 240 μg/kg dose of plerixafor and, using conventional criteria, were classified as poor mobilizers (Online Supplementary Figure S1).21 High-dose plerixafor resulted in significantly better CD34+ cell mobilization compared to plerixafor 240 μg/kg in both the poor mobilizer and the good mobilizer subgroups (Figure 3C,D and Figure 4). Remarkably, as shown in the subgroup analysis in Figure 4, six of the seven (86%) poor mobilizers achieved higher peak CD34+ cell numbers, corresponding to a relative increase of 28% (95% CI: 11–46%, P=0.014), and all seven poor mobilizers achieved higher CD34+ AUC over 24 h, corresponding to a relative increase of 36% (95% CI: 18–53%, P=0.005) after the 480 μg/kg dose compared with the 240 μg/kg dose. This increase also represented a relative improvement of 27% (95% CI: 9–44) and 30% (95% CI: 13–46) in the CD34+ cell AUC over an optimal 4-h and 6-h collection time, respectively, after the 480 μg/kg dose. Furthermore, there was a trend towards peak CD34+ cell counts and CD34+ AUC increasing more in poor mobilizers than in good mobilizers after high-dose versus conventional-dose plerixafor. In addition to the greater increase in CD34+ counts, there was a significant increase in circulating total white blood cells, lymphocytes, monocytes, and granulocytes, over time following administration of the 480 μg/kg dose of plerixafor compared with the 240 μg/kg dose (Figure 5).
Figure 3.
CD34+ cell counts. (A) Mean CD34+ cell counts in the blood over time with one standard error of the mean (SEM) in all subjects who received both doses of plerixafor. The shaded regions indicate when the mean CD34+ counts were significantly different between the two dose cohorts. (B) Mean paired difference in the CD34+ count following administration of the 480 μg/kg and 240 μg/kg dose with 95% CI. Mean CD34+ cell counts with 1 SEM for (C) poor mobilizers and (D) good mobilizers, defined as those with peak CD34+ counts ≤ 20 cells/μL and > 20 cells/μL after the 240 μg/kg dose of plerixafor, respectively.
Figure 4.
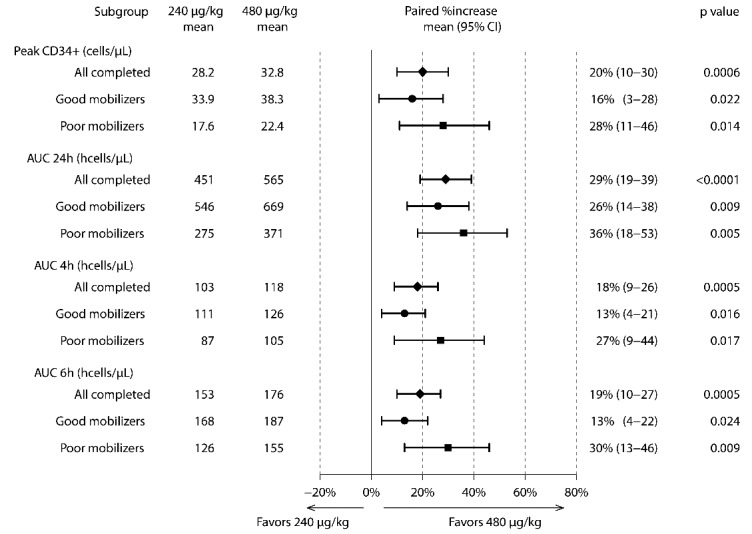
Subgroup analyses of relative differences in CD34+ cell mobilization. “All completed” included all subjects who received both doses of plerixafor. AUC 4h (or AUC 6h) was calculated from 6–10 h (or 6–12 h) for the 240 μg/kg dose of plerixafor, and from 8–12 h (or 8–14 h) for the 480 μg/kg dose of plerixafor, respectively, as the optimal collection time would be expected to start 2 h prior to CD34+ cells peaking in the circulation to achieve the maximum CD34+ AUC time window.
Figure 5.
Mean circulating white blood cells, absolute lymphocytes, absolute granulocytes, and absolute monocytes over time with one standard error of mean for both dose cohorts. The shaded regions indicate when the mean circulating cell counts were significantly different between the two dose cohorts.
Colony-forming units
The comparison of blood erythroid (E) or granulocyte-macrophage (GM) CFU colonies is shown in Online Supplementary Figure S2. Compared to baseline CFU counts immediately before dosing, the mean number of CFU-E colonies was slightly increased following each dose of plerixafor. In contrast, there was a significant increase in CFU-GM colonies following each dose of plerixafor. The increases in either CFU-E or CFU-GM colonies were comparable based on samples collected 6 h following the high dose and conventional dose of plerixafor.
Safety
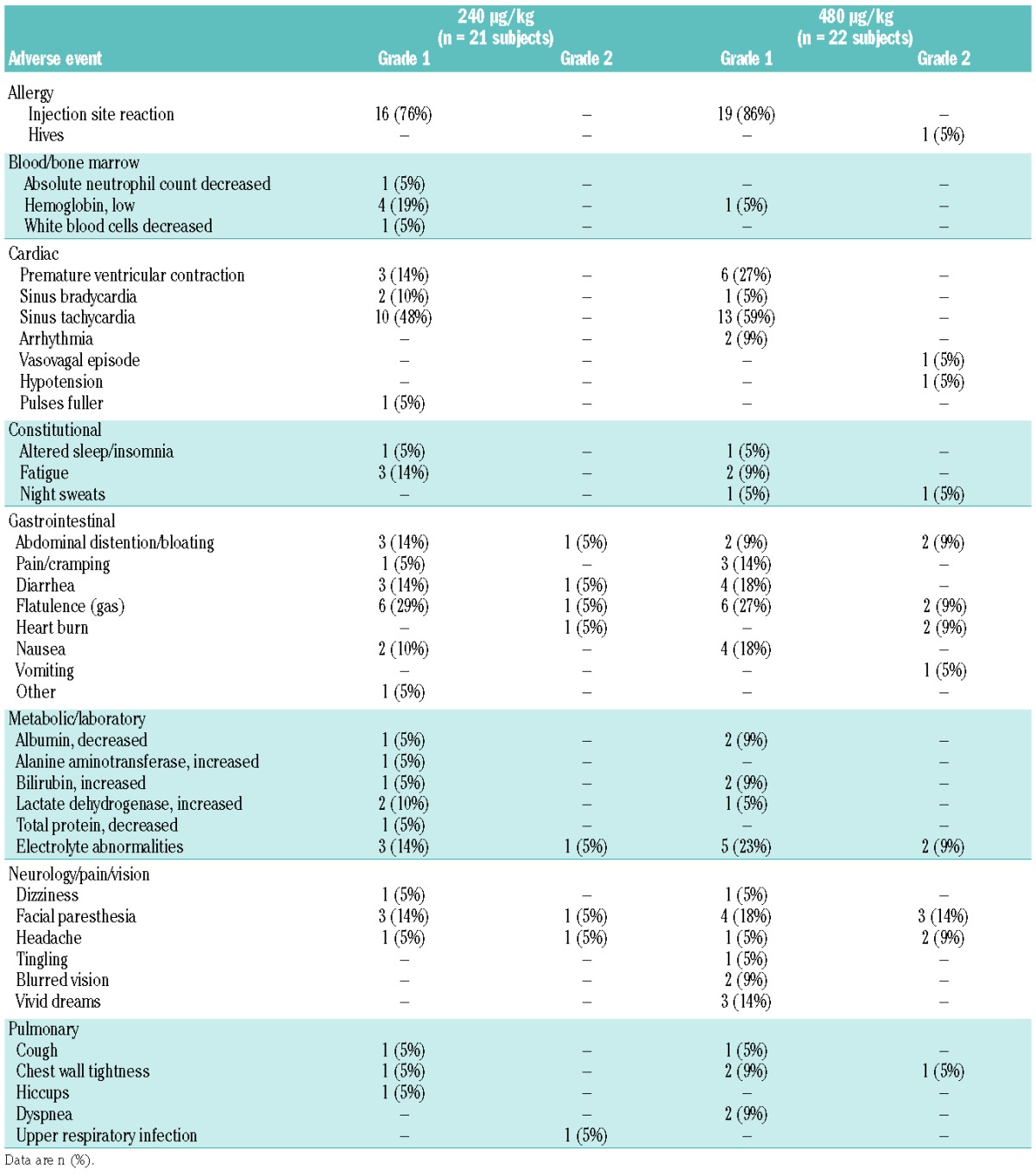
The high dose of plerixafor (480 μg/kg) was well-tolerated. Among all 23 study participants, only one grade 3 serious adverse event occurred in one subject (night terrors and palpitations), which led to overnight hospitalization for observation 3 days after the plerixafor had been administered; this event was attributed to the subject working in a stressful work environment. No treatment-related serious adverse event or adverse events of CTCAE ≥grade 3 occurred. There were eight grade 2 adverse events in six subjects who received the 240 μg/kg dose, and 19 grade 2 adverse events in ten subjects who received the 480 μg/kg dose. The percentages of subjects who had grade 2 adverse events were not statistically significantly different between the two doses (P= 0.29). Table 3 shows all adverse events that were considered at least possibly related to plerixafor. Commonly reported adverse events consisted of local self-limiting erythema or induration at the site of injection, tachycardia, and the sensation of gastrointestinal bloating, flatulence, nausea, and diarrhea. Most adverse events were mild (grade 1), occurred within 30 min to 4 h after plerixafor administration, and resolved without intervention. The overall incidences of all adverse events and of adverse events in each grade or category were not significantly different between subjects receiving the high dose or conventional dose of plerixafor.
Table 3.
Treatment-related adverse events in all 23 subjects.
Discussion
This study establishes that high-dose plerixafor can be safely administered to healthy donors and mobilizes greater numbers of CD34+ cells than conventional-dose plerixafor.
The ability to collect a large quantity of peripheral blood progenitor cells following a single injection of plerixafor without the need for G-CSF makes it an attractive agent for mobilizing donors for allogeneic hematopoietic stem cell transplantation.14,16 Despite its ease of use, we and others have observed that about one-third of healthy donors will fail to mobilize ≥20 CD34+ cells/μL after a single 240 μg/kg dose of plerixafor. These donors, conventionally classified as poor mobilizers, are less likely to have a single apheresis that yields at least 2 × 106 CD34+ cells/kg14,22 and would require one or more additional injections of plerixafor or additional apheresis procedures to collect an adequate number of CD34+ cells. Determining the most efficacious dose of plerixafor in healthy donors would improve allograft CD34+ cell yields and reduce the number of apheresis procedures. Initial phase I studies suggested that peak CD34+ cell mobilization occurred at the highest administered dose of 240 μg/kg plerixafor.10 In a recent dose escalation study, we reported that a single 480 μg/kg dose of plerixafor is safe and effective in mobilizing CD34+ cells.16 However, that study enrolled only a limited number of subjects and did not randomize the order of dosing, thus its design was inadequate to determine whether the efficacy of CD34+ cell mobilization was improved with high-dose plerixafor.
Therefore, to evaluate whether high-dose plerixafor is more efficient in mobilizing CD34+ stem cells compared to conventional-dose plerixafor, we conducted the current study that utilized a randomized crossover trial design with over 95% power to compare CD34+ mobilization in subjects mobilized with a single dose of plerixafor of either 480 μg/kg or 240 μg/kg. We observed that peak circulating CD34+ cell numbers, circulating CD34+ cell numbers at 24 h, and the CD34+ cell AUC over 24 h were, on average, all significantly higher following the administration of 480 μg/kg compared to 240 μg/kg of plerixafor. Remarkably, the peak circulating CD34+ counts were higher in 16 of 20 of subjects following the administration of plerixafor 480 μg/kg compared to plerixafor 240 μg/kg. Perhaps most importantly, improvements in CD34+ cell mobilization with high-dose plerixafor were most pronounced in those who were poor mobilizers with conventional-dose plerixafor. In this context, all seven subjects who mobilized poorly with plerixafor 240 μg/kg achieved a significantly higher CD34+ AUC after the 480 μg/kg dose (relative increase 36%) and six of seven (86%) had higher peak CD34+ cell numbers (relative increase 28%) following high-dose plerixafor (Figure 4 and Online Supplementary Figure S1). These increases represented relative improvements of 27% and 30% in AUC over an optimal 4-h and 6-h collection time, respectively, after the 480 μg/kg dose. This difference would be expected to make a significant clinical impact as those who would mobilize poorly and subsequently fail to yield enough CD34+ cells at the 240 μg/kg dose would be more likely to mobilize an adequate number of CD34+ cells at the higher 480 μg/kg dose of plerixafor. We noted high-dose plerixafor was well-tolerated in the 22 subjects who received this agent, and was not associated with treatment-related serious adverse events or adverse events of grade 3 or higher. Although the absolute number of grade 2 adverse events was slightly higher in subjects who received the 480 μg/kg dose than in those who received the 240 μg/kg dose, there was no significant difference in the severity and overall incidence of adverse events between subjects receiving high-dose and conventional-dose plerixafor. Most adverse events were mild, transient, and resolved without treatment.
A limitation of our study is that we compared CD34+ mobilization in the peripheral blood without performing an apheresis procedure. However, numerous reports have shown that the number of circulating CD34+ cells in peripheral blood correlates strongly with CD34+ graft yield and predicts the number of CD34+ cells that will be collected during apheresis.21,23,24 Achieving higher peak circulating CD34+ cell numbers and a higher circulating CD34+ AUC are clinically important outcomes that would be expected to improve the yield of hematopoietic progenitor cells collected during apheresis procedures. The target for collection for unmanipulated allogeneic peripheral blood progenitor cells is frequently 5×106 CD34+ cells/kg recipient body weight with a minimally acceptable number of 2×106 CD34+ cells/kg.23,25 Most collection centers do not have real-time information on the number of CD34+ cells mobilized in individual subjects at the time the apheresis collection is initiated. In this context, apheresis volumes are fixed, and in subjects who mobilize poorly, often fail to collect an adequate number of CD34+ cells needed for transplantation. Utilization of higher-dose plerixafor would likely increase the probability that the target dose of CD34+ progenitor cells can be successfully collected following a single apheresis procedure. It is important to consider that the efficiency with which CD34+ cells are collected directly affects the yield of CD34+ cells in apheresis collections. At our center, CD34+ cell efficiencies typically average between 30–40%, which is consistent with reports from other apheresis centers.24 Based on a validated formula24 and the within-subject comparison data in this study (Online Supplementary Figure S3A), for a 24 liter apheresis processing volume, a recipient with a 70 kg body weight, and a 30% collection efficiency, 7/20 (35%) poor mobilizers would be predicted to fail to yield the minimally acceptable 2×106 CD34+ cells/kg after a 240 μg/kg dose of plerixafor while only 3/20 (15%) of those donors would fail to mobilize adequately after the 480 μg/kg of plerixafor. With a higher collection efficiency of 35–40%, 2/20 (10%) donors would still fail to yield a minimally acceptable CD34+ number following the 240 μg/kg dose, while all of the 20 donors would be expected to have an adequate CD34+ cell collection after the 480 μg/kg dose. Importantly, regardless of the CD34+ cell collection efficiency, the proportion of donors achieving a minimally acceptable apheresis collection would predictably be higher across the spectrum of apheresis volumes typically processed following stem cell mobilization (Online Supplementary Figure S3B). Furthermore, the total number of CD34+ cells collected following apheresis would be expected to be 20% greater after the 480 μg/kg dose than after the 240 μg/kg dose, with higher percentages of donors yielding grafts containing both >2×106 and >5×106 CD34+ cells/kg across typical collection efficiencies and volumes. These differences are clinically meaningful and could be the determining factor obviating the need to proceed with a second apheresis collection. For cost, donor safety and improved collection efficiency, a single collection is preferable to multiple collections, even if the duration of the procedure needs to be extended.26,27
Interestingly, in our study we observed that the time to achieve a higher peak peripheral CD34+ count was, on average, 2 h longer after high-dose plerixafor. Compared to the 240 μg/kg dose, the mean CD34+ counts in the circulation remained significantly higher between 8 and 24 h after the 480 μg/kg dose of plerixafor (Figure 3). These differences may be the consequence of altered pharmacokinetics that are observed with high-dose plerixafor, as we previously showed the plerixafor Cmax and AUC were higher after the 480 μg/kg dose than after the 240 μg/kg dose.16 Therefore, should a decision be made to utilize a higher dose of plerixafor to mobilize an allograft, this delay in peak circulating CD34+ cells would need to be factored into decisions related to the timing of apheresis procedures to optimize progenitor cell yield. Assuming cell harvesting is scheduled for 08:00, a 480 μg/kg dose of plerixafor would optimally be administered 8 h (24:00) the night before the apheresis procedure to allow for a collection that spans the time when CD34+ cells are peaking in the circulation. As shown in Figure 3B, the sustained increase in circulating CD34+ cells during the 8 to 24 h after high-dose plerixafor is administered would also provide advantages should circumstances lead to an unforeseen delay in initiating the apheresis collection. In this context, given these altered mobilization kinetics, an adequate CD34+ cell yield could still be collected even if the procedure was delayed 10 to 14 h after the 480 μg/kg dose of plerixafor had been administered.
Despite a significant increase in all measures of peripherally circulating CD34+ cell counts following the administration of the 480 μg/kg dose of plerixafor, we found no difference between conventional-dose or high-dose plerixafor with respect to mobilized CFU-E or CFU-GM measured from peripheral blood samples. This lack of observed difference was likely due to the fact that blood samples for this analysis were collected 6 h after plerixafor administration, which was found, retrospectively from this study, to be a time-point when circulating CD34+ cell numbers were similar between both dose cohorts, as opposed to later time-points when the number of circulating CD34+ progenitor cells was significantly higher in the blood following high-dose plerixafor administration (Figure 3B). In our study, the enhanced efficacy of high-dose plerixafor on CD34+ cell mobilization was not affected by the donor’s age. The median age of the study subjects was 31 years (range, 19–48) and age, when analyzed in a multivariate analysis, did not correlate significantly with CD34+ cell mobilization. Besides CD34+ cells, we and others have reported that neutrophils, monocytes, B cells, and T cells are also mobilized with plerixafor.22 In this study, we also noted that total white blood cells, lymphocytes, monocytes, and granulocytes were significantly higher in the circulation over time following administration of the 480 μg/kg dose of plerixafor compared with the 240 μg/kg dose.
Recent data from our group have shown that alterations in T-cell phenotype and cytokine gene expression profiles characteristic of G-CSF mobilization do not occur after mobilization with plerixafor.22 G-CSF mobilization has been shown to skew T cells toward a Th-2 type phenotype, which may increase the incidence of chronic graft-versus-host disease when G-CSF-mobilized allografts are used compared with bone marrow transplants.28–30 In contrast, T cells mobilized with plerixafor do not display any changes in Th-1, Th-2, or Th-3 type cytokines compared to non-mobilized T cells. Recent studies of murine allogeneic transplantation models have also shown that the incidence of graft-versus-host disease may differ in recipients of allo-grafts mobilized with plerixafor alone or plerixafor plus G-CSF compared to G-CSF alone.22,31 These data suggest that T-cell-mediated events which occur after allogeneic transplantation may differ depending on whether donor allografts are mobilized with plerixafor or G-CSF. Given that high-dose plerixafor mobilized even greater numbers of lymphocytes than did conventional-dose plerixafor, it is possible that graft-versus-tumor effects, immune reconstitution, graft-versus-host disease, and other variables affected by engrafting donor lymphoid cells could also differ in recipients of allogeneic transplants mobilized with high-dose versus conventional-dose plerixafor.
In conclusion, this study demonstrates that high-dose plerixafor can be administered safely and is superior to conventional-dose plerixafor in mobilizing CD34+ cells in healthy donors. The enhanced mobilizing effect of high-dose plerixafor was most evident in subjects who had the greatest need for this effect, namely those who mobilized poorly with conventional-dose plerixafor. Our data suggest that mobilization of allogeneic stem cell donors with high-dose plerixafor would improve the chances of using a single apheresis procedure to collect a sufficient number of CD34+ cells for allo-grafting and would likely result in graft collections containing higher CD34+ cell numbers compared to those of donors mobilized with conventional-dose plerixafor. Our findings warrant further studies to explore the clinical impact of high-dose plerixafor use for allogeneic stem cell transplantation.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the National Heart, Lung and Blood Institute and the Clinical Center, National Institutes of Health. Sanofi US supported this trial by supplying the study drug.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/3/600
References
- 1.Gyger M, Stuart RK, Perreault C. Immunobiology of allogeneic peripheral blood mononuclear cells mobilized with granulocyte-colony stimulating factor. Bone Marrow Transplant. 2000;26(1):1–16. [DOI] [PubMed] [Google Scholar]
- 2.Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3(7): 687–694. [DOI] [PubMed] [Google Scholar]
- 3.Brissot E, Chevallier P, Guillaume T, et al. Factors predicting allogeneic PBSCs yield after G-CSF treatment in healthy donors. Bone Marrow Transplant. 2009;44(9):613–615. [DOI] [PubMed] [Google Scholar]
- 4.Visani G, Lemoli RM, Tosi P, et al. Fludarabine-containing regimens severely impair peripheral blood stem cells mobilization and collection in acute myeloid leukaemia patients. Br J Haematol. 1999;105(3):775–779. [DOI] [PubMed] [Google Scholar]
- 5.Pastore D, Specchia G, Mestice A, et al. Good and poor CD34+ cells mobilization in acute leukemia: analysis of factors affecting the yield of progenitor cells. Bone Marrow Transplant. 2004;33(11):1083–1087. [DOI] [PubMed] [Google Scholar]
- 6.Goldschmidt H, Hegenbart U, Wallmeier M, Hohaus S, Haas R. Factors influencing collection of peripheral blood progenitor cells following high-dose cyclophosphamide and granulocyte colony-stimulating factor in patients with multiple myeloma. Br J Haematol. 1997;98(3):736–744. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Dispenzieri A, Lacy MQ, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21(9):2035–2042. [DOI] [PubMed] [Google Scholar]
- 8.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185(1): 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devine SM, Flomenberg N, Vesole DH, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22(6):1095–1102. [DOI] [PubMed] [Google Scholar]
- 10.Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102(8): 2728–2730. [DOI] [PubMed] [Google Scholar]
- 11.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brave M, Farrell A, Ching Lin S, et al. FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology. 2010;78(3–4):282–288. [DOI] [PubMed] [Google Scholar]
- 13.Donahue RE, Jin P, Bonifacino AC, et al. Plerixafor (AMD3100) and granulocyte colony-stimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and microRNA expression signatures. Blood. 2009;114(12):2530–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devine SM, Vij R, Rettig M, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112(4):990–998. [DOI] [PubMed] [Google Scholar]
- 15.Larochelle A, Krouse A, Metzger M, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107(9): 3772–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemery SJ, Hsieh MM, Smith A, et al. A pilot study evaluating the safety and CD34+ cell mobilizing activity of escalating doses of plerixafor in healthy volunteers. Br J Haematol. 2011;153(1):66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lack NA, Green B, Dale DC, et al. A pharmacokinetic-pharmacodynamic model for the mobilization of CD34+ hematopoietic progenitor cells by AMD3100. Clin Pharmacol Ther. 2005;77(5):427–436. [DOI] [PubMed] [Google Scholar]
- 18.Stewart DA, Smith C, MacFarland R, Calandra G. Pharmacokinetics and pharmacodynamics of plerixafor in patients with non-Hodgkin lymphoma and multiple myeloma. Biol Blood Marrow Transplant. 2009;15(1):39–46. [DOI] [PubMed] [Google Scholar]
- 19.Hubel K, Liles WC, Broxmeyer HE, et al. Leukocytosis and mobilization of CD34+ hematopoietic progenitor cells by AMD3100, a CXCR4 antagonist. Support Cancer Ther. 2004;1(3):165–172. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5(3):213–226. [DOI] [PubMed] [Google Scholar]
- 21.Armitage S, Hargreaves R, Samson D, Brennan M, Kanfer E, Navarrete C. CD34 counts to predict the adequate collection of peripheral blood progenitor cells. Bone Marrow Transplant. 1997;20(7):587–591. [DOI] [PubMed] [Google Scholar]
- 22.Lundqvist A, Smith AL, Takahashi Y, et al. Differences in the phenotype, cytokine gene expression profiles, and in vivo alloreactivity of T cells mobilized with plerixafor compared with G-CSF. J Immunol. 2013;191(12): 6241–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.To LB, Levesque JP, Herbert KE. How I treat patients who mobilize hematopoietic stem cells poorly. Blood. 2011;118(17):4530–4540. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum ER, O’Connell B, Cottler-Fox M. Validation of a formula for predicting daily CD34(+) cell collection by leukapheresis. Cytotherapy. 2012;14(4):461–466. [DOI] [PubMed] [Google Scholar]
- 25.Singhal S, Powles R, Treleaven J, et al. A low CD34+ cell dose results in higher mortality and poorer survival after blood or marrow stem cell transplantation from HLA-identical siblings: should 2 × 10(6) CD34+ cells/kg be considered the minimum threshold? Bone Marrow Transplant. 2000;26(5):489–496. [DOI] [PubMed] [Google Scholar]
- 26.Winters JL. Complications of donor apheresis. J Clin Apher. 2006;21(2):132–141. [DOI] [PubMed] [Google Scholar]
- 27.Bolan CD, Carter CS, Wesley RA, et al. Prospective evaluation of cell kinetics, yields and donor experiences during a single large-volume apheresis versus two smaller volume consecutive day collections of allogeneic peripheral blood stem cells. Br J Haematol. 2003;120(5):801–807. [DOI] [PubMed] [Google Scholar]
- 28.Sloand EM, Kim S, Maciejewski JP, et al. Pharmacologic doses of granulocyte colony-stimulating factor affect cytokine production by lymphocytes in vitro and in vivo. Blood. 2000;95(7):2269–2274. [PubMed] [Google Scholar]
- 29.Franzke A, Piao W, Lauber J, et al. G-CSF as immune regulator in T cells expressing the G-CSF receptor: implications for transplantation and autoimmune diseases. Blood. 2003;102(2):734–739. [DOI] [PubMed] [Google Scholar]
- 30.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arbez J, Saas P, Lamarthee B, et al. Impact of donor hematopoietic cells mobilized with G-CSF and plerixafor on murine acute graft-versus-host-disease. Cytotherapy. 2015;17(7):948–955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.